Abstract
Background & Aims
Hepatocellular adenomas (HCA) are benign tumors that can lead to medical complications. Chronic inflammation and mutations in β-catenin, HNF1α or GP130 are potential causes for human HCA. However, additional causes may exist due to heterogeneity of HCA. We investigated whether HCA are caused by endoplasmic reticulum (ER) stress.
Methods
Mice with a liver specific deletion of the major chaperone BiP (LGKO) were used. Liver tumor occurrence was examined in LGKO with or without feeding of a high fat diet (HFD) and characterized with immunohistochemistry with molecular markers of proliferation/malignancy. Molecular changes were analyzed with immunoblotting.
Results
Spontaneous monoclonal liver tumors were observed in 34% of LGKO females with constitutive hepatic ER stress. Lack of portal tracks or central veins, dilated sinusoidal spaces, hemorrhagic areas, active proliferation and Lipid deposits were observed in the liver tumors. HFD feeding induced multi-clonal liver tumors in 83% of the LGKO females versus zero in wild type females. Hepatocytes reactive to anti-glypican-3 antibodies were detected in the HFD-induced but not spontaneous tumors. In the liver tumors, inhibition of cyclin D and increase of the 36kD estrogen receptor variant (ERα36), active ATF4/6, GSK3β and ERK1/2 were detected whereas no change of HNF1α, β-catenin, p-53, androgen receptor α or estrogen receptor α was detected. HFD activated JAK and STAT3.
Conclusions
Our evidence supports a possible link of HCA with ER stress and altered expression of cyclin D and ERα36. Additional stress such as HFD may promote malignant transformation of HCA through the JAK-STAT pathway.
Keywords: metabolic stress, unfolded protein response (UPR), high fat diet (HFD), abnormal function of cyclin D, sex hormone receptor variants, hepatocellular tumorigenesis
INTRODUCTION
Protein folding is an intracellular process essential for protein maturation and function which occurs mainly in the endoplasmic reticulum (ER). Under normal physiological conditions, there is a balance between the unfolded proteins and ER folding machinery. Disruption of the balance results in accumulation of unfolded and/or misfolded proteins, which triggers an adaptive stress response, the unfolded protein response (UPR). The UPR initially attenuates protein translation, increases protein folding capacity, and promotes degradation of unfolded proteins, which collectively restore ER homeostasis and minimize injury. However, prolonged UPR may lead to fat accumulation, inflammation and cell death injuries. Many human diseases such as obesity, diabetes, inflammatory bowel disease, and hepatosteatosis involve impaired UPR signaling.1–5 Molecular chaperones play a vital role in maintaining the protein homeostasis inside the ER. The glucose-regulated protein 78 (GRP78/BiP) is a major molecular chaperone in the ER. BiP associates with three ER membrane resident sensors: inositol-requiring enzyme-1 (IRE1α), transcription factor-6 (ATF6) and PKR-like eukaryotic initiation factor 2α kinase (PERK). Dissociation of BiP from the three sensors upon ER stress triggers UPR signaling pathways. Increased expression of BiP in solid tumors has been shown to be associated with tumor growth and drug resistance.6 Over-expression of BiP in the liver inhibits insulin and ER stress–induced hepatic steatosis in ob/ob mice.7 We previously deleted BiP specifically from the liver and created a mouse model (LGKO) with constitutive perturbation of UPR signaling in the liver.8 The mouse model allowed us to test the direct role of ER stress in liver diseases. The BiP deletion resulted in hepatic cell death, inflammation, insulin resistance, and spontaneous fat accumulation.8 The BiP deletion also sensitized the liver to a variety of injuries by drugs, toxins, alcohol, or high fat diets. However, it is not known whether there are any additional consequences from the BiP deletion and subsequent long term ER stress in these animals as they grow older. In this study, we hypothesized that the animals under the constitutive stress would develop advanced liver diseases such as tumor due to a continuous disruption of protein homeostasis. We tested the hypothesis in the LGKO animal model and document our evidence in this report.
MATERIALS AND METHODS
Experimental Animals
Mice with a liver-specific Grp78/BiP deletion were previously created by the LoxP-Cre strategy and detailed animal breeding, genotyping, daily inspection and maintenance of the colonies were described.8 Briefly, the established Grp78 floxed females (Grp78f/f) were crossed with male mice carrying the Cre transgene under the control of the rat albumin promoter (Alb-CreTg/Tg). The resulting heterozygous mice carrying one floxed allele and half copies of the Alb-Cre gene (Grp78f/w; Alb-CreTg/0) were back-crossed with the Grp78 floxed founders to yield mice with liver-specific Grp78 deletion (Grp78f/f; Alb-CreTg/0 or termed LGKO) and three different groups of littermates. The group carrying homozygous floxed alleles without Alb-Cre (Grp78f/f; Alb-Cre0/0) was used as wild type littermate controls. PCR genotyping with tail or liver genomic DNA was performed to distinguish Grp78 alleles of wild-type and deleted. The presence of the Alb-Cre transgene was determined by duplex quantitative PCR using Cre-specific primers. For spontaneous liver tumor development, the knockouts were maintained in the animal facility for 9 to 18 months. The following criteria were used for euthanasia of animals with suspected spontaneous liver tumors: animals were moribund, unable to move or failure to respond to gentle stimuli, with labored breathing or diarrhea, and inability to eat and drink. Littermates of wild type or knockouts without tumors were killed simultaneously as age-matched controls. For high fat diet experiments, 4 month old animals were fed the modified AIN-93G with purified high fat diet (Dyets #180529) for 12 months. All animals were treated in accordance with the Guide for Care and Use of Laboratory Animals approved by a local committee for animal care and use.
