Abstract
The risk for neuropsychiatric illnesses has a strong sex bias, and for major depressive disorder (MDD), females show a more than 2-fold greater risk compared to males. Such mood disorders are commonly associated with a dysregulation of the hypothalamo-pituitary-adrenal (HPA) axis. Thus, sex differences in the incidence of MDD may be related with the levels of gonadal steroid hormone in adulthood or during early development as well as with the sex differences in HPA axis function. In rodents, organizational and activational effects of gonadal steroid hormones have been described for the regulation of HPA axis function and, if consistent with humans, this may underlie the increased risk of mood disorders in women. Other developmental factors, such as prenatal stress and prenatal overexposure to glucocorticoids can also impact behaviors and neuroendocrine responses to stress in adulthood and these effects are also reported to occur with sex differences. Similarly, in humans, the clinical benefits of antidepressants are associated with the normalization of the dysregulated HPA axis, and genetic polymorphisms have been found in some genes involved in controlling the stress response. This review examines some potential factors contributing to the sex difference in the risk of affective disorders with a focus on adrenal and gonadal hormones as potential modulators. Genetic and environmental factors that contribute to individual risk for affective disorders are also described. Ultimately, future treatment strategies for depression should consider all of these biological elements in their design.
Keywords: stress, depression, antidepressants, estrogens, androgens, glucocorticoids
Introduction
Mood disorders primarily include 3 common types of depression that diverge in their severity of symptoms and persistence: 1) major depression in which symptoms interfere with daily life; 2) dysthymia, which is chronic but nondisabling; and 3) bipolar disorder, characterized by wide mood swings varying from deep troughs to manic peaks [1]. The lifetime risk of experiencing a major depressive episode is estimated to be over 16 % for the population in the United States and well over 10 % of the population in many Latin American countries [2] making it one of the most prevalent neuropsychiatric disorders. In all populations, the incidence in young adult women is consistently greater than in men and such a sex difference is even larger in middle-aged groups. The most common antidepressants currently used for the treatment of major depression are the selective serotonin or serotonin/noradrenaline reuptake inhibitors (SSRIs or SNRIs), tricyclics, and monoamine oxidase inhibitors (MAOIs). SSRIs or SNRI were developed to specifically target the serotonin or serotonin/norepinephrine transporters whereas tricyclics work by inhibiting norepinephrine and serotonin reuptake and by antagonizing many neurotransmitter receptors, which is the cause of their multiple side effects. MAOIs prevent the degradation of monoamines.
In order to achieve a better understanding of the neuroendocrine substrates underlying depressive behavior and with the aim of generating novel therapies, basic science investigators have developed a number of novel animal models [3]. However, animals lack most of the hallmarks of the disorder such as depressed mood, low self-esteem or suicidality, making it impossible to find an animal model that perfectly resembles all the clinical depressive symptoms. Yet, depression, as other mental disorders, presents endophenotypes that can be reproduced and evaluated in animals such as physiologic, neuroendocrine and neuroanatomic alterations as well as behavioral traits that include anhedonia, anxiety-related behavior, and despair [4]. Exposure to stress or to traumatic life events has a strong impact on the manifestation of depressive disorders suggesting an impairment of proper stress coping strategies in depressed patients [5]. Taking this into account, most of the animal models of depression are based on the exposure to various types of acute or chronic stressors. These paradigms generate changes evocative of the symptoms of depression, which can be reversed by antidepressant treatment [3, 4]. The most commonly used animal models of depression are chronic restraint and chronic unpredictable stress paradigms or the forced swimming test. Others include prolonged periods of maternal separation during the first weeks of life and are proposed to have high face validity with certain forms of depression related with child inattention or abuse.
In this review, we examine some potential factors contributing to the sex difference in the risk of mood disorders with a focus on adrenal and gonadal hormones as potential modulators of behaviors, in both clinical populations and animal models. In addition, we also describe genetic and environmental factors that can contribute to individual risk for mood disorders. Ultimately, effective treatment strategies should consider all of these biological elements to support future therapeutic designs.
The Hypothalamo-Pituitary-Adrenal Axis
A dysregulation of the hypothalamo-pituitary-adrenal (HPA) axis is one of the most commonly described alterations that correlate with symptoms of mood disorders and other neurospsychiatric diseases. Therefore, an understanding of the neurobiological mechanisms controlling the HPA axis is important for deciphering potential changes that can impact the risk for such disorders. Under basal conditions, glucocorticoids are secreted from the adrenal cortex under control of a circadian regulatory system [6]. As a result, glucocorticoid levels are lowest during the non-active period and rise prior to the onset of activity or awakening to ready numerous physiological systems for activity and metabolic challenge [7]. The diurnal rise in glucocorticoids is allowed through a reduction in inhibitory tone from the suprachiasmatic nuclei of the hypothalamus (SCN), projecting to the paraventricular hypothalamic nucleus (PVN) [8, 9], coupled with an increased adrenal sensitivity to adrenocorticotropic hormone (ACTH) through autonomic inputs, thereby enhancing corticosterone secretion [10].
In contrast, following acute stress exposure the HPA axis is activated and the secretion of corticotropin releasing hormone (CRH) from neuroendocrine neurons of the PVN is increased [11]. CRH causes the release of pituitary adrenocorticotrophic hormone (ACTH) by binding to CRH-R1 receptors on corticotrophs [12]. In turn, ACTH stimulates the biosynthesis and release of glucocorticoids (cortisol in human, corticosterone in mouse and rat) by the adrenal cortex [5].
Negative feedback by circulating glucocorticoid levels keep basal and stress reactive secretion of the HPA axis under tight control. Basal levels of CRH and ACTH secretion are controlled by the actions of a high affinity type I corticosteroid receptor, or mineralocorticoid receptor (MR). In contrast, elevations that occur following stress are controlled predominantly by activation of the type II corticosteroid receptor, or glucocorticoid receptor (GR). Since the GR has a lesser affinity for corticosteroids than does the MR, this allows it to respond to elevated corticosteroid levels to reduce the stress-induced secretions of CRH and ACTH. Given that glucocorticoids can affect many behaviors, a negative feedback mechanism involving the MR and GR is essential for keeping the balance of the HPA axis activity during both basal conditions and in response to stress [13].
CRH is the main regulator of anterior pituitary ACTH secretion; however, the role of vasopressin (AVP) as a co-secretagogue has been also recognized [14]. Although originally described as regulators of osmotic balance and parturition, respectively, AVP and its related peptide, oxytocin (OT), co-localize with CRH in discrete PVN neuronal populations and are thought to be coreleased with CRH [15, 16] to potentiate CRH’s secretogogue activity at the level of the corticotroph [17, 18]. Nonetheless, both AVP and OT can stimulate ACTH secretion even in the absence of CRH [18, 19] through actions on the anterior pituitary. In contrast, when applied to the PVN, or injected into the 3rd ventricle, OT and AVP inhibit HPA responses [20, 21] thereby supporting the possibility that these neuropeptides can be released locally from PVN neurons, to modify function in a paracrine action. This may occur through dendritic release of the peptide [22] resulting in responses in PVN that differ from their actions at the pituitary as ACTH secretogogues.
HPA Axis Dysregulation in Depressive Disorders
Studies of patients with major depressive disorder (MDD) provide strong evidence linking the dysregulation of the HPA axis with depressive neuropathology. In depressed patients, this dysregulation is characterized by increased cortisol secretory responses, an altered diurnal rhythm of cortisol secretion, especially at the afternoon nadir [23], enlarged adrenal gland volume [24] and elevated CRH in cerebrospinal fluid [25]. Interestingly, norepinephrine and serotonin, both neurotransmitters implicated in the pathogenesis of depression, are regulators of HPA axis activity [26]. Furthermore, 20–40 % of depressed patients are dexamethasone (DEX) nonsuppressors in the dexamethasone suppression test (DST) and the inability of DEX to suppress cortisol release following CRH stimulation can distinguish greater than 90 % of depressed patients from nondepressed controls [27]. Neurobiological evidence for HPA involvement in MDD is demonstrated by results of studies examining the PVN of postmortem depressed patients, which contain 4 times the number of CRH expressing cells [28] and a 3-fold increase in CRH neurons showing co-localization with AVP [28]. CRH mRNA levels are correspondingly increased in depressed patients [29] suggesting a hyperactivation of PVN CRH neurons as an underlying cause. Increases in the number of OT and AVP-immunoreactive neurons in the PVN have been reported as well [28, 30]. Further, the elevated levels of CRH in the PVN of depressed patients [28] are accompanied by a downregulation of CRH receptor 1 (CRHR1) in the pituitary, indicating the activation of compensatory mechanism to drive the ACTH and cortisol secretion [31]. Results from animal studies support similar correlations where CRHR1-deficient mice exhibit a compensatory activation of the AVP system, enabling the animals to maintain normal levels of basal ACTH [32]. Thus, the higher levels of cortisol in depressed patients may be induced by the activation of the AVP system, correlating with the increased AVP gene expression that has been observed in human hypothalamus [33]. In agreement, plasma AVP levels are elevated in MDD patients [34]. Further, ACTH and cortisol secretion can be induced by the administration of the AVP analogue, desmopressin (1-deamino-8-d-arginine vasopressin) with higher levels achieved in depressed patients compared with controls [31]. In the search for mechanisms underlying HPA dysfunction in depression, a number of laboratories have focused on identifying genes involved in HPA axis regulation, mainly the GR [35]. Glucocorticoid receptor function is reportedly reduced in the lymphocytes of depressed patients and has been shown to recover after tricyclic antidepressant treatment [35]. Similar to depressed patients, mice with an acquired deficit of forebrain GR [36] show hyperactivity of the HPA axis. These animals also display despair- and anxiety-related behaviors [36], suggesting that depressive symptoms are in part related to the rise in circulating levels of glucocorticoids. Changes in the OT system have also been implicated in the etiology of depression. Nocturnal plasma OT levels have been reported to be elevated in individuals with major depression [37]. Pulsatile patterns of OT release have also been reported to be more variable in depressed women [38]. However, the involvement of OT remains unclear since earlier studies show a negative correlation between circulating OT levels and depressive symptoms [39].
