Abstract
Chemically-inducible dimerization (CID) is a powerful tool that has proved useful in solving numerous problems in cell biology and related fields. In this review, we focus on case studies where CID was able to provide insight into otherwise refractory problems. Of particular interest are the cases of lipid second messengers and small GTPases, where the “signaling paradox” (how a small pool of signaling molecules can generate a large range of responses) can be at least partly explained through results gleaned from CID experiments. We also discuss several recent technical advances that provide improved specificity in CID action, novel CID substrates that allow simultaneous orthogonal manipulation of multiple systems in one cell, and several applications that move beyond the traditional CID technique of moving a protein of interest to a specific spatiotemporal location.
Keywords: Chemically-inducible dimerization, Rapamycin, Signaling paradox
Introduction
Chemically-inducible dimerization (CID) systems have been used as tools in cell biology for nearly 20 years. CID systems have the advantage of allowing manipulation of target molecules in a very spatially and temporally confined locale within the cell, which allows determination of interactions and pathways that would otherwise be difficult to study. In particular, CID allows us to probe the “signaling paradox”, that is, how a cell is able to generate a very large repertoire of signaling pathways using only a relatively limited set of proteins and other signaling molecules. The key to this paradox lies in the ability of cells to activate specific signaling molecules in a limited region of the cell and for a short amount of time; CID allows us to recapitulate such localized events in ways that would be difficult otherwise. In the following review, we will focus on several important papers that highlight this ability of CID to illuminate cellular signaling pathways (see Table 1 for a list of studies discussed in this paper). We will also discuss several recent technical advances in CID systems that allow even greater specificity or that allow multiple CID systems to operate within the same cell at once. Unfortunately, a complete history of the field is beyond the scope of this article (for thorough recent reviews, see Fegan et al. [5] and Putyrski and Schultz [21]).
Table 1.
CID case studies resolving biological problems
| Biological system studied | Biological insights | Technique(s) used | Publication |
|---|---|---|---|
| Lipids | |||
| KCNQ ion channels | PI(4,5)P2 sufficient to drive current through KCNQ channels | Plasma membrane targeting | Suh et al. Science 314, 1454 (2006) |
| Ca2+ influx, receptor-mediated endocytosis | Loss of PI(4,5)P2 disrupts Ca2+ influx, endocytosis | Plasma membrane targeting | Varnai et al. J Cell Biol 175, 377 (2006) |
| Clathrin-coated endocytic pits | Loss of PI(4,5)P2 disrupts clathrin-coated pits, dissociates Arp2/3 from plasma membrane | Plasma membrane targeting | Zoncu et al. Proc Nat Acad Sci USA 104, 3793 (2007) |
| Receptor-mediated endocytosis | Loss of PI(4,5)P2 dissociates AP-2 from plasma membrane at endocytic pits | Plasma membrane targeting | Abe et al. J Cell Sci 121, 1488 (2008) |
| PTEN tumor suppressor | Binding of PTEN to plasma membrane requires PI(4,5)P2 | Plasma membrane targeting | Rahdar et al. Proc Nat Acad Sci USA 106, 480 (2009) |
| Rab5a-positive endosomes | Loss of PI3P in maturing endosomes delays maturation, disrupts receptor recycling | Endosomal membrane targeting | Fili et al. Proc Nat Acad Sci USA 103, 15473 (2006) |
| Small GTPases | |||
| Cell-surface receptor CD25 | Recruitment of Rac1 to CD25 causes actin reorganization, receptor phagocytosis | Plasma membrane targeting | Castellano et al. J Cell Sci 113, 2955 (2000) |
| Axonal growth cone | HRas and PI3K form positive feedback loop, required for symmetry breaking | Plasma membrane targeting | Fivaz et al. Curr Biol 18, 44 (2008) |
| Neutrophil migration | PI3K-Rac-actin polymerization feedback loop for neutrophil polarization, migration; Rac activation alone insufficient | Plasma membrane targeting | Inoue and Meyer. PLoS ONE 3, e3068 (2008) |