Serum alanine aminotransferase and liver histology
At the time of killing, serum samples were collected and liver tissues were either snap frozen in liquid nitrogen and stored at −80°C or fixed immediately for histological staining. Serum alanine aminotransferase (ALT) and liver histology for Hematoxylin and Eosin (H&E) and Sirius Red staining were described previously.8–10 Histological changes were checked by a pathologist blinded to the genotypes. The following histological criteria were individually or collectively used to categorize a benign liver tumor into hepatocellular adenoma (HCA): presence of well-circumscribed hyperplastic nodules that consist of sheets of two to three layers of hepatocytes with a bubbly vacuolated cytoplasm, presence of cells that resemble normal hepatocytes and are traversed by blood vessels but lack portal tracts or central veins inside the nodules, and presence of hemorrhagic areas, dilated sinusoidal spaces, and lipid deposits.11–15
Liver immunohistochemistry and molecular analyses
The Betazoid DAB Chromogen kit and ancillary reagents (BioCare Medical, CA, USA) were used for immunohistochemistry.8,9 Primary antibodies against the proliferating cell nuclear antigen (PCNA) and Ki-67 and against glypican-3 (GPC3; a glycosylphosphatidyl inositol-anchored membrane protein) were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Hepatocytes stained positive with anti-molecular markers of proliferation (PCNA and Ki-67) were counted under a microscope at 100× magnification. Presence of diffuse cytoplasmic expression of GPC3 in the hepatocytes was used as a histochemical marker for malignant transformation of a benign tumor or a possible development of hepatocellular carcinoma.16 For molecular analyses, RNAs and proteins (whole and nuclear) were extracted from wild type liver, knockout liver portion and knockout tumor portion and were analyzed according to the method described previously.8,9 Immunoblotting was conducted using horseradish peroxidase-labeled secondary antibodies (1:2000 dilutions), in which the intensity of protein bands on the immunoblots was quantified with the NIH software, Image J. Primary antibodies (1: 200–500 dilutions) against the glucose regulated protein 78 (BiP), cysteine-rich with EGF-like domains 2 (Creld2), hepatocyte nuclear factor 1α (HNF1α), GP130, p-53, cyclin D, Cyclin E, Cyclin G, estrogen receptor α and β (ERα and β), androgen receptor α (ARα), extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), phosphorylated ERK1/2 (p-ERK1/2), phosphorylated Janus kinase (p-JAK), and phosphorylated signal transducers and activators of transcription 3 (p-STAT3) were from Santa Cruz Biotechnology Inc. Primary antibodies against the transcription activator 4 (ATF4) and 6 (ATF6) were from Aviva System (San Diego, CA). Primary antibodies against glycogen synthase kinase 3β (GSK3β) (Tyr-216) were from Cell Signaling (Beverly, MA). Primary antibodies against β-catenin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were from Millipore (Billerica, MA).
Statistical Analysis
Values are expressed as means ± SD unless otherwise indicated. Statistical analyses were performed using ANOVA for comparison of multiple groups or the Student’s t test for paired if same littermates were in each group and for unpaired data if combined littermates in each group. P < 0.05 or less was considered statistically significant.
RESULTS
Features of spontaneous liver tumors in female mice under ER stress
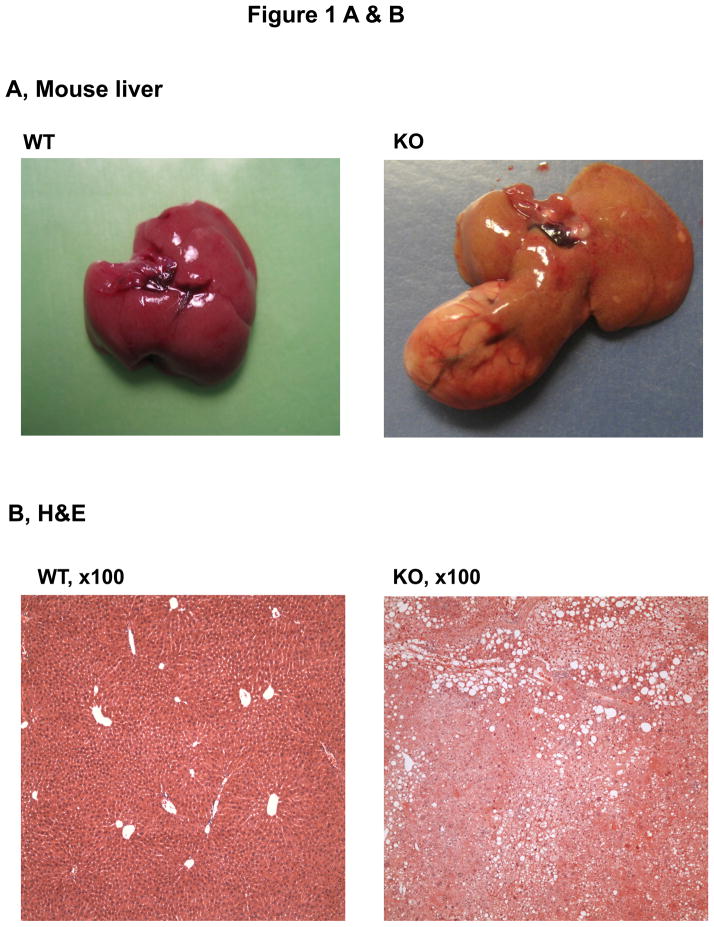
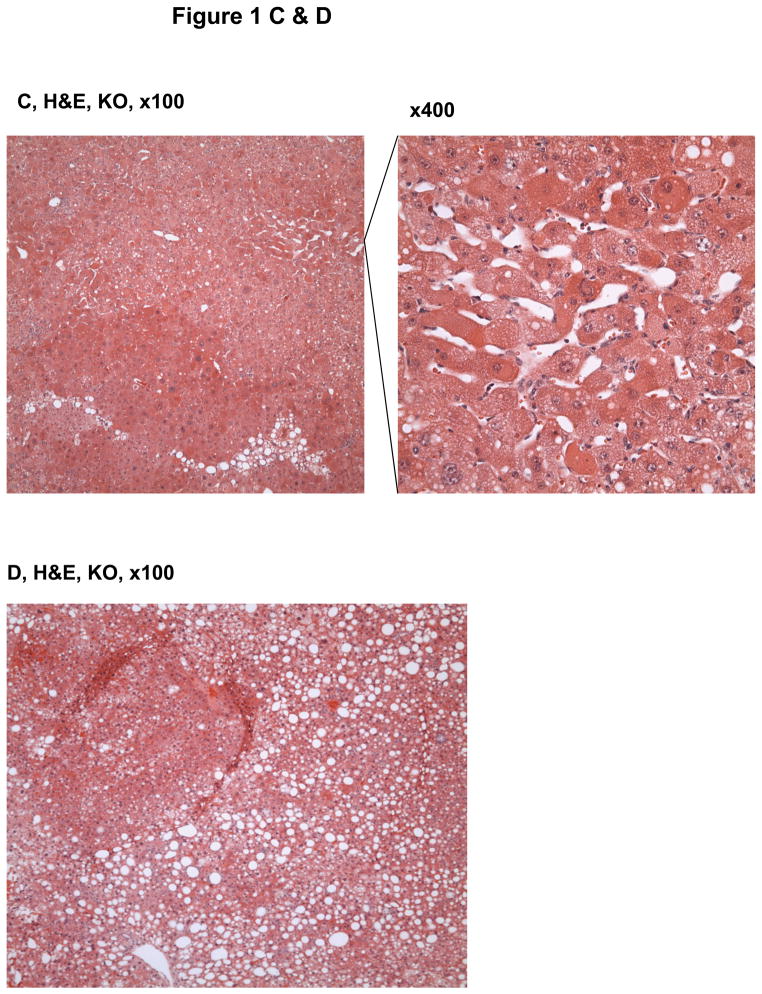
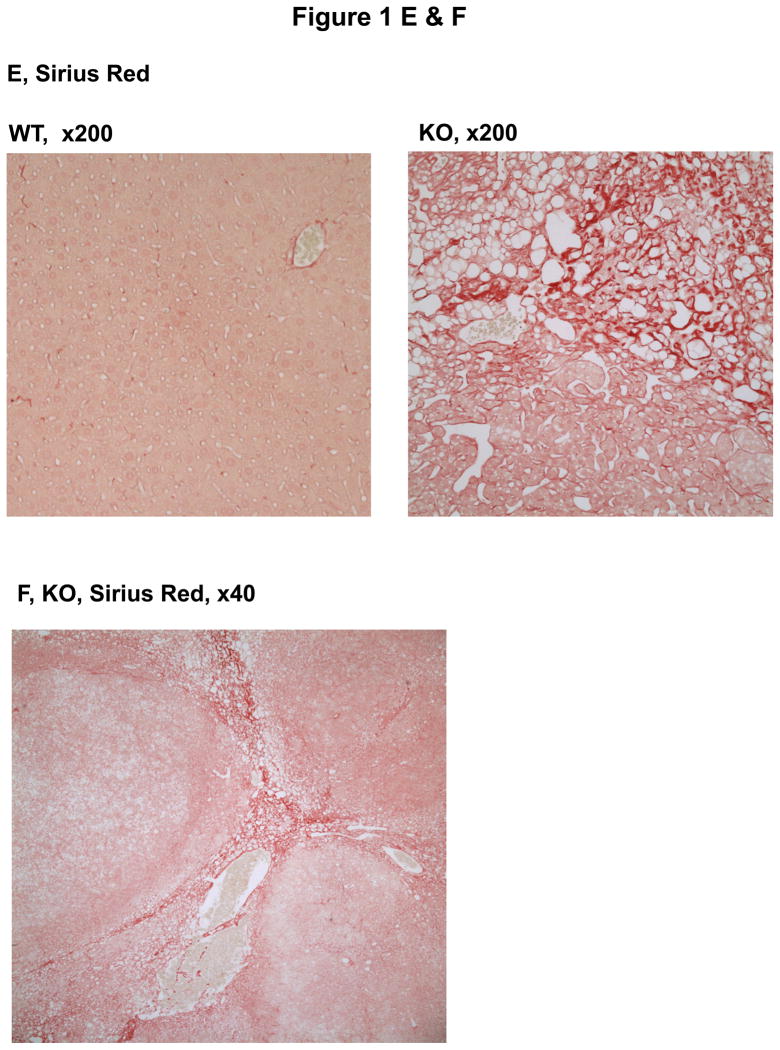