Glucocorticoids, Behavior, and Antidepressants
Corticosteroids affect brain functioning through both genomic and nongenomic mechanisms [40]. Corticosteroid receptors are potent modulators of cognitive processes, such as learning, memory, and retrieval. Thus, glucocorticoid secretion induced by acute stress may positively or negatively affect the memory processes, depending on the time of its occurrence in relation to the learning situation [41]. In particular, the glucocorticoid increases that are part of the stress response induced by learning a new task have been implicated in memory consolidation processes [5]. This phenomenon is rooted in brain regions targeted by glucocorticoids that include the hippocampus, amygdala and prefrontal cortex. These structures play a key role in integrating physiological and behavioral responses during stress and adaptation to subsequent stressful events [42]. In contrast to acute stress, the repeated exposure to unpredictable and uncontrollable stressors may result in abnormal changes in brain plasticity that impairs the ability to respond properly to subsequent stressors [43].
Cognitive impairments, especially those related to hippocampal and prefrontal function, are associated with altered cortisol levels in depressed patients [44]. Correspondingly, normalization of HPA activity has been observed following antidepressant treatment [35]. The mechanisms underlying the effects of antidepressants on the hyperactive HPA axis are still unclear. Nonetheless, a proposed mechanism is that antidepressants, mainly tricyclics, increase GR levels rendering the HPA axis more susceptible to feedback inhibition by cortisol [45]. Thus, it is important to determine how high levels of cortisol are related to depressive symptoms. In support of previous observations [46, 47], studies from the Fiedler laboratory have found that chronic restraint stress [48] or chronic corticosterone administration [49] promotes impairment in associative learning; an effect that is fully sensitive to antidepressant treatments [48, 49]. In contrast to corticosterone treated animals, stressed animals display anxiety-like behaviors that are not prevented by administration of sertraline, an SSRI antidepressant [49]. Similarly, it has been reported that chronic unpredictable stress elicits anxiety-like behaviors that are prevented by desipramine, a tricyclic norepinephrine reuptake inhibitor, but not by an SSRI antidepressant [46]. These results suggest that the anxiety component observed in depressed patients [50] is probably not mediated by a direct action of glucocorticoids.
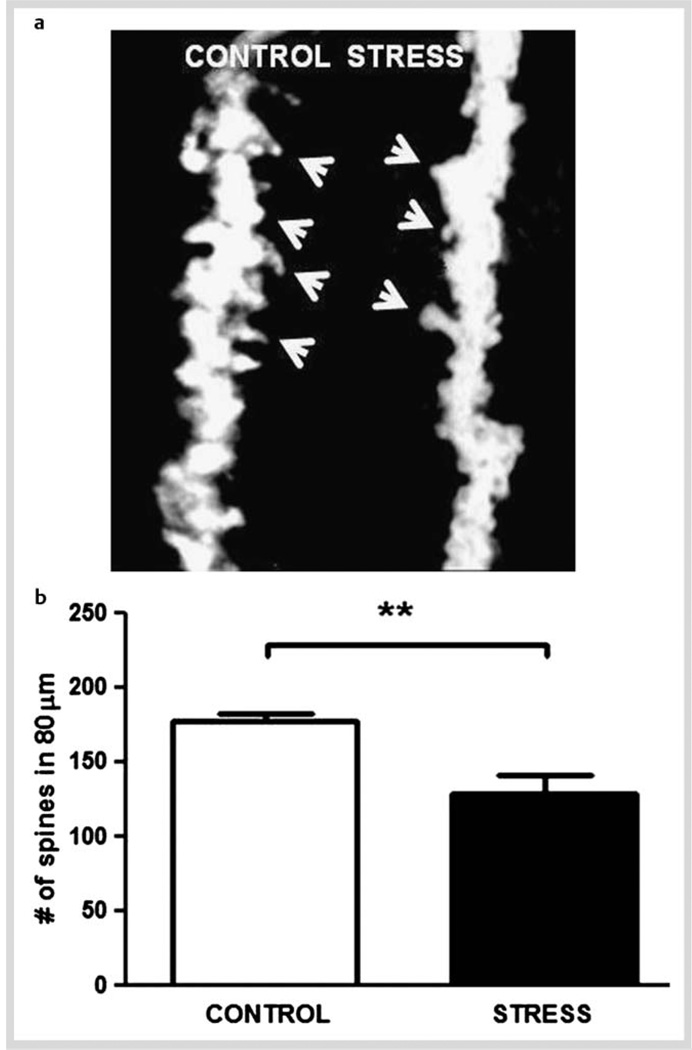
Chronic stress and depression may result in neuroplastic alterations in relevant brain areas. For example, acute glucocorticoid administration [51] and chronic restraint stress [48] have been shown to reduce Bcl-2 mRNA levels in the hippocampus. Bcl-2 is an antiapoptotic protein with known neuroprotective actions [52]. Consistent with this observation, repeated stress or glucocorticoid administration induces a reduction in dendrite branching in the CA3 hippocampal area, an effect that can be blocked by glutamate receptor antagonists [53] and some antidepressants [54]. Further, it has been shown that chronically stressed male animals exhibited a depressive-like state (anhedonic behavior and learned helplessness in the chronic restraint model) [48]. This behavioral alteration is accompanied by a reduction in spine density of primary dendrites from hippocampal CA1 pyramidal neurons (Fig. 1a). Quantitative analyses showed a significant reduction in the number of dendritic spines along the shafts of neurons sampled from stressed animals in comparison to controls (Fig. 1b). Hence, it may be plausible that the decreased spine density on hippocampal neurons is related to impaired behavior performance promoted by chronic stress. In addition to spine density, some other factors, such as the shape of spines have been postulated to play a pivotal role in synaptic plasticity models of memory formation and storage [55]. Thus, it is important to further evaluate the effect of stress and antidepressants treatment on dendritic spine morphology.
Fig. 1.
Chronic restraint stress in male rats reduces spine number of apical primary dendrites from hippocampal CA1 pyramidal neuron. After 14 days of restraint stress (2.5 h/day), brains were processed for rapid Golgi staining and the number of spines along a segment of primary apical dendrite (80 µm) from pyramidal neurons of the CA1 hippocampal area was scored. Protrusions, irrespective of their morphological characteristics were counted as spines only if they were in direct connection with the dendritic shaft. a Representative images of the primary branch of hippocampal CA1 pyramidal neurons under 100 × magnifications to show spines arrows) from control and stressed animals. Note the greater density of spines along the shafts in control compared to stressed animal. b Total number of spines in an 80 µm segment from the origin of the branch, was then averaged across all neurons in control and stressed animals (4–5 neurons per animal were used in each experimental group). Data show means ± S.D. Unpaired t-test: **p < 0.005.
Several lines of evidence have related neurotrophic factors with chronic stress and with the formation and shape variation of existing dendritic spines [56]. In the rat, increased levels of glucocorticoids or chronic stress have also been shown to reduce Brain Derived Neurotrophic Factor (BDNF) mRNA levels [48, 57]. The neurotrophic factor, BDNF, participates in dendritic remodeling, membrane receptor trafficking, neurotransmitter release, synapse connection, and promotes neuronal survival [58]. Additionally, its gene expression and secretion are regulated by neuronal activity [58]. Moreover, it has been recently reported that BDNF promotes the release of the excitatory neurotransmitter, glutamate; an effect that is suppressed by a GR agonist [59]. Taken together, these findings support the hypothesis that glucocorticoids impair BDNF signaling and could be causally related to the incidence of depressive disorder. Further evidence in rat models show that the stress-induced reduction in BDNF mRNA in hippocampal area CA3 is prevented by chronic administration of desipramine, a tricyclic antidepressant [48]. In agreement, BDNF injection into the rat hippocampus has behavioral antidepressant actions [60], which can be blocked by inhibiting ERK1/2 phosphorylation [60]. Moreover, several clinical studies have shown that the levels of serum BDNF are significantly lower in antidepressant-naive depressed patients compared to treated patients or healthy control subjects [61]. More recently, it has been reported that the early increase of serum BDNF levels in response to an antidepressant therapy predicts success of the treatment in depressed patients [62].
Fetal and Early Life Antecedents to Depression and HPA Axis Dysregulation
Adverse experiences during early life can have long-term consequences on the organization of the developing brain and thereby predispose some individuals to the development of neuropsychiatric disorders in adulthood [63]. Convincing evidence shows that exposure to early life events increases vulnerability to risk for depressive disorder in adult life [64]. In fact, individuals who experience early life trauma, such as parental loss or sexual abuse in childhood, have increased risk for suffering depression later in life [65, 66]. Similarly, rodents submitted to prolonged periods of maternal separation during the first weeks of life, showed altered behavior and high stress reactivity in adulthood [67, 68]. These associations support the concept that early adverse experiences program epigenetic modifications in the brain that persist throughout the lifetime to increase risk of an individual to mental disorders like depression [69].
