Abstract
Purpose
This review summarizes research on disorders of speech production in Down Syndrome (DS) for the purposes of informing clinical services and guiding future research.
Method
Review of the literature was based on searches using Medline, Google Scholar, Psychinfo, and HighWire Press, as well as consideration of reference lists in retrieved documents (including online sources). Search terms emphasized functions related to voice, articulation, phonology, prosody, fluency and intelligibility.
Conclusions
The following conclusions pertain to four major areas of review: (a) Voice. Although a number of studies have been reported on vocal abnormalities in DS, major questions remain about the nature and frequency of the phonatory disorder. Results of perceptual and acoustic studies have been mixed, making it difficult to draw firm conclusions or even to identify sensitive measures for future study. (b) Speech sounds. Articulatory and phonological studies show that speech patterns in DS are a combination of delayed development and errors not seen in typical development. Delayed (i.e., developmental) and disordered (i.e., nondevelopmental) patterns are evident by the age of about 3 years, although DS-related abnormalities possibly appear earlier, even in infant babbling. (c) Fluency and prosody. Stuttering and/or cluttering occur in DS at rates of 10 to 45%, compared to about 1% in the general population. Research also points to significant disturbances in prosody. (d) Intelligibility. Studies consistently show marked limitations in this area but it is only recently that research goes beyond simple rating scales.
Speech production in Down Syndrome (DS) is associated with significant impairments in spoken language (Fawcett & Peralego, 2009; Leddy, 1999; Miller & Leddy, 1999; Rondal & Comblain, 1996; Timmins, Cleland, Rodger, Wishart, Wood & Hardcastle, 2009). As shown in Figure 1, the number of studies on speech, voice, fluency/prosody, and intelligibility in DS has increased fairly steadily since the 1970s, with a substantial increase in the last decade. Studies focused on speech intelligibility have been reported only relatively recently and account for a major part of the increase in reports published since 1990. Figure 1 indicates that there is a reasonably sized literature on speech communication in DS.
Figure 1.
The number of research articles on speech production in DS covered in this review, grouped in decade intervals since 1950. The number of articles is shown by category on the vertical axis and decade intervals are shown on the horizontal axis. The four categories are Voice, Speech, Fluency and prosody, and Intelligibility.
Unlike earlier reviews, the present review covers articles published in the last 6 decades, offers systematic summaries of research participants (DS and comparison groups) and research methods, and analyzes research progress in the four major aspects of speech production: (a) voice, (b) speech sounds (including articulation, phonology and resonance), (c) fluency and prosody, and (d) intelligibility. The combination of fluency and prosody is based on the principle that both are most effectively expressed in units larger than the phone (e.g., as a syllable or multisyllabic strings). The last category, intelligibility, can be regarded as a joint product of the previous three and is the core of communication ability and disability. Although the relevant research in these areas overlaps, the categories are sufficiently distinct that they delineate the primary facets of speech difficulty in DS. The primary goal of this review is to inform clinical services and guide future research.
We used Medline, Google Scholar, Psychinfo, and HighWire Press to search the literature published since 1950 and considered reference lists in retrieved documents (including online sources). The main search terms were Down Syndrome, Down’s Syndrome, Downs Syndrome, mongolism, mongoloid, and Trisomy 21 linked to additional terms including: articulation, babbling, cluttering, communication, consonants, conversation, cry, diadokinesis, disfluency or dysfluency, formants, infant vocalizations, intelligibility, nasality, phonation, phonology, phonological, prosody, speech, speech development, speech production, stuttering, voice, voice quality, vowels.
We compiled methods and results of studies in each of the four areas of speech production (voice, speech sound disorders, fluency/prosody, and intelligibility) for a given age group of participants (Tables 1 – 4). When possible, the tables are arranged in a developmental perspective, to show the results for adults and children (and, data permitting, children of different ages).
Table 1a.
Voice in infants, children and adolescents
Summary of studies of voice in infants, children and adolescents with DS. Studies are arranged in order corresponding to approximate age of participants (youngest first).
| Source | Participants | Method | Summary of results |
|---|---|---|---|
| Vuorenkoski, Lind, Wasz-Hockert, & Partanen (1971) | N= 30 DS (infants and neonates) N= 120 TD (infants and neonates) N= 90 ATD with various pathologies excluding DS Participants aged 0 days to 8 months |
Acoustic: Derivation of a cry score (ranging from 1 to 4) calculated from 13 spectrographic features |
93% of DS participants had an abnormal cry score compared to only 6% of TD; distinguishing abnormalities included stuttering and melody. |
| Vuorenkoski, Wasz-Hockert, Lind, Koivisto, & Partanen (1971) | N= 3 DS (newborns) N= 8 TD (newborns) N= 9 ADT (not DS) (newborns) |
Perceptual: Auditory judgments of pain cries by a group of pediatricians and a group of medical students |
Acoustic information from spectrograms improved the ability to identify medical status of newborns, especially for DS |
| Lind, Vuorenkoski, Rosberg, Partanen, & Wasz-Hockert (1970) | N= 30 DS (infants) N= 120 TD (infants) |
Acoustic: Spectrographic features of pain cry |
DS had abnormal features of pain cry, including: long duration, low pitch, monotonous with flat melody form, nasal, and stuttering. |
| Weinberg & Zlatin (1970) | N= 27 DS (5;01 → 6;11) N= 66 TD (5;00 → 6;10) |
Acoustic: Analyses of mean, standard deviation and range of speaking f0, as determined with the Fundamental Frequency Indicator |
DS had a higher mean f0 compared to controls. |
| Moura, Cunha, Vilarinho, Cunha, Freitas, Palha, Pueschel, & Pais-Clemente (2008) | N= 66 DS (36M, 30F) (3 → 8 yrs) (5.8 mean age yrs) N= 204 TD (104M, 100F) (Mean age 5.7 yrs) |
Acoustic: Voice assessments using Praat (software; Boersma & Weenink, 2010) Perceptual rating: Modified GRBAS rating scale (Hirano, 1981) |
DS had lower f0 with elevated dispersion, greater measures of perturbation and noise higher, and lower value of spectral tilt. DS significantly different for all variables. |
| Pentz & Gilbert (1983) | N= 14 DS (6M, 8F) (7 → 10 yrs) (Mean age 9.42 yrs) N= 14 TD (6M, 8F) (7 → 10 yrs) (Mean age 9.25 yrs) |
Acoustic: Voice assessments using a Kay Visi-pitch, Kay spectrograph and an oscillograph. Perceptual rating: Ratings with Wilson Voice Profile (Wilson, 1972) |
DS group had increased frequency perturbation, amplitude perturbation and noise-to-harmonic ratios. DS different only on the severity subscale. |
| Pentz (1987) | N= 14 DS (6M, 8F) (7 → 10 yrs) N= 14 TD (6M, 8F) (7 → 10 yrs) |
Acoustic: Measurement of formant amplitudes using a spectrum analyzer | DS had significantly reduced formant amplitude intensity levels. |
| Michel & Carney (1964) | N= 8 DS (All M) (8.5 → 10.5 yrs) N= 42 TD (All M) (7, 8 & 10 years old |
Acoustic: Determination of speaking f0 using a phonellograph | DS group did not differ from TD. DS pitch normal with respect to age. |
|
Albertini, Bonassi, Dall’Armi, Giachetti, Giaquinto, & Mignano (2010) Also in Table 1a |
N= 48 DS Children* 27M (mean age 9.6 yrs) 21F (mean age 9.8 yrs) N= 46 TD 28M (mean age 9.2 yrs) 18F (mean age 9.4 yrs) * Adults in Table 1a |
Acoustic: Analyses with the KayPENTAX Real Time Pitch Model 5121 and Praat (software; Boersma & Weenink, 2010) |
DS group differed from TD only in the Coefficient of Variation. * See Table 1b-Adults |
| Hollien & Copeland (1965) | N= 9 DS (All F) (10 yrs) N= 36 TD (All F) (7, 8 & 11 yrs) |
Acoustic: Determination of speaking f0 using a phonellogram | DS girls and TD girls had comparable mean speaking f0. No significant differences. |
| Montague & Hollien (1973) | N= 20 DS (10M, 10F) (7.8 → 13.5 yrs) N= 20 TD (10M, 10F) (8.0 → 13.2 yrs) |
Perceptual rating: Judgments of presence of voice quality disorders by 16 listeners (8 native listeners and 8 SLP listeners) |
DS had significantly higher ratings of breathiness and roughness. Also, DS higher but variable nasality ratings also. |
| Montague, Hollien, H., Hollien, P.A., & Wold (1978) | N= 20 DS (10M, 10F) (7.8 → 13.5 yrs) N= 20 TD (10M, 10F) (8.0 → 13.2 yrs) *Same participants selected in Montague & Hollien (1973) |
Perceptual rating: Judgments of vocal pitch by 16 paid undergraduate college listeners. |
DS had lower pitch ratings as a group (60.2%) but a minority had higher pitch ratings (24.8%); differences in perceived pitch were not explained by f0, which was not different between groups. |
| Moody, Montague, & Bradley (1979) | N= 20 DS N= 20 TD *Same participants as in Montague & Hollien (1973) (reliability study) |
Perceptual rating: Ratings of voice using the Wilson Voice Profile System (Wilson, 1972) by 11 graduate students in communicative disorders (1977) |
DS had higher ratings of deviations in severity, pitch, tension and air loss. |
| Rodger (2009) | N= 22 DS (13M, 9F) (10.008 → 20.33 yrs) (Mean 14.36 yrs) N= 52 TD (34M, 18F) (1.01 → 18.67 yrs) (mean 13.97 yrs) N = 8 TD (7M, 1F) (10.0 → 15.0 yrs) (Median age 12.17 yrs) |
Acoustic: Analyses of voice using Praat (software; Boersma & Weenink, 2010) Perceptual rating: Ratings of voice using the Vocal Profile Analysis Scheme (Laver, Wirz, Mackenzie, & Hiller (1991) Other: Questionnaire-based analysis of judgments of voice |
DS did not differ from controls in f0, jitter, shimmer or S/N, but DS had higher values of spectral tilt. DS had lower pitch ratings. |
|
Novak (1972) Also in Table 2 – Speech Sounds |
N = 32 DS 19M (mean age 13.3) 13F (mean age 12.8) (7 → 19 yrs) N = 20 ATD*(11M, 9F) (7 → 20 yrs) *ATD participants with cognitive delay but not DS |
Acoustic: Measures of vocal f0 using a spectrograph See Table 2 for Speech Sounds findings |
DS did not differ in f0 but had reduced voice range and “increased rustle” of voice attributed to squeezing of the larynx and irregularity of vocal fold vibration. |
DS=participants with Down syndrome; F= female; M= male, MPT= mean phonation time, TD=typically developing participants, AD=atypically developing participants.
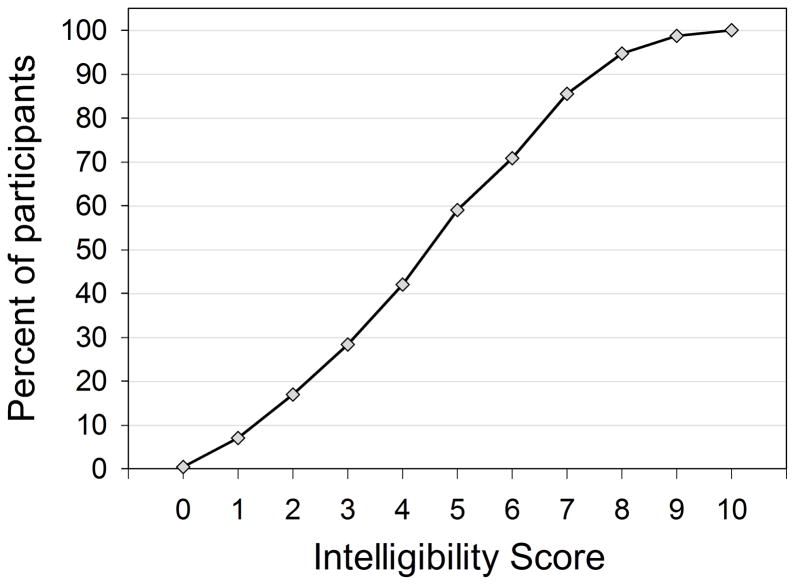
Table 4.
Speech intelligibility
Summary of studies of speech intelligibility in individuals with DS. See captions for Tables 1a, 1b and 2 for identification of abbreviations and terms.
| Source | Participants | Method | Summary of results |
|---|---|---|---|
| Barnes et al. (2009) | See Table 2 |
Phonological Assessment: Perceptual and acoustic measures of phonological accuracy and processes |
DS scored lower in accuracy and processes and used fewer intelligible words. |
| van Bysterveldt (2009) | See Table 2 |
Transcription: Determination of percentage of intelligible utterances in narratives and connected speech |
DS had mean intelligibility scores of 83.1% for narratives and 80% for connected speech. |
| Parsons, Iacono, & Rozner (1987) | N= 18 DS (9M, 9F) (5.08 → 19.60 yrs) (mean age 11.50 yrs) Children who had tongue-reduction surgery N= 9 DS (7M, 2F) (5.33 → 18.66 yrs) (mean age 9.50 yrs) Comparison group who did not have tongue-reduction surgery |
Perceptual transcription & parental questionnaire: calculation of the ratio of total number of consonant substitutions and omissions divided by the total number of consonants in words attempted by the participant |
Ratio of consonant errors about 0.40 for both groups, pre and post-treatment. No significant difference in intelligibility across time (i.e. not attributed to surgery or maturity); no significant difference in intelligibility between surgery group and non-surgery group although parents in both groups rated their children as showing improvement. |
| Chapman, Sueng, Schwartz, & Kay-Raining Bird (1998) | N= 47 DS (29M, 18F) (5;06 → 20;06) N= 47 TD (22M, 25F) (2;02 → 6;01) |
Transcription: MLU and total number of words spoken analyzed by SALT |
DS had more utterance attempts and spoke with more word tokens, types and longer MLU; Omissions more common in older children with DS; poorer intelligibility. |
| Chapman, Sueng, Schwartz, & Kay-Raining Bird (2000) | See Chapman et al. (1998) above |
Transcription: MLU analyzed by SALT; two models compared language comprehension to language production Model I (without comprehension) Model II (with comprehension) |
For DS, Model II explained 68% of the variability in number of different words, 80% in MLU and 32% in intelligibility. |
| Cleland, Timmins, Wood, Hardcastle, & Wishart (2009) | N= 6 DS (5M, 1F) (10;01 → 18;09) (mean age 12.74 yrs) All DS part of EPG group. 27 participants were randomly assigned to one of three groups: EPG therapy, speech therapy, or control. |
Standardized testing, EPG, oral-motor exam: Speech, language & cognitive tests, EPG, oral-motor exam (Robbins & Klee, 1987), DEAP phonology test (Dodd, Hua, Crosbie & Holm, 2002) |
Post-treatment, all participants showed qualitative and quantifiable differences in EPG patterns and improvements in DEAP percentage consonants correct. |
| Dodd & Thompson (2001) | N= 15 DS (12M, 3F) (5;07 → 15;02) N= 15 AD* (12M, 3F) (3;07 → 5;05) *Children with phonological disorder |
Phonological Assessment: Perceptual assessments of speaking characteristics-phonological errors-using the 25-Word Inconsistency Test (Burt et al. 1999). |
DS not significantly different from comparison group; trend for DS to use fewer phonemes. AD group made more errors involving addition or deletion of consonants. |
| Roberts et al. (2005) | See Table 2 |
Transcription: Determination of PCC (Shriberg & Kwiatkowski, 1982), phonological processes and proportion of whole word proximity (see Table 2) |
DS had phonological patterns that were delayed relative to TD controls but also differed in some respects from TD patterns; word shapes in DS were reduced because of omitted syllables, reduced consonant clusters and deletion of consonant singletons. |
| Kennedy & Flynn (2003) | N= 3 DS (7;02, 8;04, & 8;10) No gender mentioned |
Mainly perceptual: Perceptual assessments and comprehension detection using a phonological awareness based intervention |
DS improved phonological awareness targeted in intervention; overall percentage consonants correct did not significantly improve following intervention. |
| Rosin et al. (1988) | See Table 2 |
Standardized tests & transcription: SALT transcription program (Miller & Chapman, 1990) |
DS less intelligible especially in areas such as percent of consonants correct, MLU, developmental level and amount of cueing needed. |
| Wood, Wishart, Hardcastle, Cleland, & Timmins (2009) | N= 2 DS (1M, 1F) F= 11 yrs M= 14 yrs Participants received EPG therapy. |
Standardized tests: Assessment of cognition (WPPSI-III; Wechsler, 2003), language (BPCS-II; Dunn, Whetton, & Burley, 1997) and CELF-P; Wiig, Secord, & Semel, 1992), and speech (DEAP; Dodd et al., 1992). |
DS intelligibility increased from 72%–76% and from 59%–65% for female and male participant respectively; variability decreased for female although remains higher than typically-developing children. |
| Yoder, Hooshyar, Klee, & Schaffer (1996) | N= 8 DS (mean age 83 mos) N= 8 ATD* (mean age 44 mos) Matched to DS group on MLU. No DS, but language delay |
Perceptual assessment: Intelligibility and length determined with SALT transcription program (Miller & Chapman, 1990) |
DS had over 3 times as many multi-word partially intelligible utterances. However, overall there were no significant differences in intelligibility. |
| Bunton, Leddy, & Miller (2007) | N= 5 DS (5M) (26 → 39 yrs) |
Perceptual assessment: Intelligibility test and perceptual scoring by listeners and transcribers |
DS had wide range of intelligibility scores (41–75%); Errors that were ranked more highly than others: cluster-singleton production word initial and word final, vowel errors and place of production for stops and fricatives. |
Given the phenotypic variation in DS (Reeves, Baxter, and Richtsmeier, 2001; Wiseman, Alford, Tybulewicz, and Fisher, 2009), it is important that sample size and participant characteristics be considered in generalizing the results of individual studies, so we have estimated the aggregate number of participants in each of the four areas of review. Both typically developing (TD) and atypically developing (AD) individuals have been used as controls in studies of DS, and the abbreviations TD and AD are used in both the text and tables to indicate these two general categories of participants. Case reports are not included in this review, unless they provide methodological details relevant to group investigations. Treatment studies are excluded unless they present pre-treatment participant data on the categories listed earlier. Parental surveys are discussed and are summarized in Appendix 1.
The discussion highlights significant points of agreement and disagreement among the studies, relates the speech abnormalities to anatomic anomalies and other pathophysiology, and considers current perspectives on the etiology and nature of speech disturbances in DS.
1. Voice (Table 1)
1.1. Review of Literature
Data on voice in DS have been collected from a total of nearly 600 individuals, including children and adults (Tables 1a and 1b, respectively). The exact aggregate number is difficult to determine because some of the earlier studies may have reported on the same group of participants more than once. Research on vocal characteristics has focused mainly on vocal fundamental frequency (f0) level and voice quality, often with the hypothesis that DS is associated with a characteristic dysphonia. Low vocal pitch and hoarse, harsh or raucous voice have frequently been ascribed to individuals with Down syndrome (Benda, 1949; Novak, 1972; Shprintzen, 1977; Strazzulla, 1953). These reports motivated research on vocal characteristics of children and adults with DS.