| Organelle targeting motifs, Ras signaling | Expanded repertoire of target organelles for CID | Plasma membrane, organelle targeting, membrane cross-linking | Komatsu et al. Nat Methods 7, 206 (2010) |
| Others | |||
| ZAP70 tyrosine kinase | Recruitment of ZAP70 to plasma membrane in correct orientation induces signaling through MAPK and calcineurin pathways | Plasma membrane targeting | Graef et al. EMBO J 16, 5618 (1997) |
| Akt kinase in apoptosis | Recruitment of Akt to plasma membrane activates GSK3, NF-κB, sufficient to rescue cells from apoptosis-inducing stimuli | Plasma membrane targeting | Li et al. Gene Therapy 9, 233 (2002) |
| β-arrestin2 and vasopressin receptors | Association of β-arrestin 2 to GPCR causes internalization; recruitment of β-arrestin alone to plasma membrane sufficient to induce Erkl/2 signaling | Receptor targeting | Terrillon and Bouvier EMBO J 23, 3950 (2004) |
| Ca2+ channels at plasma membrane-ER junctions | Oligomerization of STIM1 causes accumulation at plasma membrane-ER junctions, CRAC channel formation | Ca2+-sensor oligomerization | Luik et al. Nature 454, 538 (2008) |
| Postsynaptic density (PSD) of neurons | CaMKIIa translocation to PSD acts to recruit proteasome to dendritic spines | Postsynaptic density (PSD) targeting | Bingol et al. Cell 140, 567 (2010) |
| UV photocaging | Photocaged rapamycin can be activated intracellularly | Photocaged rapamycin (“direct” route) | Karginov et al. J Am Chem Soc 133, 420 (2011) |
| UV photocaging | Localized photouncaging of rapamycin gives local results | Photocaged rapamycin (“indirect” route) | Umeda et al. J Am Chem Soc 133, 12 (2011) |
| Plant hormone-based CID | Abscisic acid (ABA)-based CID system to activate transcription | Abscisic acid (ABA)-based CID system | Liang et al. Sci Signaling 4, rs2 (2011) |
| Plant hormone-based CID | Gibberellin A3 (GA3)-based CID system combined with rapamycin system for intracellular logic gates | Gibberellin A3 (GA3)-based CID system | Miyamoto et al. Nat Chem Bio 8, 465 (2012) |
| Inducible protein activity | CID-activatable kinase activity independent of localization | CID-activatable kinase activity | Karginov et al. Nat Biotechnology 28, 743 (2010) |
| ER-mitochondrial contact regions | ER-mitochondrial membrane contact at many discrete points | Membrane cross-linking | Csordas et al. Mol Cell 39, 121 (2010) |
| Plasma membrane-ER contact regions | STIM1-Orail complex requires other members for productive interaction | Membrane cross-linking | Varnai et al. J Biol Chem 282, 29678 (2007) |
| Nuclear proteins in yeast | Rapid protein inactivation can be used to establish conditional mutant phenotypes | “Anchor-Away” for rapid protein inactivation | Haruki et al. Mol Cell 31, 925 (2008) |
| Adaptor protein complexes of clathrin-coated vesicles | AP-1 involved in retrograde trafficking, not anterograde | Inactivation by rerouting to mitochondria | Robinson et al. Dev Cell 18, 324 (2010) |
| GTPase activity | Initial sequestration of protein at Golgi minimizes background activity of target GTPases | Initial sequestration of protein at Golgi to minimize background | Phua et al. ACS Chem Biol (2012) |
| Phosphoinositide signals leading to actin reorganization | PI(4,5)P2 increase and PI(4)P depletion have separate effects on actin reorganization | “Lipid liberation” to increase lipid without depleting precursors | Ueno et al. Sci Signaling 4, ra87 (2011) |
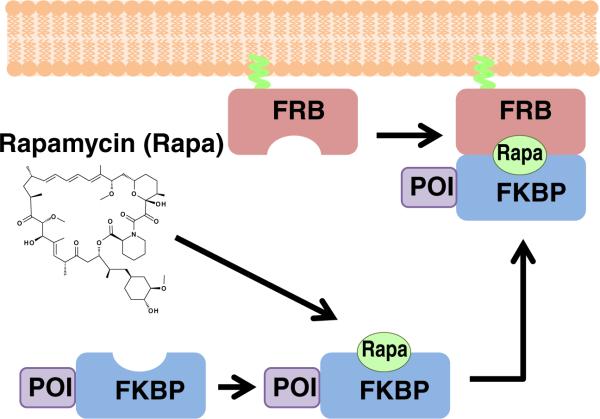
A typical CID system is shown in Fig. 1. Initially, one component (in this case, FRB) is localized and anchored at a specific cellular localization, while the other protein component (FKBP) is fused to a protein of interest (POI) and is diffusing freely in the cytoplasm. Upon addition of the dimerizer (rapamycin), a ternary complex of FKBP–rapamycin–FRB is formed, which has the result of bringing the POI to the target site. This translocation is typically rapid, on a timescale of seconds to minutes, and in current systems is essentially irreversible. Through careful choice of POI and targets, a wide variety of systems may be studied, as will be apparent in the following cases.
Fig. 1.