Evidence that early life adversity can similarly affect the developing HPA axis comes from studies showing that depressed patients with a history of childhood trauma exhibit impairment in the inhibitory feedback regulation of the HPA axis [70]. Similarly, in animal models, fetal stress can impact the developing HPA axis resulting in elevated circulating glucocorticoid levels in adulthood. Reports also suggest a sex difference in this response, with females being more sensitive to fetal stress than males [71]. Similarly, the effect of prenatal stress on adult behaviors also appears to preferentially target females [72].
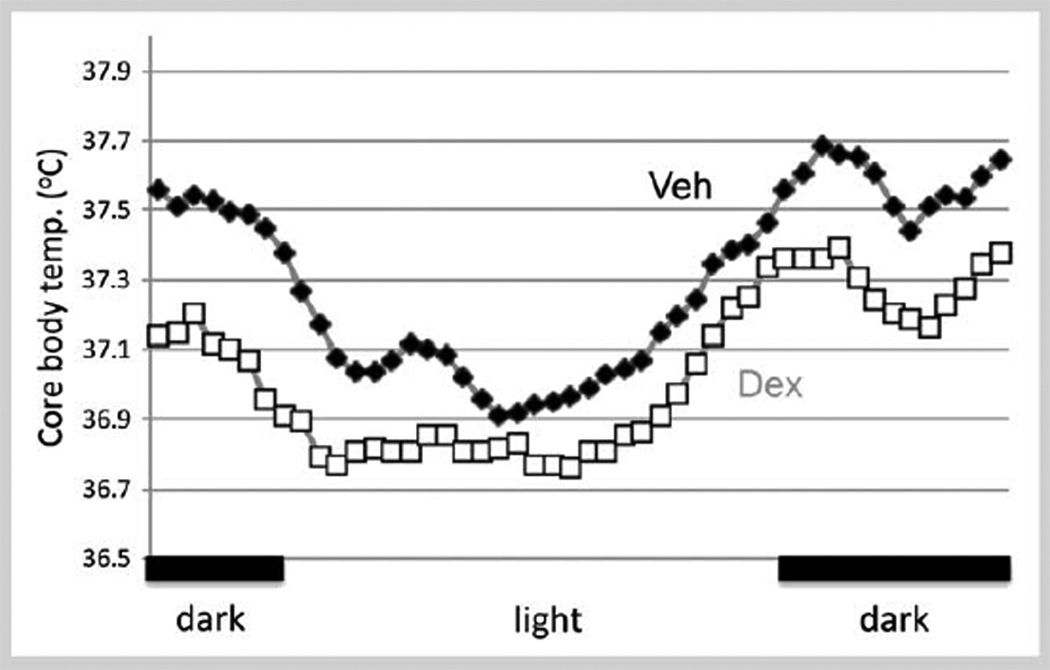
The long-term alterations in hypothalamic function following prenatal stress may be associated with prenatal overexposure to glucocorticoids. Given that the hypothalamus is a central location for preautonomic neurons, data indicate that prenatal exposure to the synthetic glucocorticoid, dexamethasone, can alter some autonomic responses in adulthood. Similar to the sex differences in neuroendocrine responses to stress, autonomic changes also occur in a sexually dimorphic pattern. For example, our studies show that parameters of autonomic function such as the daily rhythm in core body temperature (CBT) are decreased in adult female rats, but not in males that were exposed to DEX during the last 4 days of gestation (Fig. 2). Matching decreases in CBT have been reported in depressed patients [73, 74] suggesting the potential for prenatal stress or glucocorticoids to increase risk of mood disorders in adulthood. In rodents, prenatal stress and prenatal exposure to glucocorticoids can also increase neuronal death in limbic brain regions [75] resulting in permanent changes in adult neuroendocrine function and in behavior [76]. Corresponding effects have been reported in humans [77, 78]. Since neuroendocrine responses to stress and autonomic regulation are controlled by neurons residing in the PVN, this suggest that the PVN is a critical player for these organizational actions of fetal stress or overexposure to glucocorticoids [79]. To date, the cellular and molecular mechanisms mediating the long-term effect of environmental insults on fetal brain development have not been adequately explored, and is an area ripe for investigation.
Fig. 2.
Daily rhythm in core body temperature (CBT) in prenatal dexamethasone (DEX)-treated adult female rats. Pregnant dams were treated with DEX (0.4 mg/kg BW) from gestation age 18–21. Offspring were raised to adulthood. CBT was determined by in vivo telemetry (Mini mitter, Bend, OR) and temperature was recorded throughout the Dark:Light (D:L) cycle. Data shown are 30-min means from 4 females in each group recorded over a 24-h period on a day of diestrus. Controls are shown in black, DEX treated in gray. A 2-h moving average was used to plot the data. Prenatal DEX-treated females showed a significant 0.2–0.5 °C temperature decrease throughout the day compared to vehicle treated females (p < 0.05). All individuals tested arose from different litters. DEX- and vehicle-treated males were not different from each other and not different from vehicle treated-females (data not shown) indicating a sex-difference in susceptibility to hypothalamic disruption by prenatal glucocorticoids. Black bars on bottom indicate the dark period of the D:L cycle.
Genetic Differences Influence Responses to Antidepressants
Genetic influences in the vulnerability to develop MDD have been demonstrated by different strategies, including the classic twin and familial studies. In order to identify the genes involved in this disorder, association studies with candidate genes and linkage and genome wide association studies have been carried out (reviewed in [80]). Like in many other complex disorders, these studies have not been consistently replicated; however, all of them show that there is no one major vulnerability gene predisposing the development of MDD, but rather, in the etiology of depression there seems to be a group of genes that interact with environmental factors. Furthermore, genetic factors also influence the antidepressant response.
Approximately 30 % of patients do not respond to currently available antidepressant drugs; therefore they must deal with a prolonged “trial and error” period before finding an effective pharmacological treatment. Although the most common antidepressants target the monoaminergic systems, they also decrease HPA axis hyperactivity, alleviating depressive symptoms (reviewed in [81]). The effects of antidepressants on monoamine levels are exerted rapidly, within few hours; however, the therapeutic response is only observed several weeks after the initiation of treatment. This temporal discrepancy indicates that something other than monoamine normalization is required to achieve mood stabilization in depressed patients. On the other hand, post-treatment nonsuppression of cortisol on the DST has been associated with worse antidepressant outcome [82]. Thus, treatment resistant patients may constitute a biologically distinctive group. In fact, significant intra-individual stability has been observed in serum cortisol concentrations and subjects with high basal levels also exhibit higher cortisol after low doses of DEX, suggesting that the feedback sensitivity to DEX depends on the basal HPA activity [83]. Moreover, it has been recently shown that depressed patients displaying high baseline levels of ACTH show a delayed response to antidepressant treatment [84]. These data suggest a genetic contribution influencing the set point of the HPA axis [82], which could involve genes important in HPA regulation. These may include the GR gene, important in the feedback regulation; the FKBP5 gene encoding a co-chaperone of GR; and CRH and AVP genes and their receptor’s genes, all of which are involved in the control of ACTH secretion. Within the GR gene (Nuclear Receptor Subfamily 3, Group C, Member 1; NR3C1), a number of mutations related with glucocorticoid resistance syndrome and functional single nucleotide polymorphisms (SNPs) associated with more subtle effects have been identified [85]. Some of the SNPs have been associated with HPA-related clinical states, including depression and antidepressant effects [86]. For instance, the ER22/23EK polymorphism is associated with a reduced sensitivity to glucocorticoids, and higher risk to develop depression [87, 88]. In relation to antidepressant response, the same polymorphism showed a significant association with faster clinical response as it was shown in groups of patients under a range of antidepressant therapies [87]. On the other hand, a 3 SNP haplotype that includes the BclI SNP was associated with an increased sensitivity to glucocorticoids [89]. Also, carriers of the BclI allele had higher ACTH levels than noncarriers and showed a trend towards lower decrease of the scores in the Hamilton Rating Scale for Depression (HAM-D) than noncarriers in response to the SSRI, paroxetine antidepressant treatment [90]. Interestingly, a subgroup of BclI carriers with higher ACTH levels exhibited a lower reduction in HAM-D score and lower response rates than patients with the lower ACTH levels [90]. The same polymorphism has been described to interact with early stressful situations raising the risk for depression and bulimia nervosa [90–92]. Brouwer et al. could not associate the ER22/23EK polymorphism with the response rate to paroxetine, although the allele frequency for the minor allele was low in that sample [90]. It is not clear why the more sensitive allele to glucocorticoids (Bcl1) had worse outcome to antidepressant response and coincidentally why the most resistant to glucocorticoids ER22/23EK respond more rapidly.
