Abstract
Cells rely on a complex, interconnected network of signaling pathways to sense and interpret changes in their extracellular environment. The development of genetically encoded fluorescent protein (FP)-based biosensors has made it possible for researchers to directly observe and characterize the spatiotemporal dynamics of these intracellular signaling pathways in living cells. However, detailed information regarding the precise temporal and spatial relationships between intersecting pathways is often lost when individual signaling events are monitored in isolation. As the development of biosensor technology continues to advance, it is becoming increasingly feasible to image multiple FP-based biosensors concurrently, permitting greater insights into the intricate coordination of intracellular signaling networks by enabling parallel monitoring of distinct signaling events within the same cell. In this review, we discuss several strategies for multiplexed imaging of FP-based biosensors, while also underscoring some of the challenges associated with these techniques and highlighting additional avenues that could lead to further improvements in parallel monitoring of intracellular signaling events.
Keywords: co-imaging, FRET, signal transduction, microscopy, biosensor
Introduction
Cells communicate with their extra- and intracellular environments using signal transduction pathways that relay messages into functional activities. These pathways consist of diverse arrays of tightly controlled biochemical events allowing cells to decipher various stimuli and produce specific responses. A multitude of components are responsible for the distinct biochemical events that take place, including ligands, receptors, channels, G proteins, second messengers, kinases, and phosphatases. The substantial number of components, combined with the fact that many of them participate in more than one signaling pathway and that a plethora of signaling events occur simultaneously within cells, underpins the complex nature of signal transduction. Proper transduction is achieved through temporal regulation and spatial compartmentalization of pathway constituents, permitting signaling events to occur rapidly and specifically, despite the complex cellular milieu. Conversely, dysregulation of signaling pathways contributes to many illnesses, such as cancer, diabetes, cardiovascular disease, inflammatory diseases, and neurodegenerative disorders [9, 22, 30]. A collection of different techniques have been developed to study the behavior of signaling pathways. Traditional biochemical approaches, including immunohistochemical and in vitro assays, frequently rely on fixed or lysed cells and only provide static glimpses into cell signaling, a process that is constantly in flux. Rather, the ever-changing world of dynamic interactions within cells needs to be observed in the native, live cell context. Recently, genetically encoded biosensors have been developed allowing dynamic signaling events to be monitored with high spatial and temporal resolution. Many of these sensors rely on fluorescent proteins (FPs), and can be used to detect a myriad of signaling events, such as post-translational modifications, second messenger accumulation/degradation, enzyme activity, membrane potential, and pH changes in real time within the native cellular environment [45]. Though FP-based genetically encoded biosensors reveal details of signaling dynamics, when they are used separately precise information on the interrelationship between two or more signaling events can become lost, specifically, information that brings to light whether or not certain events are synergistic, antagonistic, upstream or downstream of one another. To delve into the aforementioned intricacies of signaling networks, investigation as to how various events are coordinated and integrated within cells must occur. Multiplexed visualization of two or more biosensors in the same cell allows more than one biochemical event to be monitored in parallel, thus helping further our understanding of cell signaling.
In this review, we briefly discuss the basic principles governing the use of genetically encoded FP-based biosensors to study intracellular signaling in the native cellular context. We then explore some of the common strategies being used to perform multiparameter imaging with these biosensors, while also offering some perspectives on possible directions for the future of these techniques.
Genetically encoded biosensors for signaling
The ability to study real-time signaling dynamics in living cells has been greatly advanced due to genetically encoded fluorescent biosensors [18, 40, 45]. A series of biosensors have been generated to study a broad assortment of signaling molecules including various phosphoinositides (e.g., PIP2, PIP3) [4, 8, 53, 64-66], diacylglycerol [53, 66], Ca2+ [39, 41, 43, 43, 62], cAMP [15, 50, 52, 70], cGMP [26, 44, 51, 57, 60], nitric oxide [55, 59], ATP [27], glucose [13, 17], several GTPases [6, 29, 42, 69], a number of kinases [3, 11, 24, 28, 32, 33, 63, 66], and many more not noted herein. FP-based biosensors feature a modular design that contains two functional units: a sensing unit, which recognizes a specific biochemical event, and a reporting unit, which produces a fluorescent output. The reporting unit consists of either a single FP or a pair of FPs capable of undergoing fluorescence resonance energy transfer (FRET), whereas the sensing unit is typically derived from endogenous cellular proteins.
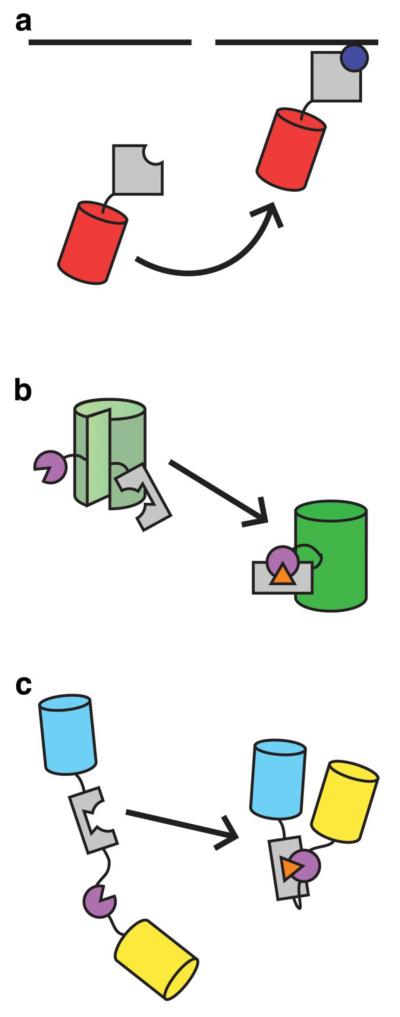
Translocation-based FP biosensors are composed of a single FP linked to a protein domain that is capable of binding a specific signaling molecule, coupling changes in the concentration of a molecule at a particular location - usually the plasma membrane - to changes in the distribution of biosensor fluorescence within the cell (Fig. 1a). For example, various pleckstrin homology (PH) domains have been tagged with green fluorescent protein (GFP) to study phosphoinositide dynamics in living cells [23]. These biosensors exhibit an increase in fluorescence at the plasma membrane, along with a corresponding decrease in biosensor fluorescence in the cytoplasm, or vice versa, in response to production or degradation of their target molecule.