The animals with a liver specific deletion of Grp78/BiP(LGKO) had moderate liver injury with a 3–4 fold increase in serum ALT levels. They were smaller than their wild type (WT) littermates8 and spontaneous liver tumors were not detected in any of the LGKO mice younger than 8 month old. However, spontaneous liver tumors were observed in LGKO starting at 9 month old (Table 1; Figure 1A). Interestingly, 34% (eight out of 23) of female LGKO had the tumor-like masses in the liver whereas only one out of 22 male LGKO had liver tumor. Compared to the female WT control, ALT values were increased by nearly 9 fold in the female LGKO containing tumors, indicating severe liver injury in these mice. There are generally three types of tumors in the liver: focal nodular hyperplasia (FNH), hepatocellular adenoma (HCA), and hepatocellular carcinoma (HCC).13–15, 17, 18 To identify the nature of the tumors observed, we conducted H&E and Sirius Red staining of the liver tissues. Histologically, we observed well-circumscribed nodules with 2–3 layers of hepatocytes, lack of normal portal tracts or central veins within the hyperplastic nodules, hemorrhagic areas, dilated sinusoidal spaces, lipid accumulation, and fibrotic or cirrhotic changes in the liver tumors (Fig. 1B–F). The tumors caused rupture inside the abdomen and three out of eight mice with the spontaneous liver tumors bled severely inside. Collectively, these observations are indicative of HCA-like features rather than non-tumorous hepatic lesions,11, 14,15 which could be a result of ER stress and fatty liver syndrome in LGKO. To determine further whether the tumor is benign or malignant, we performed immunohistochemistry with molecular markers for proliferation and malignancy. We found that the number of hepatocytes that were anti-PCNA or Ki-67 positive were moderately increased in the benign tumors of LGKO mice (Fig. 2A–C), suggesting an active proliferation in the tumors. To determine malignancy of these tumors, we further conducted immunohistochemistry with anti-glypican-3 (GPC3) antibodies since GPC3 is a member of the glypican family of glycosyl-phosphatidylinositol–anchored cell-surface heparan sulfate proteoglycans and is used as a diagnostic marker for hepatocellular carcinoma.16 However, no positive signals were detected by the immunohistochemistry with GPC3 (data not shown), suggesting that the spontaneous liver tumors were not malignant.
TABLE 1.
Liver Tumor Occurrence and Injury
| PARAMETERS | WILD TYPE | KNOCKOUT | ||
|---|---|---|---|---|
|
| ||||
| Male | Female | Male | Female | |
|
| ||||
| Number of mice | 25 | 29 | 22 | 23 |
| Number of mice with tumor | 0 | 0 | 1 | 8 |
| Mouse age (month) | 9–17 | 9–17 | 9 | 9–17 |
| ALT±SD (U/L) | 20±3.5 | 19±2.1 | 76±70.2* | 113±105.6* |
| ALT±SD (U/L) in mice with tumor | 234 | 174±114.1** | ||
p<0.05;
p<0.01 compared to wild type of same sex.
Figure 1.
Histology of hepatic tumors in female mice with a liver specific deletion of the major chaperone BiP. (A) Liver image showing a representative adenoma-like mass in the BiP deleted mice (KO). WT, wild type. (B)–(D) H&E staining of the liver tissue reveal nodular formation, hepatic hemorrhages, dilated sinusoidal spaces, and lipid deposits in KO. (E)& (F) Sirius Red staining of the liver tissue reveal lipid deposit, dilated sinusoidal spaces and fibrotic/cirrhotic changes with a map-like pattern.
Figure 2.
Benign nature of the liver tumors in female mice with the liver specific BiP deletion. (A) Liver immunohistochemistry with the proliferating cell nuclear antigen (PCNA) shows increased anti-PCNA positive hepatocytes in the BiP deleted mice (KO) versus wild type (WT). (B) Liver immunohistochemistry with Ki-67 confirms increased proliferation in KO. (C) Quantitation of the immunohistochemistry with PCNA and Ki-67. *, p<0.05; n=6.