The FK506-Binding Protein 51 (FKBP5) is a co-chaperone of heat shock protein 90, which in turn regulates the GR sensitivity. Binder et al. reported that 3 polymorphisms in the FKBP5 gene are associated with rapid response to antidepressant treatment [93]. In fact, the SNP rs1360780 was associated with a higher intracellular FKBP51 protein expression with T/T homozygous individuals expressing levels 2-fold greater than those with the other 2 genotypes [93]. Moreover, they also found associations of the same marker with the antidepressant response, faster response to antidepressant treatment, lower activity of the HPA axis during a depressive episode, and a higher recurrence of depressive episodes in their lifetime, with an over-representation of T/T homozygotes among the responders [93]. These results have been replicated in some studies [81], but not in others [94]. The causes of this discrepancy may reside in genetic differences in the ethnic populations tested, lifetime recurrence of depressive episodes, or differences in the phenotypes and drugs used. The allele associated with more expression of FKBP51 (i. e., the one involved in more glucocorticoid resistance) is also involved in higher risk to develop depression and a faster antidepressant response, similar to the results observed with the ER22/23EK polymorphism in GR.
As previously mentioned, the CRH and AVP systems have both been implicated in the pathophysiology of depression as well as in the antidepressant response [28, 31, 33, 34]. In fact, various antidepressants suppress CRH gene expression and SSRIs exert their therapeutic action by reducing the activity of CRH neurons [95]. In addition, 3 polymorphisms in the CRH-R1 gene (rs1876828, rs242939, and rs242941) have been associated with better response in a high anxiety depressed group of Mexican-Americans [96], and in a Chinese patient sample. Further, in the Chinese group, the antidepressant response to the SSRI, fluoxetine (FLX) has been associated with the G/G genotype of the rs242941 SNP [97]. In relation to the CRH-R2 gene, CRH-R2-deficient mice display a stress-sensitive and anxiety-like phenotype, suggesting that CRHR2 is also a candidate gene influencing the reactivity of the HPA axis [98]. The CRHR2s183 polymorphism of the CRHR2 gene has been associated with increased risk for major depression [99]. Additionally, allele G carriers of the rs2270007 polymorphism, show less of an overall response to citalopram (SSRI) after several weeks [100].
An involvement of AVP in the etiology of depression is evidenced by studies using rat models with extreme anxiety phenotype. In these animals AVP over-expression was observed in the PVN, similar to stressed rats or those showing depressive-like behaviors [101]. AVP overexpression can be caused by a SNP A(− 1276) G in the promoter of the AVP gene, that reduces the binding of the transcriptional repressor CBF-A [101]. Interestingly, chronic FLX treatment significantly reduced AVP release from rat hypothalamic organ culture in vitro [102]. In humans, similar polymorphisms, to the one mentioned above, have not been described. On the other hand, under exposure to chronic stress and to glucocorticoids increased the mRNA levels of the AVP1b receptor gene, AVPR1b [103]. Moreover, FLX or desipramine administration significantly attenuated stress-induced increases in plasma ACTH and corticosterone levels in male and female AVPR1b knockout mice, when compared to their wild-type counterparts [104]. These data indicate that AVPR1b plays a role in controlling stress-induced cortisol secretion. Two polymorphisms of AVPR1b gene (Lys65Asn and AVPR1b-s4) have been associated with childhood-onset mood disorders [105] and 2 SNPs (AVPR1b-s3 and AVPR1b-s5) are correlated with recurrent major depression [106]. However, to our knowledge, no association studies of genes involved in AVP actions have been performed in order to relate them to antidepressant responses.
Sex and Age Differences in Affective Disorders and Their Treatment
Epidemiological studies have observed significant gender-specific differences among patients with depression, with adult young women outnumbering men at a rate of 2:1. This high prevalence in women may be exacerbated to greater than 5:1 during particular periods of life (reviewed in [107]). In general, women have a higher prevalence of most affective disorders, whereas, men have higher rates of substance use disorders [108]. Chronic stress, low sense of mastery, and rumination are more common in women than in men, and may underlie the gender differences in depressive symptoms. These 3 factors interact and synergize with one another to produce depressive symptoms and these symptoms contribute to more rumination and less mastery over time. Studies on the role of personality factors in gender differences in depression have shown that the level of neuroticism, which is significantly higher among women, also increases the risk of depression. Whether gender differences in depression could be explained by differences in comorbid anxiety is still controversial, but attempts to explain these gender differences could benefit from the understanding that women are more likely to experience life stress. For example, somatic depression, which is associated with high rates of stress, is much higher among women than men (for review, see [107]).
It has been hypothesized that cyclic changes in hormone levels in women are a contributing factor for the incidence of depression across a woman’s lifetime [109]. During early adulthood, depression becomes increasingly prevalent, with a typical onset between the second and third decades of life. Women of childbearing age are at heightened risk and women in the perimenopausal transition are at the highest risk for suffering depressive episodes [109]. After 65 years of age, data fail to demonstrate an increase in rates of MDD. In addition, symptom profiles that characterize depression in later life differ from those earlier in the life span, with older adults less likely to experience dysphoria. A constellation of symptoms, more frequent in older adults and older women specifically, characterize the depletion syndrome, where symptoms include loss of interest and energy, hopelessness, helplessness and psychomotor retardation [110]. In support of these observations, in patient populations it has been reported in animal models of depression that aged female rats are more susceptible than young adults to develop stress-induced experimental depression, and that estrogens produce antidepressant-like actions [4, 111, 112]. In clinical studies, estrogens have proven to alleviate various perimenopause associated symptoms [113]. These observations support the idea that the gender differences in depression may have biological bases that, among others, include variations in gonadal steroid hormones. It is still unclear if the women’s response to SSRIs is greater than men’s; one study has suggested greater sensitivity in women whereas others have not [114]. In addition, women have a greater response than men to SSRIs compared to tricyclic antidepressants or to SNRI [115]. These slight gender differences in treatment response suggest that they may guide the clinical use of SSRI and SNRI antidepressants. These findings also raise the possibility that antidepressants may work differently in men and women. A source of this difference may rely on the clear action of ovarian hormones on the serotonergic system. Thus, in young premenopausal women ovarian steroids, primarily estrogens, may be modulating the action of some antidepressants, inviting the study of these gender differences in middle-aged patients with relatively low levels of gonadal steroids. Additionally, in males, the role of testicular hormones is usually disregarded simply because it is relatively constant and decreases linearly with age. However, the literature reveals that aged male rats and men, when compared with young adults, respond less well to antidepressant treatments [116, 117]. Thus, the optimal sex comparison in the response to antidepressants must consider aged-matched populations.
Sex and Gonadal Hormone Effects on Experimental Depressive-like Behaviors
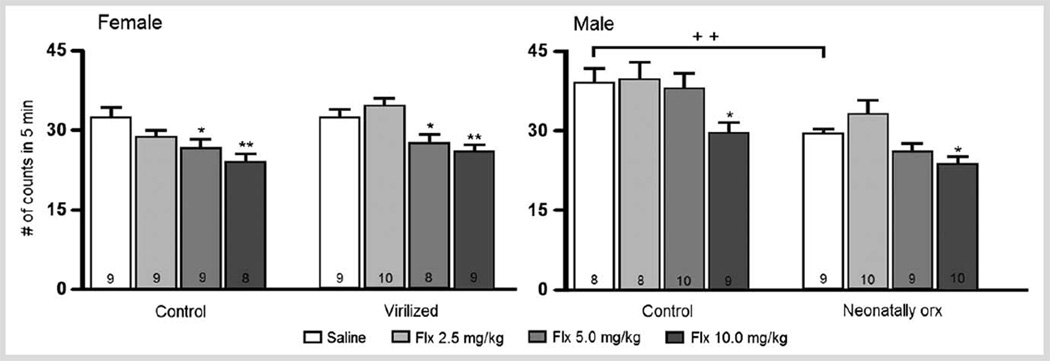
Sex differences in depressive-like behaviors have been described in various animal models of depression [118]. In the forced swimming test, some authors have found sex differences in the expression of immobility behavior [119]. However, others [107, 120, 121] have found no statistical significant differences between male and female rats, although females trend to show lower levels of immobility than males (Fig. 3). It is worth clarifying that in this study, all females were tested in the estrous phase of the cycle under the influence of 17β-estradiol (E2) and progesterone, which reduce immobility [111]. In relation to the antidepressant response [122], females in estrus exhibit a greater response to drug treatment than males. This observation was confirmed since subchronic treatment with FLX reduced immobility at the dose of 5 mg/kg in female rats, while males did not respond to this dose (Fig. 3). After 10 mg/kg both sexes showed reduced immobility. These data reveal that female rats are more sensitive than males to the antidepressant-like effect of FLX. Similar findings have been reported in mice [123] and in humans (vide supra). Therefore, it is possible that neurochemical changes evoked in the female’s serotonergic system after forced swimming test (FST) underlie the more prominent effect of FLX in this sex.
Fig. 3.
Effect of subchronic treatment with fluoxetine (2.5, 5 and 10 mg/kg) on females’ (left panel) and males’ (right panel) immobility in the forced swimming test. Control- and neonatally-virilized (testosterone propionate, 60 µg/rat subcutaneously (s.c.) at day 5) females were tested in estrus. Neonatally-orchidectomized (orx) males were castrated on day 5 of life under cryo-anesthesia. Data show means ± S.E.M. of number of counts of 8–10 rats per group (within bars). One way ANOVAs for: control females: F(3,31) = 4.01, p < 0.05; virilized females: F(3,32) = 6.8, p < 0.001; control males: F(3,31) = 2.93, p < 0.05, neonatally castrated males: F(3,34) = 5.15, p < 0.01, Fisher Least Significant Difference (LSD): *p < 0.05; **p < 0.01 vs. its respective nontreated control group. t-test: ++ p < 0.01 between control and neonatally orchidectomized groups. Note that without treatment under control conditions females in estrus display lower levels of immobility than males.