Fig. 1.
General designs of FP-based biosensors. (a) Schematic of a translocation-based biosensor reporting on changes in the level of a signaling molecule at the plasma membrane through redistribution of fluorescence from the cytosol (left) to the membrane (right). (b) A single-FP based biosensor featuring a molecular switch inserted into a circularly permuted FP. Upon detection of a specific biochemical event (orange triangle), a conformational change in the molecular switch results in increased fluorescence intensity. (c) A FRET-based biosensor with an engineered molecular switch. As depicted here, the conformational change results in increased FRET
Changes in the intensity of biosensor fluorescence can also serve as an alternative readout for FP-based biosensors (Fig. 1b). For example, the Ca2+ indicator GCaMP consists of a circular permutant of GFP, in which the native N- and C-termini have been fused together and new termini, along with a 4 amino acid gap, introduced within the β barrel of GFP. Calmodulin (CaM) is attached to the new C-terminus, while the CaM-binding M13 peptide is fused to the new N-terminus. Together, CaM and the M13 peptide function as a molecular switch; in the presence of Ca2+, CaM binds the M13 peptide, resulting in a conformational change that closes the gap and increases fluorescence intensity [2, 62].
The conformational change produced by a molecular switch can also be readily used to alter the relative distance and/or orientation of a pair of FPs, leading to changes in FRET (Fig. 1c). One class of FRET-based biosensors are the Kinase Activity Reporters (KARs). The general design of KARs utilizes a two-component switch, comprised of a kinase-specific substrate peptide and a phosphoamino acid binding domain (PAABD), inserted between a FRET pair. Upon phosphorylation by the kinase of interest, the PAABD binds the phosphorylated residue, inducing a conformational change and altering FRET [12]. Molecular switches can also be derived from intrinsic conformational switches found in native cellular proteins. For example, the calcineurin (CaN) activity reporter, CaNAR, sandwiches a fragment from the N-terminal regulatory domain of the nuclear factor of activated T-cells (NFAT), a specific substrate of CaN, between cyan (CFP) and yellow fluorescent protein (YFP). This domain undergoes an intrinsic conformational change upon dephosphorylation by CaN, which translates into a FRET increase in CaNAR [46].
A major advantage of using these biosensors is their nondestructive delivery into cells. Additionally, given their reliance on the endogenous cellular machinery for expression, these biosensors can easily be targeted to different subcellular regions, offering a view of local signaling events within discrete environments. For example, Gallegos et al monitored distinct protein kinase C (PKC) activity dynamics throughout the cell using C Kinase Activity Reporter (CKAR). Specifically, they targeted CKAR to the plasma membrane, mitochondria, cytoplasm, Golgi complex, and nucleus. The various reporters revealed that basal, stimulated, and phosphatase-suppressed PKC activities differ for each subcellular region. Further investigation of these differences indicated that they are controlled by variations in Ca2+ release, localized DAG production, and the level of phosphatase activity present within in each region [19].
Employing FRET-based biosensors has helped scientists unearth detailed information on many signal transduction pathways. For instance, researchers previously found that mutually negative interactions between the PIP3 and RhoA GTPase signaling pathways were responsible for governing spontaneous polarization in neutrophils responding to uniform concentrations of a chemoattractant [68]. In cells expressing either a translocation-based PIP3 biosensor or a FRET-based reporter for RhoA activation, PIP3 accumulation could be observed at the leading edge of cells, along with the corresponding exclusion of RhoA activity to the back and sides of cells. Similarly, cAMP and cAMP-dependent protein kinase (PKA) signaling was recently shown to antagonize the effects of cGMP signaling on dendrite and axon formation. Specifically, cAMP accumulation and PKA activation were found to promote axon growth and inhibit dendrite formation in undifferentiated neurons, whereas heightened levels of cGMP had a reciprocal effect [61]. These and other studies, which capitalize on the use of different biosensors to monitor multiple signaling events, have significantly broadened our understanding of how cells integrate diverse signal transduction pathways. However, far more precise characterization of the interplay between these signaling pathways, such as analysis of kinetic interrelationships, could be afforded by visualizing multiple signaling events within the same cell.
Parallel monitoring of multiple cell signaling events with genetically encoded biosensors
Recent advances in FPs, molecular switch development, and imaging equipment provide the opportunity for the concurrent visualization of multiple biochemical processes in living cells. However, there are challenges associated with co-imaging that stem from a limited number of spectrally distinct fluorophores, as well as the practical limitations associated with using many filter sets. Despite these limitations, various methodologies can be implemented to work within co-imaging constraints.
