Abstract
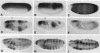
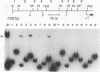
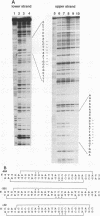
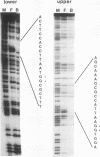
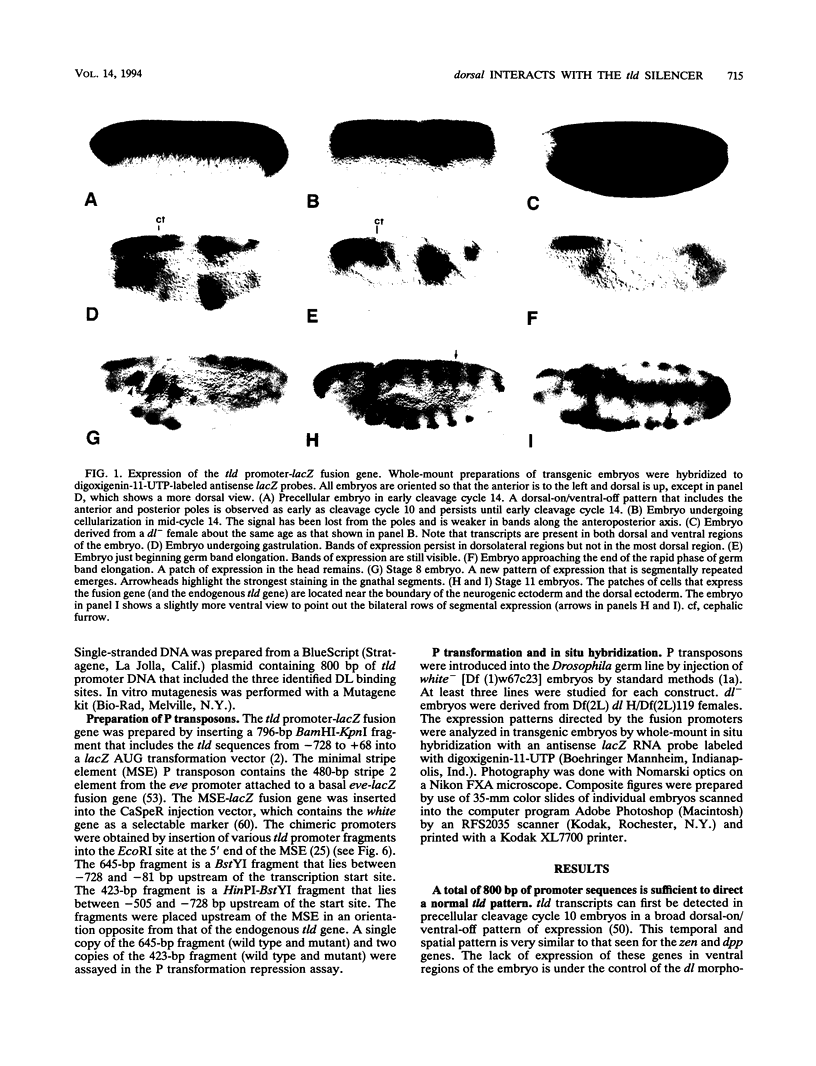
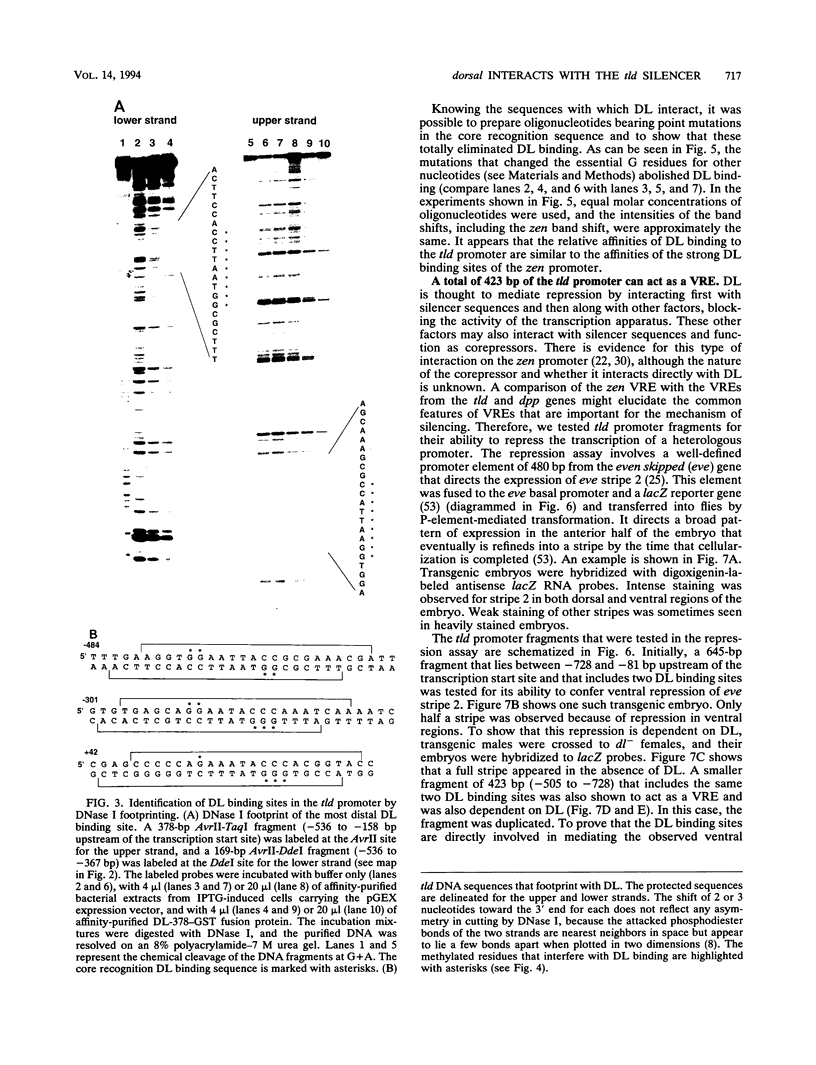
The dorsal protein (DL) regulates the transcriptional activity of several genes that determine cell fate along the dorsoventral axis of the Drosophila melanogaster embryo. DL is present at high levels in ventral nuclei, where it activates some genes (twi and sna) and represses others (zen, dpp, and tld). DL shows homology to the Rel family of proteins and interacts with specific DNA sequences in the regulatory regions of its target genes. The distal portion of the zen gene acts as a silencer that can mediate the repression of a heterologous promoter in ventral