Table 1b.
Voice in adults
Summary of studies of voice in adults with DS. Studies are arranged in order of date of publication (earliest first).
| Source | Participants | Method | Summary of results |
|---|---|---|---|
|
Schlanger & Gottsleben (1957) * Also in Table 2 |
N= 44 DS participants (ages not specified) N= 472 ATD* Mean age of total group (DS and ATD) was 28.9 yrs. *ATD individuals with other etiologies of mental retardation; all were residents of a training school |
Perceptual ratings: Assessments of speech articulation, voice, and stuttering See Table 2 for Speech Sounds assessment/findings |
72% (32 participants) of DS were judged to have a voice disorder. |
| Moran & Gilbert (1978) | N= 16 DS 8M (mean age 38;04) 8F (mean age 41;02) (institutionalized adults) N= 16 TD 8M (mean age 37;08) 8F (mean age 41;02) (adults with no cognitive impairment |
Acoustic: Analysis of speaking f0 using an oscillograph |
DS (both males and females) had higher mean f0 than controls. |
| Wold & Montague (1979) | N= 51 DS (16 yrs and older) |
Perceptual ratings: Identification of voice qualities by 2 trained listeners |
Most DS voices rated as breathy; pitch was rated as either low or high. |
| Moran & Gilbert (1982) | N= 16 DS (8M, 8F) (Adults) |
Acoustic: Analysis of f0 and other voice features (f0 perturbation, and noise to harmonic ratio) using oscillograph and spectrograph Perceptual ratings: Ratings with Wilson Voice Profile (Wilson, 1972) by 17 graduate students in Communicative Disorders |
DS had variable patterns across individuals; authors concluded that abnormal voice quality reflected the interaction of several factors. DS had abnormal profiles, with breathiness being the most frequent voice quality disorder. |
| Moran (1986) | N= 14 DS (8M, 6F) (20 → 43 yrs) (institutionalized adults) N= 14 TD (8M, 6F) (19 → 54 yrs) (adults with hoarse voices but without cognitive impairment) |
Acoustic: Measures of SFF of three prolonged vowels using Kay Visi-pitch and measures of vowel formants using a Voice Identification Inc. Series 700 spectrograph Perceptual ratings: Judgments of whether a speaker had DS and ratings of nasality by 16 listeners (8 faculty in Special Education & 8 faculty in Speech Pathology) |
DS did not differ in f0 or vowel formants. DS identified at better than chance and received higher nasality ratings. |
| Pryce (1994) | N= 30 DS (16M, 14F) N= 30 ATD learning disabilities (15M, 17F) N= 30 ATD with functional dysphonia (8M, 11F) N= 30 TD normal voice (15M, 14F) |
Physiologic: Level of laryngeal EMG needed to initiate phonation. |
DS had higher levels (almost two times greater) of EMG to initiate phonation. |
| Lee, Thorpe, & Verhoeven (2009) | N= 9 DS (4M, 5F) (17 → 29 yrs) (Mean age 24.7 yrs) N= 9 TD (Matched for age and gender, speaking Standard British English) (Mean age 23.5 yrs) |
Acoustic: Analysis of organic and linguistic pitch ranges, voice compass and declination; acoustic analyses of phonation including maximum phonation time, jitter and shimmer |
DS had (1) normal respiratory capacity, reduced organic pitch range, and reduced linguistic pitch range; (2) intonation patterns with a high f0 and reduced dynamics, (3) reduced jitter and normal shimmer. |
|
Albertini, Bonassi, Dall’Armi, Giachetti, Giaquinto, & Mignano (2010) * Also in Table 1a |
N= 30 DS Adults* 17M (mean age 28.7 yrs) 13F (mean age 23.2 yrs) N= 60 TD Adults 30M (mean age 48.7 yrs) 30F (mean age 44.7 yrs) * Children in Table 1a |
Acoustic: Analyses with the KayPENTAX Real Time Pitch Model 5121 and Praat (software; Boersma & Weenink, 2010) Same as in Table 1a |
DS had higher mean vocal f0 with reduced f0 variation and lower energy. See Table 1a |
| Seifpanahi, Bakhtiar, & Salmalian (2011) | N= 22 DS (14M, 8F) (20 → 28 yrs, mean 25) N= 22 TD Adults (matched for age and gender) |
Acoustic: Analyses of voice using Dr. Speech 4.3U from Tiger Electronics Inc. |
DS group had higher f0 and lower jitter; no difference in MPT and shimmer. |
DS= Participants with Down syndrome; F= female; M= male, MPT=mean phonation time, TD= typically developed participants, ATD= atypically developed individuals.
1.1.1. Newborn and infant cry
Research in this area was published in the 1970s when there was a keen interest in the diagnostic significance of the newborn and infant cry (especially the pain cry, which could be elicited reliably). The cries of babies with DS were distinguished from those of healthy babies on the basis of spectrographic abnormalities such as “stuttering,” “flat melody” and low pitch (Lind et al., 1970). Stuttering was defined as “a special kind of tenseness which is periodically heightened during the cry, when attacks of glottal pressure are superimposed on the phonation” (Lind et al., 1970, p. 479). Vuorenkoski et al. (1971) developed a cry score based on 13 acoustic characteristics that distinguished the pain cries of infants with DS from those of healthy infants. These studies indicate that the underlying disturbed infant cry in DS is most likely due to abnormalities in respiratory and laryngeal function, which is not surprising given that the cry is formed largely by phonatory activity with relatively little participation of the vocal tract except to maintain an open airway.
1.1.2. Vocal pitch and fundamental frequency (f0)
The variable of f0 is the primary acoustic correlate of perceptual judgments of vocal pitch. If vocal pitch is judged to be low in DS, then f0 is expected to be lower in DS than in age-matched TD controls. Perceptual ratings of vocal pitch in DS are mixed (Montague, Hollien, Hollien & Wold, 1978).
Quantitative studies based on acoustic methods, summarized in Table 1, offer mixed results on vocal f0, with the majority of studies reporting no difference between individuals with DS and TD controls, although a difference may exist when age is taken into account. One study demonstrated a low f0 in the pain cry of infants with DS (Lind et al., 1970). In children with DS, one study showed a higher f0 (Weinberg & Zlatin, 1970), while another study showed a lower f0 compared to TD controls (Moran & Gilbert, 1978). Four studies showed a higher f0 in adults with DS compared to TD controls (Albertini et al, 2010; Lee et al., 2009; Moura et al., 2008; Seifpanahi, 2010).
A discrepancy between perceptual judgments of pitch level and acoustic measures of f 0 has been noted (Rodger, 2009), which may mean that perception of low vocal pitch is influenced by factors other than the actual frequency of vocal fold vibration. It may help to resolve this discrepancy by examining a range of acoustic and perceptual factors associated with voice production in individuals with DS, taking into account a developmental perspective that covers the period from infancy to adulthood.
1.1.3. Voice quality
Vocal quality has been studied with both perceptual and acoustic methods, as detailed in Table 1. The perceptual studies of voice in DS note especially breathiness and roughness. Published studies are by no means in complete agreement, but acoustic studies report increased frequency perturbations (e.g., higher values of jitter), amplitude perturbations (e.g., higher values of shimmer) and increased noise in phonation (e.g., reduced signal-to-noise ratio, S/N). Discrepant results also have been reported for spectral tilt (Moura et al. 2008; Rodger, 2009) The variability in results among studies may be due in part to differences in participant samples, speaking task differences, language differences, or differences in the algorithms or equipment used to calculate the acoustic values. No single acoustic correlate of voice quality in DS consistently emerges in the published literature, nor is it clear if a particular voice quality persists in individuals with DS across various speaking tasks and if voice quality in DS changes with development. Despite frequent comments in the clinical literature on voice quality differences in DS, there has not been a satisfactory convergence on perceptual features or on acoustic correlates of voice quality.
1.1.4. Anatomic Anomalies and Pathophysiology Related to Voice in DS
Some researchers have suggested that vocal features in DS are associated with anatomic and physiologic abnormalities such as hypothyroidism, absence of facial sinuses, or anomalies in laryngeal structures (Benda, 1949; Novak et al., 1972; Leddy, 1996). Endoscopic studies have shown that airway obstruction, which occurs in a significant proportion of individuals with DS, is often associated with laryngomalacia, tracheomalacia or bronchomalacia (Bertrand, Navarro, Caussade, Holmgren, & Sanchez, 2003; Mitchell, Call & Kelly, 2003).
Laryngomalacia may affect the epiglottis, the arytenoid cartilages, or both (Prescott, 1991; Roger, Denoyelle, Triglia & Garabedian, 1995). Epiglottal involvement often appears as an elongation, with an inward folding of the walls that can obstruct the airway. The epiglottis is often omega-shaped in cross section. This feature is by no means unique to DS as it has been described in a significant percentage of TD children (Ferguson, 1970; Solomons & Prescott, 1987). With involvement of the arytenoid cartilages, enlargement is the most prominent feature. The cartilage is generally soft and pliable and is prone to dynamic prolapse over the larynx during inspiration, often resulting in inspiratory noise or stridor. Thompson (2009) has presented evidence that laryngomalacia is associated with altered laryngeal tone and sensorimotor integration, which may help to explain some of the cry abnormalities described in section 1.1.1.
1.2. Discussion
It is likely that dysphonia of at least a mild degree is a common feature of speech in DS, although prevalence data have not been reported. (Prevalence is estimated to be about 6% for 8-year-old children in the general population; Carding, Roulstone, Northstone, & the ALPAC Study Team, 2006). Research on pain cry in neonates and infants with DS (reviewed in section 1.1.1) points strongly to the conclusion that vocal abnormalities are evident in the earliest stages of phonation.
Acoustic studies in adults indicate that vocal f0 is generally higher in DS than in healthy controls possibly because of the smaller body size in DS compared to TD controls (Myrelid, Gustafsson, Ollars, & Annerén, 2002: Rosenbloom, McGregor, Chen, An, & Dupont, 2010). Because of the documented reduced body size in DS, growth curves specific to DS have been developed (Myrelid et al., 2002). If the size of the larynx is related to body size, individuals with DS may have a relatively small larynx compared to age- and sex-matched TD controls, and therefore would have a higher vocal f0. This hypothesis would be supported if it could be established that laryngeal structures are smaller in DS than in TD.
Perceptual studies of voice point to disturbances in vocal quality that are typically judged as breathiness and roughness. Acoustic studies often show increased perturbations and a reduced S/N, which are consistent with the results of perceptual studies. In general, vibratory aperiodicity, as measured by jitter and shimmer, has been attributed to four sources: (a) neurologic, (b) biomechanical or structural, (c) aerodynamic and (d) source-filter (source-resonator) interaction (Titze, Horii & Scherer, 1987). Any or all of these factors could account for vocal perturbation in DS and different combinations of these factors could account for the variation in the results of studies on voice. A complicating factor in interpreting acoustic data for shimmer and S/N for children with DS is that typically developing children can have results for these parameters that would be considered as pathological values for adults (Glaze, Bless, Milenkovic, & Susser, 1988).
The larger picture of voice quality includes oral/nasal resonance as well as characteristics derived from vocal fold function. As reviewed in section 2 (speech sound disorders), resonance is altered in at least some individuals with DS, so that the overall perception of voice quality could be a combination of abnormalities in vocal fold vibration and atypical vocal tract resonances. Phonatory function may be affected by abnormal vocal fold behavior, loss of acoustic energy due to nasalization, and their interaction. Abnormalities of voice may have a significance that goes beyond a perceived difference in voice quality, as they may signal inefficiencies in voice production that contribute to an overall difficulty in producing speech. The discordant results in published studies may be resolved by further study of age-related phenotypic variation in voice.
Another important question at the functional level is whether the vocal characteristics in DS are a result of laryngeal hyperadduction or hypoadduction. Pryce (1994) observed higher levels of electromyography (EMG) to initiate phonation in individuals with DS which is indicative of increased muscular activation of the larynx. If the laryngeal muscles are typically hypotonic, then it is possible that higher levels of muscular activation are needed to initiate and sustain phonation. Developmental factors may be relevant as well. Laryngeal hyperfunction in typically developing children has been described by Sapienza, Ruddy and Baker (2004), who comment on the likelihood of false vocal fold adduction and the compression of the arytenoid cartilages to the petiole (stalk of the epiglottis).
1.3. Indications for Future Research and Clinical Services
Despite a long history of research, the nature of the phonatory disorder in DS is not clearly established. Results of acoustic studies have been mixed, so that it is difficult to draw firm conclusions or even to identify the most sensitive acoustic measures (e.g., jitter, shimmer, S/N) to be used in future research. The inconsistent results of efforts to identify acoustic correlates of perceived vocal abnormalities may mean that the vocal quality disorders are associated with a combination of acoustic characteristics that contribute in varying degrees to vocal quality among individuals with DS. Future research should be directed toward both structural (micro- and macro-anatomic features of the laryngeal tissues) and functional objectives, taking into account developmental factors. New insights may be gained by pursuing methods of the kind described by Mehta and Hillman (2008). These include: (1) perceptual assessment (use of the new consensus auditory-perceptual evaluation of voice inventory for the auditory-perceptual assessment of voice quality [CAPE-V]; Kempster, Gerratt, Verdolini Abbott, Barkmeier-Kraemer, & Hillman, 2009), (2) acoustic assessment (use of new algorithms that are more robust across varieties of dysphonia and are capable of deriving voice quality-related measures from conversational speech), (3) aerodynamic assessment (methods and devices for measuring phonation threshold air pressures and air flows), and (4) endoscopic imaging (high rates of image capture enhance the capabilities to examine the dynamics of vocal fold behavior). These research methods could be paired with a developmental perspective aimed toward the study of how laryngeal function changes with maturity and with the natural history of DS.
2. Speech Sound Disorders (Table 2)
Table 2.
Speech sound disorders
Summary of studies of speech sound disorders (articulation, phonology and resonance) along with related oral motor functions in individuals with DS. Information on age and gender is included, whenever available. Studies involving children are listed according to approximate age (youngest first). Within age groups (e.g., infants and adults), studies are listed in chronological order of publication. Unless stated otherwise, the participants were speakers of English (or, in the case of infants, having English as the ambient language). PCC-R is Percentage of Consonants Correct-Revised and PVR is Percentage of Vowels Correct. See Tables 1a and 1b for definitions of other abbreviations.