Multiplexed imaging with single-fluorophore biosensors
At the very least, parallel imaging of multiple signaling events within a single cell requires the use of two or more biosensors with good spectral separation of their fluorophores. This can be achieved relatively easily using multiple single-fluorophore-based biosensors, as each reporter emits at only a single wavelength. A red fluorescent protein (RFP)-based pH sensor, pHTomato, was recently developed and fused to the vesicle membrane protein synaptophysin (sypHTomato), in order to permit visualization of activity-dependent exocytosis of synaptic vesicles [37]. By co-imaging sypHTomato with the GFP-based Ca2+ indicator GCaMP3, expressed in the same cell, it was possible to track neurotransmitter release in parallel with presynaptic Ca2+ transients occurring within individual nerve termini. Furthermore, co-imaging of sypHTomato and GCaMP3 expressed in different cells enabled simultaneous monitoring of pre- and post-synaptic behaviors between connected neurons.
Using FRET-based biosensors for multiplexed imaging
Parallel imaging of multiple signaling events in the same cell is also possible using genetically encoded FRET-based biosensors. By definition, each of these reporters already contains two spectrally distinct fluorophores. This limits the number of spectral FP variants which can be used together in an experiment, and presents a somewhat greater challenge for co-imaging. Of particular concern is spectral bleed-through between FRET pairs with insufficient spectral separation, which in some cases can significantly obscure the signal. Despite this, a number of methods have been devised to allow co-imaging using FRET-based reporters. Below, we focus on co-imaging techniques associated with the most common fluorescence intensity based FRET measurements.
Spatial separation of biosensors via subcellular localization
A simple strategy for co-imaging multiple FRET-based biosensors involves targeting them to different subcellular regions, thereby spatially separating the reporters within the cell (Fig. 2a). This largely avoids the problem of spectral bleed-through and, provided the regions are distinct enough to allow the fluorescent signals to be easily distinguished, can be performed using reporters that have spectrally similar FRET pairs. This approach is convenient when investigating how one signaling event affects another, monitoring signaling events in discrete subcellular compartments, and tracing the path of a signaling event as it unfolds through cellular space. Previously, a plasma membrane-localized Epac1-based cAMP biosensor (pmICUE) was co-imaged with a nuclear-localized PKA activity reporter (AKAR-NLS), both of which have a CFP/YFP (CY) FRET pair. These reporters were used to investigate the temporal relationship between the production of cAMP at the plasma membrane and PKA activity in the nucleus. In response to β-adrenergic receptor stimulation, cAMP accumulation at the plasma membrane was instantaneous, while the onset of nuclear PKA activity was delayed by 5 – 10 minutes. To determine the source of this delay, pmICUE was co-imaged with ICUE-NLS. In contrast to nuclear PKA, nuclear cAMP accumulates more or less as quickly as plasmalemmal cAMP. This study implies that nuclear PKA signaling following receptor stimulation is temporally controlled by translocation the catalytic domain of PKA into the nucleus [16, 58]. Similarly, this strategy was used to examine the relative timing of PKC and calcium/calmodulin-dependent protein kinase IIα (CaMKIIα) activation upon elevation of intracellular Ca2+ levels. PKC activity was monitored at the plasma membrane using pmCKAR, and CaMKIIα activity was monitored throughout the cytoplasm using CYCaMIIα. Co-imaging of these CY-FRET biosensors showed that PKC activity precedes CaMKIIα activation [56].
Fig. 2.
Representative illustrations of co-imaging strategies using FRET-based biosensors. (a) Spatial separation of spectrally similar biosensors to co-image signaling dynamics in distinct cellular compartments, e.g. plasma membrane and nucleus. (b) Spectrally separated biosensors can be used to co-image multiple signaling events in the same region of the cell. One strategy involves using biosensors with distinct FRET donors and a shared acceptor
As demonstrated by these studies, concurrent imaging of multiple signaling events can be achieved using spectrally similar FRET-based biosensors. As many biosensors utilize the CY FRET pair, targeting these reporters to distinct subcellular regions provides a convenient method for co-imaging that does not require any additional imaging accessories or equipment.
Spectrally distinct fluorophores
Co-imaging using multiple, spectrally distinct biosensors (Fig. 2b) represents a more versatile and generalizable multiplexing strategy, as opposed to relying on identical FRET pairs targeted to distinct locations, especially when the question under investigation involves temporal relationship of two signaling events in the same cellular compartment. If the individual reporters can be directly distinguished by the imaging equipment, it is possible to visualize multiple signaling events in the same location. Though this approach does require the use of additional equipment beyond standard 2-color (i.e., cyan/yellow) FRET imaging, such as added filter sets, it nevertheless serves as a powerful technique for performing multiparameter imaging. Commonly used strategies include imaging a FRET-based biosensor with a single-fluorophore reporter, imaging two FRET-based biosensors with a shared acceptor, and imaging FRET-based biosensors with distinct FRET pairs.
FRET-based biosensor in conjunction with a single-fluorophore reporter
Imaging a FRET-based biosensor in parallel with a reporter that utilizes only a single additional fluorophore is the simplest method for performing multiparameter imaging with spectrally distinct biosensors. For example, BKAR, a CY-FRET-based biosensor for monitoring protein kinase B (PKB) activity, was previously co-imaged in conjunction with the PH domain of PKBα fused to red fluorescent protein (RFP), which functions as a PIP3 biosensor. Concurrent imaging of these two biosensors revealed that the production of PIP3 at the plasma membrane, which coincides with PKB activation at the membrane, precedes the activation of cytosolic PKB [34].