Schematic illustration of a typical CID experiment. Initially, one protein component (in this case, FRB) is anchored at a target site (in this case plasma membrane), while the other protein component (FKBP) is fused to a protein of interest (POI). In the absence of dimerizer, the POI-FKBP fusion protein diffuses freely in the cytoplasm. Upon addition of the dimerizer rapamycin, a ternary complex is formed of FKBP–rapamycin–FRB, which brings the POI to the target site
Lipids as targets of CID
The lipids of the plasma membrane have proved to be an attractive target for manipulation by CID. Given the importance of lipids as second messengers, and the involvement of PM in such fundamental processes as endocytosis, receptor trafficking, and cell migration, its potential interest as a target is obvious. Conventional techniques, such as the use of engineered kinase and phosphatase enzymes, including constitutively active and dominant negative versions, has yielded significant insight, but these techniques have limitations in the amount of time required for induction of an effect (timescale of hours) compared to the short period (seconds) that lipid signaling takes, and the difficulty of extrapolating from global effects of lipid manipulation to the highly localized signaling events often seen natively. Fortunately, the ready accessibility of plasma membrane to cytoplasmic proteins has allowed the development of a large number of CID probes targeted to this region.
Researchers have used CID to look at effects of the important second messenger phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2). Suh et al. [24] targeted Inp54p and PI(4)P 5-kinase to the plasma membrane to deplete or enhance, respectively, plasma membrane concentration of PI (4,5)P2. The potassium channel KCNQ was rapidly inactivated by depletion of PI(4,5)P2, even in the absence of other second messengers, and could be rapidly activated by increasing PI(4,5)P2. Importantly, when a PI 3-kinase was used to increase PI(3,4,5)P3 levels, no effect was seen on current through KCNQ channels, demonstrating once again the specificity of phosphoinositide effects. Another key study was performed by Varnai et al. [28]. PI(4,5)P2 was known to be a crucial molecule in the plasma membrane. PI (4,5)P2 was known to regulate many important proteins such as ion channels, phospholipases C and D, and also as the source of the second messengers diacylglycerol and inositol 1,4,5-triphosphate. A type IV 5-phosphatase domain was fused to FKBP, while plasma membrane-targeted FRB served as the anchor. Prior to addition of rapamycin, the 5-phosphatase fusion is in the cytoplasm, far from its target substrate, and thus effectively inactive. Addition of rapamycin induced rapid translocation to the PM and dephosphorylation of PI(4,5)P2. This had a number of effects, including loss of ATP-induced Ca2+ influx, decreased activity of TRPM8 channels, and loss of receptor-mediated endocytosis (assayed by transferrin receptor uptake). As the depletion of PI(4,5)P2 is rapid, and specific at the plasma membrane, these effects can be presumed to be directly due to PI(4,5)P2 depletion, in contrast to previous results relying on long-term disruption of PI(4,5)P2 levels which could cause secondary effects and were thus potentially difficult to interpret. This set the stage for further studies to characterize the effects of rapid gain or loss of specific phosphoinositides.
A different 5-phosphatase was used for several subsequent studies of PI(4,5)P2 depletion and its effect on endocytosis. Zoncu et al. [30] reported that rapid loss of PI(4,5)P2 caused disappearance of clathrin-coated pits. A follow-up study by Abe et al. [1], in contrast, found only a modest effect on clathrin assembly, although they did see serious disruption in receptor-mediated endocytosis. Specifically, transferrin receptor recycling was decreased, as was LDL receptor endocytosis, while plasma membrane localization of AP-2, epsin, and CALM adapters was disrupted.
Rahdar et al. [22] examined the plasma membrane binding of the important tumor suppressor gene PTEN. Depletion of PI(4,5)P2 in the plasma membrane by Inp54p resulted in rapid loss of PTEN binding to the inner leaflet of the plasma membrane. Finally, Inp54p was used in conjunction with a pharmacologic inhibitor of PI 3-kinase to test the plasma membrane phospholipid-binding specificity of proteins with polybasic clusters. Both PI(4,5)P2 and PI(3,4,5)P3 depletion was required for loss of membrane targeting of proteins with polybasic motifs, demonstrating that both phosphoinositides are used in targeting of these proteins to the plasma membrane.
The plasma membrane is not the only target for specific phosphoinositide depletion. Fili et al. [6] studied Rab5a-positive endosomes. With Rab5a-2xFKBP as the anchor, FRB-myotubularin phosphatase 1 (MTM1) could be recruited specifically to endosomes, where the MTM1 degraded PI3P specifically. This loss of PI3P resulted in delays in endosome maturation, giving a tubularized structure, and prevented recycling of transferrin receptor. EGF receptor cycling was delayed but not entirely lost. The crucial importance of specific phosphoinositides was thus shown.