| Source | Participants | Method | Summary of Results |
|---|---|---|---|
| Legerstee, Bowman, & Fels (1992) | N = 8 DS (4M, 4F) (56 → 66 days old when study began) |
Perceptual rating: Longitudinal study of infant reactions to different situations, with vocalizations categorized as melodic (speech-like), vocalic (nonspeech-like), or emotional. |
DS produced more vocalic (nonspeech-like) sounds and fewer melodic sounds than TD infants studied previously. |
| Dodd (1972) | N= 10 DS (5M, 5F) (Infants 9 → 13 months) N= 10 TD (5M, 5F) (Infants 9 → 13 months) |
Acoustic and transcription: Measures of utterance frequency and duration; counts of phonetic constituents of utterances |
DS did not differ from control group on any measure. |
| Smith & Oller (1981) | N= 10 DS (infants) N= 9 TD (infants) |
Transcription: Determination of age of reduplicated babbling, developmental trends for place of consonant articulation, and developmental aspects of vocalic productions |
DS, like controls, began to produce canonical, reduplicated babble at 8 to 8.5 months. DS had patterns that were highly similar to those in the control group. |
| Steffens, Oller, Lynch, & Urbano (1992) | N= 13 DS infants (4 → 18 months)* N= 27 TD infants (4 → 18 months)* *Longitudinal study over the age period |
Perceptual: Categorization of vocalizations into 4 types: Quasi-vowel, Full vowel, Marginal syllable, and Canonical syllable |
DS developmental patterns not significantly different from TD. Large variability noted in both groups. |
| Lynch, Oller, Steffens, & Buder (1995) | N= 8 DS infants (2 → 12 months) * N= 8 TD infants *Longitudinal study from 2 to 12 months of life |
Acoustic/Perceptual: Judgments by non-trained adults of phrasing in infant vocalizations were made between nonvegetative utterances, temporal utterances, and utterance durations. |
DS rhythmic units longer in DS, but there were no differences between groups in overall vocal output or in the complexity of the rhythmic units. |
| Lynch, Oller, Steffens, Levine, Basinger, & Umbel (1995) | N= 13 DS infants (4M, 9F) N= 17 TD infants (17M, 10F) |
Perceptual judgment: Categorization of vocalizations including syllable type (canonical, marginal, quasi-resonant, fully resonant) |
DS were delayed by about 2 months in onset of canonical babbling relative to reported onset for TD infants; DS infants also had less stable babbling patterns. |
| Smith & Stoel-Gammon (1983) | N= 5 DS (2M, 3F) (longitudinal observations from 3–6 yrs) N= 4 TD (3M, 1F) (longitudinal observations from 18–36 months) |
Transcription Longitudinal observations of singleton stops consonants and clusters |
DS similar to controls, but had a considerable delay in sound acquisition. |
| Bleile & Schwarz (1984) | N= 3 DS 1M= 4;06 2F=3;04 & 3;06 |
Transcription: Analysis of free-play speech using 3 methods: phonological oppositions, phone acquisition and phonological processes |
DS had developmental delays; the 3 methods provided complementary information on phonological development. |
| Stoel-Gammon (1980) | N= 4 DS* (3;10 → 6;03) *Comparisons to typical development in literature |
Transcription Analyses of spontaneous speech to determine phonetic inventory, accuracy of target phonemes and characterization of errors in terms of phonological processes |
In DS, correct sound production tended to be limited to particular word positions; DS had phonological patterns similar to those reported for TD children. |
| van Bysterveldt (2009) | N = 77 DS (5;08 → 4:11) |
Assessment battery: Articulation, phonological awareness, letter knowledge, real word decoding |
In DS, PCC-R scores ranged from 55.2 to 93.5% (M= 78.2) and PVC scores ranged from 69.9 to 100&% (M=92.8). Evidence was seen for both developmental and nondevelopmental speech errors. |
| van Bysterveldt, Gillon, & Foster-Cohen (2010) | N= 10 DS (5M, 5F) (4;04 → 5;05) (Mean age 4;11) |
Assessment battery: A number of receptive/expressive language and phonological awareness tests were used to determine pre-treatment and post-treatment status |
In DS, PCC-R scores ranged from 22.4 to 76.1% and PVC scores ranged from 84.6 to 100%. |
| Moura, Cunha, L., Vilarinho, Cunha, M., Freitas, Palha, Pueschel, & Pais-Clemente (2008) | N= 66 DS (36M, 30F) (3 → 8 yrs) (mean age 5.8 yrs) N= 204 TD (104M, 100F) (Mean age 5.7 yrs) Speakers of Portuguese |
Acoustic: F1–F2 frequencies for the 5 main Portuguese vowels |
DS had smaller value of the ratio between F2 for /i/ and F2 for /u/ (termed the “DS vocalic anatomical functional ratio”); DS also had smaller F1–F2 area. |
|
Perceptual rating: Modified to Portuguese GRBAS rating scale by two expert speech therapists. |
All parameters showed significant differences between the two groups. | ||
| Kumin, Councill, & Goodman (1994) | N= 60 DS (31M, 29F) (9 mo → 9 yrs) |
Transcription: Emergence of phonemes in transcriptions obtained from structured therapy sessions in a play environment |
DS had considerable variation in age of emergence of individual sounds; DS had a different order of emergence compared to published norms for TD children. |
| Borghi (1990) | N= 50 DS (25M, 25F) (5.0 → 19.1 yrs) (Mean age 9.2 yrs) Participants divided into 3 age categories (5.0–7.11 yrs, 8.0–11.9, & 12.0–19.1) |
Articulation testing: Fischer-Logemann Test of Articulation Competence (Fischer & Logemann, 1971) |
DS had persistent articulation errors noted across the 3 age ranges; 7 phonemes were determined to be the most error-prone. |
| Crosley & Dowling (1989) | N= 22 DS (10M, 12F) (6;06 → 12;07) (Mean age 9;08) |
Transcription and coding: Analysis of phonological processes |
For DS, sentence length was a primary predictor of cluster reduction and liquid simplification; liquid /r/ was more difficult than liquid /l/. |
| Crosley & Dowling (1989–90) | N= 22 DS (10M, 12F) (6;06 → 12;07) |
Transcription and coding: Analysis of phonological processes |
DS had phonological patterns similar to those of younger TD children. |
| Roberts, Long, Malkin, Barnes, Skinner, Hennon, & Anderson (2005) | N= 32 DS (32M) (4 → 13 yrs) N= 50 AD* (50M) (3 → 14 yrs) N= 33 TD, (2 → 6 yrs) (Matched to DS & Fragile X groups on developmental age) *Fragile X |
Articulation testing: Goldman Fristoe Test of Articulation-2nd Edition (GFTA-2; Goldman & Fristoe, 2000) |
DS had more consonant errors than either of the other 2 groups. |
| Dodd (1976) | N= 5 DS, home-reared (6;06 → 8;05) N= 5 DS, residential (12;04 → 14;09) N= 10 TD (5 home-reared and 5 residential) N= 10 severely subnormal (5 home-reared and 5 residential) |
Transcription: Phonological analyses of oral responses to picture identification (spontaneous and imitative) |
DS had more errors than comparison groups; DS performed better on imitation than spontaneous naming. |
| Brown-Sweeney & Smith (1997) | N= 8 DS (mean age 7.0 yrs) N= 8 DS (mean age 12.0 yrs) 2 groups of TD children age-matched to DS groups |
Acoustic: Measurements of voice onset time, vowel duration and word duration from oscillographic tracings |
DS had greater temporal variability, poorer articulatory accuracy and slower syllable repetition rate; speech timing and maximum syllable repetition rates were good predictors of single-word accuracy. |
| Hohoff, Seifert, Ehmer, & Lamprecht-Dinneson (1998) | N= 10 DS (8M, 2F) (mean age 7.0 yrs) N= 10 TD (5M, 5F) (mean age 7.1 yrs) |
Acoustic: Spectrographic analyses of the test word “tasse” (including temporal and spectral features); compared to peripheral factors including Angle class, overbite, oral motor ability, hearing disorder and logopedics |
DS had a longer and more variable duration of the test word and a less sharp production of the fricative /s/. (Acoustic features not correlated to the peripheral factors under study). |
| Dodd & Thompson (2001) | N= 15 DS (12M, 3F) (5.6 → 15.8 yrs) N= 15 AD* *Phonological disorder characterized by inconsistent errors |
Transcription: 25-word Inconsistency Test (Burt, Holm & Dodd, 1999) |
DS did not differ in the number of whole words produced inconsistently, but there were differences in the quality of the inconsistent errors. |
| Rupela & Manjula (2007) | N= 7 DS (3M, 4F) (11.5 → 14.5 yrs) N= 7 mental-age matched children with mental retardation but without DS N= 6 TD (3M, 4F) (4 → 5 yrs) Speakers of Kannada |
Transcription: Analysis of phonotactic patterns in conversational speech |
DS had a higher percentage of the occurrence of simpler phonotactic patterns. |
|
Schlanger & Gottsleben (1957)* * Also in Table 1b |
N= 44 DS (ages not specified) N= 472 Controls ATD* (mean age 30 yrs) See Table 1b for detail |
Perceptual: Assessments of speech articulation, voice, and stuttering See Table 1b for Voice assessment/findings |
95% of participants with DS were judged to have an articulatory disorder. |
| Van Borsel (1988) | N= 5 DS (All F) (16;05 → 19;09) Speakers of Dutch |
Transcription: Phonetic and phonological analyses of speech |
DS speech errors were highly similar to those reported in young TD children. |
| Timmins, Hardcastle, Wood & Cleland (2011) | N= 26 DS (15M, 11F) (8;3 → 18;9) (Mean age 13.4) N= 10 TD matched for cognitive age (3;8 → 7;1) (Mean age 5.9) |
Electropalatographic: Articulatory contact for the obstruent /t/ in the word toe |
DS differed from TD in type of contact, with most frequent atypical patterns being forward movement, increasing contact, and minimal contact. |
| Cleland, Wood, Hardcastle, Wishart, & Timmins (2010) | N= 15 DS (12M, 3F) (9 → 18 yrs) (Mean age 14.3 yrs) |
Standardized Testing: Standardized speech, language and cognitive assessments |
DS had atypical and often unusual errors co-occurring with developmental errors; speech measures were not correlated with language or cognitive measures. |
| Rosin, Swift, Bless, & Vetter (1988) | N= 10 DS (All M) (10.6 → 17.5 yrs) N=10 ATD (All M) with mental retardation of unknown etiology (12.5 → 18.7 yrs) N= 10 TD (All M) with chronologic age matched to DS (12.2 → 18.6 yrs) N= 10 TD (All M) with mental age matched to DS (5.1 → 6.11 yrs) |
Transcription & Standardized Testing: Speech assessments with intelligibility rating, Goldman-Fristoe Test of Articulation, oral motor evaluation. |
DS had more articulatory errors and more abnormalities of oral structure than other groups. |
|
Aerodynamic: Intraoral air pressure for bilabial stop /p/ |
DS had higher intraoral air pressures for /p/ in different phonetic contexts. | ||
| McCann & Wrench (2007) | N= 12 DS (10.08 → 18.75 yrs) (Mean age 15.02 yrs) N= 4 TD (5.4 → 7.1 yrs) (Mean age 4.63 yrs) |
Acoustic & electropalatographic: Analysis of diadochokinesis (DDK) rate and accuracy |
DS and TD had similar DDK rates but DS group was more inaccurate. |
| Timmins, Cleland, Wood, Hardcastle, & Wishart (2009) | N= 20 DS (11M, 9F) (8 → 19 yrs) (Mean age 13;01) N= 8 TD (6M, 2F) (4 → 8 yrs) (Mean age 6;01) |
Transcription and electropalatography (EPG): Study of the production of the palatal fricative in “a sheep” |
DS had inconsistent production, with more errors observed in EPG than in perceptual judgment. |
|
Novak (1972) Also in Table 1a |
N= 32 DS (19M, 13F) (7 → 19 yrs) N= 20 Controls ATD* (11M, 9F) (7 → 20 yrs) N= 10 Controls TD** * ATD participants with cognitive delay but no DS ** TD participants for X-ray portion of study only |
Acoustic: Measures of vowel formant frequencies |
DS had overlapping F1/F2 areas for different vowels. |
|
Pneumographic: breathing patterns Imaging: X-rays of vocal tract Other: Otolaryngologic exam See table 1a for voice findings |
DS had shallow breathing, frequently abdominal. DS had altered shape of resonating cavities. DS had rough, over-large tongue, hypertrophy of tonsils, small and narrow epipharynx. |
||
| Fourakis, Karlsson, Tilkens, & Shriberg (2010) | N= 8 DS (gender not specified) (15 → 17 yrs) N= 8 Fragile X (All M) (15 → 19 yrs) N= 5 TD (gender not specified) (14 yrs) N= 5 TD (All M) (16 yrs) |
Acoustic: Measures of F1 and F2 in an effort to determine the acoustic correlates of nasopharyngeal resonance, which was judged to characterize the majority of the samples in DS, some of the samples in Fragile X, and none of the samples in TD |
DS had reduced F2 frequencies for the high vowels /i/ and /u/. |
| Rolfe, Montague, Tirman, & Vandergrift (1979) | N= 6 DS (5 M, 1 F) (non-institutionalized adults) (26 → 30 yrs) Participants were perceived as having hypernasal speech by 2 speech and language therapists |
Perceptual ratings: Ratings by 2 groups of listeners who differed in clinical experience |
DS had essentially normal ratings of nasality. |
| Kline & Hutchinson (1980) | N= 20 DS (10M, 10F) (15 → 35 yrs) N= 20 Controls ATD* (10M, 10F) N= 20 TD (10M, 10F) All groups 15 → 35 yrs * ATD with idiopathic mental retardation |
Acoustic: Measures of nasalance using TONAR II (Fletcher, 1972) |
DS had larger nasalance values. |
|
Perceptual ratings: Ratings of nasality |
DS had higher ratings of nasality. | ||
| Beckman, Wold, & Montague, Jr. (1983) | N= 2 DS (1M, 1F) (adults) *Both subjects had perceived voice disorders |
Acoustic: Analysis of first three formants with computer-generated vocal tract shapes. Measures of sustained vowels, f0, F1–3, and jitter. |
In DS, the pharynx cavity is lengthened and the oral cavity is shortened. |
| Moran (1986) | See Table 1 |
Acoustic: Measures of F1 and F1 for vowels /i/, /u/ and /a/ |
DS not different from TD in F1/F2 ratio. |
| Sommers, Reinhart, & Sistrunk (1988) | N= 22 DS* (15;02 → 22;02) N= 24 DS* (13;06 → 17;01) * Same participants as in Sommers, Patterson & Wildgen (1988) |
Coding of articulatory errors: Articulation assessed in spontaneous picture-naming test, imitation test, and a sample of spontaneous conversational speech |
Both groups of DS had patterns of delayed and deviant productions. |
| Sommers, Patterson, & Wildgen (1988) | N= 24 DS (10M, 14F) (15;02 → 22;02) |
Orthographic transcription: Presence of natural phonological processes determined from connected speech, picture-naming and imitation |
In DS, patterns of both delayed and disordered phonology were observed. |
| Hamilton (1993) | N= 3 DS (2M, 1F) (17, 17 & 20 yrs) N= 1 TD (F) (adult) |
Electropalatographic: Analysis of diadochokinetic (DDK) performance |
DS had various irregularities in EGP patterns, including excessive contact areas and reduced contact areas, asymmetrical contacts, prolonged contacts; slow DDK rates. |
| Van Borsel (1996) | N= 20 DS (10M, 10F) (mean age 20;10) (15;04 → 28;03) N= 20 TD (10M, 10F) (mean age 3;00) (2;06 → 3;04) Speakers of Dutch |
Transcription: Examination of consonant, vowel and diphthong production to determine sounds in error, error rate and nature of errors (error type). |
DS had similar patterns to TD group, which was interpreted as evidence of developmental delay to account for speech patterns in DS. |
| Bunn, Simon, Welsh, Watson, & Elliott (2002) | N= 14 DS (6M, 8F) (22 → 36 yrs) (Mean age 29.2 yrs) N= 15 ATD* (5M, 10F) (21 → 41 yrs) (Mean age 29.1 yrs) *Developmental delays |
Transcription: Reading, repeating and formulating speech from a picture following presentation of word and picture sequences |
DS had more memory errors and also had more speech production errors in the repetition and formulation tasks (but not in reading). |
| Carlstedt, Henningsson, & Dahllöf (2003) | N= 9 DS* (6M, 3F) (Mean age 5.6 yrs) N= 11 DS** (6M, 5F) (Mean age 5.6 yrs) *PPT Treatment Group ** Control Group |
Articulation testing & Oral Exam: Consonants, nasals, and vowels perceptually assessed, questionnaire, and intraoral exam. |
DS round lips more during spontaneous speech. |
| Barnes, Roberts Mirrett, Sideris, & Misenheimer (2006) | N= 34 DS (Males) (4.3 → 15.9 yrs) (mean age 7.9 yrs) N= 59 ATD (Males) Fragile X (2.9 → 14.0 yrs) (mean age 9.1 yrs) N= 36 TD (Males) (2.5 → 6.6 yrs) (mean 4.6 yrs) TD boys developmentally matched to DS and FX |
Oral-motor exam: Assessment of structure and function using an adapted version of Robbins & Klee’s (1987) Oral Motor Speech Protocol |
Structure: Boys with DS had more atypical oral structures than the 2 comparison groups. Oral and speech function: Boys with DS performed more poorly than TD boys. |
| Barnes, Roberts, Long, Martin, Berni, Mandulak, & Sideris (2009) | N= 34 DS (Males) (4.5 → 16.0 yrs) (mean age 9.7 yrs) N= 31 ATD (Males) Fragile X and ASD (5.0 → 15.4 yrs) (mean age 10.1 yrs) N= 32 ATD (Males) Fragile X Only (3.2 → 14.5 yrs) (mean age 10.9 yrs) N= 45 TD (Males) (2.8 → 7.8 yrs) (mean age 5.0 yrs) Developmentally matched to other groups |
Phonological Assessment: Measures of phonological accuracy, phonological process occurrence, and intelligibility determined for connected speech samples |
Boys with DS scored lower than other groups on phonological accuracy and occurrence of phonological processes. DS had greater delays in all phonological measures. |
| Saz, Simon, Rodriguez, Lleida, & Vaquero (2009) | N= 3 DS (1M, 2F) (2 F=13 yrs, 1 M= 18 yrs) N= 11 ATD* (6M, 5F) (11 → 21 yrs) * Individuals with cognitive or physical impairments affecting speech Compared to a reference corpus of 13–14 year old females. |
Acoustic: Analysis of how vowel production varies using LPC formant frequencies, f0, (tone), intensity, and duration. |
No specific conclusions on DS. |
|
Perceptual: Judgments of vowel production |
2 of 3 DS had substantial vowel errors | ||
| Bunton & Leddy (2011) | N= 2 DS (Males) (29 & 26 yrs) N = 2 TD (Males) (29 & 26 yrs) |
Acoustic and radiographic: Analysis of vowel formant frequencies using LPC; kinematic studies of tongue articulation using X-ray microbeam |
DS had smaller acoustic vowel space (F1 & F2), reduced articulatory working space, and slower articulatory movements. |
Light grey indicates an instrumental methodology.
2.1. Review of Literature
Studies in speech sound disorders in DS disclose a variety of problems affecting speech sound articulation, timing of syllable sequences, and phonological patterns. As shown in Table 2, research in this category involved a total of more than 700 participants and the number of participants in individual studies generally ranged from fewer than 10 to 66, with a mean of about 16.
2.1.1. Ontogeny of Speech Disorder
This section is concerned with the phonetic properties of speech-like vocalizations such as babbling, which involves supraglottal adjustments such as those of the jaw, lips and tongue. Divergence in speech patterns between children with DS and typically developing children is clearly evident between the ages of 3 and 6 years (Bliele & Scharz, 1984; Moura et al., 2006; Smith & Stoel-Gammon, 1983). The stage of development at which differences in phonetic behavior emerge is less clear, but speech patterns may begin to diverge as early as the first year of life. Studies on early speech development in DS appear in the first section of Table 2.
Although some studies did not find any remarkable differences in vocal development in infants with DS compared to TD infants (Dodd, 1972, Smith and Oller, 1981, Steffens et al., 1992), differences between DS and TD infants have been observed. For example, studies have shown that infants with DS produced more nonspeech sounds and fewer speech-like sounds than TD infants (Legerstee et al., 1992) and that the onset of canonical babbling was delayed by about 2 months in infants with DS and was less stable than in TD infants (Lynch, Oller, Steffens, Levine, Basinger and Umbel, 1995). As discussed by Oller (2000), these conflicting results may be attributable in part to different sampling intervals. Oller also noted that the delay in babbling onset in infants with DS is surprisingly small, especially when compared to the delays in gross motor skills such as sitting, crawling, standing and walking (Palisano et al., 2001). Similarly, Cobo-Lewis et al. (1996) concluded that although attainment of canonical babbling was delayed in subjects with DS, the delay was smaller than that for other milestones in motor and vocal development they considered.
Smith and Stoel-Gammon (1996) reported no major differences in the development of specific types of babbling (e.g., reduplicated versus variegated) in infants with DS aged between 6 months and 2 years of age, when compared to TD age-matched infants. Research on phrasing in infant vocalizations showed that infants with DS have longer rhythmic units than infants with TD, but there were no differences in overall vocal output or in the complexity of the rhythmic units (Lynch, Oller, Steffans & Buder, 1995).
From these rather disparate findings we can conclude that: (1) the occurrence of babbling is typical but not universal in infants with DS (the same appears to be true of TD infants, but relevant data at the population level are surprisingly meager), (2) the age of onset of canonical babbling in infants with DS overlaps that in TD infants, but may be somewhat delayed in infants with DS, (3) there may be differences in the features of babbling between infants with DS and TD infants, (4) the delays in babbling are much less conspicuous than delays in gross motor skills such as crawling and walking.
2.1.2. Perceptual studies of vowel and consonant errors
An overall indication of vowel and consonant errors is expressed in the two measures of percentage of vowels correct (PVC) and percentage of consonants correct-revised (PCC-R). (In the calculation of PCC-R, both clinical and nonclinical distortions are counted as correct, so that only substitutions and omissions are counted as error sounds.) Bysterveldt (2009), reporting on 77 children with DS, obtained a mean percentage of vowels correct (PVC) of 92.8 and a mean percentage of consonants correct-revised (PCC-R) of 78.2. In an intervention study of 10 children with DS in the age range of 4 to 5 years, Bysterveldt et al (2010) observed a PVC mean of 91.3 compared to a mean PCC-R of 50.6. These values of PCC-R in DS exceed those for TD children compiled in Bernthal, Bankson and Flipsen (2009) except for one study of children with a mean age of 1;6.
Several studies of speech in DS have noted vowel errors (Bunton, et al., 2007; Bysterveldt, Gillion & Foster-Cohen, 2010; Van Borsel, 1996). In their study of phonetic contrasts impaired in adults with DS, Bunton et al. (2007) reported frequent errors with high versus low vowel and front versus back vowel. These errors indicate a limitation in the regulation of tongue height and advancement, which can occur because of anatomic factors, motor limitations, or both. This issue is revisited in a subsequent discussion of acoustic studies of vowel articulation (section 2.1.4).