Another approach involves co-imaging a FRET-based biosensor with a dye-based fluorescent indicator, such as fura-2, which is a widely used fluorescent Ca2+ indicator. By co-imaging fura-2 with the CY-FRET-based cAMP biosensor Epac1-camps, Landa and colleagues were able to simultaneously monitor Ca2+ and cAMP dynamics in MIN6 β cells. They demonstrated a highly coordinated interplay between these second messengers, specifically in the form of Ca2+-dependent cAMP oscillations in response to membrane depolarization [36]. Though fura-2 can be successfully co-imaged with CY-FRET-based biosensors, its spectral properties are not ideally suited to this purpose, particularly due to contamination of the FRET channel by the fura-2 signal [21]. One way to avoid this is by co-imaging using Fura red [10, 35]. Using this approach, it was possible to directly observe coupling between histamine-induced PKC oscillations, monitored using pmCKAR, and Ca2+ oscillations in HeLa cells [66]. Alternatively, fura-2 can be co-imaged with a non-CY FRET pair. For example, fura-2 was recently co-imaged with a green/red-FRET-based PKA activity reporter, as well as with a yellow/red-FRET-based cAMP indicator. These studies shed additional light on the interplay between Ca2+, cAMP, and PKA in MIN6 β cells, specifically the PKA-mediated potentiation of Ca2+ influx [47].
Two FRET pairs with a shared acceptor
An additional, simple approach that can be utilized to co-image multiple parameters is to use two spectrally distinct FRET-based biosensors that share a common acceptor. In order to image two FRET pairs with a common acceptor and differentiate FRET between each pair, the individual channels must be acquired sequentially, in rapid succession. Moreover, along with the basic requirement that the donor and acceptor excitation spectra do not overlap, both donors must also not have overlapping excitation spectra. For example, CFP and YFP are already known to fulfill the latter requirement, while RFP offers adequate spectral separation and has been shown to serve as a FRET acceptor for both CFP and YFP (Fig. 3a). Furthermore, as concomitant imaging of CFP/RFP (CR) and YFP/RFP (YR) FRET pairs only requires the addition of an RFP emission filter to an existing imaging setup, this is a very accessible technique. For example, in order to understand the temporal relationship between the activation of initiator and effector caspases within cells, Kawai et al generated a YR-FRET biosensor for initiator caspase and a CR-FRET biosensor for effector caspase. Their parallel tracking experiments revealed that initiator and effector caspases are activated virtually simultaneously during TNF-α-induced cell death in HeLa cells [31].
Fig. 3.
Excitation/emission spectra for various FRET pairs used in co-imaging. (a) CFP and YFP excitation spectra are sufficiently separated, minimizing cross-excitation, while their emission spectra overlap the excitation spectrum of RFP, which serves as a common FRET acceptor. (b) Despite having distinct excitation and emission maxima, the spectra for OFP and RFP overlap significantly, potentially leading to cross-excitation and contamination of the FRET channel, resulting in a decreased signal-to-noise ratio. OFP is also not well separated from YFP, which may also lead to contamination of CY FRET measurements. (c) Although the emission spectrum for mAmetrine is virtually identical to that of Citrine, its long Stokes-shift allows it to serve as the donor for tdTomato, while Citrine serves as the acceptor for mTFP. However, overlap between the excitation spectra of the two donors leads to significant cross-excitation. In all panels, boxes are shown to illustrate the excitation (ex) and emission (em) filters that were used to image these FRET pairs.
Similarly, this strategy has also been used to investigate the temporal relationship between cAMP and PKA following the stimulation of various receptors in HEK293 cells. Using CR-AKAR to monitor PKA activity and YR-ICUE to monitor cAMP accumulation, we recently revealed that stimulation of EP receptors induces a relatively small, transient increase in cAMP, which corresponds to a large, sustained PKA response. Global β-adrenergic receptor stimulation was also shown to result in sustained PKA activity, though the response appeared to track more closely with the brief burst of cAMP accumulation [5]. In a separate study, a new twist on multiparameter imaging with a shared FRET acceptor was presented with a single-chain, dual-specificity biosensor for parallel tracking of PKA and cAMP dynamics, called ICUEPID. This reporter was used to correlate membrane depolarization-induced cAMP and PKA oscillations in MIN6 β cells [47].
Two spectrally distinct FRET pairs
The alternative to using a shared acceptor is to co-image FRET-based biosensors with orthogonal FRET pairs. Given the relatively broad spectra of FP color variants, the principle challenge associated with this technique is the identification of suitable FRET pairs that can be spectrally distinguished. This typically presents the need to tailor the imaging setup to the particular FRET pairs being used. Nevertheless, this technique has been explored for studying cell signaling. For example, a CY-FRET-based Src activity reporter was co-imaged with an orange/red (OR)-FRET-based biosensor for membrane type 1 matrix metalloproteinase (MT1-MMP), although the spectral separation between the latter FPs is minimal and thus not ideal for FRET (Fig. 3b). These biosensors were targeted to the plasma membrane in HeLa cells, and used to study the temporal relationship between Src and MT1-MMP activity in response to oncogenic stimulation by epidermal growth factor (EGF). Despite functioning in the same signaling pathway, these enzymes were found to exhibit distinct spatiotemporal activity dynamics. Specifically, upon EGF stimulation, Src activity was immediate, rapid, and dispersed throughout the plasma membrane, whereas MT1-MMP activity increased slowly and was largely confined to the cell periphery [54].