The main line of research on sex differences relates to the organizational and activational role of gonadal hormones that appears to impact all neurotransmitter systems. Therefore, the possibility that these sex differences were due to the organizational action of gonadal steroids was addressed. It is generally thought that in the male rat, early castration (within the first 5 days of life) results in a brain with a largely female pattern of cyclic hypothalamic and hypophyseal hormone secretion [124]. Conversely, administration of T or E2 to neonatal females induces a male-like brain characterized by a tonic production of reproductive hormones [124]. Thus, the antidepressant-like effect of FLX in the FST in neonatally-T-treated females and in neonatally-castrated males was tested. It was observed that the females’ higher sensitivity to the antidepressant-like effect of FLX (5 mg/kg) was also found in virilized females suggesting that such increased sensitivity does not depend upon the postnatal sexual differentiation process (Fig. 3, left panel). For control and comparison purposes both groups of females were tested in estrus (virilized females showed constant vaginal estrus), and thus were under the influence of ovarian hormones secretions, which may contribute to their higher sensitivity to FLX (vide supra). When tested in adulthood, male rats that were neonatally castrated show much lower levels of immobility (similar to those of females) than intact males, suggesting that the lower levels of immobility in females are not only related to the activational effects of ovarian steroids during adulthood but also may depend upon the organizational effect of these hormones during development (Fig. 3, right panel). In neonatally-castrated males, FLX produced an antidepressant-like effect similar to that observed in intact males. That is, the only effective dose was 10 mg/kg. However, neonatally-castrated males lack the testicular production of E2 and T in adulthood, which is known to mediate the antidepressant-like action of this SSRI and of desipramine, a tricyclic antidepressant primarily acting on the noradrenergic transporter [121, 125]. Males castrated in adulthood do not respond to the antidepressant-like action of FLX and the tricyclics, clomipramine and desipramine even if used at very high doses [121, 125]. Therefore, neonatally castrated males showed a sui generis response: they behave as females in terms of their response to the antidepressant-like actions of FLX and as males since they were affected only by the highest dose. Future experiments using neonatal and adult castrated animals should be undertaken to broaden our knowledge of the role of gonadal hormones in experimental depression and its clinical impact of treatment.
Sex Differences in Depression and HPA Axis Regulation
As previously mentioned, clinical studies of patients with MDD reveal that the sex differences in incidence arise at adolescence and more closely coincide with androgen and estrogen levels rather than physical changes associated with puberty [126, 127]. As a result, changing patterns of hormones and hormone sensitivity may be implicated as potential etiological factors for the onset of depressive symptoms in women. Similarly, estrogen and androgen signaling in limbic brain may influence the regulation of HPA axis function, perhaps contributing to the dysregulation seen in patients with affective disorders and underlying susceptibility of some individuals to affective disorders. Numerous brain changes occur across the lifespan, including reorganization occurring at puberty, and although these invariably interact to regulate HPA reactivity and behaviors in adulthood, a discussion of this nature is beyond the scope of this review (for a recent review on this topic, see [128]).
Actions of Gonadal Hormones and Receptors in the Regulation of the HPA Axis and Anxiety- and Depressive-like Behaviors in Rodents
Sex differences in HPA axis reactivity and the impact of gonadal steroid hormones on HPA axis function have been studied in animal models for several decades [129, 130]. This sex difference is characterized by higher basal and stress responsive ACTH and corticosterone levels in intact females. Further, gonadectomy of male rats increases neuroendocrine responses to stress and correspondingly, c-fos mRNA expression, an indicator of neuronal activity, is elevated in PVN neurons [131, 132] indicating an involvement of testosterone. These effects of androgens in reducing the reactivity of the HPA axis are not through the aromatization to estrogens since the nonaromatizable androgen, dihydrotestosterone (DHT) also reduces ACTH and corticosterone responses to stress [132, 133].
Androgens also affect CRH-ir [134] and vasopressin mRNA expression within the PVN. However, androgen receptors (AR) are not expressed by neuroendocrine CRH or AVP neurons within the PVN [135], but are found in non-neuroendocrine neurons of the PVN that project to spinal cord and brainstem preautonomic nuclei [136]. As a consequence, androgens might regulate PVN neuropeptide expression and secretion trans-synaptically, through projections from the preoptic area and bed nucleus of the stria terminalis, with the caveat that the direction of this regulation is conflicted by studies showing both enhancement and inhibition of the expression of neuropeptides that control the drive of PVN neuroendocrine neurons [137, 138].
Estrogen receptors have also been shown to impact HPA function. Initial studies indicated that E2 treatment enhanced, and T treatment inhibited HPA reactivity [139–141]. However, the directions of E2 effects have not always been consistent, as both enhancement [142] and inhibition [143] of HPA activity following E2 have also been reported. This may be a consequence of the amplitude and duration of hormone exposure that differentially influences the actions of gonadal steroid hormones on HPA axis function [143].
With the discovery of a novel estrogen receptor (ER), termed ERbeta [144], several groups showed that its mRNA and immunoreactivity (ir) were highly expressed by PVN neurons [145, 146], whereas the original ER, now termed ERalpha, was not. A large number of ERbeta-ir cells in PVN are AVP or OT positive [147, 148] but only few CRH neurons express ERbeta [146, 148]. These receptors are functional in controlling stress-related activity since the administration of ERbeta agonists to rats inhibits stress-induced corticosterone secretion [149, 150], and increases depressive- and anxiolytic-like behaviors [150]. To determine the site for the neuroendocrine actions of ERbeta, Lund and coworkers [149] placed ERbeta agonists into an area adjacent to the PVN of ovariectomized rats. This reduced stress-responsive corticosterone and ACTH secretion. In contrast, ERalpha agonistshave an enhancing effect on corticosterone and ACTH [151]. Thus, a direct action of estrogens on the PVN that is mediated by ERalpha to regulate stress reactivity appears unlikely.
The effects of steroid hormones on HPA function are not only activational in nature, but can be tracked in part to the organizational effects of steroid hormones on brain development. Recent studies show that adult female rats display a larger and longer lasting rise in plasma ACTH following acute ether stress as compared to males, but females treated with testosterone at birth display male-like ACTH secretory patterns [152]. Conversely, prenatal aromatase inhibition in males results in a female-typical pattern of ACTH secretion in response to an adult ether stress [153]. Together, these data indicate that the HPA axis is a developmental target of perinatal steroid hormones and provide another layer of complexity to the mechanisms underlying sex differences in the etiology of neuropsychiatric disorders and the accompanying dysregulation of the HPA axis.
Summary and Conclusions
In summary, the incidence of depression has a strong sex bias with females showing enhanced risk compared to males. Affective disorders are commonly associated with a dysregulation of the HPA axis. Thus, sex differences in the incidence of MDD correlate with sex differences in HPA axis function. Organizational and activational effects of gonadal steroid hormones have been shown for the regulation of HPA axis function and may underlie increased risk of affective disorders in women. Further, prenatal stress and prenatal overexposure to glucocorticoids can impact adult behaviors and neuroendocrine responses to stress. Sex differences in responses to glucocorticoids and to antidepressants that normalize glucocorticoid responses have also been demonstrated in animal models and a strong influence of estrogen and androgens have been demonstrated. In humans, the clinical benefits of antidepressants are also associated with the normalization of the dysregulated HPA axis, and genetic polymorphisms have been found in some genes involved in controlling the stress response. However, information about the precise mechanism underlying the antidepressant response is still incomplete in both humans and animal models. Other factors, such as environment, age, sex and development should be considered. Thus, although it is still not possible to translate the pharmacogenetic information available for the generation of strong and safe predictors with clinical relevance, the impact of gender and age must be taken into consideration when considering any therapeutic approach.
Acknowledgements
The authors’ research programs have been funded by: CONACYT J162020 and 104659 (AFG), FONDECYT 104-0937 and 108-0489 (JF), FONDECYT 109-0219 (LH), NIH NS039951, and MH082679 (RJH). The authors would also like to thank the organizers of the US-Latinoamerican workshop in Neuroendocrinology that took place in Viña del Mar, Chile, in August 2011.