Studies of both children and adults point to a higher than normal frequency of articulatory errors, with substantial involvement of consonants (Brown-Sweeney & Smith, 1992; Bunn et al., 2002; Bysterveldt, 2009; Bysterveldt et al., 2010; Kumin, 1994; Roberts et al., 2005; Rosin et al., 1988; Schlanger & Gottsleben, 1957; Sommers et al., 1988; Timmins et al., 2009). Both the emergence and mastery of consonant phonemes in children with DS appear to be protracted processes, with substantial inter-individual variability. The emergence of phonemes in the speech of children with DS does not seem to follow the order of published norms for TD children (Kumin, Councill & Goodman, 1994). The most frequently misarticulated consonants may differ between DS and TD children. For example, Sommers et al. (1988) reported that for their group of 15- to 22 year old participants, the ten most frequently misarticulated sounds were (in descending order): /s/, /d/, /t/, /r/, /z/, /l/, /s/ blends, /r/ blends, /n/ and /v/. Errors on /d/, /t/, /n/ and /v/ are not common in TD children and these sounds usually are mastered at an early age, with most children mastering /d t n/ by about 3 years of age (Bernthal, Bankson, & Flipsen, 2009, p. 96). Of the 10 sounds listed by Sommers et al., seven involve the alveolar place of articulation, which is the most frequently used place of articulation in English and carries a significant intelligibility load (see Section 4). Bunton et al. (2007) identified phonetic contrasts that were most affected in DS. These included, in addition to the vowel contrasts mentioned earlier: (1) simplification of clusters in both the word initial and word final position, and (2) contrasts involving tongue-posture, control, and timing (place of articulation for stops and fricatives).
2.1.3. General conclusions from perceptual studies of articulation
A condition is properly viewed as developmental delay if the features of the condition follow the typical developmental course but with an overall delay in progress. The term disorder is applied if the features deviate from the pattern of typical development. Although developmental errors of articulation are prominent in DS, articulation errors of a non-developmental (“disordered”) nature also have been noted (Cleland et al, 2010; Dodd & Thompson, 2001; Kumin, Councill & Goodman, 1994; Sommers, Reinhart & Sistrunk, 1988).
2.1.4. Acoustic and physiologic studies of speech in DS
Studies involving acoustic and/or physiologic methods are shaded in Table 2 to distinguish them from the more commonly used perceptual or transcription methods. Several studies examined vowel production acoustically by examining formant frequencies. Novak (1972) commented that the overlap of F1–F2 areas for different vowels may explain listener difficulties in distinguishing vowels in DS, although Moran (1986) found no difference between DS and controls. Similarly, Saz et al. (2009) concluded from a study of Spanish speakers that errors in vowel identification were related to the confusability of vowel formant patterns, as well as to poor control over the energy in stressed versus unstressed vowels and excessive variability in vowel duration.
Moura et al. (2006) reported that individuals with DS had a smaller ratio of the F2 frequencies for vowels /i/ and /u/ and called this ratio the “DS vocalic anatomical functional ratio,” implicating anatomy as the underlying basis of the formant-frequency abnormality. However, this ratio may reflect either anatomic or motor factors (or both), since it is also a robust discriminator of dysarthric vs. healthy speech (Sapir, Ramig, Spielman & Fox, 2010). In a combined acoustic-articulatory study of two adults with DS, Bunton and Leddy (2010) reported a reduced range of F2 frequencies for the vowels /i/ and /u/, in agreement with Moura et al. (2006). Their data also show a smaller acoustic vowel area and a reduced articulatory working space compared to two age- and sex-matched healthy controls. Their most striking finding, markedly low F1 frequencies for the low vowels, could be explained by reduced mouth opening (and probably jaw lowering) in the participants with DS. In an acoustic study designed to identify the correlates of nasopharyngeal voice quality (presumably a frequent characteristic of DS), F2 frequencies for the high vowel sounds were shown to be reduced in adolescent participants with DS, compared to TD children (Fourakis, Karlsson, Tilkens, & Shriberg, 2010). This feature was interpreted as evidence of backing of the tongue. The difference in F2 between /i/ and /u/ was virtually identical between the DS group and the TD group, which means that this dimension of the vowel space was not compressed in DS, contrary to the results of Moura et al. (2006).
Although it is reasonable to expect that vowel working space tends to be reduced in DS, studies on vowel formant frequencies in children and adults have been very limited and somewhat contradictory. More extensive data are needed from children and adults with DS. These could be compared against normative data on acoustic vowel area that have been compiled for various age-sex groupings of speakers (Vorperian & Kent, 2007).
In a study of speech timing patterns, Brown-Sweeney and Smith (1997) did not find significant differences between DS and TD children for durational measures, but the DS group was significantly more variable in 2 of 7 segment measurements. Variability of word duration in children with DS also was reported by Hohoff et al. (1998), whose results pertained to production of a single German word (Tasse, meaning cup). These limited data point to increased variability in some temporal structures but not to abnormalities in the durations of segmental structure.
Physiologic methods are shedding new light on speech articulation in DS. Patterns of lingual contact have been studied with electropalatography (EPG) (Gibbon et al., 2003; Hamilton, 1993; Timmins, Cleland, Wishart, Wood, & Hardcastle, 2009; Timmins, Hardcastle, Woods & Cleland, 2011). Abnormalities observed in DS included both excessive and reduced areas of articulatory contact, moving contact, extended closure durations for occlusive consonants, and lengthened consonant transition times within clusters. Articulatory abnormalities were sometimes seen even when production of a speech sound was judged perceptually to be correct. Aerodynamic data on speech production in DS have seldom been reported, but Rosin et al. (1988) noted an increased intraoral air pressure for /p/ in speakers with DS. One interpretation of this result is that individuals with DS produce speech with greater respiratory pressures than healthy controls. This possibility, together with the indication of increased muscular activation for phonation (Novak, 1972; Pryce, 1994; Section 1.2) could mean that individuals with DS expend more energy in speech production than do TD speakers.
2.1.5. Phonological patterns
Articulation as a process is focused on physical production of sounds and the articulation data reviewed above answer questions such as: When are individual speech sounds mastered? In contrast, phonology pertains to sound patterns such as those used to form words (for example, the shapes of syllables within words) and phonological data are suited to questions such as: When are the phonological patterns of the language reliably produced to form words? Studies of phonology in DS are summarized in Table 2.
Phonological patterns in DS have been described for English speakers (Barnes et al., 2009; Cleland et al., 2010; Crosley & Dowling, 1989–1990; Dodd, 1976; Dodd & Thompson, 2001; Roberts et al., 2005; Sommers, Patterson & Wildgen, 1988; Stoel-Gammon, 1980; van Bysterveldt, 2009); Cantonese speakers (So & Dodd, 1994); Dutch speakers (Van Borsel, 1988); and Kannada speakers (Rupela & Manjula, 2007). As with studies of articulation (Section 2.1.4), phonological studies support a conclusion of combined developmental and disordered patterns in children with DS (Cleland et al., 2010; Dodd, 1976; Roberts et al., 2005; So and Dodd, 1994; Sommers, Patterson & Wildgen; 1988). For example, Sommers et al. (1988) observed the following nondevelopmental or disordered patterns: persistence of final consonant deletion processes, unusual difficulty with the acquisition of the liquids /r/ and /l/ and the nasals, and frequent errors with stop consonants. Unusual or atypical processes noted by van Bysterveldt (2009) included: syllable reduction, glottal substitutions, epenthesis, matathesis, coalescence, and idiosyncratic substitutions. Nondevelopmental errors may be characteristic of a subtype of DS and may not necessarily occur in all individuals with DS.
2.1.6. Nasality and nasalance
Nasality is a perceived resonance quality that is related to velopharyneal function. Nasalance is a physical measure of the ratio of nasally emitted acoustic energy to orally emitted energy. Nasality and nasalance are complementary measures but they are not necessarily correlated in all speakers and speaking tasks.
Although nasality has been mentioned in some descriptions of speech in DS, very few studies have directly assessed this aspect of speech production. In their study of pain cry in neonates and infants, Lind et al. (1970; Table 1) remarked that hypernasality was a common feature in DS. Rolfe et al. (1979) noted that nasality was normal in most of their participants but that inconsistent hypernasality appeared in six children with DS. Hypernasality was not a prominent feature of speech reported in a parental report survey (Kumin, 2006), but lay individuals are not particularly discriminating when judgments of nasality are concerned. Kline and Hutchinson (1980) observed a marked increase in both perceptually judged nasality and acoustically determined nasalance in individuals with DS. Further study of oral/nasal resonance is needed, given that nasalization may contribute to abnormal voice quality, reduced energy levels in speech (because of increased damping in sound transmission through the vocal tract), and reduced intelligibility (because nasalization can interfere with the production of phonetic contrasts). It is also possible that oral/nasal resonance balance is affected by abnormalities in the nasal cavities, sinuses and the tissue boundaries between the oral and nasal passages. As mentioned earlier, Fourakis et al. (2010) reported on the acoustic correlates of a voice quality they termed nasopharyngeal resonance. The origin of this quality is unclear but it may be related to reports of hypernasality in DS.
2.1.7. Oral motor control in simplified speaking tasks
Diadochokinesis (DDK), also known as maximum syllable repetition rate or alternating motion rate, is commonly used to assess oral movement skills in a task that makes modest demands on language ability and memory. Most studies of DDK in DS report a decreased rate (Brown-Sweeny & Smith, 1977; Hamilton, 1993; Rosin et al., 1998) but McCann and Wrench (2007) observed a DDK rate similar to that in typically developing children although they noted that the participants with DS were more inaccurate in performing the task.
The generally slow DDK rates reported for DS stand in contrast to some reports of an overall normal or even rapid speaking rate. Fawcett and Peralego (2009) commented, “Probably one of the most striking characteristics of the speech of people with Down syndrome is a rapid rate” (p. 111). But rapid rate has not been uniformly confirmed in DS, with at least one study reporting a slower speaking rate in words per minute for DS compared to TD controls (Chapman, Seung, Schwartz & Kay-Raining Bird, 1998). Brown-Sweeny and Smith (1997) found that temporal segment durations in word production were not significantly different between speakers with DS and TD speakers even though the speakers with DS had slower DDK rates. Additional studies of speaking rate for both syllable repetition and meaningful speech are needed before firm conclusions can be drawn. The issue of speaking rate is revisited in the discussion of disfluency (Section 3), where rate is potentially related to the disorder of cluttering.
2.1.8. Anatomic Anomalies and Pathophysiology
Description of craniofacial anomalies is complicated by phenotypical variation and by developmental changes of specific features. Some characteristics of DS, including brachycephaly and the absence of nasal bone ossification, can be identified prenatally (Stempfle et al. 1999). Craniofacial dysplasia is evident at birth and increases in severity with age until at least 14 years (Fischer-Brandies, 1988), although the rates and directions of growth appear to be similar to typical development (Frostad, Cleall & Melosky, 1971).
2.1.8.1. Overall craniofacial anatomy
In an MRI study, Uong et al. (2001) noted that, compared to controls, participants with DS had reduced volumes of the airway, mandible, adenoid and tonsil and a smaller mid- and lower-face skeleton and hard palate. The tongue, soft palate, pterygoid and parapharyngeal fat pads seemed unaffected. It was concluded that the reduction in upper airway size is the result of soft tissue crowding within a smaller mid- and lower-face skeleton. An anthropometric study of craniofacial features showed a relatively small maxilla but a normal mandible (Allanson, O’Hara, Farkas & Nair, 1993). A number of dental abnormalities have been reported (Cohen & Winer, 1965; Shapiro, Gorlin, Redman & Bruhl, 1967). Anatomic studies have shown poorly differentiated midface muscles and the presence of muscles not seen in healthy individuals (Bersu, 1976, 1980).
2.1.8.2. Hypotonia
It is repeatedly asserted in the literature on DS that affected individuals have a hypotonic musculature (Desai, 1977). However, assessments of stiffness do not necessarily support the contention that hypotonia is a pervasive characteristic (Connaghan, 2004). To the extent that hypotonia is present, it could explain some the speech features that resemble the dysarthrias, with the expectation that these features would resemble those in flaccid or ataxic dysarthria, both of which are associated with hypotonia. Generalized hypotonia could help to explain altered function in the subsystems of speech production-- especially the larynx, velopharynx, and the oral articulators.
2.1.8.3. The tongue
Macroglossia has historically been assumed to be a common feature of DS. This thinking led to surgical intervention by lingual resection, but it appears that an enlarged tongue in DS is more apparent than real. Adran, Harker and Kemp (1972) concluded from a radiographic study that none of the 16 children with DS had a generalized enlargement of the tongue, although regional enlargement was noted in five individuals. Similarly, Guimaraes, Donnelly, Shott, Amin, & Kalra (2008) concluded that children with DS do not have true macroglossia but rather have relatively large tongues compared to the bony confines of the oral cavity. Evidence also has been reported on abnormalities of the myofibers of the tongue (Yarom, Sagher, Havivi, Peied & Wexler, 1986).
2.1.8.4. The palate
Abnormalities in palatal anatomy have been recognized for decades (Benda, 1960, Oster, 1953). In one early study, it was concluded that the palates of individuals with DS were narrower but not higher than the palates of controls (Oster, 1953). More recently, however, Dellavia et al. (2007) reported no differences in the sagittal plane but observations of the frontal plane showed a higher palate. Similarly, Bhagyalakshmi (2007) concluded that individuals with DS had smaller values than age- and sex-matched controls for measures of palatal width, length and volume, but they had greater values for the measure of average palatal height.
Skrinjari (2004) found that shelf-like or “stair palate” palatal shape was more than three times as likely to occur in participants with DS than in a control group. It was also noted that the frequency of shelf-like palate diminished with age, which was attributed to the growth of craniofacial structures and increased tonus of the tongue and other orofacial muscles.
Beck (1997) suggested that the short, narrow palate with an essentially normal tongue would lead to fronted articulations of the tongue tip and blade, along with a fronting and raising of the tongue body setting. Brunner, Fuchs and Perrier (2009), concluded that flat palates are associated with a greater acoustic sensitivity and therefore a smaller tolerance in articulatory positioning than arched palates. The acoustic effects of shelf-like palatal shape apparently have not been studied.
2.1.8.5. Vocal tract and laryngeal configuration
Beck (1997) described significant differences in the “vocal setting” in DS compared to healthy controls including protruded mandible; fronted tongue body; pharyngeal constriction; harshness; whisperiness; lax vocal tract; minimal range of lip, tongue and jaw motion; nasality and open jaw. Evidence of a relatively small oral cavity in the presence of apparently normal pharyngeal length, pharyngeal volume and vocal tract length was reported by Xue, Kaine and Ng (2010), who used an acoustic reflection technique.
2.1.8.6. Auditory function
Reports on the prevalence of hearing loss in DS vary considerably, but some degree of hearing loss has been noted in audiometric studies of children (Balkany, Downs, Jafek, & Krajicek, 1979; Park, Wilson, Stevens, Harward & Hohler, 2011; Roizen, Wolters, Nicol & Blondis, 1993; Shott, Joseph & Heithaus, 2001) and adults (Buchanan, 1990; Evenhuis, Van Zanten, Brocaar & Roerdinkholder, 1992). Survey studies show moderate prevalence of hearing impairment (Kumin, 2006; Schreve et al., 2009). Hearing impairment certainly must be considered in explanations of delayed or disordered development of articulation but, as Vicari (2006) observed, “there is no definitive evidence that language impairment in DS is merely a consequence of the hearing loss” (p. 356).
2.1.8.7. Summary
The craniofacial anatomy in DS is characterized by a compact mid-and lower-face skeleton, a tongue of average size, and a palate that is high and often shelf-like. The developmental trajectory of orofacial characteristics is not well established. Developmental instabilities have been implicated in fluctuating dental asymmetry (Barden, 1980), which is an example of a more general pattern of developmental instability manifest as decreased developmental and physiological buffering against genetic and environmental forces (Shapiro, 1975; Shapiro, Herman & Opitz, 1983).
2.2. Discussion
Speech production in DS is compromised by several types of impairment. The relationship among these multiple impairments is not clear, because the full range of impairments has rarely been examined in the same set of participants. There is reasonable agreement on the following general points:
Speech difficulties are not highly correlated with language or cognition, which may indicate that problems in speech are rooted in other factors such as anatomy and motor control.
Reports are mixed on the extent to which infants with DS have atypical patterns of vocal development, but there appears to be some delay in the appearance of canonical babbling. Any such delay is modest compared to delays in gross motor skills.
Articulatory and phonological studies show both delayed (i.e., developmental) and disordered (i.e., nondevelopmental) patterns in children with DS by the age of about 3 years, although other effects may appear at earlier ages.
Articulatory and phonological patterns in DS show inconsistent errors and possibly increased variability at the acoustic level, at least for some segments. This fluidity of disordered patterns is an important clue to their etiology and a factor to be considered in assessment and treatment.
Although peripheral factors such as anatomic anomalies are not likely to explain all aspects of the speech disorder in DS, the deviations may impose some limitations on articulatory performance (Beck, 2010; Leddy, 1999; Bunton & Leddy, 2010). It is not well established how developmental changes in anatomy and physiology relate to articulatory and resonance features of speech.
2.3. Indications for Future Research and Clinical Services
Perceptual methods such as articulation testing and transcriptions of speech samples have provided a general description of speech sound disorders in DS. As indicated in Table 2, the error patterns are complex and may be understood more fully from the use of instrumental methods, such as acoustic analysis, aerodynamic recordings, EPG, and movement transduction. It may be particularly informative to use combined methodologies to study speech production in DS, e.g., combining acoustic measures of speech with physiologic recordings. In addition, electromagnetic articulography (EMA) may be suitable to the study of speech movements in adults and children with DS. This method has been used successfully to study speech articulation in children with dysarthria (Murdoch & Goozee, 2003). Reports of increased variability in speech production could be examined further with the spatiotemporal index (STI), a measure of variability in the production of several tokens of an utterance (Smith, Goffman, Zelaznik, Ying, & McGillem, 1995). It is also particularly important to study micro- and macro-anatomic development of the craniofacial system with respect to its motoric capabilities to determine structure-function relationships.
3. Fluency and Prosody (Table 3)
Table 3.