Other FPs are also being used in FRET pairs for multiparameter imaging. For example, a FRET pair consisting of mAmetrine as the donor and tdTomato as the acceptor was found to be suitable for co-imaging with an orthogonal pair consisting of teal fluorescent protein (mTFP1) as the donor for mCitrine (Fig. 3C) [1]. These two FRET pairs were recently used to simultaneously visualize Ca2+ in the nucleus and cytoplasm of HeLa cells. Although, the reporters were spatially segregated, the nucleus and cytoplasm are not visually distinct when illuminated with the same fluorophores. Therefore it is necessary to use spectrally distinct biosensors to co-image signaling events within these compartments. In addition, these FRET pairs have also been used for multiparameter imaging within the same subcellular compartment, as shown by concurrent imaging of Ca2+ dynamics and caspase-3 activity together in the cytoplasm [14].
It is important to stress, however, that all strategies for multiparameter imaging using spectrally distinct biosensors share certain inherent caveats. Despite the number of FP color variants, it remains challenging to find multiple FRET pairs that can be used to generate biosensors with enough dynamic range to detect physiologically relevant signaling events in cells [5]. For example, orange and red FPs have been found to suffer from poor sensitized emission, regardless of the donor [1], affecting the dynamic range in emission ratio-based measurements. Even when multiple FRET pairs can be found, the fact that all FPs are characterized by relatively broad excitation/emission spectra poses additional problems for co-imaging. Given the amount of spectrum taken up by each FP, in general no two FRET pairs will be perfectly orthogonal. Although selective filter sets are used during co-imaging experiments, cross-excitation of FPs and contamination of emission cannot be fully eliminated. Instead, mathematical correction factors often need to be applied during data analysis. Furthermore, co-imaging two FRET pairs leaves little of the visible spectrum for monitoring additional parameters.
Combining approaches allows visualization of more parameters
At present, parallel imaging of more than two parameters entails combining multiple co-imaging techniques into a single experiment. For example, concurrent imaging of cytosolic CaMKIIα, along with plasma membrane PKC and annexin A4 dynamics was achieved by combined monitoring of spatially separated CY-FRET-based biosensors for PKC activity and CaMKIIα activation with an OR-FRET-based biosensor for annexin A4 self-association in neuroblastoma cells. Adding Fura red to the cells further enabled tracking of Ca2+ as well, though this precluded the observation of OR-FRET from the annexin A4 reporter. By instead monitoring the quenching of orange fluorescence directly, the authors were able to overcome this and achieve parallel imaging of four distinct biochemical events [56]. Similarly, a biosensor for cGMP was developed by pairing blue fluorescent protein with a nonfluorescent-YFP quenching acceptor. This reporter was co-imaged with a CY-FRET cAMP biosensor and Fura red, allowing cAMP, cGMP, and Ca2+ signaling dynamics to be visualized concurrently [48].
Future perspectives and concluding remarks
Genetically encoded biosensors permit the visualization of a variety of biochemical events within living cells. Parallel imaging of multiple biosensors is increasingly being used to delve into the organization of intracellular signaling networks, providing insights into how cells coordinate and integrate multiple inputs into specific biological outputs. Co-imaging techniques continue to improve, and below we mention several strategies that could lead to more powerful multiparameter imaging.
As discussed above, single-fluorophore-based biosensors are suitable for performing multiparameter fluorescence imaging, either on their own or in conjunction with FRET-based biosensors. Currently, a number of genetically encoded single-fluorophore biosensors have been developed, yet, so far these reporters have been predominantly generated for use in monitoring the dynamics of various second messengers and cellular analytes [45, 62]. Furthermore, most of these reporters have, to date, utilized only a limited color palette. Neither of these limitations is inherent to the design of single-fluorophore biosensors, however, as their modular architecture can easily accommodate almost any molecular switch, as well as any circularly permuted FP color variant. This would allow significantly expanded opportunities for co-imaging of single-fluorophore biosensors alongside FRET-based reporters, while also leading to increased co-imaging of multiple single-fluorophore biosensors together.
Quantum dots (QDs) represent another avenue for the expansion of co-imaging methodologies. In particular, they have narrower excitation/emission spectra compared to FPs, in addition to superior brightness and photostability. Despite the noted advantages inherent to QDs, they are rarely used to study intracellular signaling events, as they do not readily cross the plasma membrane. However, recent advances have led to the development of polymer-coated QDs (pcQDs) that harness the cell-penetrating TAT-peptide, which facilitates internalization and avoids endosomal sequestration. Moreover, pcQDs were successfully targeted to intracellular proteins via conjugation of the pcQD to a specific antibody [7]. This serves as a proof-of-principle for the targeting of QDs to intracellular proteins, and potentially anticipates the development of QD-based biosensors.
In addition to experimental improvements gained through biosensor evolution, novel computational approaches also offer a way towards enhanced multiparameter imaging. For example, linear unmixing is a computational method allowing for spectral separation of the individual signals from a mixture of fluorophores [20, 25, 71]. This technique was recently used to perform multiparameter imaging using a Sapphire/RFP-FRET-based Ca2+ biosensor and a CY-FRET-based cAMP biosensor [49]. As this involves simultaneous excitation of the FRET donors, the temporal resolution of co-imaging experiments can be increased. However, this also requires the use of more elaborate imaging equipment capable of simultaneously separating and recording the individual emission channels.