Small GTPases as targets of CID
Small GTPase molecules have proved another major class of cellular molecules whose interactions can be teased apart by careful use of CID systems, in addition to such standard techniques as dominant negative and constitutively active mutants or pharmacological inhibition. One early such study, by Castellano et al. [3], examined the GTPase Rac1 in a model of phagocytosis. A cell-surface receptor, CD25, was fused to FKBP and introduced into cells along with FRB-tagged constitutively active Rac1. Latex beads were used to cluster the CD25 receptors, and thus lead to concentrated patches of Rac1 upon rapamycin stimulation. Recruitment of the active Rac1 caused phagocytosis of the CD25-bound latex beads in an actin-dependent fashion.
The involvement of the small GTPase HRas in neuronal development was also studied using CID by Fivaz et al. [7]. During neuronal development, polarity develops via a process of symmetry breaking resulting in one neurite being selected to grow rapidly to form the axon. The small GTPase HRas was known to activate PI3K in the developing neuron, and the hypothesis was formed that the two form a positive feedback loop. CID was used to target a cytosolic PI3K to the plasma membrane, which resulted in increased HRas activation (as seen by increase in the GTP-bound form). The precise mechanism of this feedback was not determined, although it is possible that the enhanced production of PI(3,4,5)P3 at the plasma membrane as a result of PI3K activity may serve as a signal to HRas.
CID also proved useful in testing a positive feedback model of the polarization of migrating neutrophils [10]. According to the model, PI3K (activated by a chemoattractant), acting through a downstream effector Rac (a small GTPase), induces actin remodeling; the actin remodeling then feeds back to the PI3K, creating a positive feedback loop. CID was used to bring PI3K to the plasma membrane in the absence of chemoattractant, and neutrophils showed rapid PI(3,4,5)P3 production, actin remodeling, and polarization. When Rac was directly activated, however (using the guanine nucleotide exchange factor Tiam1), there was no PI(3,4,5)P3 production or polarization observed. This, combined with pharmacologic data, suggested the existence of some other input besides chemoattractant signaling through G-protein-coupled receptor (GPCR) is necessary, in what the authors compared to an AND-gate. These CID results have supported the compartmentalization model of GTPase signaling, whereby the compartment- and time-specific activation of GTPases is key to their signaling specificity [13]. For example, activation of Ras specifically at the plasma membrane can induce membrane ruffling, while activation of Ras at the Golgi has no effect on cell morphology [13]. This compartmentalization is in agreement with earlier models of Ras and MAPK signaling pathways [17, 19].
Other applications
An interesting early use of CID by Graef et al. [8] was to study requirements of membrane recruitment and orientation in activation of the non-receptor tyrosine kinase ZAP70. Recruitment of ZAP70 to the plasma membrane led to its phosphorylation and the activation of Ras/MAPK and Ca2+/calcineurin signaling. A series of different synthetic dimerizers (rapalogs) was also used in bringing ZAP70 to the membrane that would provide different conformations and different degrees of rotational freedom for the ZAP70 relative to the plasma membrane. It was found that merely bringing ZAP70 to the PM was not in itself sufficient to induce its activity. Rapamycin, which locks FKBP-FRB into a rigid structure, did not lead to activation of ZAP70 although it did bring it to the plasma membrane; only those rapalogs that allowed greater conformational freedom led to activation. Additionally, this study was the first report that the SH2 domain was involved only in membrane binding and not kinase activity, as the entire SH2 domain could be replaced with the CID system without affecting kinase activity.
Membrane recruitment of another kinase, Akt, was performed by Li et al. [14] to uncover the importance of membrane localization in the activation of this important anti-apoptotic factor. The PH domain of Akt was replaced with FKBP, which could then be recruited to a myristoylated FRB. Recruitment of the recombinant Akt to plasma membrane caused its phosphorylation and the activation of its downstream target NF-κB. The true functional ability of this reconstituted system was proved by exposing Jurkat cells with the induced Akt to apoptotic factors including staurosporine, anti-Fas antibody, and the DNA damage induced by etoposide.
Another early study was done by Terrillon and Bouvier [25], who studied the role of β-arrestin2 in GPCR signaling. It was known that β-arrestin could associate with activated GPCRs, and that these β-arrestin-bound receptors were then internalized, but it was not clear whether β-arrestin bonding was sufficient to induce receptor uptake, or whether some additional signal was sent by the active (ligand-bound) receptor. By using CID, Terrillon and Bouvier were able to force β-arrestin to associate with two different vasopressin receptors, V2R or V1aR. Association of β-arrestin to the receptors was sufficient to induce their endocytosis in the absence of ligand, demonstrating that β-arrestin was acting as a signaling molecule for receptor uptake. Interestingly, when FRB-β-arrestin2 was recruited directly to PM via a myristoylated FKBP, this was sufficient to induce signaling leading to ERK1/2 phosphorylation, even without the involvement of GPCR receptors.