Fluency and prosody
Summary of studies of fluency and prosody in individuals with DS. Studies are listed in approximate order of age of participants. See caption for Table 1 for definition of abbreviations.
| Source | Participants | Method | Summary of Results |
|---|---|---|---|
| Reichle, Siegel, & Rettie (1985) | N = 8 DS (4M, 4F) (2.2 → 3.75) (mean age 2.73 yrs) |
Perceptual ratings: Imitations of adult vocalizations that were systematically varied in pitch, duration and loudness |
No relationship between imitative performance for prosodic features and speech sounds; no particular prosodic feature was more likely to be imitated. |
| Stojanovik (2010) | N= 9 DS (8;03 → 12;05) (mean age 9;09) N= 8 TD (4;02 → 5;07) (mean age 5;05) *MA matched controls N= 8 TD (8;00 → 11;00) (mean age 9;08) Age matched to DS *CA matched controls No gender mentioned. |
Standardized testing: Assessment of prosody with the computerized battery, Profiling Elements of Prosody for Speech and Communication (PEPS-C) (Peppe, McCann & Gibbon, 2003) |
DS had significantly lower scores than the CA matched group on all aspects of prosody. DS had significantly lower scores than the MA group on the production of affect and the production of pre-final narrow focus, and on all four tasks assessing prosody. DS receptive language abilities unrelated to prosodic abilities. |
| Nash & Snowling (2008) | N= 17 DS (7M, 10F) (9;05 → 17;00) (mean age of 14 yrs) N= 17 TD* (6M, 11F) (5;06 → 9;05) (mean age 7;02) *Matched pairwise to DS participants for receptive vocabulary age. |
Verbal fluency task: Semantic and phonological representations observed in a verbal fluency task. |
DS had reduced productivity in both semantic and phonological tasks, which was interpreted to reflect less efficient retrieval strategies. DS produced fewer clusters in phonological task. Reduced productivity in semantic/ phonological fluency is due to impaired processing. |
| Willcox (1988) | N= 5 DS (3M, 2F) Considered non-fluent (10;10 → 15;01) N= 5 TD (5M) (2;00 → 2;08) TD children matched for Language |
Perceptual ratings: Analysis of frequency and type of disfluencies |
Similarities and differences observed in the disfluency types of the 2 groups The mean number of non-fluencies for DS was 7.4 (per 100 words) and 3.6 for TD. Questionable results because of individual differences. Repetitions most common for both groups. Percentages of prolongations much lower in the TD group. |
| Pettinato & Verhoeven (2008) | N= 16 DS (10M, 6F) (11 → 20 yrs) N= 12 *TD (4.06 → 7 yrs) *Matched on receptive vocabulary level with gender balance similar to that for DS group |
Perceptual ratings: Examination of the production (using a non-word repetition task) and perception of word stress (using XAB discrimination task) |
DS had processing difficulties in both the production and perception of more difficult and later acquired stress patterns as well as weak word-initial syllables. |
| Van Borsel & Vandermuelen (2008) | N= 76 DS (51M, 24F, 1 Unknown) (3.8 → 57.3 yrs) (mean age 22.8 yrs) |
Perceptual ratings: Used Predictive Cluttering Inventory (Daly, 2006) administered by 26 speech-language therapists |
78.9% of DS had scores that classified them as clutterers, and 17.1% had scores that classified them as clutterer-stutterers. |
| Gottsleben (1955) | N= 36 DS (23M, 13F) (8;11 → 51;07) (Mean age 27;03) N= 36 *ATD (23M, 13F) (9;07 → 76;05) (mean age 28;03) *Individuals with mental retardation but not DS |
Perceptual ratings: Judgments of stuttering by 3 individuals |
33% of DS were identified as stutterers. |
|
Schlanger & Gottsleben (1957)* * Also in Table 1b |
N=44 DS participants (ages not specified) N= 472 ATD* For all participants (DS and ATD), mean CA was 28.9 yrs *ATD individuals with other etiologies of mental retardation; all were residents of a training school |
Perceptual ratings: Assessments of speech articulation, voice, and stuttering |
45% of DS were judged to stutter. |
| Rohovsky (1965) | N= 9 DS (2M, 7F) (10;07 → 19;03) (mean age 15;10) (institutionalized) N= 18 DS (9M, 9F) (9;04 → 19;07) (mean age 14;06) (non-Institutionalized) |
Perceptual ratings: Fluency judgments (severity, incidence, and reactions of stuttering) by 10 graduate students enrolled in the study of speech and hearing |
Stuttering identified in 35% of the institutionalized group and 19% of the non-institutionalized group (both high and low verbal groups). Greater incidence of stuttering found in females than males. |
| Preus (1972) | N= 47 DS (21M, 26F) (7+ yrs) (Participants part of a day-home for mentally deficient individuals in Oslo.) |
Perceptual ratings & Transcription: Analysis of stuttering and cluttering behaviors by 10 judges familiar with DS individual based on a spontaneous speech sample. Articulation Testing: Articulation test (by L. Backe) used to screen for articulatory disorders |
Stuttering on 5 percent of words observed in 34%, secondary symptoms observed in 29.8%, and cluttering observed in 31.9%. 52% of DS were judged to be stutterers. 46.8% showed no signs of cluttering. 10.6 % had a pronounced tendency to stutter, and 31.9% were clutterers. |
| Otto & Yairi (1974) | N= 19 DS* (9M, 10F) (14 → 31 yrs) (mean age 21;00) N= 19 TD (15 → 32 yrs) (mean age 22;04) TD group matched to DS group on sex, age, and race. *Institutionalized individuals |
Perceptual ratings: Analysis of 7 disfluency categories for samples of spontaneous speech |
DS were more disfluent on categories that are regarded as most typical of developmental stuttering. |
| Devenny & Silverman (1990) | N= 31 DS (20M, 11F) (30.0 → 57.5 yrs) (M mean age= 40 yrs) (F mean age=41.5 yrs) |
Transcription and standardized testing: Analysis of the relationship between speech disfluency and manual lateralization |
42% of DS were judged to be stutterers; increased disfluency was associated with increased non-right-handedness. |
| Devenny, Silverman, Balgley, Wall, & Sidtis (1990) | N= 8 DS (8M) Stutterers (40.0 → 39.5 yrs) N= 8 DS (8M) Fluent (40.0 → 39.5 yrs) |
Electropalatographic: Verbal and manual motor production tasks at two levels of complexity (simple and complex) Simple: Diadiochokinetic rate and finger tapping Complex: imitation of sentences and pegging a pegboard |
Compared to the fluent controls, the individuals who stutter were faster on the simpler tasks but slower on the more complex tasks. |
| Ferrier, Bashir, Meryash, Johnston, & Wolff (1991) | N= 18 DS (mean age 19.55 yrs) N= 18 ATD Fragile X (mean age 21.63 yrs) N= 18 ATD Autism (mean age 16.68 yrs) *All groups had 10 adults and 8 children. Mean ages are for the total group. For children, the mean ages were 9.31 yrs for DS, 9.2 yrs for FX and 9.17 for ASD No gender mentioned |
Transcription and coding: Analysis of conversational roles, conversational skills, and articulatory fluency (among others). |
DS had significantly more disfluencies (6.1%) than the group with Autism (1.6%) but not significantly different from the group with Fragile X (4.9%). |
| Flipsen (1999) | N= 6 DS (2M, 4F) (21;00 → 39;00) |
Perceptual ratings: Determination of intelligibility and segmental accuracy |
In DS, prepausal rhythm groups were more intelligible. |
| Shriberg & Widder (1990) | N= 8 DS (part of a larger group of 40 20- to 50-yr-old non-institutionalized adults with mental retardation) |
Transcription: Narrow phonetic transcription of recorded speech samples to determine segmental and suprasegmental (prosodic) characteristics |
DS had problems with most prosodic variables, including rate, phrasing, stress, and voice quality. |
3.1. Review of Literature
As noted in the introduction, fluency and prosody are grouped together in this review because they pertain to speech behaviors that are best expressed in units larger than the phone (i.e., the syllable or multisyllabic strings). Studies of fluency disorders have used several different terms, including dysfluency, disfluency, stuttering, cluttering and stuttering/cluttering. For present purposes, the word disfluency is a general term that includes all varieties of interruption in the flow of speech. Some of the reported disfluencies may be similar to those that occur in typical speech development.
3.1.1. Disfluency
Studies of speech disfluency in more than 300 participants have demonstrated that stuttering and/or cluttering occurs in DS at rates of 10 to 45% (Table 3), compared to the incidence of about 1% in the general population (Guitar, 1998). It is generally not possible to distinguish normal developmental disfluencies from genuine stuttering or cluttering in this literature. Presumably, stuttering and cluttering were judged to be clinically significant. The published data do not permit conclusions on the persistence or developmental pattern of fluency disorders in individuals with DS.
In studies, stuttering has been demonstrated 10% to 45% of children with DS, with a mean of about 31%, or 1 in every 3 individuals with DS (Devenny & Silverman, 1990; Gottsleben, 1955; Keane, 1970; Preus, 1972; Rohovsky, 1965; Schlanger & Gottsleben, 1957). Rohovsky (1965) observed a rate of 36% in institutionalized individuals with DS, compared with 19% in those individuals with DS who were not institutionalized. Survey data confirm a rather high incidence of stuttering in DS: 17% in Kumin’s (1994) parent report survey and 15.6% in Schrieve et al.’s (2009) analysis of data from the NHIS household survey.
Other studies have provided information on the topography of stuttering. Otto and Yairi (1974) found statistically significant differences in disfluencies between 19 institutionalized individuals with DS compared to an equivalent number of healthy controls. Analysis of the disfluencies with respect to seven categories of disfluency showed that the participants with DS had patterns similar to those observed in developmental stuttering. Willcox (1988) observed both similarities and differences in the types of disfluency in the speech of children with DS compared to language-matched children without DS. She concluded that “it is clinically more appropriate to consider the speech non-fluencies of Down’s syndrome individuals as part of a global language deficit rather than as a symptom of the syndrome” (p. 169).
The disfluencies in DS may take forms other than developmental stuttering. Cluttering may be even more frequent than stuttering. One of the first authors to note the possibility of cluttering was Cabanas (1954), who asserted that the rhythm disorders in the individuals he studied should be called “cluttering” because of their restricted vocabularies, rapid speech patterns, and “lack of ideomotor equilibrium” (p. 36). Van Borsel & Vandermuelen (2008) classified a very large percentage of their 76 participants with DS as being either clutterers (about 80%) or clutterer-stutterers (about 17%). Preus (1972) noted that both stuttering and cluttering occur in DS and are not correlated.
3.1.2. Prosody
Prosody is a general term for the rhythmic and intonational aspects of language and includes rhythm, intonation, lexical and emphatic stress. As can be seen in Table 3, only a handful of studies, involving almost 50 participants, have examined prosodic features in the speech of individuals with DS, but they all indicate that individuals with DS have limitations in the perception, imitation and spontaneous production of prosodic features (Pettinato & Verhoeven, 2008; Reichle et al. 1985; Shriberg & Widder, 1990; Stojanivik, 2010). Shriberg and Widder (1990) found that participants with a higher probability of being able to live independently also had better speech and prosodic capabilities. Prosodic features may have a bearing on intelligibility, insofar as increased intelligibility has been reported for prepausal rhythmic groups (Flipsen, 1999).
3.2. Discussion
Disfluency (either stuttering or cluttering) is highly likely to occur in DS but it is by no means a universal characteristic of the syndrome. The types of disfluency are similar to those seen in developmental stuttering, which may be a sign of similarities in the origin of the disorder. The diagnosis of cluttering, as in the study of Van Borsel & Vandermuelen (2008), emphasizes the need to consider disfluency in relation to speaking rate, given that a rapid rate is frequently implicated in cluttering. Results on speaking rate in DS are mixed. The few studies reporting on prosody indicate that prosodic disturbance is a common feature of DS.
It is difficult to determine the degree to which stuttering or cluttering is comorbid with other speech and voice problems. It is also unclear if the nature and severity of the fluency disorder changes over the lifespan, or if the “stuttering” in infant pain cry (Lind et al., 1970) is related to the later appearance of disfluencies in childhood. Disfluent speech in DS has been attributed either to dysfunction in motor control or to dysfunction in language processes such as utterance formulation or word finding (Leddy, 1999). Both kinds of dysfunction may need to be recognized in an integrated model, such as the model, EXPLAN, proposed to account for developmental stuttering (Howell, 2011; Howell & Au-Yeung, 2002). This model assumes that language planning (PLAN) and speech-motor programming and execution (EX) are independent processes, and it is the interface between these processes that determines the fluency of speech. An advantage of the EXPLAN model is that it can account for both language and motor influences on disfluent speech.
Limitations in prosody could be the result of motor difficulties, problems in coordinating speech motor control with phonological or other higher level representations, or even serious segmental (articulatory) errors that impede the effective production of speech across multisyllabic sequences. Prosodic abnormalities may have their origin in limitations of phonological processing (Pettinato and Verhoeven, 2008, Shriberg and Widder, 1990). It is also possible that prosodic difficulties contribute to problems in other domains. For example, Pettinato and Verhoeven (2008) concluded that “Our findings are in accord with studies which suggest that underlying difficulties with the rhythmic and prosodic structure of speech are driving dysfluencies and reduced speech intelligibility in the speech of individuals with Down syndrome” (p. 11).
3.3. Indications for Future Research and Clinical Services
Disfluencies and dysprosody are fairly common in DS and constitute one part of a larger profile of communication disorder. A challenge for future research is to determine the interactions between disfluencies and dysprosody with other aspects of communication, including syntactic, lexical and phonological processes, in an effort to identify causal relations. In addition, research that combines methodologies (e.g., acoustics, EMA and perceptual scaling) should be used in an effort to describe motor patterns associated with disturbances in fluency and prosody.
4. Intelligibility (Table 4)
4.1. Review of Literature
While the investigations in Table 2 address speech articulation or phonology, those in Table 4 specifically provide estimates of overall intelligibility. Definitions of intelligibility differ across published articles, as do the methods of assessing it. As Leddy (1999) pointed out, many reports assessed intelligibility incidental to other research goals, such as determining aspects of language formulation or vocabulary. Omitting parent surveys and intervention research, the total number of participants in studies that directly assessed intelligibility approaches 150 (Table 4) but the number is larger if related measures such as some reported in Table 2 are included. When data from parental surveys are aggregated (Kumin, 1994; 2006), the number of participating units swells to more than 2500. Several published intervention studies are not included in Table 4 because they reported only a change in intelligibility between pre-treatment and post-treatment rather than explicit pre- and post-treatment ratings. Table 4 includes a small number of studies in which intelligibility was assessed relative to an intervention.
4.1.1. Studies reporting intelligibility estimates
Reduced intelligibility results in difficult communication and can interfere with a variety of activities in everyday life (Barnes et al., 2009; Bray & Woolnough, 1988; Bunton et al., 2007; Kumin, 1994, 2006; Price & Kent, 2008; Rosin et al., 1988). Research that focuses on intelligibility per se is limited in the literature on DS. Diminished intelligibility is substantiated by parental report (Appendix 1) and clinical or laboratory testing (Table 4). The underlying causes of this problem can only be surmised from studies that examine aspects of speech production, as reviewed in the previous sections, along with studies of other domains of spoken language. It appears that intelligibility reduction is exacerbated by increased length of utterance (Kumin, 1994; Yoder et al., 1996) and nonfamiliarity of the listener (Kumin, 1994).
A variety of procedures are used to estimate intelligibility (Price & Kent, 2008), but the main methods that have been used in DS are scaling procedures (such as percentage estimate of intelligibility; Kumin, 2006), word identification (Bunton et al., 2007), and scoring from transcriptions (Chapman et al., 1998; Chapman et al., 2000; Rosin et al., 1988). Regarding the last of these, Chapman et al. (1998) wrote that “Intelligibility was scored as the proportion of complete and intelligible utterances over total utterances” (p. 864). Another approach is to measure correlates of intelligibility, for example, the percentage of consonants correct (PCC) (Barnes et al., 2009; Bysterveldt, 2009; Bysterveldt et al., 2010; Kennedy & Flynn, 2003; McCann & Wrench, 2007; Roberts et al., 2005). As noted in Section 2.1.2, PCC values in DS are markedly reduced compared to values reported for TD children. We discovered only two studies of DS that reported Percentage of Vowels Correct (PVC) (van Bysterveldt, 2009; van Bysterveldt et al., 2010). The results indicate that production of vowels and diphthongs is more accurate than consonants.
Figure 2 shows a cumulative plot of intelligibility scores derived from the data of Kumin’s (1994) study. Note that 60% of the participants had an intelligibility rating of 5 or lower and that 89% of participants had a rating of 7 or lower. These result, based on parental ratings, agree with estimates of intelligibility reported by Chapman et al. (1988) and van Bysterveldt (2009), both of whom reported an average intelligibility score of about 80%.
Figure 2.
Plot of cumulative percentage of participants receiving a given intelligibility score in Kumin (1994). The horizontal axis is the intelligibility rating and the vertical axis is the cumulative percentage. For example, about 60% of participants had scores of 5 or less.
4.1.2. Related measures
Measures of intelligibility are complemented by other measures including comprehensibility, listener comprehension and communicative participation. Comprehensibility is defined as “contextual intelligibility,” or intelligibility when contextual information is present in different forms, such as semantic cues, syntactic cues, orthographic cues, and gestures (Yorkston, Strand & Kennedy, 1996). Measures of listener comprehension evaluate listeners’ ability to interpret the meaning of messages without regard for accuracy of phonetic and lexical parsing (Hustad & Beukelman, 2002). Communicative participation is defined as communication in social contexts (Eadie et al., 2006). These latter three measures have been used only infrequently in the study of communication in DS, but Camarata et al. (2006) used a measure of speech-comprehensibility defined as the percentage of utterances that are comprehensible. The advantage of this measure is that it is sensitive to communication success or failure whether or not individual words are accurately identified by the listener.
4.2. Discussion
Several studies substantiate that intelligibility is a serious problem in DS, that persists throughout life for many individuals and may have negative effects on social and vocational pursuits. Very few of these studies have reported a detailed analysis of factors underlying reduced intelligibility, although it can be assumed that disturbances in voice, articulation and resonance, fluency and prosody all contribute to the problem. It is not known how difficulties in each of these areas contribute to an overall deficit in intelligibility. It is also not clear if the presence of unusual or atypical articulatory or phonological errors, as reviewed in Section 2, increases the risk of impaired intelligibility.
4.3. Indications for Future Research and Clinical Services
Reduced intelligibility in DS has been well documented, but the reasons for it have not been sufficiently explored. Impaired intelligibility is probably based to some degree on all the other functions considered in this review (voice, speech sound production, fluency and prosody), but a satisfactory study of their interrelationships would require many participants and several research methods. It is likely that progress could be made with less ambitious methods, such as acoustic studies of speech in DS. It may suffice to examine a set of acoustic features that appear to be related to speech intelligibility. One such set was described by Amano-Kusumoto and Hosom (2011) in a review of clear (highly intelligible) versus conversational (less intelligible) speech in healthy adults: formant transitions, temporal envelope, F1 and F2 ranges, formant bandwidth, and voice onset time (VOT). These features should be studied systematically in DS. As reviewed in Section 2, more data have been published on F1 and F2 ranges than any other acoustic aspect of speech, but even these studies are not in agreement. Future studies could examine all the acoustic features mentioned, preferably in the same group of participants and with suitable TD controls. A better understanding of the bases of reduced intelligibility would help to guide clinical intervention. These bases may vary across individuals with DS, which is further reason to develop profiles of speech disorder that are linked to intervention strategies.
5. General Discussion
Given the evidence reviewed here, individuals with DS have difficulties in the domains of voice, speech sound production, fluency and prosody, and intelligibility. Children and adults with this syndrome face serious challenges in spoken communication which may substantially interfere with participation in social, educational and vocational activities. The difficulties in communication are rooted in virtually all aspects of speech production, making it difficult to identify domains of strength that might be leveraged in the design of effective interventions. Although not every individual with DS will experience the full range of abnormalities noted in this review, multiple involvements are likely and comprehensive assessments should be considered. with due consideration of the results in treatment planning.