As the inevitable march of progress leads to improved biosensors and more advanced imaging technologies, it is important to keep in mind that there will always be practical limitations on the number of parameters that can realistically be imaged within the same cell. Additional approaches are therefore being developed in order to generate a truly comprehensive model of signaling pathways, such as the ability to integrate and correlate the data from independent multiparameter imaging experiments [67]. For example, biosensors for the Rho GTPases Rac1, Cdc42, and RhoA were expressed in randomly migrating cells, and imaged individually as well as co-imaged in pairs. In this study, cell protrusions provided a morphological reference for uniform pathway function and for making spatiotemporal comparisons between experiments. Cross-correlation analyses were then used to determine the relationships between cell protrusions and spontaneous fluctuations in biosensor activity, showing that RhoA plays a role in initiating cell protrusions, whereas Rac1 and Cdc42 stabilize newly formed protrusions [38]. Further application of these “computational multiplexing” approaches will help lift multiparameter imaging beyond its practical limitations. Thus, parallel tracking of different signaling activities via concurrent imaging of multiple fluorescent biosensors should allow researchers to directly observe and elucidate the intricate interconnections that underlie intracellular signaling networks.
Acknowledgements
We wish to thank members of the Zhang lab for helpful discussions, in particular Fabian Hertel for comments on the manuscript. This work is supported by the National Institutes of Health (R01DK073368 and DP1OD006419 to J.Z.).
Footnotes
The authors declare they have no competing financial interests.
References
- 1.Ai HW, Hazelwood KL, Davidson MW, Campbell RE. Fluorescent protein FRET pairs for ratiometric imaging of dual biosensors. Nat Methods. 2008;5:401–403. doi: 10.1038/nmeth.1207. [DOI] [PubMed] [Google Scholar]
- 2.Akerboom J, Rivera JD, Guilbe MM, Malave EC, Hernandez HH, Tian L, Hires SA, Marvin JS, Looger LL, Schreiter ER. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J Biol Chem. 2009;284:6455–6464. doi: 10.1074/jbc.M807657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananthanarayanan B, Fosbrink M, Rahdar M, Zhang J. Live-cell molecular analysis of Akt activation reveals roles for activation loop phosphorylation. J Biol Chem. 2007;282:36634–36641. doi: 10.1074/jbc.M706227200. [DOI] [PubMed] [Google Scholar]
- 4.Ananthanarayanan B, Ni Q, Zhang J. Signal propagation from membrane messengers to nuclear effectors revealed by reporters of phosphoinositide dynamics and Akt activity. Proc Natl Acad Sci U S A. 2005;102:15081–15086. doi: 10.1073/pnas.0502889102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aye-Han NN, Allen MD, Ni Q, Zhang J. Parallel tracking of cAMP and PKA signaling dynamics in living cells with FRET-based fluorescent biosensors. Mol Biosyst. 2012;8:1435–1440. doi: 10.1039/c2mb05514g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu VK, Bivona T, Hach A, Sajous JB, Silletti J, Wiener H, Johnson RL, 2nd, Cox AD, Philips MR. Ras signalling on the endoplasmic reticulum and the Golgi. Nat Cell Biol. 2002;4:343–350. doi: 10.1038/ncb783. [DOI] [PubMed] [Google Scholar]
- 7.Choi Y, Kim K, Hong S, Kim H, Kwon YJ, Song R. Intracellular protein target detection by quantum dots optimized for live cell imaging. Bioconjug Chem. 2011;22:1576–1586. doi: 10.1021/bc200126k. [DOI] [PubMed] [Google Scholar]
- 8.Cicchetti G, Biernacki M, Farquharson J, Allen PG. A ratiometric expressible FRET sensor for phosphoinositides displays a signal change in highly dynamic membrane structures in fibroblasts. Biochemistry. 2004;43:1939–1949. doi: 10.1021/bi035480w. [DOI] [PubMed] [Google Scholar]
- 9.Cohen P. Protein kinases--the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 10.DeMarinis RM, Katerinopoulos HE, Muirhead KA. New tetracarboxylate compounds as fluorescent intracellular calcium indicators. 1990;112:381. [Google Scholar]
- 11.Depry C, Allen MD, Zhang J. Visualization of PKA activity in plasma membrane microdomains. Mol Biosyst. 2011;7:52–58. doi: 10.1039/c0mb00079e. [DOI] [PubMed] [Google Scholar]
- 12.Depry C, Zhang J. Visualization of kinase activity with FRET-based activity biosensors. Curr Protoc Mol Biol. 2010 doi: 10.1002/0471142727.mb1815s91. Chapter 18:Unit 18.15. [DOI] [PubMed] [Google Scholar]
- 13.Deuschle K, Okumoto S, Fehr M, Looger LL, Kozhukh L, Frommer WB. Construction and optimization of a family of genetically encoded metabolite sensors by semirational protein engineering. Protein Sci. 2005;14:2304–2314. doi: 10.1110/ps.051508105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding Y, Ai HW, Hoi H, Campbell RE. Forster resonance energy transfer-based biosensors for multiparameter ratiometric imaging of Ca2+ dynamics and caspase-3 activity in single cells. Anal Chem. 2011;83:9687–9693. doi: 10.1021/ac202595g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiPilato LM, Zhang J. The role of membrane microdomains in shaping beta2-adrenergic receptor-mediated cAMP dynamics. Mol Biosyst. 2009;5:832–837. doi: 10.1039/b823243a. [DOI] [PubMed] [Google Scholar]