Stromal interaction molecule 1 (STIM1) is a Ca2+ sensor in the ER that plays an important role in Ca2+ release at ER-plasma membrane junctions. Luik et al. [16] used CID to induce oligomerization of STIM1 in the absence of Ca2+ signals. Oligomerization of STIM1 led to STIM1 accumulation at ER-plasma membrane junctions, and this oligomerization was sufficient to drive Ca2+ entry through CRAC channels.
Another example of localization driving function was shown by Bingol et al. [2]. The protein kinase CaMKIIα is abundant in the postsynaptic density of neurons, and was known to be important in synaptic plasticity. Its extremely high concentration in dendritic shafts led to speculation that it might be playing a scaffolding role. Using PSD95 as the anchor in a CID system, CaMKIIα was induced to translocate to postsynaptic sites. This localization of CaMKIIα was shown to drive recruitment of proteasomes to the postsynaptic density. This finding is consistent with the involvement of CaMKIIα in synaptic plasticity, as proteasomes are expected to be present at a site of extensive cellular remodeling, but this requirement would have been difficult to determine directly without the use of CID.
New technologies
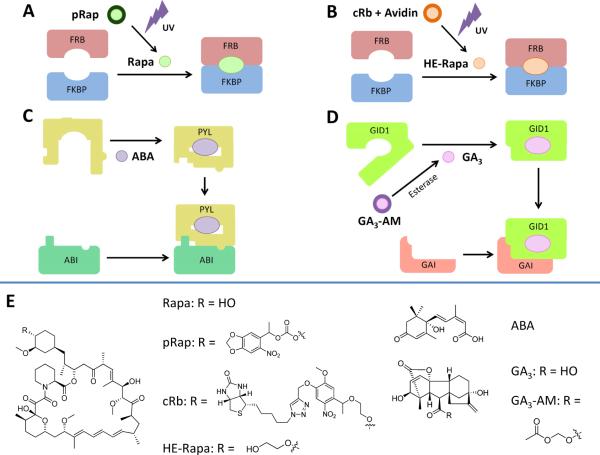
Photocaging of the dimerizer in a CID system potentially offers greater selection in the ability to induce dimerization in very specific regions of a cell, as the photoactivating light can be focused on only a small area of the target cell. Recently, two different groups have published different approaches to the same problem. Karginov et al. [12] modified rapamycin at the C40 hydroxyl position with a bulky acyl group to create pRap (Fig. 2a). However, pRap was able to induce dimerization between FKBP and FRB; a modified version of FKBP (iFKBP) was needed to discriminate between caged (pRap) and uncaged (Rap) dimerizer. Treatment of cells with pRap did not induce dimerization of iFKBP and FRB; irradiation with UV light at 365 nm for 1 min was sufficient to induce robust dimerization, as assayed by IP pulldowns, translocation of fluorescent protein-labeled FRB, and activation of kinase activity. While the ability to induce dimerization on only a small subcellular region was not demonstrated in this paper, and the requirement to replace FKBP with iFKBP in existing constructs may slightly hinder further applications, this remains an exciting technique.
Fig. 2.
Variations on the CID theme: caged rapamycin and plant hormone CID systems. a Direct UV photocaging: the caged rapamycin derivative pRap is unable to induce FKBP-FRB dimerization. Activation by UV light releases rapamycin, which can then induce dimerization as normal. b Indirect UV photocaging: the cRb-A complex is unable to cross the cell membrane, and thus unable to interact with intracellular FRB or FKBP proteins. Activation by UV light releases HE-Rapa, which is able to cross the plasma membrane and induce FRB-FKBP dimerization. c Plant hormone CID: the plant hormone abscisic acid (ABA) is able to induce complex formation between the ABI and PYL protein moieties, in a manner analogous to rapamycin-induced dimerization of FRB and FKBP. d Plant hormone CID: The gibberellic acid derivative GA3-AM, upon crossing the plasma membrane, is rapidly cleaved by cytosolic esterases to release GA3, which can induce complex formation between GID1 and GAI proteins
A different strategy was used by Umeda et al. [27]. Modification of rapamycin at the C40 group was again performed, but this time, the caging group includes biotin, which has an extremely tight binding affinity for the protein streptavidin (Fig. 2b). The caged rapamycin–biotin–streptavidin conjugate (cRb-A) is excluded from entry into cells due to the bulky streptavidin protein, which does not cross the plasma membrane. UV irradiation releases the biotin–streptavidin conjugate, leaving free rapamycin which can then freely diffuse into the cell to induce CID. Importantly, highly localized spatial control was demonstrated: irradiation of a small portion of a cell was able to induce localization of a Tiam1 fusion protein (as assayed by induction of membrane ruffling) in only the irradiated quadrant of the cell, with very little spill-over into adjacent regions of the cell. This high specificity is a valuable asset, though unfortunately, due to the nature of the caging moiety, it is only useful if the protein of interest is active at or very near the plasma membrane.