Malignant transformation under ER stress and high fat diet feeding
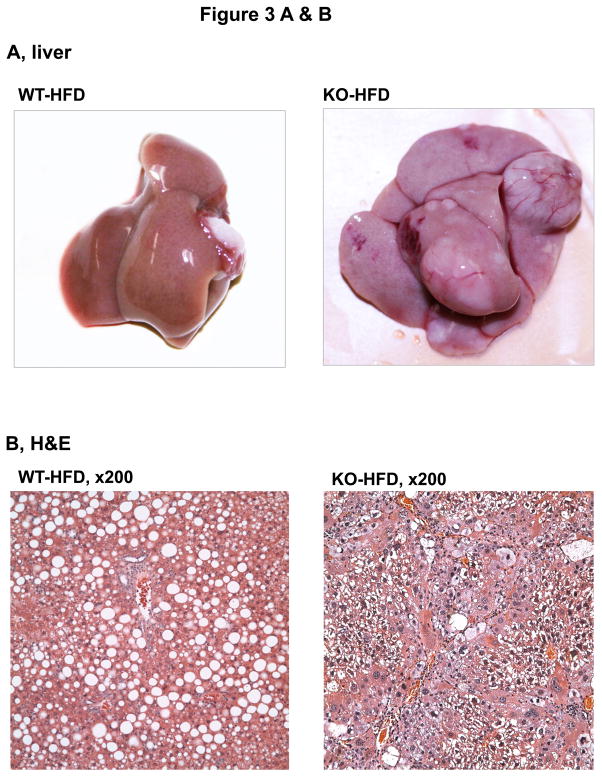
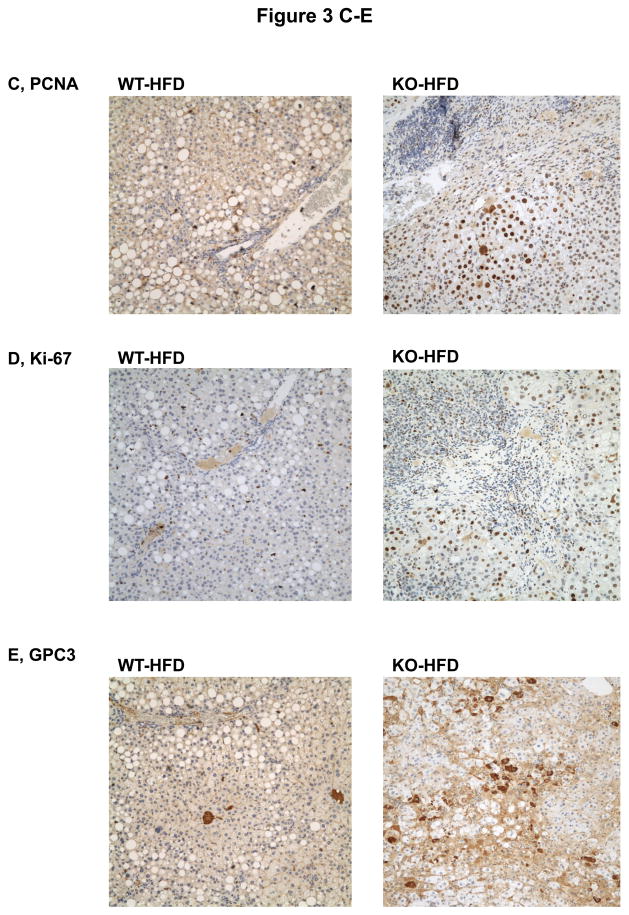
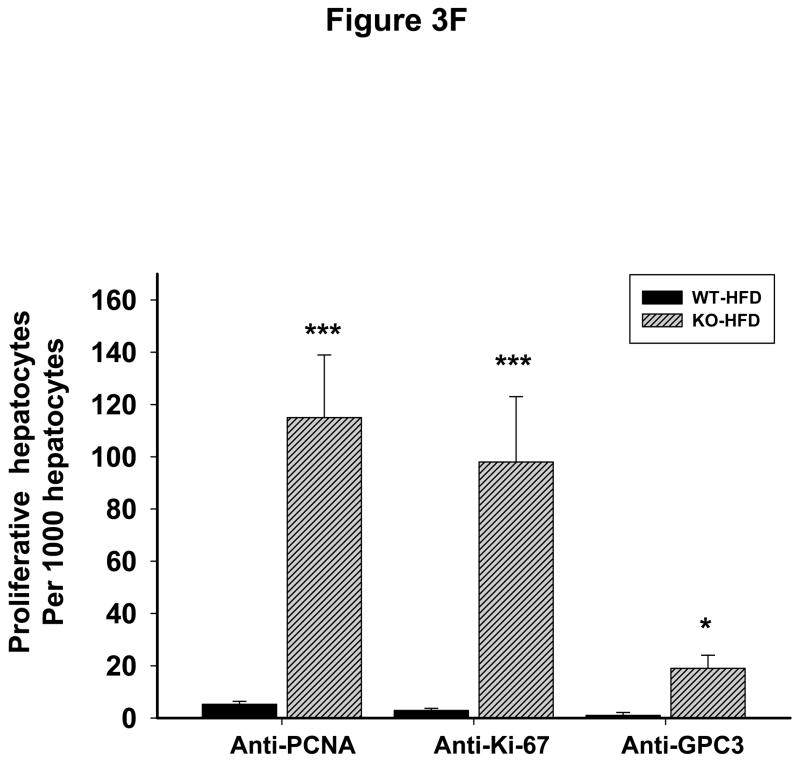
To know whether additional stress promotes liver tumorigenesis in the LGKO under constitutive ER stress, we fed the animals a high fat diet (HFD) for one year. Remarkably, HFD induced liver tumors in 83% (5 out of 6) of female LGKO (Fig. 3A) whereas under the same feeding conditions no liver tumors were found in male LGKO or female WT (data not shown). Two or more tumor masses or multiple proliferative nodules were observed in the livers of the female LGKO fed HFD (Fig. 3A and 3B). Massive neutrophil infiltration was also observed in the liver tumors. ALT levels in the female LGKO fed HFD and female WT fed HFD were 217 ± 79.9 U/L and 56 ± 31.2 U/L respectively, indicating that HFD induced much more severe liver injury in the female LGKO. HFD promoted proliferation in the LGKO liver dramatically (Fig. 3C and 3D). The number of PCNA was increased by 22 fold in the LGKO compared to the WT and the number of Ki-67 positive hepatocytes was increased by 23 fold in the LGKO compared to the WT (Fig. 3F). Notably positive hepatocytes with diffuse cytoplasmic expression of GPC3 were detected in the liver of the LGKO fed HFD but not in the WT fed HFD (Fig 3E). The number of GPC3 positive cells was significantly increased in the LGKO (Fig. 3F), indicating a possible malignant transformation under constitutive ER stress and HFD.
Figure 3.