- 16.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci U S A. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehr M, Lalonde S, Lager I, Wolff MW, Frommer WB. In vivo imaging of the dynamics of glucose uptake in the cytosol of COS-7 cells by fluorescent nanosensors. J Biol Chem. 2003;278:19127–19133. doi: 10.1074/jbc.M301333200. [DOI] [PubMed] [Google Scholar]
- 18.Frommer WB, Davidson MW, Campbell RE. Genetically encoded biosensors based on engineered fluorescent proteins. Chem Soc Rev. 2009;38:2833–2841. doi: 10.1039/b907749a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallegos LL, Kunkel MT, Newton AC. Targeting protein kinase C activity reporter to discrete intracellular regions reveals spatiotemporal differences in agonist-dependent signaling. J Biol Chem. 2006;281:30947–30956. doi: 10.1074/jbc.M603741200. [DOI] [PubMed] [Google Scholar]
- 20.Garini Y, Young IT, McNamara G. Spectral imaging: principles and applications. Cytometry A. 2006;69:735–747. doi: 10.1002/cyto.a.20311. [DOI] [PubMed] [Google Scholar]
- 21.Gerbino A, Ruder WC, Curci S, Pozzan T, Zaccolo M, Hofer AM. Termination of cAMP signals by Ca2+ and G(alpha)i via extracellular Ca2+ sensors: a link to intracellular Ca2+ oscillations. J Cell Biol. 2005;171:303–312. doi: 10.1083/jcb.200507054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant SK. Therapeutic protein kinase inhibitors. Cell Mol Life Sci. 2009;66:1163–1177. doi: 10.1007/s00018-008-8539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halet G. Imaging phosphoinositide dynamics using GFP-tagged protein domains. Biol Cell. 2005;97:501–518. doi: 10.1042/BC20040080. [DOI] [PubMed] [Google Scholar]
- 24.Harvey CD, Ehrhardt AG, Cellurale C, Zhong H, Yasuda R, Davis RJ, Svoboda K. A genetically encoded fluorescent sensor of ERK activity. Proc Natl Acad Sci U S A. 2008;105:19264–19269. doi: 10.1073/pnas.0804598105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiraoka Y, Shimi T, Haraguchi T. Multispectral imaging fluorescence microscopy for living cells. Cell Struct Funct. 2002;27:367–374. doi: 10.1247/csf.27.367. [DOI] [PubMed] [Google Scholar]
- 26.Honda A, Sawyer CL, Cawley SM, Dostmann WR. Cygnets: in vivo characterization of novel cGMP indicators and in vivo imaging of intracellular cGMP. Methods Mol Biol. 2005;307:27–43. doi: 10.1385/1-59259-839-0:027. [DOI] [PubMed] [Google Scholar]
- 27.Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci U S A. 2009;106:15651–15656. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh RE, Kurokawa K, Fujioka A, Sharma A, Mayer BJ, Matsuda M. A FRET-based probe for epidermal growth factor receptor bound non-covalently to a pair of synthetic amphipathic helixes. Exp Cell Res. 2005;307:142–152. doi: 10.1016/j.yexcr.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol. 2002;22:6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson LN. Protein kinase inhibitors: contributions from structure to clinical compounds. Q Rev Biophys. 2009;42:1–40. doi: 10.1017/S0033583508004745. [DOI] [PubMed] [Google Scholar]
- 31.Kawai H, Suzuki T, Kobayashi T, Sakurai H, Ohata H, Honda K, Momose K, Namekata I, Tanaka H, Shigenobu K, Nakamura R, Hayakawa T, Kawanishi T. Simultaneous real-time detection of initiator- and effector-caspase activation by double fluorescence resonance energy transfer analysis. J Pharmacol Sci. 2005;97:361–368. doi: 10.1254/jphs.fp0040592. [DOI] [PubMed] [Google Scholar]
- 32.Komatsu N, Aoki K, Yamada M, Yukinaga H, Fujita Y, Kamioka Y, Matsuda M. Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol Biol Cell. 2011;22:4647–4656. doi: 10.1091/mbc.E11-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunkel MT, Toker A, Tsien RY, Newton AC. Calcium-dependent regulation of protein kinase D revealed by a genetically encoded kinase activity reporter. J Biol Chem. 2007;282:6733–6742. doi: 10.1074/jbc.M608086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunkel MT, Ni Q, Tsien RY, Zhang J, Newton AC. Spatio-temporal dynamics of protein kinase B/Akt signaling revealed by a genetically encoded fluorescent reporter. J Biol Chem. 2005;280:5581–5587. doi: 10.1074/jbc.M411534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurebayashi N, Harkins AB, Baylor SM. Use of fura red as an intracellular calcium indicator in frog skeletal muscle fibers. Biophys J. 1993;64:1934–1960. doi: 10.1016/S0006-3495(93)81564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landa LR, Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem. 2005;280:31294–31302. doi: 10.1074/jbc.M505657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Tsien RW. pHTomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity. Nat Neurosci. 2012;15:1047–1053. doi: 10.1038/nn.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mank M, Griesbeck O. Genetically encoded calcium indicators. Chem Rev. 2008;108:1550–1564. doi: 10.1021/cr078213v. [DOI] [PubMed] [Google Scholar]
- 40.Mehta S, Zhang J. Reporting from the field: genetically encoded fluorescent reporters uncover signaling dynamics in living biological systems. Annu Rev Biochem. 2011;80:375–401. doi: 10.1146/annurev-biochem-060409-093259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 42.Mochizuki N, Yamashita S, Kurokawa K, Ohba Y, Nagai T, Miyawaki A, Matsuda M. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature. 2001;411:1065–1068. doi: 10.1038/35082594. [DOI] [PubMed] [Google Scholar]