The authors wish to thank the following students: Martha Isabel Luna Gómez, Damaris Arancibia, and Anthony Lacagnina for their research effort, as well as Rebeca Reyes Serrano for editing the manuscript.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- ANOVA
Analysis of variance
- AR
Androgen receptors
- BDNF
Brain derived neurotrophic factor
- CBT
Core body temperature
- CRH
Corticotropin releasing hormone
- DEX
Dexamethasone
- DST
Dexamethasone suppression test
- DHT
Dihydrotestosterone
- E2
17β-estradiol
- ER
Estrogen receptor
- FLX
Fluoxetine
- GR
Glucocorticoid receptors
- HPA
Hypothalamo-pituitary-adrenal
- ir
Immunoreactivity
- MDD
Major depressive disorder
- MAOIs
Monoamine oxidase inhibitors
- SNRIs
Serotonin-Noradrenaline reuptake inhibitors
- OT
Oxytocin
- PVN
Paraventricular hypothalamic nucleus
- SSRIs
Selective serotonin reuptake inhibitors
- SCN
Suprachiasmatic nuclei of the hypothalamus
- T
Testosterone
- AVP
Vasopressin
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington D.C: American Psychiatry Press Inc; 2000. [Google Scholar]
- 2.Garcia-Alvarez R. Epidemiology of depression in Latin America. Psychopathlogy. 1986;19(Suppl 2):22–25. doi: 10.1159/000285128. [DOI] [PubMed] [Google Scholar]
- 3.Willner P, Mitchel PJ. The validity of animal models of predisposition to depression. Behav Pharmacol. 2002;13:169–188. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Recamier-Carballo S, Fernández-Guasti A. Forced swimming and chronic mild stress as animal models of depression. In: Cruz-Morales SE, Pedro Arriaga-Ramírez JC, editors. Behavioral animal models. Kerala, India: Research Signpost; 2012. pp. 123–137. [Google Scholar]
- 5.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 6.Chung S, Son GH, Kim K. Adrenal peripheral oscillator in generating the circadian glucocorticoid rhythm. Ann NY Acad Sci. 2011;1220:71–81. doi: 10.1111/j.1749-6632.2010.05923.x. [DOI] [PubMed] [Google Scholar]
- 7.Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhyths in the hypothalamo-pituitary-adrenal (HPA) axis. Mol Cell Endocrinol. 2012;349:20–29. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 8.Szafaraczyk A, Ixart G, Alonso G, Malaval F, Nouguier-Soule J, Assenmacher I. CNS control of the circadian adrenocortical rhythm. J Steroid Biochem. 1983;19:1009–1015. doi: 10.1016/0022-4731(83)90047-x. [DOI] [PubMed] [Google Scholar]
- 9.Kalsbeek A, Van Der Vliet J, Buijs RM. Decrease of endogenous vasopressin release necessary for expression of the circadian rise in plasma corticosterone: a reverse microdialysis study. J Neuroendocrinol. 1996;8:299–307. doi: 10.1046/j.1365-2826.1996.04597.x. [DOI] [PubMed] [Google Scholar]
- 10.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Dunn AJ, Berridge CW. Is corticotropin-releasing factor a mediator of stress responses? Ann NY Acad Sci. 1990;579:183–191. doi: 10.1111/j.1749-6632.1990.tb48360.x. [DOI] [PubMed] [Google Scholar]
- 12.Aguilera G, Nikodemova M, Wynn PC, Catt KJ. Corticotropin releasing hormone receptors: two decades later. Peptides. 2004;25:3319–3329. doi: 10.1016/j.peptides.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Ratka A, Sutanto W, Bloemers M, de Kloet ER. On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology. 1989;50:117–123. doi: 10.1159/000125210. [DOI] [PubMed] [Google Scholar]
- 14.Herman JP, Wiegand SJ, Watson SJ. Regulation of basal corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid expression in the paraventricular nucleus: effects of selective hypothalamic deafferentations. Endocrinology. 1990;127:2408–2417. doi: 10.1210/endo-127-5-2408. [DOI] [PubMed] [Google Scholar]
- 15.Bondy CA, Whitnall MH, Brady LS, Gainer H. Coexisting peptides in hypothalamic neuroendocrine systems: some functional implications. Cell Mol Neurobiol. 1989;9:427–446. doi: 10.1007/BF00712791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raadsheer FC, Sluiter AA, Ravid R, Tilders FJ, Swaab DF. Localization of corticotropin-releasing hormone (CRH) neurons in the paraventricular nucleus of the human hypothalamus; age-dependent colocalization with vasopressin. Brain Res. 1993;615:50–62. doi: 10.1016/0006-8993(93)91113-7. [DOI] [PubMed] [Google Scholar]
- 17.Rivier C, Vale W. Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology. 1983;11:939–942. doi: 10.1210/endo-113-3-939. [DOI] [PubMed] [Google Scholar]
- 18.Schlosser SF, Almeida OF, Patchev VK, Yassouridis A, Elands J. Oxytocin stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology. 1994;135:2058–2063. doi: 10.1210/endo.135.5.7956927. [DOI] [PubMed] [Google Scholar]
- 19.Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the neew CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- 20.Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action iwthin the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 21.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 22.Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans. 2007;35:1252–1257. doi: 10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- 23.Deuschle M, Schweiger U, Weber B, Gotthardt U, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82:234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- 24.Rubin RT, Phillips JJ, McCracken JT, Sadow TF. Adrenal gland volume in major depression: relationship to basal and stimulated pituitary-adrenal cortical axis function. Biol Psychiatry. 1996;40:89–97. doi: 10.1016/0006-3223(95)00358-4. [DOI] [PubMed] [Google Scholar]
- 25.Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreacitivty in depressed patients. Science. 1984;226:21342–21344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 26.Feldman S, Weidenfeld J. The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res Bull. 1998;45:389–393. doi: 10.1016/s0361-9230(97)00384-5. [DOI] [PubMed] [Google Scholar]
- 27.Heuser Il, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 28.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinolog. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 29.Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 30.Purba JS, Googendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- 31.Dinan TG, Scott LV. Anatomy of melancholia: focus on hypothalamic-pituitary-adrenal axis overactivity and the role of vasopressin. J Anat. 2005;207:259–264. doi: 10.1111/j.1469-7580.2005.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller MB, Keck ME, Zimmermann S, Holsboer F, Wurst W. Disruption of feeding behavior in CRH receptor 1-deficient mice is dependent on glucocorticoids. Neuroreport. 2000;11:1963–1966. doi: 10.1097/00001756-200006260-00031. [DOI] [PubMed] [Google Scholar]
- 33.Meynen G, Unmehopa UA, Heerikhuize JJ, Hofman MA, Swaab DF, Hoogendijk WJ. Increased Arginine Vasopressin mRNA Expression in the Human Hypothalamus in Depression: A Preliminary Report. Biol Psychiatry. 2006;60:892–895. doi: 10.1016/j.biopsych.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Van Londen L, Goekoop JG, van Kempen GM, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, De Wied D. Plasma levels of arginine-vasopressin elevated in patients with major depression. Neuropsychopharmacology. 1997;17:284–292. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- 35.Pariante CM. The glucocorticoid receptor: part of the solution or part of the problem? J Psychopharmacol. 2006;20:79–84. doi: 10.1177/1359786806066063. [DOI] [PubMed] [Google Scholar]
- 36.Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker KJ, Kenna HA, Zeitzer JM, Kelelr J, Blasey CM, Amico JA, Schatzberg AF. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res. 2010;178:359–362. doi: 10.1016/j.psychres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med. 2008;70:965–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scantamburlo G, Hansenn M, fuchs S, Pitchot W, Marechal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels an danxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Groeneweg FL, Karst H, de Kloet ER, Joels M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J Endocrinol. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- 41.Kellendonk C, Gass P, Kretz O, Schutz G, Tronche F. Corticosteroid receptors in the brain: gene targeting studies. Brain Res Bull. 2002;57:73–83. doi: 10.1016/s0361-9230(01)00638-4. [DOI] [PubMed] [Google Scholar]
- 42.Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev. 2001;25:117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 43.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann NY Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinkelmann K, Moritz S, Botzenhardt J, Riedesel K, Wiedemann K, Kellner M, Otte C. Cognitive impairment in major depression: association with salivary cortisol. Biol Psychiatry. 2009;66:879–885. doi: 10.1016/j.biopsych.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 45.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29:185–193. [PMC free article] [PubMed] [Google Scholar]
- 46.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 47.Gourley SL, Taylor JR. Recapitulation and reversal of a persistent depression-like syndrome in rodents. Curr Protoc Neurosci. 2009;Chapter 9(Unit 9):32. doi: 10.1002/0471142301.ns0932s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bravo JA, Diaz-Veliz G, Mora S, Ulloa JL, Berthoud VM, Morales P, Arancibia S, Fiedler JL. Desipramine prevents stress-induced changes in depressive-like behavior and hippocampal markers of neuroprotection. Behav Pharmacol. 2009;20:273–285. doi: 10.1097/FBP.0b013e32832c70d9. [DOI] [PubMed] [Google Scholar]
- 49.Ulloa JL, Castaneda P, Berrios C, Diaz-Veliz G, Mora S, Bravo JA, Araneda K, Menares C, Morales P, Fiedler JL. Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol Biochem Behav. 2010;97:213–221. doi: 10.1016/j.pbb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annu Rev Psychol. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- 51.Cardenas SP, Parra C, Bravo J, Morales P, Lara HE, Herrera-Marschitz M, Fiedler JL. Corticosterone differentially regulates bax, bcl-2 and bcl-x mRNA levels in the rat hippocampus. Neurosci Lett. 2002;331:9–12. doi: 10.1016/s0304-3940(02)00744-9. [DOI] [PubMed] [Google Scholar]