5.1. Population sampling and criteria for selection of control groups
Shin et al. (2009) estimated that in 2002, there were 83,400 individuals with DS under the age of twenty living in the United States. As noted in this review, the aggregate number of participants in each of the four areas of research related to speech communication in DS is in the low hundreds, which probably is not sufficient to assess phenotypic variation, especially because the majority of published studies focused on a small set of measures within any of the four research areas.
Control groups used in studies of speech in DS, include mental-age matches of TD individuals, chronological-age matches of TD individuals, and participants with other types of disorder (e.g., fragile-X and children with phonological disorders). Characteristics of control groups can strongly affect the validity of conclusions reached in studies of speech abilities. With mental-age matching, there is no control for physical development and body size, both of which can substantially affect aspects of speech (particularly acoustic measures of f0 and formant frequencies). Chronological-age matching provides a better control over physical development, but offers limited control over physical size and little or no control over language or cognitive capabilities or general experience (such as social interactions in different settings). Comparison with other types of developmental disorders can be revealing but questions arise as to the need for matching body size, chronological age, and mental age. No single control group is satisfactory for all aspects of research on speech production but a particular control group can be justified for studies of a highly specific nature.
5.2. Co-occurrence and impairment profiles
Considering the broad spectrum of speech disturbances in DS, it is important to know patterns of co-occurrence. Unfortunately, this information is not easily extracted from the literature. It has been established that many types of speech disorders in DS have high rates of co-occurrence or comorbidity in populations other than DS. For example, it has been estimated that developmental stuttering has a comorbidity of about 60% with speech, language and other disorders (with articulation and phonological disorders being the most frequently co-occurring; Blood, Ridenour, Qualls, & Hammer, 2003). Similarly, Arndt and Healey (2001) reported from a survey of 241 speech-language pathologists that 44% of 467 children who stuttered had a verified concomitant phonological and/or language disorder. Future studies of DS should examine the co-occurrence of voice, articulatory-phonological, fluency and prosodic disorders, and various aspects of language disorders. Identification of profiles of impairment may be an important step in selecting treatment strategies.
The multidimensional character of the speech disorder in DS is central to determinations of symptomatology and pathophysiology. A profile of impairments is one way to register the dimensions of the speech disorder in individuals with DS and can be used to identify general patterns of disorder in the population. Individual differences can be described relative to these general patterns. The classification of speech production difficulties into the four major classifications used in this review cannot capture the interaction among these categories. Intelligibility, the most critical outcome with respect to communicative success or failure, is moderately to severely compromised in DS yet it is one of the most poorly quantified aspects of speech production. Given the breadth of the difficulties in speech production, a hierarchy could be established to guide efficient assessment and treatment. Comprehensive testing allows the identification of co-occurring problems as well as the identification of areas of relative strength or competence.
5.3. Speech Disorders in Relation to Language, Cognitive and Memory Functions
Speech cannot be isolated from other aspects of communication or cognition. Although this review focuses on speech production, problems with speech must be viewed in a larger context of perceptual, motor and linguistic abilities. Speech problems in DS may be related to peripheral factors such as anatomic differences in the vocal tract, impaired hearing acuity during recurrent otitis media, and impaired motor function (dysarthria and/or apraxia) or to central factors such as language and cognitive dysfunctions. It is likely that several factors interact in the development and persistence of speech disorders in DS, each with a developmental trajectory that contributes to the overall interaction. Causal relationships among the various speech and language impairments are not easily determined. For example, it has been suggested that disfluencies are the result of: language impairment (Willcox, 1988), underlying difficulties in the control of rhythm and prosody (Pettinato & Verhoeven, 2008) or a combination of language and motor limitations (Cabanas, 1954). Longitudinal studies may shed light on the relationships among the impairments noted in this review, but these studies are nearly non-existent.
Short-term memory impairments have been noted in DS (Bunn, Roy & Elliott, 2007; Jarrold, Baddeley & Phillips, 2002; Kanno & Ikeda, 2002; Laws, 1998; Vicari, 2006), and appear largely independent of speech articulation or speech perception abilities. Performance on certain speech and language tasks is likely affected by limitations in short-term memory.
5.4. Childhood Apraxia of Speech: A component of DS?
More than thirty years ago, Dodd (1976) posited that the articulatory disorder in DS is rooted at least partly in “difficulties in programming the motor movements of speech” (p. 41). This implies that the motor disorder in speech is not only a dysarthria (typically defined as a disorder of execution) but perhaps also an apraxia (typically defined as a disorder of motor programming or sequencing). More recently, it has been proposed that children with DS have childhood apraxia of speech (CAS) (Kumin, 2006; Rupela & Manjula, 2007). This proposal was based on similarities between speech behaviors in DS and those in CAS.
A diagnosis of CAS can be difficult, especially when this disorder is comorbid with other speech and language abnormalities associated with DS. CAS has been defined as “a neurological childhood (pediatric) speech sound disorder in which the precision and consistency of movements underlying speech are impaired in the absence of neuromuscular deficits (e.g., abnormal reflexes, abnormal tone)” (American Speech-Language-Hearing Association, 2007). There are only three features of CAS with widely acknowledged diagnostic validity: (1) inconsistent error production on both vowels and consonants across repeated productions of syllables and words, (2) lengthened and impaired coarticulatory transitions between sounds and syllables, and (3) inappropriate prosody. The diagnosis of CAS is usually made on the assumption that there is no evidence of craniofacial anomalies or of neurologic abnormality in the speech musculature. Obviously, this assumption cannot be made in individuals with DS, who are considered to have, at the minimum, a hypotonic musculature and fairly distinctive craniofacial features, some of which affect the oropharyngeal structures involved in speech. The high prevalence of cluttering or cluttering-stuttering further complicates a confident diagnosis of CAS. This is not to say that CAS is unlikely to occur, but rather that confident diagnosis of this condition must take into account the combination of articulatory errors, abnormal muscle tone, and fluency disorders that appear to be common in individuals with DS. To some degree, CAS is a diagnosis by exclusion which is obviated in DS. The challenge, then, is to distinguish features of CAS from co-occurring abnormalities related to neurological, structural, and perhaps other domains.
There is evidence of a general difficulty in praxis skills in DS (Bunn et al., 2007; Fidler, Hepburn, Mankin & Rogers, 2005). Bunn et al. (2007) proposed that movement organization deficiencies in DS could reflect a difficulty in generating actions from memory. If this limitation is general across motor systems, then some aspects of speech production disorders would be based on deficiencies in central processes. Vulnerability of praxis skills is evident throughout life, as older individuals with DS appear to exhibit increased praxis disturbances (Daunhauer & Fidler, 2011).
5.5. Neural abnormalities
Neural dysfunctions likely underlie many of the disorders considered in this review. Abnormalities of neuroanatomy and neural function have been described in several recent articles (Fidler, 2005; Nadel, 2003; Pinter et al., 2001; Vicari, 2006) and these could well be the basis for apractic and dysarthric characteristics of speech in DS. An important step in this effort is the systematic description of speech disorders in DS, including their natural history, comorbidity, and response to intervention.
5.6. Cross-linguistic research
The great majority of studies in this review pertain to speakers of English. Cross-linguistic studies are important to establish features that are universal versus those that are specific to individual languages or families of languages. Unfortunately, it is very difficult to determine from the published studies if there are strong cross-language correspondences in problems with voice, speech sound production, fluency, prosody, or intelligibility. The conclusions of this review may be used to form hypotheses for research on DS in other languages.
5.7. Future research
Perceptual methods such as ratings of voice quality and articulation tests have provided basic information on characteristics of spoken language in DS but the use of the instrumental techniques of acoustic and physiological methods has been limited. EPG is one of the most frequently used of these techniques and has contributed especially to an improved understanding of lingual articulation. Acoustic methods have potential for refined analyses of articulation and prosody. Aerodynamic recordings may reveal important aspects of voice and speech dysfunctions. A major direction for future research is the application of instrumental techniques in a lifespan perspective to answer questions such as:
How do the air pressures and air flows for speech production in DS compare with those of TD controls at various times of development? For example, if intraoral air pressures are higher in DS than TD controls (Rosin et al., 1988) then do individuals with DS drive the speech production system with unusually high pressures? If so, how does this feature relate to disturbances in voice, articulation, fluency and prosody?
Assuming that vowel articulation is often impaired in DS (as perceptual studies indicate), what is the characteristic acoustic (F1–F2) space for vowels produced by children and adults with DS, and how does this result relate to reduced intelligibility? If atypical results are found in DS, are they the consequence of anatomic anomalies, motor control deficiencies, or both?
Despite longstanding comments on voice quality abnormalities in DS, no consistent acoustic correlates have emerged. What are the acoustic patterns of phonation in DS in different phonation tasks, including sustained phonation, single-word production, and sentence recitation? How does phonation change during development and maturation? Do abnormalities in voice contribute to dysprosody?
Given the considerable evidence to date that DS is associated with prosodic abnormalities, what are the acoustic correlates of prosody in DS and how do these differ from the correlates in TD controls?
Different conclusions have been reached on how speaking rate in DS varies across tasks. Acoustic and physiologic methods are well suited to the quantitative study of speaking rate. What is the effect of rate changes on segmental durations? Is rate a potent variable in intervention for speech?
One of the most productive approaches to address the foregoing and other questions listed in the conclusion of each major section in this review,, would be to use combined methodologies (perceptual judgments, acoustic measures, physiologic recordings) to obtain detailed information on how voice, speech sound articulation, fluency, and prosody interact to determine the intelligibility of speech in DS. It may be particularly fruitful to use such methods to determine speech production capabilities as a function of development. Such an approach to understand speech functions in DS may benefit the study of other complex disorders, such as childhood dysarthria, which involve a constellation of atypical patterns that interact to reduce speech intelligibility.
Acknowledgments
This work was supported in part by Research Grant R01 DC006282 from the National Institute on Deafness and Other Communicative Disorders (NIDCD), National Institutes of Health (NIH) and Core Grant P-30 HD0335 to the Waisman Center from the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH). We thank Erin Henigan Douglas, Jennifer Lewandowski and Kathryn Lester for assistance with the review of the literature and the compilation of information for the summary tables. We also thank Jacqueline Houtman for comments on earlier versions of this paper.
References
- Ardran GM, Harker P, Kemp FH. Tongue Size in Down’s Syndrome. Journal of Mental Deficiency Research. 1972;16:160–166. doi: 10.1111/j.1365-2788.1972.tb01172.x. [DOI] [PubMed] [Google Scholar]
- Albertini G, Bonassi S, Dall-Armi V, Giachetti I, Giaquinto S, Mignano M. Spectral analysis of the voice in Down syndrome. Research in Developmental Disabilities. 2010;31:996–1001. doi: 10.1016/j.ridd.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Allanson JE, O’Hara P, Farkas LG, Nair RC. Anthropometric craniofacial pattern profiles in Down syndrome. American Journal of Medical Genetics. 1993;47:748–752. doi: 10.1002/ajmg.1320470530. [DOI] [PubMed] [Google Scholar]
- Amano-Kusumoto A, Hosom J-P. Center for Spoken Language Understanding (CSLU) Technical Report CSLU-11-002. Department of Biomedical Engineering, Oregon Health & Science University; 2010. A review of research on speech intelligibility and correlations with acoustic features; pp. 1–16. [Google Scholar]
- American Speech-Language-Hearing Association. Childhood Apraxia of Speech [Position Statement] 2007 Available from www.asha.org/policy.
- Arndt J, Healey EC. Concomitant disorders in school-age children who stutter. Language, Speech & Hearing Services in the Schools. 2001;32:68–78. doi: 10.1044/0161-1461(2001/006). [DOI] [PubMed] [Google Scholar]
- Balkany TJ, Downs MP, Jafek BW, Krajicek MJ. Hearing loss in Down’s syndrome: A treatable handicap more common than generally recognized. Clinical Pediatrics. 1979;18:116–118. doi: 10.1177/000992287901800207. [DOI] [PubMed] [Google Scholar]
- Barden HS. Fluctuating dental asymmetry: a measure of developmental instability in down syndrome. American Journal of Physical Anthropology. 1980;52:169–173. doi: 10.1002/ajpa.1330520203. [DOI] [PubMed] [Google Scholar]
- Barnes E, Roberts J, Long SH, Martin GE, Berni MC, Mandulak KC, Sideris J. Phonological accuracy and intelligibility in connected speech of boys with fragile X syndrome or Down syndrome. Journal of Speech, Language, and Hearing Research. 2009;52:1048–1061. doi: 10.1044/1092-4388(2009/08-0001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes EF, Roberts J, Mirrett P, Sideris J, Misenheimer J. A comparison of oral structure and oral-motor function in young males with fragile X syndrome and Down syndrome. Journal of Speech, Language, and Hearing Research. 2006;49:903–917. doi: 10.1044/1092-4388(2006/065). [DOI] [PubMed] [Google Scholar]
- Beck JM. Organic variation in the vocal apparatus. In: Hardcastle WJ, Laver J, editors. A handbook of phonetic sciences. Oxford, England: Blackwell; 1997. pp. 256–297. [Google Scholar]
- Beckman DA, Wold DC, Montague JCJ. A noninvasive acoustic method using frequency perturbations and computer-generated vocal-tract shapes. Journal of Speech, Language, and Hearing Research. 1983;26:304–314. doi: 10.1044/jshr.2602.304. [DOI] [PubMed] [Google Scholar]
- Benda CE. Mongolism and cretinism. New York: Grune and Stratton; 1949. [Google Scholar]
- Benda CE. The child with Mongolism (congenital acromicria) New York: Grune & Stratton; 1960. [Google Scholar]
- Bernthal JE, Bankson NW, Flipsen P., Jr . Articulation and phonological disorders. Boston: Allyn & Bacon; 2009. [Google Scholar]
- Bersu ET. Unpublished doctoral dissertation. University of Wisconsin-Madison; 1976. An analysis of the anatomic variations in human trisomy based on dissections of 21- and 18-trisomes. [Google Scholar]
- Bersu ET. Anatomical analysis of the developmental effects of aneuploidy in man: the Down syndrome. American Journal of Medical Genetics. 1980;5:399–420. doi: 10.1002/ajmg.1320050411. [DOI] [PubMed] [Google Scholar]
- Bertrand P, Navarro H, Caussade S, Holmgren N, Sanchez I. Airway anomalies in children with Down syndrome: Endoscopic findings. Pediatric Pulmonology. 2003;36:137–141. doi: 10.1002/ppul.10332. [DOI] [PubMed] [Google Scholar]
- Bhagyalakshmi G, Renukarya A, Rajangam S. Metric analysis of the hard palate in children with Down syndrome - a comparative study. Down Syndrome Research and Practice. 2007;12:55–59. doi: 10.3104/reports.1999. [DOI] [PubMed] [Google Scholar]
- Bleile KM, Schwarz I. Three perspectives on the speech of children with Down’s syndrome. Journal of Communication Disorders. 1984;17:87–94. doi: 10.1016/0021-9924(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Blood GW, Ridenour VJ, Qualls CD, Hammer CS. Co-occurring disorders in children who stutter. Journal of Communication Disorders. 2003;36:427–448. doi: 10.1016/s0021-9924(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat (5.1.32) Amsterdam, The Netherlands: Publisher; 2010. Available from http://www.fon.hum.uva.nl/praat. [Google Scholar]
- Borghi RW. Consonant phoneme, and distinctive feature error patterns in speech. Clinical perspectives in the management of Down syndrome. 1990:147–152. [Google Scholar]
- Bray M, Woolnough L. The language skills of children with Down’s syndrome aged 12 to 16 years. Child Language Teaching and Therapy. 1988;4:311–324. [Google Scholar]
- Brown-Sweeney G, Smith BL. The development of speech production abilities in children with Down syndrome. Clinical Linguistics & Phonetics. 1977;11:345–362. [Google Scholar]
- Brown-Sweeney SG, Smith BL. The development of speech production abilities in children with Down syndrome. Clinical Linguistics and Phonetics. 1997;11:345–362. [Google Scholar]