- 43.Nagai T, Sawano A, Park ES, Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+ Proc Natl Acad Sci U S A. 2001;98:3197–3202. doi: 10.1073/pnas.051636098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nausch LW, Ledoux J, Bonev AD, Nelson MT, Dostmann WR. Differential patterning of cGMP in vascular smooth muscle cells revealed by single GFP-linked biosensors. Proc Natl Acad Sci U S A. 2008;105:365–370. doi: 10.1073/pnas.0710387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newman RH, Fosbrink MD, Zhang J. Genetically encodable fluorescent biosensors for tracking signaling dynamics in living cells. Chem Rev. 2011;111:3614–3666. doi: 10.1021/cr100002u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman RH, Zhang J. Visualization of phosphatase activity in living cells with a FRET-based calcineurin activity sensor. Mol Biosyst. 2008;4:496–501. doi: 10.1039/b720034j. [DOI] [PubMed] [Google Scholar]
- 47.Ni Q, Ganesan A, Aye-Han NN, Gao X, Allen MD, Levchenko A, Zhang J. Signaling diversity of PKA achieved via a Ca2+-cAMP-PKA oscillatory circuit. Nat Chem Biol. 2011;7:34–40. doi: 10.1038/nchembio.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niino Y, Hotta K, Oka K. Blue fluorescent cGMP sensor for multiparameter fluorescence imaging. PLoS One. 2010;5:e9164. doi: 10.1371/journal.pone.0009164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niino Y, Hotta K, Oka K. Simultaneous live cell imaging using dual FRET sensors with a single excitation light. PLoS One. 2009;4:e6036. doi: 10.1371/journal.pone.0006036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res. 2006;99:1084–1091. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- 51.Nikolaev VO, Gambaryan S, Lohse MJ. Fluorescent sensors for rapid monitoring of intracellular cGMP. Nat Methods. 2006;3:23–25. doi: 10.1038/nmeth816. [DOI] [PubMed] [Google Scholar]
- 52.Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 53.Nishioka T, Aoki K, Hikake K, Yoshizaki H, Kiyokawa E, Matsuda M. Rapid turnover rate of phosphoinositides at the front of migrating MDCK cells. Mol Biol Cell. 2008;19:4213–4223. doi: 10.1091/mbc.E08-03-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ouyang M, Huang H, Shaner NC, Remacle AG, Shiryaev SA, Strongin AY, Tsien RY, Wang Y. Simultaneous visualization of protumorigenic Src and MT1-MMP activities with fluorescence resonance energy transfer. Cancer Res. 2010;70:2204–2212. doi: 10.1158/0008-5472.CAN-09-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearce LL, Gandley RE, Han W, Wasserloos K, Stitt M, Kanai AJ, McLaughlin MK, Pitt BR, Levitan ES. Role of metallothionein in nitric oxide signaling as revealed by a green fluorescent fusion protein. Proc Natl Acad Sci U S A. 2000;97:477–482. doi: 10.1073/pnas.97.1.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piljic A, Schultz C. Simultaneous recording of multiple cellular events by FRET. ACS Chem Biol. 2008;3:156–160. doi: 10.1021/cb700247q. [DOI] [PubMed] [Google Scholar]
- 57.Russwurm M, Mullershausen F, Friebe A, Jager R, Russwurm C, Koesling D. Design of fluorescence resonance energy transfer (FRET)-based cGMP indicators: a systematic approach. Biochem J. 2007;407:69–77. doi: 10.1042/BJ20070348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sample V, DiPilato LM, Yang JH, Ni Q, Saucerman JJ, Zhang J. Regulation of nuclear PKA revealed by spatiotemporal manipulation of cyclic AMP. Nat Chem Biol. 2012;8:375–382. doi: 10.1038/nchembio.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato M, Nakajima T, Goto M, Umezawa Y. Cell-based indicator to visualize picomolar dynamics of nitric oxide release from living cells. Anal Chem. 2006;78:8175–8182. doi: 10.1021/ac061791b. [DOI] [PubMed] [Google Scholar]
- 60.Sato M, Hida N, Ozawa T, Umezawa Y. Fluorescent indicators for cyclic GMP based on cyclic GMP-dependent protein kinase Ialpha and green fluorescent proteins. Anal Chem. 2000;72:5918–5924. doi: 10.1021/ac0006167. [DOI] [PubMed] [Google Scholar]
- 61.Shelly M, Lim BK, Cancedda L, Heilshorn SC, Gao H, Poo MM. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- 62.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsou P, Zheng B, Hsu CH, Sasaki AT, Cantley LC. A fluorescent reporter of AMPK activity and cellular energy stress. Cell Metab. 2011;13:476–486. doi: 10.1016/j.cmet.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Wal J, Habets R, Varnai P, Balla T, Jalink K. Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J Biol Chem. 2001;276:15337–15344. doi: 10.1074/jbc.M007194200. [DOI] [PubMed] [Google Scholar]
- 65.Varnai P, Balla T. Live cell imaging of phosphoinositide dynamics with fluorescent protein domains. Biochim Biophys Acta. 2006;1761:957–967. doi: 10.1016/j.bbalip.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 66.Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welch CM, Elliott H, Danuser G, Hahn KM. Imaging the coordination of multiple signalling activities in living cells. Nat Rev Mol Cell Biol. 2011;12:749–756. doi: 10.1038/nrm3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong K, Pertz O, Hahn K, Bourne H. Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc Natl Acad Sci U S A. 2006;103:3639–3644. doi: 10.1073/pnas.0600092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, Nagashima K, Matsuda M. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162:223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat Cell Biol. 2000;2:25–29. doi: 10.1038/71345. [DOI] [PubMed] [Google Scholar]
- 71.Zimmermann T, Rietdorf J, Pepperkok R. Spectral imaging and its applications in live cell microscopy. FEBS Lett. 2003;546:87–92. doi: 10.1016/s0014-5793(03)00521-0. [DOI] [PubMed] [Google Scholar]