It would be useful to have multiple, orthogonal dimerization systems available that could potentially be used in the same cell simultaneously to generate complex outputs. Two different groups have recently published novel CID systems that rely on plant hormones and their cognate binding proteins. Liang et al. [15] utilized the plant hormone S-(+)-abscisic acid (ABA) and its binding proteins PYL1 and ABI1 (Fig. 2c). When utilized in a manner equivalent to rapamycin and FKBP-FRB, the ABA system was able to induce protein localization in mammalian cells. The ABA system was shown to be orthogonal to the rapamycin system, as both could be used simultaneously in the same cells without interference. Miyamoto et al. [18] utilized a different phytohormone, gibberellic acid 3 (GA3) and its cognate binding proteins GAI and GID1 (Fig. 2d). The GA3 system was also fully orthogonal to rapamycin in mammalian cells; this was exploited to produce simple proof-of-concept AND and OR logic gates using GA3 and rapamycin as inputs. Combination of one of the plant hormone-derived CID systems with rapamycin CID should allow the ability to probe multiple portions of signal transduction pathways simultaneously.
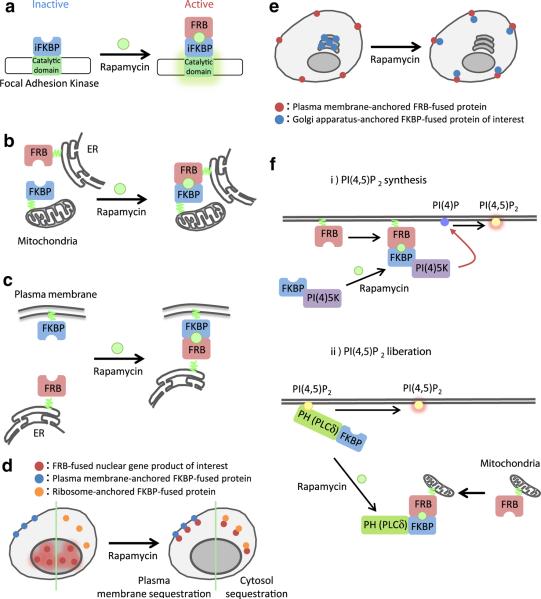
One intriguing application of CID is the selective activation of a protein kinase via an inserted CID site (Fig. 3a). Karginov et al. [11] demonstrated that insertion of iFKBP into the catalytic domain of focal adhesion kinase causes the resultant protein to be catalytically inactive. Rapamycin-induced dimerization with FRB, however, causes a conformational change that restores catalytic activity without affecting other properties of the protein. This ability to specifically activate a kinase was shown to be generalizable when insertions of iFKBP in Src and p38 behaved similarly. This may be a useful strategy for induction of catalytic activity in any desired kinase.
Fig. 3.
Further variations on the CID theme. a Kinase activation: Insertion of the iFKBP into the kinase domain of a protein can cause the protein to be catalytically inactive. Binding of the iFKBP to rapamycin and FRB induces a conformational change that restores kinase activity. b and c Membrane cross-linking: FRB and FKBP proteins are anchored to different cellular membranes. Addition of rapamycin leads to cross-linking of the membranes. d “Anchor-Away” technique: The POI is initially at its active location. Addition of rapamycin induces translocation to a different domain where is sequestered and inactive. e Inactive reservoir: POI is initially sequestered at a location where it is inactive (in this case the Golgi) rather than floating freely in the cytoplasm where it can have background activity. Addition of dimerizer induces translocation to the active site without membrane cross-linking. f Lipid synthesis versus lipid liberation: standard targeting of a PI(4) 5-kinase to plasma membrane induces formation of PI(4,5)P2, but also depletes the plasma membrane supply of PI(4)P. If instead the PI(4,5)P2 at the plasma membrane is initially “hidden” with a PH domain, which is then removed by sequestration to the mitochondrial membrane, the concentration of available PI(4,5)P2 at the plasma membrane is suddenly increased without affecting concentrations of other membrane lipids
One application of CID is to cross-link different membrane-bound organelles via FRB and FKBP that are tethered specifically to different organelles in the same cell (Fig. 3b, c) [13]. As this cross-linking requires that the organelles in question must already come into very close proximity, this is useful for studying cases where membranes from different organelles are naturally tethered already. This was utilized by Csordas et al. [4] to study the ER–mitochondrial junction, which is important for recruitment of Ca2+ stores from ER into mitochondrial matrix. A similar strategy was also used [29] to examine the ER–plasma membrane junction during Ca2+ release. Variation of linker sizes revealed an unsuspected requirement for some space between the ER protein STIM1 and the plasma membrane Ca2+ channel Orai1, implying that a previously unknown protein complex must bridge these two components. This result would have been difficult to achieve without the use of a CID to stabilize the relatively transient interaction between the two membranes.