- Brunner J, Fuchs S, Perrier P. On the relationship between palate shape and articulatory behavior. Journal of the Acoustical Society of America. 2009;125:3936–3949. doi: 10.1121/1.3125313. [DOI] [PubMed] [Google Scholar]
- Buchanan LH. Early onset of presbyacusis in Down syndrome. Scandinavian Audiology. 1990;19:103–110. doi: 10.3109/01050399009070760. [DOI] [PubMed] [Google Scholar]
- Bunn L, Simon DA, Welsh TN, Watson C, Elliott D. Speech production errors in adults with and without Down syndrome Following verbal, written, and pictorial cues. Developmental Neuropsychology. 2002;21:157–172. doi: 10.1207/S15326942DN2102_3. [DOI] [PubMed] [Google Scholar]
- Bunn L, Roy EA, Elliott D. Speech perception and motor control in children with Down syndrome. Child Neuropsychology. 2007;13:262–275. doi: 10.1080/09297040600770738. [DOI] [PubMed] [Google Scholar]
- Bunton K, Leddy M, Miller J. Phonetic intelligibility testing in adults with Down syndrome. Down’s syndrome, research and practice: the journal of the Sarah Duffen Centre/University of Portsmouth. 2007;12:1–24. [Google Scholar]
- Bunton K, Leddy M. An evaluation of articulatory working space area in vowel production of adults with Down syndrome. Clinical Linguistics & Phonetics. 2010 doi: 10.3109/02699206.2010,535647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bysterveldt AK. Unpublished doctoral dissertation. University of Canterbury; Christchurch, New Zealand: 2009. Speech, phonological awareness and literacy in New Zealand children with Down syndrome. [Google Scholar]
- Bysterveldt AK, van Gillion G, Foster-Cohen S. Integrated speech and phonological awareness intervention for pre-school children with Down syndrome. International Journal of Language and Communication Disorders. 2010;45:320–335. doi: 10.3109/13682820903003514. [DOI] [PubMed] [Google Scholar]
- Cabanas R. Some findings in speech and voice therapy among mentally deficient children. Folia Phoniatrica. 1954;6:34–39. doi: 10.1159/000262677. [DOI] [PubMed] [Google Scholar]
- Camarata S, Yoder P, Camarata M. Simultaneous treatment of grammatical and speech-comprehensibility deficits in children with Down syndrome. Down Syndrome Research & Practice. 2006;11:9–17. doi: 10.3104/reports.314. [DOI] [PubMed] [Google Scholar]
- Carding PN, Roulstone S, Northstone K the ALSPAC Study Team. The prevalence of childhood dysphonia: a cross-sectional study. Journal of Voice. 2006;20:623–630. doi: 10.1016/j.jvoice.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Carlstedt K, Henningsson G, Dahllof G. A four-year longitudinal study of palatal plate therapy in children with Down syndrome: effects on oral motor function, articulation and communication preferences. Acta Odontologica Scandinavica. 2003;61:39–46. doi: 10.1080/ode.61.1.39.46. [DOI] [PubMed] [Google Scholar]
- Chapman RS, Seung HK, Schwartz SE, Kay-Raining Bird E. Language skills of children and adolescents with Down syndrome: II. Production deficits. Journal of Speech Language and Hearing Research. 1998;41:861–873. doi: 10.1044/jslhr.4104.861. [DOI] [PubMed] [Google Scholar]
- Chapman RS, Seung HK, Schwartz SE, Kay-Raining Bird EK. Predicting language production in children and adolescents with Down syndrome: the role of comprehension. Journal of Speech, Language, and Hearing Research. 2000;43:340–350. doi: 10.1044/jslhr.4302.340. [DOI] [PubMed] [Google Scholar]
- Cleland J, Timmins C, Wood SE, Hardcastle WJ, Wishart JG. Electropalatographic therapy for children and young people with Down’s syndrome. Clinical Linguistic and Phonetics. 2009;23:926–939. doi: 10.3109/02699200903061776. [DOI] [PubMed] [Google Scholar]
- Cleland J, Wood S, Hardcastle W, Wishart J, Timmins C. Relationship between speech, oromotor, language and cognitive abilities in children with Down syndrome. International Journal of Language and Communicative Disorders. 2010;45:83–95. doi: 10.3109/13682820902745453. [DOI] [PubMed] [Google Scholar]
- Cobo-Lewis AB, Oller DK, Lynch MP. Relations of motor vocal milestones in typically developing infants and infants with Down syndrome. American Journal on Mental Retardation. 1996;100:456–467. [PubMed] [Google Scholar]
- Cohen MM, Winer RA. Dental and facial characteristics in Down’s syndrome (Mongolism) Journal of Dental Research. 1965;44:197–208. doi: 10.1177/00220345650440011601. [DOI] [PubMed] [Google Scholar]
- Connaghan KP. Unpublished doctoral dissertation. University of Washington; Seattle, Washington: 2004. Jaw stiffness during speech by children with suspected hypo- or hypertonia. [Google Scholar]
- Crosley PA, Dowling S. The relationship between cluster and liquid simplification and sentence length, age, and IQ in Down’s syndrome children. Journal of Communication Disorders. 1989;22:151–168. doi: 10.1016/0021-9924(89)90013-0. [DOI] [PubMed] [Google Scholar]
- Crosley PA, Dowling S. The relationship between syllable reduction, final consonant deletion, and sentence length in children with Down syndrome. National Student Speech, Language, Hearing Journal. 1989–1990;17:65–71. [Google Scholar]
- Daly DA. Predictive cluttering inventory. Author; Ann Arbor, MI: 2006. [Google Scholar]
- Dellavia C, Sforza C, Orlando F, Ottolina P, Pregliasco F, Ferrario VF. Three-dimensional hard tissue palatal size and shape in Down syndrome subjects. European Journal of Othodontia. 2007;29:417–422. doi: 10.1093/ejo/cjm026. [DOI] [PubMed] [Google Scholar]
- Desai SS. Down syndrome: a review of the literature. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodotics. 1997;84:279–285. doi: 10.1016/s1079-2104(97)90343-7. [DOI] [PubMed] [Google Scholar]
- Devenny DA, Silverman WP. Speech dysfluency and manual specialization in Down’s syndrome. Journal of Intellectual Disability Research. 1990;34:253–260. doi: 10.1111/j.1365-2788.1990.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Devenny DA, Silverman W, Balgley H, Wall MJ, Sidtis JJ. Specific motor abilities associated with speech fluency in Down’s syndrome. Journal of Mental Deficiency Research. 1990;34(Pt 5):437–443. doi: 10.1111/j.1365-2788.1990.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Dodd BJ. Comparison of babbling patterns in normal and Down-syndrome infants. Journal of Mental Deficiency Research. 1972;16:35–40. doi: 10.1111/j.1365-2788.1972.tb01569.x. [DOI] [PubMed] [Google Scholar]
- Dodd B. A comparison of the phonological systems of mental age matched, normal, severely subnormal and Down’s syndrome children. British Journal of Disorders and Communication. 1976;11:27–42. doi: 10.3109/13682827609011289. [DOI] [PubMed] [Google Scholar]
- Dodd B, Hua Z, Crosbiek S, Holm A, Ozanne A. Diagnostic evaluation of articulation and phonology. London: The Psychological Corporation; 2002. [Google Scholar]
- Dodd B, Thompson L. Speech disorder in children with Down’s syndrome. Journal of Intellectual Disability Research. 2001;45:308–316. doi: 10.1046/j.1365-2788.2001.00327.x. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Whetton C, Burley J. The British picture vocabulary scales. 2. Windsor, England: Nfer-Nelson; 1997. (BPVS0II) [Google Scholar]
- Eadie TL, Yorkston KM, Klasner ER, Dudgeion BJ, Deitz JC, Baylor CR, Miller RM, Amtmann D. Measuring communicative participation: A review of self-report instruments in speech-language pathology. American Journal of Speech- Language Pathology. 2006;15:307–320. doi: 10.1044/1058-0360(2006/030). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenhuis HM, Van Zanten GA, Brocaar MP, Roerdinkholder WHM. Hearing loss in middle-age persons with Down syndrome. American Journal on Mental Retardation. 1992;97:47–56. [PubMed] [Google Scholar]
- Fawcett S, Peralego J. Speech in individuals with Down syndrome. Perspectives on Language Learning and Education. 2009;16:109–116. [Google Scholar]
- Ferguson CF. Congenital abnormalities of the infant larynx. Otolaryngologic Clinics of North America. 1970;3:185–200. [PubMed] [Google Scholar]
- Ferrier LJ, Bashir AS, Meryash DL, Johnston J, Wolff P. Conversational skills of individuals with fragile-X syndrome: a comparison with autism and Down syndrome. Developmental Medicine & Child Neurology. 1991;33:776–788. doi: 10.1111/j.1469-8749.1991.tb14961.x. [DOI] [PubMed] [Google Scholar]
- Fidler DJ, Hepburn SL, Mankin G, Rogers SJ. Praxis skills in young children with Down syndrome, other developmental disabilities, and typically developing children. American Journal of Occupational Therapy. 2005;59:129–138. doi: 10.5014/ajot.59.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Brandies H. Cephalometric comparison between children with and without Down’s syndrome. European Journal of Orthodontia. 1988;10:255–263. doi: 10.1093/ejo/10.3.255. [DOI] [PubMed] [Google Scholar]
- Fisher HB, Logemann JA. The Fisher-Logemann test of articulation competence. Boston: Houghton Mifflin; 1971. [Google Scholar]
- Fletcher SG. TONAR II: an instrument for use in management of nasality. Alabama Journal of Medical Sciences. 1972;9:333–338. [PubMed] [Google Scholar]
- Flipsen P. Prepausal accuracy in the speech of adults with down syndrome. Clinical Linguistics & Phonetics. 1999;13:55–65. [Google Scholar]
- Fourakis M, Karlsson H, Tilkens C, Shriberg L. Acoustic correlates of nasopharyngeal resonance. In: Botinis A, editor. Proceedings of the 3rd ISCA Tutorial and Research Workshop on Experimental Linguistics. Athens: University of Athens and the International Speech Communication Association (ISCA); 2010. pp. 41–44. [Google Scholar]
- Frostad WA, Cleall JF, Metosky LC. Craniofacial complex in the Trisomy 21 syndrome (Down’s syndrome) Archives of Oral Biology. 1971;16:707–722. doi: 10.1016/0003-9969(71)90116-6. [DOI] [PubMed] [Google Scholar]
- Gibbon FE, McNeill AM, Wood SE, Watson JM. Changes in linguapalatal contact patterns during therapy for velar fronting in a 10-year-old with Down’s syndrome. International Journal of Language & Communication Disorders. 2003;38:47–64. doi: 10.1080/13682820304816. [DOI] [PubMed] [Google Scholar]
- Glaze LE, Bless DM, Milenkovic P, Susser RD. Acoustic characteristics of children’s voice. Journal of Voice. 1988;2:312–319. [Google Scholar]
- Goldman R, Fristoe M. Goldman-Fristoe test of articulation. Circle Pines, MN: American Guidance Service; 1969. [Google Scholar]
- Goldman R, Fristoe M. Goldman-Fristoe test of articulation - GFTA-2. Circle Pines, MN: American Guidance Service; 2000. [Google Scholar]
- Gottsleben RH. The incidence of stuttering in a group of mongoloids. Training School Bulletin. 1955;51:209–218. [Google Scholar]
- Guimaraes CVA, Donnelly LF, Shott SR, Amin RS, Kalra M. Relative rather than absolute macroglossia in patients with Down syndrome: implications for treatment of obstructive sleep apnea. Pediatric Radiology. 2008;38:518–523. doi: 10.1007/s00247-008-0941-7. [DOI] [PubMed] [Google Scholar]
- Guitar B. Stuttering: An integrated approach to its nature and treatment. Philadelphia: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- Hamilton C. Investigation of the articulatory patterns of young adults with Down’s syndrome using electropalatography. Down Syndrome Research and Practice. 1993;1:15–28. [Google Scholar]
- Hohoff A, Seifert E, Ehmer U, Lamprecht-Dinnesen A. Articulation in children with Down’s syndrome: A pilot study. Journal of Orofacial Orthopedics. 1998;59:220–228. doi: 10.1007/BF01579166. [DOI] [PubMed] [Google Scholar]
- Hollien H, Copeland RH. Speaking fundamental frequency (SFF) characteristics of mongoloid girls. Journal of Speech and Hearing Disorders. 1965;30(4):344–349. doi: 10.1044/jshd.3004.344. [DOI] [PubMed] [Google Scholar]
- Howell P. Recovery from stuttering. New York: Psychology Press; 2011. [Google Scholar]
- Howell P, Au-Yeung J. The EXPLAN theory of fluency control and the diagnosis of stuttering. In: Fava E, editor. Pathology and therapy of speech disorders. Amsterdam, The Netherlands: John Benjamins; 2002. pp. 75–94. [Google Scholar]
- Hustad KC, Beukelman DR. Listener comprehension of severely dysarthric speech: effects of linguistic cues and stimulus cohesion. Journal of Speech, Language, and Hearing Research. 2002;45:545–558. doi: 10.1044/1092-4388(2002/043). [DOI] [PubMed] [Google Scholar]
- Jarrold C, Baddeley AD, Phillips CE. Verbal short-term memory in Down syndrome: a problem of memory, audition, or speech? Journal of Speech, Language, and Hearing Research. 2002;45:531–544. doi: 10.1044/1092-4388(2002/042). [DOI] [PubMed] [Google Scholar]
- Kanno K, Ikeda Y. Word-length effect in verbal short-term memory in individuals with Down syndrome. Journal of Intellectual Disabilities Research. 2002;46:613–618. doi: 10.1046/j.1365-2788.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- Keane VA. Unpublished doctoral thesis. University of Oregon; Eugene, OR: 1970. An investigation of disfluent speech behavior in Down’s syndrome. [Google Scholar]
- Kempster GB, Gerratt BR, Verdolini Abbott K, Barkmeier-Kraemer J, Hillman RE. Consensus Auditory-Perceptual Evaluation of Voice: Development of a standardized clinical protocol. American Journal of Speech-Language Pathology. 2009;18:124–132. doi: 10.1044/1058-0360(2008/08-0017). [DOI] [PubMed] [Google Scholar]
- Kennedy EJ, Flynn MC. Training phonological awareness skills in children with Down syndrome. Research in Development Disability. 2003;24:44–57. doi: 10.1016/s0891-4222(02)00168-3. [DOI] [PubMed] [Google Scholar]
- Kline LS, Hutchinson JM. Acoustic and perceptual evaluation of hypernasality of mentally retarded persons. American Journal of Mental Deficiency. 1980;85:153–160. [PubMed] [Google Scholar]
- Kumin L, Councill C, Goodman M. A longitudinal study of the emergence of phonemes in children with Down syndrome. Journal of Communication Disorders. 1994;27:293–303. doi: 10.1016/0021-9924(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Kumin L. Intelligibility of speech in children with Down syndrome in natural settings: parents’ perspective. Perceptual & Motor Skills. 1994;78:307–313. doi: 10.2466/pms.1994.78.1.307. [DOI] [PubMed] [Google Scholar]
- Kumin L. Speech intelligibility and childhood verbal apraxia in children with Down syndrome. Down’s Syndrome, Research, and Practice. 2006;10:10–22. doi: 10.3104/reports.301. [DOI] [PubMed] [Google Scholar]
- Laver JD, Wirz SL, Mackenzie J, Hiller S. A perceptual protocol for the analysis of vocal profiles. In: Laver JD, editor. The gift of speech. Edinburgh: Edinburgh University Press; 1991. pp. 265–280. [Google Scholar]
- Laws G. The use of nonword repetition as a test of phonological memory in children with Down syndrome. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39:1119–1130. [PubMed] [Google Scholar]
- Leddy M. Unpublished doctoral dissertation. University of Wisconsin-Madison; 1996. The relation among select vocal function characteristics of adult males with Down syndrome. [Google Scholar]
- Leddy M. The biological bases of speech in people with Down syndrome. In: Miller JF, Leddy M, Leavitt LA, editors. Improving the communication of people with Down syndrome. Baltimore: Paul H. Brookes; 1999. pp. 61–80. [Google Scholar]
- Lee MT, Thorpe J, Verhoeven J. Intonation and phonation in young adults with Down syndrome. Journal of Voice. 2009;23:82–87. doi: 10.1016/j.jvoice.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Legerstee M, Fels S, Bowman TG. People and objects affect the quality of vocalizations in infants with Down syndrome. Early Development and Parenting. 1992;1:149–156. [Google Scholar]
- Lind J, Vuorenkoski G, Rosberg G, Partanen TJ, Wasz-Hockert O. Spectrographic analysis of vocal responses to pain stimuli in infants with Down’s syndrome. Developmental Medicine & Child Neurology. 1970;12:478–486. doi: 10.1111/j.1469-8749.1970.tb01943.x. [DOI] [PubMed] [Google Scholar]
- Lynch MP, Oller DK, Steffens ML, Buder EH. Phrasing in prelinguistic vocalizations. Developmental Psychobiology. 1995;28:3–25. doi: 10.1002/dev.420280103. [DOI] [PubMed] [Google Scholar]
- Lynch MP, Oller DK, Steffens ML, Levine SL, Basinger DL, Umbel V. Onset of speech-like vocalizations in infants with Down syndrome. American Journal of Mental Retardation. 1995;100:68–86. [PubMed] [Google Scholar]
- McCann J, Wrench AA. In: Trouvain J, Barry WJ, editors. A new EPG protocol for assessing DDK accuracy scores in children : a Down’s syndrome study; 16th International Congress of Phonetic Sciences; Monday 6th August - Friday 10th August, 2007; Saarbrücken: Saarland University; 2007. [Google Scholar]
- Mehta DD, Hillman RE. Voice assessment: updates on perceptual, acoustic, aerodynamic, and endoscopic imaging methods. Current Opinion in Otolaryngology & Head and Neck Surgery. 2008;16:211–215. doi: 10.1097/MOO.0b013e3282fe96ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel JF, Carney RJ. Pitch characteristics of mongoloid boys. Journal of Speech and Hearing Disorders. 1964;21:121–125. doi: 10.1044/jshd.2902.121. [DOI] [PubMed] [Google Scholar]
- Miller JF, Chapman RS. Systematic analysis of language transcripts. Madison, WI: Language Analysis Laboratory, Waisman Center; 1990. [Google Scholar]
- Miller J, Leddy M. Verbal fluency, speech intelligibility and communicative effectiveness. In: Miller JF, Leddy M, Leavitt LA, editors. Improving the communication of people with Down syndrome. Baltimore: Paul H. Brookes; 1999. pp. 81–91. [Google Scholar]
- Mitchell RB, Call E, Kelly J. Diagnosis and therapy for airway obstruction in children with Down syndrome. Archives of Otolaryngology-Head and Neck Surgery. 2003;129:642–645. doi: 10.1001/archotol.129.6.642. [DOI] [PubMed] [Google Scholar]
- Montague JCJ, Hollien H. Perceived voice quality disorders in Down’s syndrome children. Journal of Communication Disorders. 1973;6:76–87. doi: 10.1016/0021-9924(73)90011-7. [DOI] [PubMed] [Google Scholar]
- Moody DK, Montague J, Bradley B. Preliminary validity and reliability data on the Wilson Voice Profile system. Language, Speech and Hearing Services in Schools. 1979;10:231–240. [Google Scholar]
- Moran MJ. Identification of Down’s syndrome adults from prolonged vowel samples. Journal of Communication Disorders. 1986;19:387–394. doi: 10.1016/0021-9924(86)90028-6. [DOI] [PubMed] [Google Scholar]
- Moran MJ, Gilbert HR. Speaking fundamental frequency characteristics of institutionalized adults with Down’s syndrome. American Journal of Mental Deficiency. 1978;83:248–252. [PubMed] [Google Scholar]
- Moran MJ, Gilbert HR. Selected acoustic characteristics and listener judgments of the voice of Down syndrome adults. American Journal of Mental Deficiency. 1982;86:553–556. [PubMed] [Google Scholar]
- Moura CP, Cunha LM, Vilarinho H, Cunha MJ, Freitas D, Palha M, Pueschel SM, Pais-Clemente M. Voice parameters in children with Down syndrome. Journal of Voice. 2008;22:34–42. doi: 10.1016/j.jvoice.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Murdoch BE, Goozee JV. EMA analysis of tongue function in children with dysarthria following traumatic brain injury. Journal of Medical Speech-Language Pathology. 2003;17:79–93. doi: 10.1080/0269905021000010203. [DOI] [PubMed] [Google Scholar]