Malignant liver tumors in female mice with the liver specific BiP deletion and high fat diet feeding. (A) Fatty liver of wild type (WT) fed a high fat diet (HFD) versus liver with multiple tumor masses in the BiP deleted female mice (KO) fed HFD. (B) H&E staining reveals lipid deposit, neutrophil infiltration and formation of multi-nodules in KO fed HFD but only fat accumulation is seen in WT fed HFD. (C)& (D) Liver immunohistochemistry with PCNA and Ki-67 shows severe inflammation and dramatically increased anti-PCNA or Ki-67 positive hepatocytes in KO fed HFD. Note that the positive nuclear staining of Ki-67 is prominently present in KO despite the nonspecific cytoplasmic Ki-67 staining is seen in both WT and KO. (E) Immunohistochemistry reveals positive hepatocytes with diffuse cytoplasmic expression of GPC3 protein in KO fed HFD. (F) Quantitation of immunohistochemistry with PCNA, Ki-67 and GPC3. *, p<0.05 and ***, p<0.005; n=4.
Molecular changes in the liver tumors
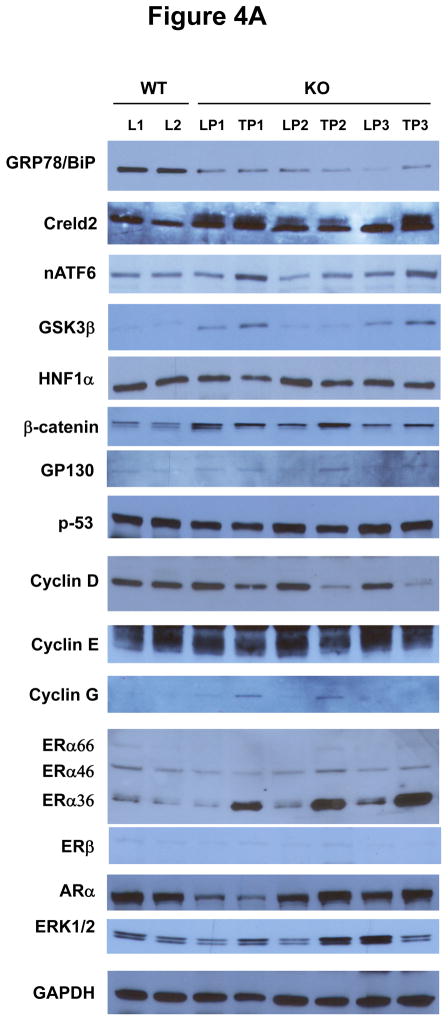
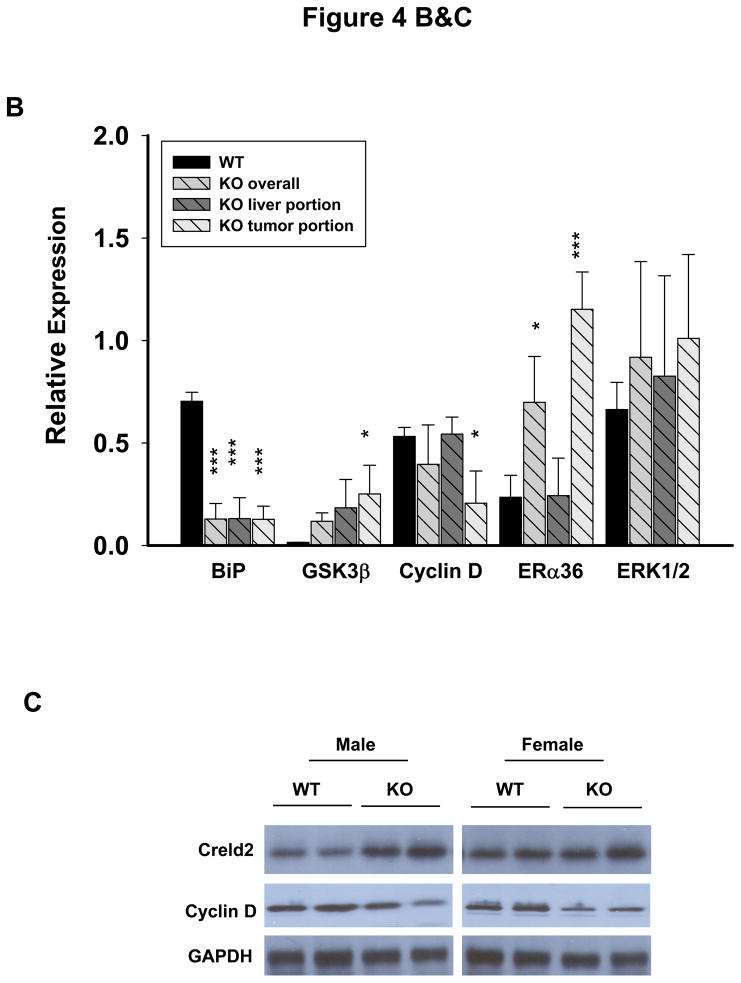
The above observed phenotypes prompted us to investigate molecular mechanisms linking chronic ER stress to liver tumorigenesis, particularly HCA. Molecular changes were revealed by immunoblotting and compared between WT liver (L) and LGKO liver and between LGKO liver portion (LP) and tumor portion (TP) (Fig. 4A). GRP78 was reduced by 70% in the LGKO mice in both the liver portion and the tumor portion (Fig. 4B) confirming a deletion of GRP78 in the hepatocytes. Three randomly selected ER stress markers: cysteine-rich with EGF-like domains 2 (Creld2), active ATF6 and GSKβ were increased in the LGKO, indicative of continuous ER stress. Significant changes of mRNA of HNF1α and β-catenin were not detected (data not shown). Proteins of HNF1α, β-catenin and GP130 were slightly increased in LGKO compared to WT, supporting the increased proliferation in the benign tumor. There were no apparent changes in p53 between the WT and LGKO or between LP and TP of the LGKO females. Interestingly, cyclin D was specifically reduced by 2.6 fold in TP of the LGKO. In contrast, cyclin E was slightly reduced and cyclin G was slightly increased in TP of the LGKO, suggesting possible compensatory responses upon the reduction of cyclin D. Since almost all the hepatocellular adenomas were found in the female LGKO not in the male LGKO, we examined expression of receptors for sex hormones including estrogen receptor α and β (ERα and β) and androgen receptor α (ARα). Three isoforms of ERα with molecular sizes of 66, 46 and 36 kD were detected in the liver (Fig. 4A). There were no apparent changes in the expression of ERα66, ERα46 or ARα between WT and LGKO or between LP and TP of the LGKO. In contrast, ERα36 was increased by 5 fold specifically in TP of the LGKO (Fig. 4A and 4B). ERβ was minimally expressed in the liver and there was an increased ERK1/2 in the LGKO (Fig. 4A). In addition, ER stress response indicated by increased expression of Creld2 and inhibition of cyclin D was similar between the male and female knockouts at the same age of 15 months (Fig. 4C).