Many proteins are only active at a specific cellular location, and can thus be inactivated by moving them to a different location within the cell. Haruki et al. [9] developed a CID system they dubbed the “Anchor-Away” technique to rapidly inactivate nuclear proteins (Fig. 3d). First, they used FKBP-fused plasma membrane proteins as the “anchor” to verify that FRB-tagged proteins that shuttle between nucleus and cytoplasm could be sequestered by rapamycin addition. Next, taking advantage of the continuous flow of ribosomal proteins into and out of the nucleus, they fused FKBP to a ribosomal protein (the “anchor”) and FRB to various nuclear proteins of interest. Addition of rapamycin would be expected to deplete the targeted gene from the nucleus, which they confirmed for over 40 different target genes involved in transcription, nuclear transport, and chromosome structure. Work by Robinson et al. [23] utilized mitochondrial-targeted FRB as the anchor and adaptor protein (AP)-complex proteins from clathrin-coated vesicles as the target. The ability to rapidly (seconds to minutes time-scale) deplete AP-1 was used to show that AP-1 is involved in retrograde transport only, a point that had been unclear in previous studies relying on much more slowly-acting knockdown effects.
Unfortunately, when the POI in a CID system is an enzyme that is active at a particular membrane, there is a problem of background activity by the enzyme prior to localization to the target site. This is particularly likely when the POI is floating free in the cytoplasm and can thus come transiently in contact with the target membrane prior to the induction of CID. One recent technique resolves this problem by initially sequestering the POI to one organelle (specifically, the Golgi apparatus) and then upon addition of dimerizer sending the POI to its target at the plasma membrane (Fig. 3e) [20]. As the initial localization to the outer surface of the Golgi membrane is via binding of an FAPP (PH) domain to PI(4)P, which is relatively weak relative to the FRB-rapamycin-FKBP interaction, the POI is targeted to the plasma membrane without inducing cross-linking of plasma membrane and Golgi. Further developments should increase the repertoire of initial sequestration sites and final targets.
One final new technique, dubbed “liberation”, is designed to get around the limitation of simply targeting phosphatases or kinases to increase or decrease specific membrane lipid concentrations (Fig. 3f) [26]. Rapid generation of PI(4,5)P2 at the plasma membrane can be achieved by using CID to target a PI(4)P 5-kinase to the plasma membrane; however, in addition to increasing PI(4,5)P2 levels, this inevitably also depletes the pool of PI(4)P, which may have unintended consequences. In the “liberation” technique, a PH domain from PLCδ is fused to FKBP. The PH domain specifically binds to PM PI(4,5)P2, essentially sequestering it from accessibility to other interacting proteins. An FRB is co-expressed that is mitochondria-anchored. Addition of rapamycin rapidly induces translocation of the PLCδ domain to mitochondria, “liberating” the PI(4,5)P2 at the plasma membrane without affecting the level of PI(4)P or other metabolites. Indeed, while increased PI(4,5)P2 synthesis (and concomitant PI(4)P loss) causes formation of actin comets, PI(4,5)P2 liberation (without affecting PI(4)P level) induces membrane ruffling but no actin comet formation. These two different signaling pathways would have remained entangled without the use of this new technology to separate the effects of two different lipid second messengers.
Perspectives
CID is a robust tool for studying proteins in a highly subcellular localization specific fashion and in a highly temporally-limited manner (on a timescale of seconds). This has allowed us to get a handle on the “signaling paradox” to understand how cells are able to generate a large variety of signals using a relatively small number of fundamental signaling molecules. While CID has been most spectacularly used to study signaling pathways, particularly those involving membrane lipids and small GTPases, it has also proved useful in many other systems. Recent advances allow even greater specificity of localization or activation and allow the control of orthogonal signals at the same time. CID is expected to remain a major tool in the cell biologist's toolbox for many years to come. It is hoped that readers will be inspired to consider how CID may be useful in their own research.
Acknowledgments
The authors' research was supported by NIH grants GM092930 to T.I. We regret that due to length considerations, we could not discuss many worthwhile papers that have appeared in the literature over the years.