- Myrelid Å, Gustafsson J, Ollars B, Annerén G. Growth charts for Down’s syndrome from birth to 18 years of age. Archives of Disease in Childhood. 2002;87:97–103. doi: 10.1136/adc.87.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L. Down’s syndrome: a genetic disorder in biobehavioral perspective. Genes, Brain and Behavior. 2003;2:156–166. doi: 10.1034/j.1601-183x.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- Nash HM, Snowling MJ. Semantic and phonological fluency in children with Down syndrome: atypical organization of language or less efficient retrieval strategies? Cognitive Neuropsychology. 2008;25:690–703. doi: 10.1080/02643290802274064. [DOI] [PubMed] [Google Scholar]
- Novak A. The voice of children with Down’s syndrome. Folia Phoniatrica (Basel) 1972;24:182–194. doi: 10.1159/000263566. [DOI] [PubMed] [Google Scholar]
- Oller DK. The emergence of the speech capacity. Mahweh, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Oster J. Mongolism. Copenhagen: Danish Science Press Ltd; 1953. [Google Scholar]
- Otto FM, Yairi E. An analysis of speech disfluencies in down’s syndrome and in normally intelligent subjects. Journal of Fluency Disorders. 1974;1:26–32. [Google Scholar]
- Palisano RJ, Walter SD, Russell DJ, Rosenbaum PL, Gemus M, Galuppi BE, Cunningham L. Gross motor function of children with Down syndrome: creation of motor growth curves. Archives of Physical and Medical Rehabilitation. 2001;82:494–500. doi: 10.1053/apmr.2001.21956. [DOI] [PubMed] [Google Scholar]
- Park AH, Wilson MA, Stevens PT, Harward R, Hohler N. Identification of hearing loss in pediatric patients with Down syndrome. Otolaryngology-Head & Neck Surgery. 2011 doi: 10.1177/0194599811425156. [DOI] [PubMed] [Google Scholar]
- Parsons CL, Iacono TA, Rozner L. Effect of tongue reduction on articulation in children with Down syndrome. American Journal of Mental Deficiency. 1987;91:328–332. [PubMed] [Google Scholar]
- Pentz ALJ. Formant amplitude of children with Down Syndrome. American Journal of Mental Deficiency. 1987;92:230–233. [PubMed] [Google Scholar]
- Pentz AL, Jr, Gilbert HR. Relation of selected acoustical parameters and perceptual ratings to voice quality in Down syndrome children. American Journal of Mental Deficiency. 1983;88:203–210. [PubMed] [Google Scholar]
- Pettinato M, Verhoeven J. Production and perception of word stress in children and adolescents with Down syndrome. Down Syndrome Research and Practice. 2008 doi: 10.3104/reports.2036. [DOI] [Google Scholar]
- Pinter JD, Eliez S, Schmitt JE, Capone GT, Reiss AL. Neuroanatomy of Down’s syndrome: a high-resolution MRI study. The American Journal of Psychiatry. 2001;158:1659–1665. doi: 10.1176/appi.ajp.158.10.1659. [DOI] [PubMed] [Google Scholar]
- Prescott CAJ. The current status of corrective surgery for laryngomalacia. American Journal of Otolaryngology. 1991;12:230–236. doi: 10.1016/0196-0709(91)90123-w. [DOI] [PubMed] [Google Scholar]
- Preus A. Stuttering in Down’s syndrome. Scandinavian Journal of Educational Research. 1972;14:89–104. [Google Scholar]
- Price JR, Kent RD. Increasing speech intelligibility in Down syndrome and fragile X syndrome. In: Warren SF, Fey ME, Roberts JE, Chapman RS, Warren SF, editors. Communication and language intervention series: Speech and language development and intervention in Down syndrome and Fragile X syndrome. Baltimore: Paul H. Brookes Publishing Co; 2008. pp. 219–231. [Google Scholar]
- Pryce M. The voice of people with Down syndrome: An EMG biofeedback study. Down Syndrome Research and Practice. 1994;2:106–111. [Google Scholar]
- Reeves RH, Baxter LL, Richtsmeier JT. Too much of a good thing: mechanisms of gene action in Down syndrome. Trends in Genetics. 2001;17:83–88. doi: 10.1016/s0168-9525(00)02172-7. [DOI] [PubMed] [Google Scholar]
- Reichle J, Siegel G, Rettie M. Matching prosodic and sound features: performance of Down’s syndrome preschoolers. Journal of Communication Disorders. 1985;18:149–159. doi: 10.1016/0021-9924(85)90017-6. [DOI] [PubMed] [Google Scholar]
- Rice ML, Warren SF, Betz SK. Language symptoms of developmental language disorders: an overview of autism, Down syndrome, fragile X, specific language impairment, and Williams syndrome. Applied Psycholinguistics. 2005;26:7–27. [Google Scholar]
- Robbins J, Klee T. Clinical assessment of oropharyngeal motor development in young children. Journal of Speech and Hearing Disorders. 1987;52:271–277. doi: 10.1044/jshd.5203.271. [DOI] [PubMed] [Google Scholar]
- Roberts J, Long SH, Malkin C, Barnes E, Skinner M, Hennon EA, et al. A comparison of phonological skills of boys with fragile X syndrome and Down syndrome. Journal of Speech, Language, and Hearing Research. 2005;48:980–995. doi: 10.1044/1092-4388(2005/067). [DOI] [PubMed] [Google Scholar]
- Rodger R. Unpublished PhD thesis. Queen Margaret University; Edinburgh, UK: 2009. Voice quality of children and young people with Down’s syndrome and its impact on listener judgement. [Google Scholar]
- Roger G, Denoyelle F, Triglia JM, Garabedian EN. Severe laryngomalacia: surgical indications and results in 115 patients. Laryngoscope. 1995;105:1111–1117. doi: 10.1288/00005537-199510000-00018. [DOI] [PubMed] [Google Scholar]
- Rohovsky KA. Unpublished master’s thesis. Ohio State University; Columbus, OH: 1965. A study of stuttering in institutional and non-institutional mongoloids. [Google Scholar]
- Roizen NJ, Wolters C, Nicol T, Blondis TA. Hearing loss in children with Down syndrome. The Journal of Pediatrics. 1993;123:S9–S12. doi: 10.1016/s0022-3476(05)81588-4. [DOI] [PubMed] [Google Scholar]
- Rolfe CR, Montague JC, Tirman RM, Vandergrift JF. Pilot perceptual and physiological investigation of hypernasality in Down’s syndrome adults. Folia Phoniatrica et Logopaedica. 1979;31:177–187. doi: 10.1159/000264164. [DOI] [PubMed] [Google Scholar]
- Rondal JA, Comblain A. Language in adults with Down syndrome. Down Syndrome Research & Practice. 1996;4:3–14. [Google Scholar]
- Rosenbloom ST, McGregor TL, Chen Q, An AQ, HS, Dupont WD. Specialized pediatric growth charts for electronic health record systems: the example of Down syndrome. American Medical Informatics Association 2010 Symposium Proceedings; 2010. pp. 687–691. [PMC free article] [PubMed] [Google Scholar]
- Rosin MM, Swift E, Bless D, Vetter DK. Communication profiles of adolescents with Down syndrome. Journal of Childhood Communication Disorders. 1988;12:49–64. [Google Scholar]
- Rupela V, Manjula R. Phonotactic patterns in the speech of children with Down syndrome. Clinical Linguistics & Phonetics. 2007;21:605–622. doi: 10.1080/02699200701416784. [DOI] [PubMed] [Google Scholar]
- Sapienza CM, Ruddy BH, Baker S. Laryngeal structure and function in the pediatric larynx: clinical applications. Language, Speech, and Hearing Services in Schools. 2004;35:299–307. doi: 10.1044/0161-1461(2004/029). [DOI] [PubMed] [Google Scholar]
- Sapir S, Ramig LO, Spielman JL, Fox C. Formant centralization ratio: A proposal for a new acoustic measure of dysarthric speech. Journal of Speech, Language, and Hearing Research. 2010;53:114–125. doi: 10.1044/1092-4388(2009/08-0184). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saz O, Simon J, Rodriguez W-R, Lleida E, Vaquero C. Analysis of acoustic features in speakers with cognitive disorders and speech impairments. EURASIP Journal on Advances in Signal Processing. 2009:Article ID 159234. doi: 10.1155/2009/159234. [DOI] [Google Scholar]
- Scherer NJ, D’Antonio LL, Rodgers JR. Profiles of communication disorder in children with velocardiofacial syndrome: Comparison to children with Down syndrome. Genetics in Medicine. 2001;3:72–78. doi: 10.1097/00125817-200101000-00016. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Sheree A, Boulet L, Boyle C, Rasmussen SA, Schendel D. Health of children 3 to 17 years of age with Down Syndrome in the 1997–2005 National Health Interview Survey. Pediatrics. 2009;123:e253–e260. doi: 10.1542/peds.2008-1440. [DOI] [PubMed] [Google Scholar]
- Schlanger BB, Gottsleben RH. Analysis of speech defects among the institutionalized mentally retarded. Journal of Speech Hearing Disorders. 1957;22(1):98–103. doi: 10.1044/jshd.2201.98. [DOI] [PubMed] [Google Scholar]
- Seifpanahi S, Bakhtiar M, Salmalian T. Objective vocal parameters in Farsi- speaking adults with Down syndrome. Folia Phoniatrica et Logopaedica. 2010;63:72–76. doi: 10.1159/000316326. [DOI] [PubMed] [Google Scholar]
- Seifpanahi MS, Salmalian T, Dehghan M. Vocal parameters of adults with Down syndrome in Zahedan/Iran. Journal of Kerman University of Medical Sciences. 2009;16:333–341. [Google Scholar]
- Shapiro BL. Amplified developmental instability in Down’s syndrome. Annals of Human Genetics. 1975;38:429–437. doi: 10.1111/j.1469-1809.1975.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Shapiro BL, Hermann J. Down syndrome--a disruption of homeostasis. American Journal of Medical Genetics. 1983;14:241–269. doi: 10.1002/ajmg.1320140206. [DOI] [PubMed] [Google Scholar]
- Shapiro BL, Gorlin RL, Redman RS, Bruhl HH. The palate and Down’s syndrome. New England Journal of Medicine. 1967;276:1460–1463. doi: 10.1056/NEJM196706292762603. [DOI] [PubMed] [Google Scholar]
- Shin M, Besser JM, Kucik JE, Lu C, Siffel C, Correa A the Congenital Anomaly Multistate Prevalence and Survival (CAMPA) Collaborative. Pediatrics. 2009;124:1565–1571. doi: 10.1542/peds.2009-0745. [DOI] [PubMed] [Google Scholar]
- Shott SR, Joseph A, Heithaus D. Hearing loss in children with Down syndrome. International Journal of Pediatric Otorhinolaryngology. 2001;61:199–205. doi: 10.1016/s0165-5876(01)00572-9. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Genetics, syndromes, and communication disorders. San Diego: Singular Publishing; 1997. [Google Scholar]
- Shriberg LD, Widder CJ. Speech and prosody characteristics of adults with mental retardation. Journal of Speech and Hearing Research. 1990;33:627–653. doi: 10.1044/jshr.3304.627. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, Pensler JM. The efficacy of tongue resection in treatment of symptomatic macroglossia in the child. Annals of Plastic Surgery. 1990;25:14–17. doi: 10.1097/00000637-199007000-00003. [DOI] [PubMed] [Google Scholar]
- Skrinjaric T, Glavina D, Jukic J. Palatal and dental arch morphology in down syndrome. Collegium Antropologicum. 2004;28:841–847. [PubMed] [Google Scholar]
- Smith A, Goffman L, Zelaznik HN, Ying G, McGillem C. Spatiotemporal stability and patterning of speech movement sequences. Experimental Brain Research. 1995;104:493–501. doi: 10.1007/BF00231983. [DOI] [PubMed] [Google Scholar]
- Smith BL, Oller DK. A comparative study of pre-meaningful vocalizations produced by normally developing and Down’s syndrome infants. The Journal of Speech and Hearing Disorders. 1981;46:46–51. doi: 10.1044/jshd.4601.46. [DOI] [PubMed] [Google Scholar]
- Smith BL, Stoel-Gammon C. A longitudinal study of the development of stop consonant production in normal and Down’s syndrome children. Journal of Speech and Hearing Disorders. 1983;48:114–118. doi: 10.1044/jshd.4802.114. [DOI] [PubMed] [Google Scholar]
- Smith BL, Stoel-Gammon C. A quantitative analysis of the reduplicated and variegated babbling in vocalizations by Down syndrome infants. Clinical Linguistics & Phonetics. 1996;10:119–130. [Google Scholar]
- So LK, Dodd BJ. Down’s syndrome and the acquisition of phonology by Cantonese-speaking children. Journal of Intellectual Disabilities Research. 1994;38:501–517. doi: 10.1111/j.1365-2788.1994.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Solomons NB, Prescott CA. Laryngomalacia: A review and the surgical management of severe cases. International Journal of Pediatric Otorhinolaryngology. 1987;13:31–39. doi: 10.1016/0165-5876(87)90005-x. [DOI] [PubMed] [Google Scholar]
- Sommers RK, Patterson JP, Widgen PL. Phonology of Down syndrome speakers, ages 13–22. Journal of Childhood Communication Disorders. 1988;12:65–90. [Google Scholar]
- Sommers RK, Reinhart RW, Sistrunk DA. Traditional articulation measures of Down syndrome speakers, Ages 13–22. Communication Disorders Quarterly. 1988;12:93–108. [Google Scholar]
- Steffens ML, Oller KD, Lynch M, Urbano RC. Vocal development in infants with Down syndrome and infants who are developing normally. American Journal on Mental Retardation. 1992;97:235–246. [PubMed] [Google Scholar]
- Stempfle N, Huten Y, Fredouille C, Brisse H, Nessmann C. Skeletal abnormalities in fetuses with Down’s syndrome: a radiographic post-mortem study. Pediatric Radiology. 1999;29:682–688. doi: 10.1007/s002470050675. [DOI] [PubMed] [Google Scholar]
- Stoel-Gammon C. Phonological analysis of four Down’s syndrome children. Applied Psycholinguistics. 1980;1:31–48. [Google Scholar]
- Stojanovik Prosodic deficits in children with Down syndrome. Journal of Neurolinguistics. 2010 doi: 10.1016/j.neuroling.2010.01.004. [DOI] [Google Scholar]
- Strazzulla M. Speech problems of the Mongoloid child. Quarterly Review of Paediatrics. 1953;8:268–273. [Google Scholar]
- Timmins C, Hardcastle WJ, Wood S, Cleland J. An EPG analysis of /t/ in young people with Down’s syndrome. Clinical Linguistics & Phonetics. 2011;25:1022–1027. doi: 10.3109/02699206.2011.616981. [DOI] [PubMed] [Google Scholar]
- Timmins C, Cleland J, Rodger R, Wishart J, Wood S, Hardcastle WJ. Speech production in Down syndrome. Down Syndrome Quarterly. 2009;11:16–22. [Google Scholar]
- Titze I, Horii Y, Scherer R. Some technical considerations in voice perturbation measurements. Journal of Speech and Hearing Research. 1987;30:252–260. doi: 10.1044/jshr.3002.252. [DOI] [PubMed] [Google Scholar]
- Thompson DM. Abnormal sensorimotor integrative function of the larynx in congenital laryngomalacia: A new theory of etiology. The Laryngoscope. 2009;117(Supp. 114):1–33. doi: 10.1097/MLG.0b013e31804a5750. [DOI] [PubMed] [Google Scholar]
- Uong EC, McDonough JM, Tayag-Kier CE, Zhao H, Haselgrove J, Mahboubi S, Schwab RJ, Pack AI, Arens R. Magnetic resonance imaging of the upper airway in children with Down syndrome. American Journal of Respiratory Critical Care Medicine. 2001;163:731–736. doi: 10.1164/ajrccm.163.3.2004231. [DOI] [PubMed] [Google Scholar]
- Van Borsel J. An analysis of the speech of five Down’s syndrome adolescents. Journal of Communication Disorders. 1988;21:409–421. doi: 10.1016/0021-9924(88)90026-3. [DOI] [PubMed] [Google Scholar]
- Van Borsel J. Articulation in Down’s syndrome adolescents and adults. European Journal of Disorders of Communication. 1996;31:415–444. doi: 10.3109/13682829609031330. [DOI] [PubMed] [Google Scholar]
- Van Borsel J, Vandermeulen A. Cluttering in Down syndrome. Folia Phoniatrica et Logopaedica. 2008;60:312–317. doi: 10.1159/000170081. [DOI] [PubMed] [Google Scholar]
- Vicari S. Motor development and neuropsychological patterns in persons with Down syndrome. Behavior Genetics. 2006;36:355–364. doi: 10.1007/s10519-006-9057-8. [DOI] [PubMed] [Google Scholar]
- Vorperian H, Kent R. Vowel acoustic space development in children: A synthesis of acoustic and anatomic data. Journal of Speech, Language, and Hearing Research. 2007;50:1510–1545. doi: 10.1044/1092-4388(2007/104). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorenkoski V, Lind J, Wasz-Hockert O, Partanen T. Cry score. a method for evaluating the degree of abnormality in the pain cry response of the newborn and young infant. Quarterly Progress & Status Report, Speech Transmission Lab (Stockholm) 1971;12:68–75. [Google Scholar]
- Vuorenkoski V, Wasz-Hockert O, Lind J, Koivisto M, Partanen TJ. Training the auditory perception of some specific types of abnormal pain cry in newborn and young infants. Quarterly Progress & Status Report, Speech Transmission Lab; Stockholm. 1971. pp. 37–48. [Google Scholar]
- Wechsler D. Wechsler pre-school & primary scale of intelligence. 3. Oxford, England: Pearson; 2003. UK edition (WPPSI-III UK) [Google Scholar]
- Weinberg B, Zlatin M. Speaking fundamental frequency characteristics of five- and six-year-old children with Mongolism. Journal of Speech and Hearing Research. 1970;13:418–425. doi: 10.1044/jshr.1302.418. [DOI] [PubMed] [Google Scholar]
- Wiig E, Secord W, Semel E. Clinical evaluation of language fundamentals--preschool UK. London: The Psychological Corporation; 1992. [Google Scholar]
- Willcox A. An investigation into non-fluency in Down’s syndrome. International Journal of Language & Communication Disorders. 1988;23:153–170. doi: 10.3109/13682828809019884. [DOI] [PubMed] [Google Scholar]
- Wilson F. Voice disorders. St. Louis: Voice Tapes; 1972. [Google Scholar]
- Wiseman FK, Alford KA, Tybulewicz VLJ, Fisher EMC. Down syndrome--recent progress and future prospects. Human Molecular Genetics. 2009;18:R75–R83. doi: 10.1093/hmg/ddp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold DC, Montague JC., Jr Preliminary perceived voice deviations and hearing disorders of adults with Down’s syndrome. Perceptual and Motor Skills. 1979;49:564–564. doi: 10.2466/pms.1979.49.2.564. [DOI] [PubMed] [Google Scholar]
- Wood S, Wishart J, Hardcastle W, Cleland J, Timmins C. The use of electropalatography (EPG) in the assessment and treatment of motor speech disorders in children with Down’s syndrome: evidence from two case studies. Developmental Neurorehabilitation. 2009;12:66–75. doi: 10.1080/17518420902738193. [DOI] [PubMed] [Google Scholar]
- Xue S, Kaine L, Ng ML. Quantification of vocal tract configurations of older children with Down syndrome using acoustic reflection technology: a pilot study. International Journal of Pediatric Otorhinolaryngology. 2010;74:378–383. doi: 10.1016/j.ijporl.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Yarom R, Sagher U, Havivi Y, Peled IJ, Wexler MR. Myofibers in tongues of Down’s syndrome. Journal of the Neurological Sciences. 1986;73(3):279–287. doi: 10.1016/0022-510x(86)90152-8. [DOI] [PubMed] [Google Scholar]
- Yoder PJ, Hooshyar N, Klee T, Schaffer M. Comparison of the types of child utterances mothers expand in children with language delays and with Down’s syndrome. Journal of Intellectual Disability Research. 1996;40(Pt 6):557–567. doi: 10.1046/j.1365-2788.1996.02929.x. [DOI] [PubMed] [Google Scholar]
- Yorkston KM, Strand EA, Kennedy MRT. Comprehensibility of dysarthric speech: Implications for assessment and treatment planning. American Journal of Speech-Language Pathology. 1996;5:55–66. [Google Scholar]
- Zink PK, Bialer I. Speech and language problems in Mongolism: A review of the literature. Journal of Speech and Hearing Disorders. 1967;32:228–241. [Google Scholar]