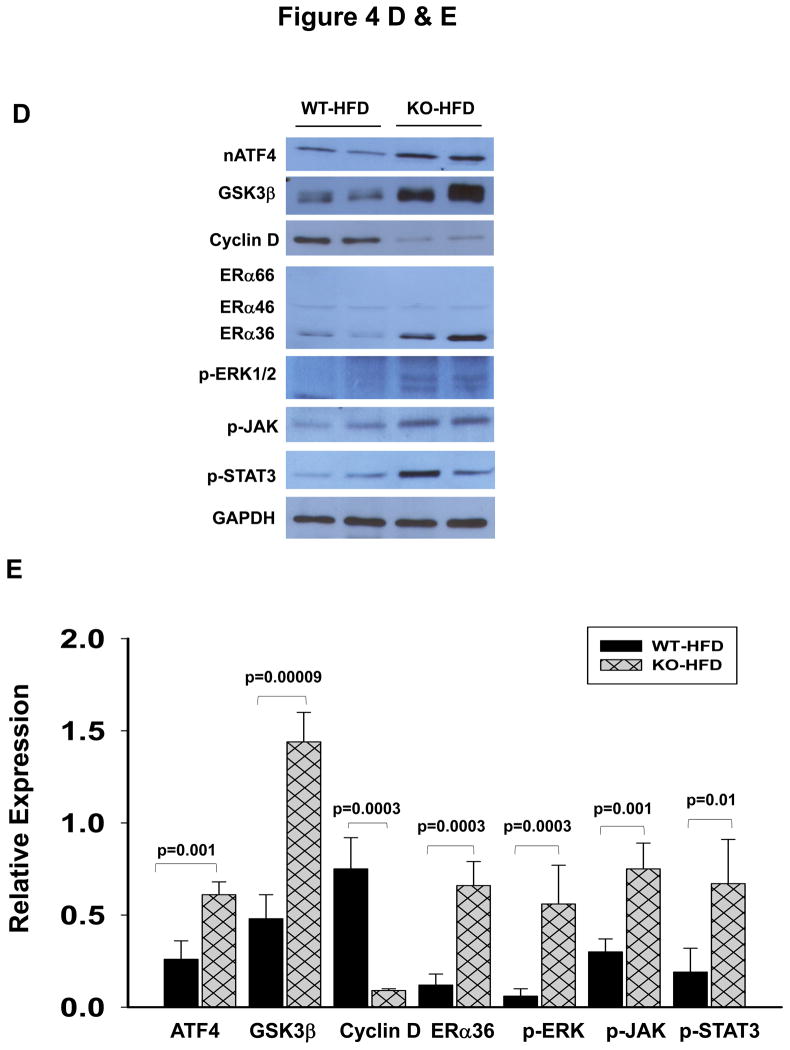
Figure 4.
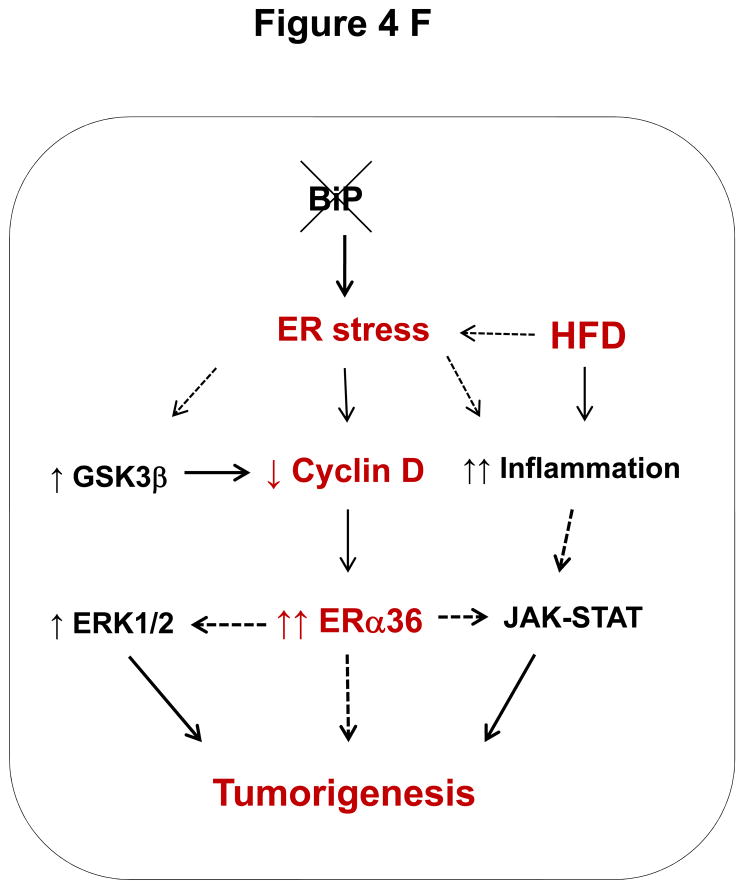
Association of liver tumorigenesis with constitutive ER stresses response, cyclin D inhibition, sex hormone receptor isoform overexpression and proliferative pathways in female mice with the liver specific BiP deletion. (A) Molecular changes revealed with immunoblotting of proteins from WT liver (L), KO liver portion (LP) and KO tumor portion (TP). Creld2, cysteine-rich with EGF-like domains 2; HNF1α, hepatocyte nuclear factor 1α; ERα and β, estrogen receptor α and β; ARα, androgen receptor α; ERK1/2, extracellular signal-regulated protein kinases 1 and 2; nATF6, active transcription activator 6; GSK3β, glycogen synthase kinase 3β; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.(B) Quantitation of levels of key protein markers normalized with GAPDH. *, p<0.05; ***, p<0.005, n=4. (C) Comparison of ER stress response between male and female knockouts at age of 15 months. (D) Molecular changes in KO liver versus WT liver in response to HFD. nATF4, active transcription activator 4; p-JAK, phosphorylated Janus kinase; p-STAT3, phosphorylated signal transducers and activators of transcription 3. (E) Quantitation of levels of proteins normalized with GAPDH. P values of each comparing group are shown above the bars. (F) A diagram depicting molecular mechanisms linking ER stress and/or HFD, cyclin D and ERα36 with hepatocellular tumorigenesis.
Activation of the JAK-STAT pathway by HFD
The potential molecular mechanisms underlying the malignant transformation of HCA by HFD were further investigated. In response to HFD, both active ATF4 and GSK3β were significantly increased in the liver of LGKO compared to the corresponding proteins in the liver of WT (Fig. 4D and 4E). Suppression of cyclin D and overexpression of ERα36 continued to be observed specifically in the female LGKO with HFD feeding. Activation of phosphorylated ERK1/2 was detected in the LGKO fed HFD but not in the WT fed HFD. Significant phosphorylation of JAK and STAT3 was detected in the LGKO fed HFD (Fig. 4D and 4E), indicating that the JAK-STAT pathway that modulates hepatocellular carcinogenesis was activated in response to HFD and subsequently exacerbated inflammation.