- 52.Xu H, Steven Richardson J, Li XM. Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology. 2003;28:53–62. doi: 10.1038/sj.npp.1300009. [DOI] [PubMed] [Google Scholar]
- 53.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 54.Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 55.Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 56.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 57.Smith MA, Cizza G. Stress-induced changes in brain-derived neurotrophic factor expression are attenuated in aged Fischer 344/N rats. Neurobiol Aging. 1996;17:859–864. doi: 10.1016/s0197-4580(96)00066-8. [DOI] [PubMed] [Google Scholar]
- 58.Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: A link between stress and mood disorders. Psychoneuroendocrinology. 2009;34S:S208–S216. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Numakawa T, Kumamaru E, Adachi N, Yagasaki Y, Izumi A, Kunugi H. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-gamma signaling for glutamate release via a glutamate transporter. Proc Natl Acad Sci USA. 2009;106:647–652. doi: 10.1073/pnas.0800888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aydemir O, Deveci A, Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:261–265. doi: 10.1016/j.pnpbp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Rojas PS, Fritsch R, Rojas RA, Jara P, Fiedler JL. Serum brain-derived neurotrophic factor and glucocorticoid receptor levels in lymphocytes as markers of antidepressant response in major depressive patients: a pilot study. Psychiatry Res. 2011;189:239–245. doi: 10.1016/j.psychres.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 63.Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 64.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 65.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 66.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 67.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 68.Ploj K, Roman E, Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience. 2003;121:787–799. doi: 10.1016/s0306-4522(03)00499-8. [DOI] [PubMed] [Google Scholar]
- 69.Murgatroyd C, Spengler D. Epigenetics of early child development. Front Psychiatry. 2011;2:16. doi: 10.3389/fpsyt.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult off-spring: sex-specific effects. J Neuroendocrinol. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- 72.Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki K, Miyamoto T, Miyamoto T, Kaji Y, Takekawa H, Hirata K. Circadian variation of core body temperature in Parkinson’s disease patients with depression: a potential biological marker for depression in Parkinson disease. Neuropsychobiology. 2007;56:172–179. doi: 10.1159/000119735. [DOI] [PubMed] [Google Scholar]
- 74.Salerian AJ, Saleri NG, Salerian JA. Brain temperature may influence mood: a hypothesis. Med Hypotheses. 2008;70:497–500. doi: 10.1016/j.mehy.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 75.Zuloaga DG, Carbone DL, Hiroi R, Chong DL, Handa RJ. Dexamethansone induces apoptosis in the developing rat amygdala in an age-, region-, and sex-specific manner. Neuroscience. 2011;199:535–547. doi: 10.1016/j.neuroscience.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hossain A, Hajman K, Charitidi K, Erhardt S, Zimmermann U, Knipper M, Canlon B. Prenatal dexamethasone impairs behavior and the activation of the BDNF exon IV promoter in the paravetnricular nucleus in adult offspring. Endocrinology. 2008;149:6356–6365. doi: 10.1210/en.2008-0388. [DOI] [PubMed] [Google Scholar]
- 77.Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr. 2001;1:1. doi: 10.1186/1471-2431-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karemaker R, Kavelaars A, ter Wolbeek M, Tersteeg-Kamperman M, Baerts W, Veen S, Samsom JF, Visser GH, van Bel F, Heijnen CJ. Neonatal dexamethasone treatment for chronic lung disease of prematurity alters the hypothalamo-pituitary-adrenal axis and immune system activity at school age. Pediatrics. 2008;121:e870–e878. doi: 10.1542/peds.2007-2454. [DOI] [PubMed] [Google Scholar]
- 79.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosc. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 80.Lohoff FW. Overview of the genetics of major depressive disorder. Curr Psychiatry Rep. 2010;12:539–546. doi: 10.1007/s11920-010-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horstmann S, Binder EB. Glucocorticoids as predictors of treatment response in depression. Harvard Rev Psychiatry. 2011;19:125–143. doi: 10.3109/10673229.2011.586550. [DOI] [PubMed] [Google Scholar]
- 82.Ribeiro SC, Tandon R, Grunhaus L, Greden JF. The DST as a predictor of outcome in depression: a meta-analysis. Am J Psychiatry. 1993;150:1618–1629. doi: 10.1176/ajp.150.11.1618. [DOI] [PubMed] [Google Scholar]
- 83.Huizenga NA, Koper JW, de Lange P, Pols HA, Stolk RP, Grobbee DE, de Jong FH, Lamberts SW. Interperson variability but intraperson stability of baseline plasma cortisol concentrations, and its relation to feedback sensitivity of the hypothalamo-pituitary-adrenal axis to a low dose of dexamethasone in elderly individuals. J Clin Endocrinol Metab. 1998;83:47–54. doi: 10.1210/jcem.83.1.4498. [DOI] [PubMed] [Google Scholar]
- 84.Araya AV, Rojas P, Fritsch R, Rojas R, Herrera L, Rojas G, Gatica H, Silva H, Fiedler JL. Early Response to Venlafaxine Antidepressant Correlates with Lower ACTH Levels Prior to Pharmacological Treatment. Endocrine. 2006;30:289–298. doi: 10.1007/s12020-006-0007-2. [DOI] [PubMed] [Google Scholar]
- 85.DeRijk RH, Schaaf M, de Kloet ER. Glucocorticoid receptor variants: clinical implications. J Steroid Biochem Mol Biol. 2002;81:103–122. doi: 10.1016/s0960-0760(02)00062-6. [DOI] [PubMed] [Google Scholar]
- 86.Derijk RH, van Leeuwen N, Klok MD, Zitman FG. Corticosteroid receptor-gene variants: Modulators of the stress-response and implications for mental health. Eur J Pharmacol. 2008;585:492–501. doi: 10.1016/j.ejphar.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 87.van Rossum EF, Binder EB, Majer M, Koper JW, Ising M, Modell S, Salyakina D, Lamberts SW, Holsboer F. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 88.van West D, Van Den Eede F, Del-Favero J, Souery D, Norrback KF, Van Duijn C, Sluijs S, Adolfsson R, Mendlewicz J, Deboutte D, Van Broeckhoven C, Claes S. Glucocorticoid receptor gene-based SNP analysis in patients with recurrent major depression. Neuropsychopharmacology. 2006;31:620–627. doi: 10.1038/sj.npp.1300898. [DOI] [PubMed] [Google Scholar]
- 89.Stevens A, Ray DW, Zeggini E, John S, Richards HL, Griffiths CE, Donn R. Glucocorticoid sensitivity is determined by a specific glucocorticoid receptor haplotype. J Clin Endocrinol Metab. 2004;89:892–897. doi: 10.1210/jc.2003-031235. [DOI] [PubMed] [Google Scholar]
- 90.Brouwer JP, Appelhof BC, van Rossum EF, Koper JW, Fliers E, Huyser J, Schene AH, Tijssen JG, Van Dyck R, Lamberts SW, Wiersinga WM, Hoogendijk WJ. Prediction of treatment response by HPA-axis and glucocorticoid receptor polymorphisms in major depression. Psychoneuroendocrinology. 2006;31:1154–1163. doi: 10.1016/j.psyneuen.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 91.Bet PM, Penninx BW, Bochdanovits Z, Uitterlinden AG, Beekman AT, van Schoor NM, Deeg DJ, Hoogendijk WJ. Glucocorticoid receptor gene polymorphisms and childhood adversity are associated with depression: New evidence for a gene-environment interaction. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:660–669. doi: 10.1002/ajmg.b.30886. [DOI] [PubMed] [Google Scholar]
- 92.Steiger H, Bruce K, Gauvin L, Groleau P, Joober R, Israel M, Richardson J, Kin FN. Contributions of the glucocorticoid receptor polymorphism (BclI) and childhood abuse to risk of bulimia nervosa. Psychiatry Res. 2011;187:193–197. doi: 10.1016/j.psychres.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 93.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Köhnlein O, Dabitz H, Brückl T, Müller N, Pfister H, Lieb R, Mueller JC, Lõhmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Müller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 94.Tsai SJ, Hong CJ, Chen TJ, Yu YW. Lack of supporting evidence for a genetic association of the FKBP5 polymorphism and response to antidepressant treatment. Am J Med Genet B Neuropsychiatr Genet. 2007;144:1097–1098. doi: 10.1002/ajmg.b.30246. [DOI] [PubMed] [Google Scholar]
- 95.Nemeroff CB, Owens MJ. Pharmacologic differences among the SSRIs: focus on monoamine transporters and the HPA axis. CNS Spectr. 2004;9:23–31. doi: 10.1017/s1092852900025475. [DOI] [PubMed] [Google Scholar]
- 96.Licinio J, O’Kirwan F, Irizarry K, Merriman B, Thakur S, Jepson R, Lake S, Tantisira KG, Weiss ST, Wong ML. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry. 2004;9:1075–1082. doi: 10.1038/sj.mp.4001587. [DOI] [PubMed] [Google Scholar]
- 97.Liu Z, Zhu F, Wang G, Xiao Z, Tang J, Liu W, Wang H, Liu H, Wang X, Wu Y, Cao Z, Li W. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett. 2007;414:155–158. doi: 10.1016/j.neulet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 98.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 99.Villafuerte SM, Del-Favero J, Adolfsson R, Souery D, Massat I, Mendlewicz J, Van Broeckhoven C, Claes S. Gene-based SNP genetic association study of the corticotropin-releasing hormone receptor-2 (CRHR2) in major depression. Am J Med Genet. 2002;114:222–226. doi: 10.1002/ajmg.10179. [DOI] [PubMed] [Google Scholar]