References
- 1.Abe N, Inoue T, Galvez T, Klein L, Meyer T. Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferring receptor. J Cell Sci. 2008;121:1488–1494. doi: 10.1242/jcs.020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKIIα acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–578. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Castellano F, Montcourrier P, Chavrier P. Membrane recruitment of Rac1 triggers phagocytosis. J Cell Sci. 2000;113:2955–2961. doi: 10.1242/jcs.113.17.2955. [DOI] [PubMed] [Google Scholar]
- 4.Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnoczky G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fegan A, White B, Carlson JCT, Wagner CR. Chemically controlled protein assembly: techniques and applications. Chem Rev. 2010;110:3315–3336. doi: 10.1021/cr8002888. [DOI] [PubMed] [Google Scholar]
- 6.Fili N, Calleja V, Woscholski R, Parker PJ, Larjani B. Compartmental signal modulation: endosomal phosphatidylinositol 3-phosphate controls endosome morphology and selective cargo sorting. Proc Nat Acad Sci USA. 2006;103:15473–15478. doi: 10.1073/pnas.0607040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fivaz M, Bandara S, Inoue T, Meyer T. Robust neuronal symmetry breaking by Ras-triggered local positive feedback. Curr Biol. 2008;18:44–50. doi: 10.1016/j.cub.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 8.Graef IA, Holsinger LJ, Diver S, Schreiber SL, Crabtree GR. Proximity and orientation underlie signaling by the non-receptor tyrosine kinase ZAP70. EMBO J. 1997;16:5618–5628. doi: 10.1093/emboj/16.18.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haruki H, Nishikawa J, Laemmli UK. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell. 2008;31:925–932. doi: 10.1016/j.molcel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Inoue T, Meyer T. Synthetic activation of endogenous PI3K and Rac identifies an AND-gate switch for cell polarization and migration. PLoS One. 2008;3(8):e3068. doi: 10.1371/journal.pone.0003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karginov AV, Ding F, Kota P, Dokholyan NV, Hahn KM. Engineered allosteric activation of kinases in living cells. Nat Biotechnol. 2010;28:743–747. doi: 10.1038/nbt.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karginov AV, Zou Y, Shirvanyants D, Kota P, Dokholyan NV, Young DD, Hahn KM, Deiters A. Light regulation of protein dimerization and kinase activity in living cells using photocaged rapamycin and engineered FKBP. J Am Chem Soc. 2011;133:420–423. doi: 10.1021/ja109630v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komatsu T, Kukelyansky I, McCaffrey JM, Ueno T, Varela LC, Inoue T. Nat Method. 2010;7:206–208. doi: 10.1038/nmeth.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Desai SA, MacCokle-Chosnek RA, Fan L, Spencer DM. A novel conditional Akt “survival switch” reversibly protects cells from apoptosis. Gene Ther. 2002;9:233–244. doi: 10.1038/sj.gt.3301641. [DOI] [PubMed] [Google Scholar]
- 15.Liang FS, Ho WQ, Crabtree GR. Engineering the ABA plant stress pathway for regulation of induced proximity. Sci Signal. 2011;4(164):rs2. doi: 10.1126/scisignal.2001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto T, DeRose R, Suarez A, Ueno T, Chen M, Sun T, Wolfgang MJ, Mukherjee C, Meyers DJ, Inoue T. Rapid and orthogonal logic gating with a gibberellin-induced dimerization system. Nat Chem Biol. 2012;8:465–470. doi: 10.1038/nchembio.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mor A, Philips MR. Compartmentalized Ras/MAPK signaling. Annu Rev Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- 20.Phua SC, Pohlmeyer C, Inoue T. Rapidly relocating molecules between organelles to manipulate small GTPase activity. ACS Chem Biol. 2012 doi: 10.1021/cb300280k. doi:10.1021/cb300280k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putyrski M, Schultz C. Protein translocation as a tool: the current rapamycin story. FEBS Lett. 2012 doi: 10.1016/j.febslet.2012.04.061. doi:10.1016/j.febslet.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 22.Rahdar M, Inoue T, Meyer T, Zhang J, Vazquez F, Devreotes PN. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Nat Acad Sci USA. 2009;106:480–485. doi: 10.1073/pnas.0811212106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson MS, Sahlender DA, Foster SD. Rapid inactivation of proteins by rapamycin-induced rerouting to mitochondria. Dev Cell. 2010;18:324–331. doi: 10.1016/j.devcel.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terrillon S, Bouvier M. Receptor activity-independent recruitment of βarrestin2 reveals specific signaling modes. EMBO J. 2004;23:3950–3961. doi: 10.1038/sj.emboj.7600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno T, Falkenburger BH, Pohlmeyer C, Inoue T. Triggering actin comets versus membrane ruffles: distinctive effects of phosphoinositides on actin reorganization. Sci Signal. 2011;4(203):ra87. doi: 10.1126/scisignal.2002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umeda N, Ueno T, Pohlmeyer C, Nagano T, Inoue T. A photocleavable rapamycin conjugate for spatiotemporal control of small GTPase activity. J Am Chem Soc. 2011;133:12–14. doi: 10.1021/ja108258d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 complex. J Biol Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 30.Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Nat Acad Sci USA. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]