DISCUSSION
In this study we found spontaneous benign hepatic tumors in female mice with a liver-specific BiP deletion and under constitutive ER stress for more than nine months. The benign tumors had a high risk of progressing into a malignancy in mice with additional stress, e.g. high fat diet feeding and the tumors caused complications such as rupture or bleed inside the abdomen. These features indicate that the benign tumors are most likely hepatocellular adenomas (HCA).11–15 Our findings are of clinical relevance since human HCA can also lead to medical complications such as internal bleeding, liver rupture and hepatocellular carcinoma transformation, and 80–85% of HCA cases are from women taking oral contraceptives for more than two years.15,19 The pathogenesis of HCA is not completely understood due to its heterogeneity. Current known potential causes for human HCA are mutations in HNF1α, β-catenin or GP130 and chronic inflammation.11–15, 18–21 Hepatocellular stress from disruption of protein homeostasis has rarely been noticed to be a potential mechanism. Our findings may reveal a novel ER stress mechanism for HCA (Fig. 4F). First, HNF1α, β-catenin or GP130 might not be the major causes for HCA in this mouse model since significant changes for these factors were not detected in the liver of LGKO with tumors. Long-term ER stress-caused inflammation could in general be responsible for the HCA development. Second, mechanistically the in vivo inhibition of cyclin D expression upon ER stress in LGKO is consistent with an earlier study showing that activation of the UPR in mouse NIH 3T3 fibroblasts with the pharmacological ER stress-inducing agent, tunicamycin led to a decline in cyclin D and subsequent G(1) phase arrest.22–24 Cyclin D has been known to interact with cyclin dependent kinases (CDK) and other factors regulating a number of cellular functions including proliferation, senescence and tumorigenesis.25,26 Increased expression of cyclin D is usually associated with proliferation and cancer. However, a number of studies have shown a surprising lack of the correlation of increased cyclin D with proliferation in tumors.26,27 For instance, in one subtype of human breast carcinoma, cyclin D1 protein expression was absent in the noninvasive cells.28, 29 Similarly, a loss of cyclin D did not inhibit the proliferative response of mouse liver to mitogenic stimuli.30 These observations of abnormal functions of cyclin D are consistent with our results that associate cyclin D inhibition with increased proliferation in HCA of the LGKO. Third, in respect with the dominant occurrence of HCA in female LGKO, we believe that it may be due to possible malfunction of estrogen receptors in the female LGKO. This notion is supported by our observations that there was no apparent difference of ER stress response between the male and female knockouts at the same age and that few, if any, aged male knockouts developed tumors. The notion is also consistent with the known mechanistic evidence that estrogen receptors are among the factors that physically interact with cyclin D.27, 31 The proliferation and tumorigenesis may thus be a consequence of an impaired long-term interaction between the suppressed cyclin D and estrogen receptor α in the female LGKO. This is also supported by our evidence that the estrogen receptor variant ERα36 was detected specifically in the female LGKO with liver tumors. Fourth, although it is not currently clear about how ERα36 mechanistically causes or regulates hepatic tumorigenesis, overexpression of ERα36 has been linked to carcinogenesis in other systems such as breast cancer and gastric cancer.32–37 Therefore, by combining the information from the literature with our results, we propose a specific link of long-term ER stress with HCA in females. In our proposed model (Fig. 4F), a deletion of the chaperone BiP leads to constitutive ER stress, which activates GSK3β. GSK3β interacts with cyclin D and increases its ubiquitin-dependent proteolysis.38 The low levels of cyclin D may affect its interactions with ERα resulting in over-expression of the variant ERα36, which activates ERK1/238, 39 and stimulates proliferation and tumorigenesis in the liver. It should be noted that the spontaneous tumors were benign because invasive tumors require the presence and function of molecular chaperones such as BiP,2, 6 which is deleted in our mouse model. Interestingly, the high fat diet feeding might ablate the effect of BiP deletion on tumor growth restriction and augment the ER stress-induced inflammation by activating the JAK-STAT3 pathway, which promotes malignant transformation of HCA.21,40–42 The detailed upstream or downstream signaling pathways for the HFD-promoted malignancy of this animal model such as transcriptional expression of C-reactive protein (CRP) or serum amyloid A (SAA) require further investigation.
In conclusion, results from this study support a novel link of HCA with chronic ER stress, cyclin D suppression and ERα36 overexpression. High fat diet feeding may promote malignant transformation of HCA through the JAK-STAT pathway. This discovery may provide a molecular basis for preventing HCA and/or subsequent complications in women through targeting ER stress and inflammatory response.
Acknowledgments
This study is supported by US NIH grants: R01AA018846 and R01AA018612 (to C. Ji). The authors thank Dr. Lydia M. Petrovic for technical assistance in liver histopathology.