- 100.Papiol S, Arias B, Gasto C, Gutierrez B, Catalan R, Fananas L. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J Aff ect Disord. 2007;104:83–90. doi: 10.1016/j.jad.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 101.Landgraf R, Kessler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M, Nussbaumer M, Czibere L, Turck CW, Singewald N, Rujescu D, Frank E. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci. Biobehav Rev. 2007;31:89–102. doi: 10.1016/j.neubiorev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 102.Altemus M, Cizza G, Gold PW. Chronic fluoxetine treatment reduces hypothalamic vasopressin secretion in vitro. Brain Res. 1992;593:311–313. doi: 10.1016/0006-8993(92)91326-a. [DOI] [PubMed] [Google Scholar]
- 103.Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 104.Stewart LQ, Roper JA, Young WS, 3rd, O’Carroll AM, Lolait SJ. The role of the arginine vasopressin Avp1b receptor in the acute neuroendocrine action of antidepressants. Psychoneuroendocrinology. 2008;33:405–415. doi: 10.1016/j.psyneuen.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 105.Dempster EL, Burcescu I, Wigg K, Kiss E, Baji I, Gadoros J, Tamas Z, Kennedy JL, Vetro A, Kovacs M, Barr CL. Evidence of an association between the vasopressin V1b receptor gene (AVPR1B) and childhood-onset mood disorders. Arch Gen Psychiatry. 2007;64:1189–1195. doi: 10.1001/archpsyc.64.10.1189. [DOI] [PubMed] [Google Scholar]
- 106.van West D, Del-Favero J, Aulchenko Y, Oswald P, Souery D, Forsgren T, Sluijs S, Bel-Kacem S, Adolfsson R, Mendlewicz J, Van Duijn C, Deboutte D, Van Broeckhoven C, Claes S. A major SNP haplotype of the arginine vasopressin 1B receptor protects against recurrent major depression. Mol Psychiatry. 2004;9:287–292. doi: 10.1038/sj.mp.4001420. [DOI] [PubMed] [Google Scholar]
- 107.Estrada-Camarena E, Martínez-Mota L, Fernández-Guasti A. Sex differences in depression and its treatment. In: de Gortari P, editor. Psychoneuroendocrinology. Kerala, India: Research Signpost; 2011. pp. 97–113. [Google Scholar]
- 108.Linzer M, Spitzer R, Kroenke K, Williams JB, Hahn S, Brody D, deGruy F. Gender, quality of life, and mental disorders in primary care: results from the PRIME-MD 1 000 study. Am J Med. 1996;101:526–533. doi: 10.1016/s0002-9343(96)00275-6. [DOI] [PubMed] [Google Scholar]
- 109.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63:375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 110.Newmann J, Engel R, Jensen J. Age differences in depressive symptom experiences. J Gerontol. 1991;46:224–235. doi: 10.1093/geronj/46.5.p224. [DOI] [PubMed] [Google Scholar]
- 111.Estrada-Camarena E, Fernández-Guasti A, López-Rubalcava C. Antidepressant like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology. 2003;28:830–838. doi: 10.1038/sj.npp.1300097. [DOI] [PubMed] [Google Scholar]
- 112.Romano-Torres M, Fernández-Guasti A. Estradiol valerate elicits antidepressant-like effects in middle-aged female rats under chronic mild stress. Behav Pharmacol. 2010;21:104–111. doi: 10.1097/FBP.0b013e328337bdfc. [DOI] [PubMed] [Google Scholar]
- 113.Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- 114.Quitkin F, Stewart J, McGrath P, Taylor BP, Tisminetzky MS, Petkova E, Chen Y, Ma G, Klein DF. Are there differences between women’s and men’s antidepressant responses? Am J Psychiatry. 2002;159:1848–1854. doi: 10.1176/appi.ajp.159.11.1848. [DOI] [PubMed] [Google Scholar]
- 115.Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA. Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol. 2005;25:318–324. doi: 10.1097/01.jcp.0000168879.03169.ce. [DOI] [PubMed] [Google Scholar]
- 116.Herrera-Pérez JJ, Martínez-Mota L, Fernández-Guasti A. Aging impairs the antidepressant-like response to citalopram in male rats. Eur J Pharmacol. 2010;633:39–43. doi: 10.1016/j.ejphar.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 117.Reynolds CF, 3rd, Kupfer DJ. Depression and aging: a look to the future. Psychiatry Serv. 1999;50:1167–1172. doi: 10.1176/ps.50.9.1167. [DOI] [PubMed] [Google Scholar]
- 118.Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in response to stress and expression of depressive-like behaviours in the rat. Curr Top Behav Neurosci. 2011;8:97–118. doi: 10.1007/7854_2010_94. [DOI] [PubMed] [Google Scholar]
- 119.Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol Toxicol. 2010;106:226–233. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 120.Alonso SJ, Castellano MA, Afonso D, Rodriguez M. Sex differences in behavioral despair: relationships between behavioral despair and open field activity. Physiol Behav. 1991;49:69–72. doi: 10.1016/0031-9384(91)90232-d. [DOI] [PubMed] [Google Scholar]
- 121.Martínez-Mota L, Cruz-Martínez JJ, Márquez-Baltazar S, Fernández-Guasti A. Estrogens participate in the antidepressant-like effect of desipramine and fluoxetine in male rats. Pharmacol Biochem Behav. 2008;88:332–340. doi: 10.1016/j.pbb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 122.Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras Sfikakis A, Papadopoulou-Daifot Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 123.David DJ, Nic Dhonnchadha BA, Jolliet P, Hascoët M, Bourin M. Are there gender differences in the temperature profile of mice after acute antidepressant administration and exposure to two animal models of depression? Behav Brain Res. 2001;119:203–211. doi: 10.1016/s0166-4328(00)00351-x. [DOI] [PubMed] [Google Scholar]
- 124.Wilson CA, Davies DC. The control of sexual differentiation of the reproductive system and brain. Reproduction. 2007;133:331–359. doi: 10.1530/REP-06-0078. [DOI] [PubMed] [Google Scholar]
- 125.Martínez-Mota L, Fernández-Guasti A. Testosterone-dependent antidepressant like effect of noradrenergic, but not of serotonergic drugs. Pharmacol Biochem Behav. 2004;78:711–718. doi: 10.1016/j.pbb.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 126.Angold A, Worthman CS. Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. J Affect Disord. 1993;29:145–158. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 127.Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- 128.Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gaskin JH, Kitay JI. Adrenocortical function in the hamster. Sex differences and effects of gonadal hormones. Endocrinology. 1970;87:779–786. doi: 10.1210/endo-87-4-779. [DOI] [PubMed] [Google Scholar]
- 130.Martínez-Mota L, Ulloa RE, Herrera-Pérez J, Chavira R, Fernández-Guasti A. Sex and age differences in the impact of the forced swimming test on the levels of steroid hormones. Physiol Behav. 2011;104:900–905. doi: 10.1016/j.physbeh.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 131.Viau V, Lee P, Sampson J, Wu J. A testicular influence on restraint-induced activation of medial parvocellular neurons in the paraventricular nucleus in the male rat. Endocrinology. 2003;144:3067–3075. doi: 10.1210/en.2003-0064. [DOI] [PubMed] [Google Scholar]
- 132.Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurons in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004;16:272–278. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- 133.Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinology. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 134.Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CHR-immunoreactivity following gonadectomy. Neuroendocrinology. 1994;59:228–234. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- 135.Bingaman EW, Baeckman LM, Yracheta JM, Handa RJ, Gray TS. Localization of androgen receptor within peptidergic neurons of the rat forebrain. Brain Res bull. 1994;35:379–382. doi: 10.1016/0361-9230(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 136.Bingham B, Williamson M, Viau V. Androgen and estrogen receptor-beta distribution within spinal-projecting and neurosecretory neurons in the paraventricular nucleus of the male rat. J Comp Neurol. 2006;499:911–923. doi: 10.1002/cne.21151. [DOI] [PubMed] [Google Scholar]
- 137.Williamson M, Bingham B, Gray M, Innala L, Viau V. The medial preoptic nucleus integrates the central influences of testosterone on the paraventriculara nucleus of the hypothalamus and its extended circuitries. J Neurosc. 2010;30:11762–11770. doi: 10.1523/JNEUROSCI.2852-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bingham B, Myung C, Innala L, Gray M, Anonuevo A, Viau V. Androgen receptors in the posterior bed nucleus of the stria terminalis increase neuropeptide expression and the stress-induced activation of the paraventricular nucleus of the hypothalamus. Neuropsychopharmacology. 2011;36:1433–1443. doi: 10.1038/npp.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during trhe estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 140.Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 142.Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of the hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–845. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- 143.Ochedalski T, Subburaju S, Wynn PC, Aguilera G. Interaction between oestrogen and oxytocin on hypothalamic-pituitary-adrenal axis activity. J Neuroendocrinology. 2007;19:189–197. doi: 10.1111/j.1365-2826.2006.01525.x. [DOI] [PubMed] [Google Scholar]
- 144.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 146.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurbiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 147.Hrabovszky E, Kallo I, Hajszan T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-beta messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 148.Suzuki S, Handa RJ. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42. doi: 10.1002/cne.20457. [DOI] [PubMed] [Google Scholar]
- 149.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–1825. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightma SL. Postnatal masculinization alters the HPA axis phenotype in the adult female rat. J Physiol. 2005;563:265–274. doi: 10.1113/jphysiol.2004.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Seale JV, Wood SA, Atkinson HC, Lightman SL, Harbuz MS. Organizational role for testosterone and estrogen on adult hypothalamic-pituitary-adrenal axis activity in the male rat. Endocrinology. 2005;146:1973–1982. doi: 10.1210/en.2004-1201. [DOI] [PubMed] [Google Scholar]