ABBREVIATIONS
- ER
endoplasmic reticulum
- UPR
unfolded protein response
- GRP78/BiP
glucose regulated protein 78
- FNH
focal nodular hyperplasia
- HCA
hepatocellular adenoma
- HCC
hepatocellular carcinoma
- Creld2
cysteine-rich with EGF-like domains 2
- HNF1α
hepatocyte nuclear factor 1α
- CRP
C-reactive protein
- ERα and β
estrogen receptor α and β
- ARα
androgen receptor α
- ERK1/2
extracellular signal-regulated protein kinases 1 and 2
- PCNA
the proliferating cell nuclear antigen
- GPC3
glypican-3
- ATF4
the transcription activator 4
- GSK3β
glycogen synthase kinase 3β
- JAK
Janus kinase
- STAT
the signal transducers and activators of transcription
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
Footnotes
CONFLICT OF INTEREST: Nothing to disclose
References
- 1.Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012;63:317–28. doi: 10.1146/annurev-med-043010-144749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang G, Yang ZQ, Zhang K. Endoplasmic reticulum stress response in cancer: molecular mechanism and therapeutic potential. Am J Transl Res. 2010;2(1):65–674. [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, Sun L, Zha W, Studer E, Gurley E, Zhou H. HIV protease inhibitors induce endoplasmic reticulum stress and disrupt barrier integrity in intestinal epithelial cells. Gastroenterology. 2010;138(1):197–209. doi: 10.1053/j.gastro.2009.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 2008;23 (Suppl 1):S16–24. doi: 10.1111/j.1440-1746.2007.05276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji C. Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int. 2012;2012:216450. doi: 10.1155/2012/216450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6(1):45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 7.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119(5):1201–15. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 2011;54(1):229–39. doi: 10.1002/hep.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kao E, Shinohara M, Feng M, Lau MY, Ji C. Human immunodeficiency virus protease inhibitors modulate Ca(2+) homeostasis and potentiate alcoholic stress and injury in mice and primary mouse and human hepatocytes. Hepatology. 2012;56(2):594–604. doi: 10.1002/hep.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji C, Kaplowitz N. Betaine decreases hyperhomocy steinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124(5):1488–99. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 11.Fauci Anthony S, Braunwald Eugene, Kasper Dennis L, Hauser Stephen L, Longo Dan L, Larry Jameson J, Loscalzo Joseph. Harrison’s principles of internal medicine. 17. Chapter 92. New York: McGraw-Hill Medical; 2008. (benign liver tumors) [Google Scholar]
- 12.Bioulac-Sage P, Taouji S, Possenti L, Balabaud C. Hepatocellular adenoma subtypes: the impact of overweight and obesity. Liver Int. 2012;32(8):1217–21. doi: 10.1111/j.1478-3231.2012.02786.x. [DOI] [PubMed] [Google Scholar]
- 13.Nault JC, Zucman-Rossi J. Genetics of hepatobiliary carcinogenesis. Semin Liver Dis. 2011;31(2):173–87. doi: 10.1055/s-0031-1276646. [DOI] [PubMed] [Google Scholar]
- 14.Katabathina VS, Menias CO, Shanbhogue AK, Jagirdar J, Paspulati RM, Prasad SR. Genetics and imaging of hepatocellular adenomas: 2011 update. Radiographics. 2011;31(6):1529–43. doi: 10.1148/rg.316115527. [DOI] [PubMed] [Google Scholar]
- 15.Lin H, van den Esschert J, Liu C, van Gulik TM. Systematic review of hepatocellular adenoma in China and other regions. J Gastroenterol Hepatol. 2011;26(1):28–35. doi: 10.1111/j.1440-1746.2010.06502.x. [DOI] [PubMed] [Google Scholar]
- 16.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125(1):89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 17.Bioulac-Sage P, Balabaud C, Zucman-Rossi J. Focal nodular hyperplasia, hepatocellular adenomas: past, present, future. Gastroenterol Clin Biol. 2010;34(6–7):355–8. doi: 10.1016/j.gcb.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Bioulac-Sage P, Cubel G, Balabaud C, Zucman-Rossi J. Revisiting the pathology of resected benign hepatocellular nodules using new immunohistochemical markers. Semin Liver Dis. 2011;31(1):91–103. doi: 10.1055/s-0031-1272837. [DOI] [PubMed] [Google Scholar]
- 19.Edmondson HA, Henderson B, Benton B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294(9):470–2. doi: 10.1056/NEJM197602262940904. [DOI] [PubMed] [Google Scholar]
- 20.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44(6):694–8. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142(2):219–32. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brewer JW, Hendershot LM, Sherr CJ, Diehl JA. Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proc Natl Acad Sci USA. 1999;96(15):8505–10. doi: 10.1073/pnas.96.15.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci USA. 2000;97(23):12625–30. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamanaka RB, Bennett BS, Cullinan SB, Diehl JA. PERK and GCN2 contribute to eIF2 alpha phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Mol Biol Cell. 2005;16(12):5493–501. doi: 10.1091/mbc.E05-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JK, Diehl JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol. 2009;220(2):292–6. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11(8):558–72. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 27.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145(12):5439–47. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 28.Oyama T, Kashiwabara K, Yoshimoto K, Arnold A, Koerner F. Frequent overexpression of the cyclin D1 oncogene in invasive lobular carcinoma of the breast. Cancer Res. 1998;58(13):2876–80. [PubMed] [Google Scholar]
- 29.Shoker BS, Jarvis C, Davies MP, Iqbal M, Sibson DR, Sloane JP. Immunodetectable cyclin D(1) is associated with oestrogen receptor but not Ki67 in normal, cancerous and precancerous breast lesions. Br J Cancer. 2001;84:1064–1069. doi: 10.1054/bjoc.2001.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ledda-Columbano GM, Pibiri M, Concas D, Cossu C, Tripodi M, Columbano A. Loss of cyclin D1 does not inhibit the proliferative response of mouse liver to mitogenic stimuli. Hepatology. 2002;36(5):1098–105. doi: 10.1053/jhep.2002.36159. [DOI] [PubMed] [Google Scholar]
- 31.Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88(3):405–15. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
- 32.Vranic S, Gatalica Z, Deng H, Frkovic-Grazio S, Lee LM, Gurjeva O, Wang ZY. ER-α36, a novel isoform of ER-α66, is commonly over-expressed in apocrine and adenoid cystic carcinomas of the breast. J Clin Pathol. 2011;64(1):54–7. doi: 10.1136/jcp.2010.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhri RA, Olivares-Navarrete R, Cuenca N, Hadadi A, Boyan BD, Schwartz Z. Membrane estrogen signaling enhances tumorigenesis and metastatic potential of breast cancer cells via estrogen receptor-α36 (ERα36) J Biol Chem. 2012;287(10):7169–81. doi: 10.1074/jbc.M111.292946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Li J, Fang R, Xie S, Wang L, Xu C. Expression of ERα36 in gastric cancer samples and their matched normal tissues. Oncol Lett. 2012;3(1):172–175. doi: 10.3892/ol.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang XT, Ding L, Kang LG, Wang ZY. Involvement of ER-α36, Src, EGFR and STAT5 in the biphasic estrogen signaling of ER-negative breast cancer cells. Oncol Rep. 2012;27(6):2057–65. doi: 10.3892/or.2012.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhri RA, Olivares-Navarrete R, Cuenca N, Hadadi A, Boyan BD, Schwartz Z. Membrane estrogen signaling enhances tumorigenesis and metastatic potential of breast cancer cells via estrogen receptor-α36 (ERα36) J Biol Chem. 2012;287(10):7169–81. doi: 10.1074/jbc.M111.292946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miceli V, Cocciadiferro L, Fregapane M, Zarcone M, Montalto G, Polito LM, et al. Expression of wild-type and variant estrogen receptor alpha in liver carcinogenesis and tumor progression. OMICS. 2011;15(5):313–7. doi: 10.1089/omi.2010.0108. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi-Yanaga F, Sasaguri T. GSK-3beta regulates cyclin D1 expression: a new target for chemotherapy. Cell Signal. 2008;20(4):581–9. doi: 10.1016/j.cellsig.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Lin SL, Yan LY, Zhang XT, Yuan J, Li M, Qiao J, et al. ER-alpha36, a variant of ER-alpha, promotes tamoxifen agonist action in endometrial cancer cells via the MAPK/ERK and PI3K/Akt pathways. PLoS One. 2010;5(2):e9013. doi: 10.1371/journal.pone.0009013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2012 doi: 10.1038/onc.2012.347. [DOI] [PubMed] [Google Scholar]
- 41.Constantinescu SN, Girardot M, Pecquet C. Mining for JAK-STAT mutations in cancer. Trends Biochem Sci. 2008;33(3):122–31. doi: 10.1016/j.tibs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 42.He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21(1):159–68. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]