regions of the embryo. It contains four DL binding sites which alone are sufficient for activation but not repression. Here we analyze the interaction of DL with another one of its repressed targets, the tolloid (tld) gene. Approximately 800 bp of 5'-flanking sequences upstream of the tld coding region were shown to drive an expression pattern indistinguishable from the wild-type pattern. A 423-bp fragment located within these sequences contains two DL binding sites and was shown to act as a silencer to mediate ventral repression. Point mutations in the sites abolish not only DNA binding but also ventral repression. We discuss a comparison of the DNA sequences from the zen and tld promoters and the possible mechanisms of transcriptional silencing.
Full text
PDF


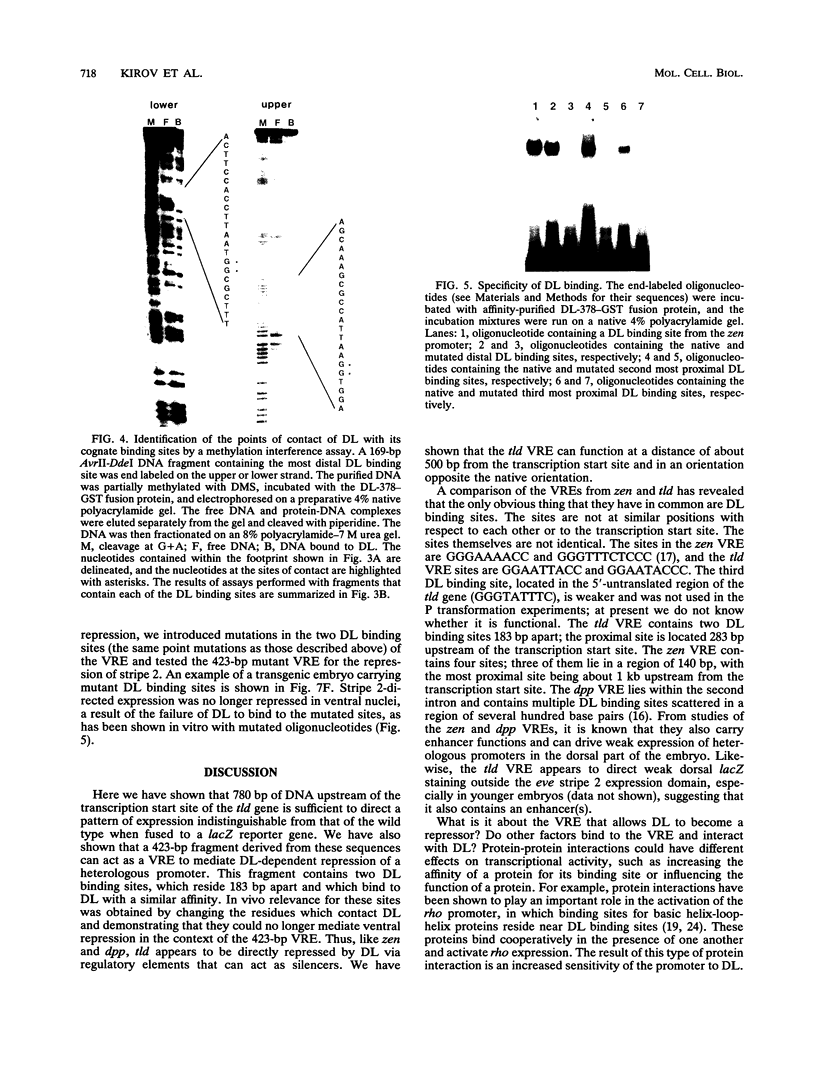
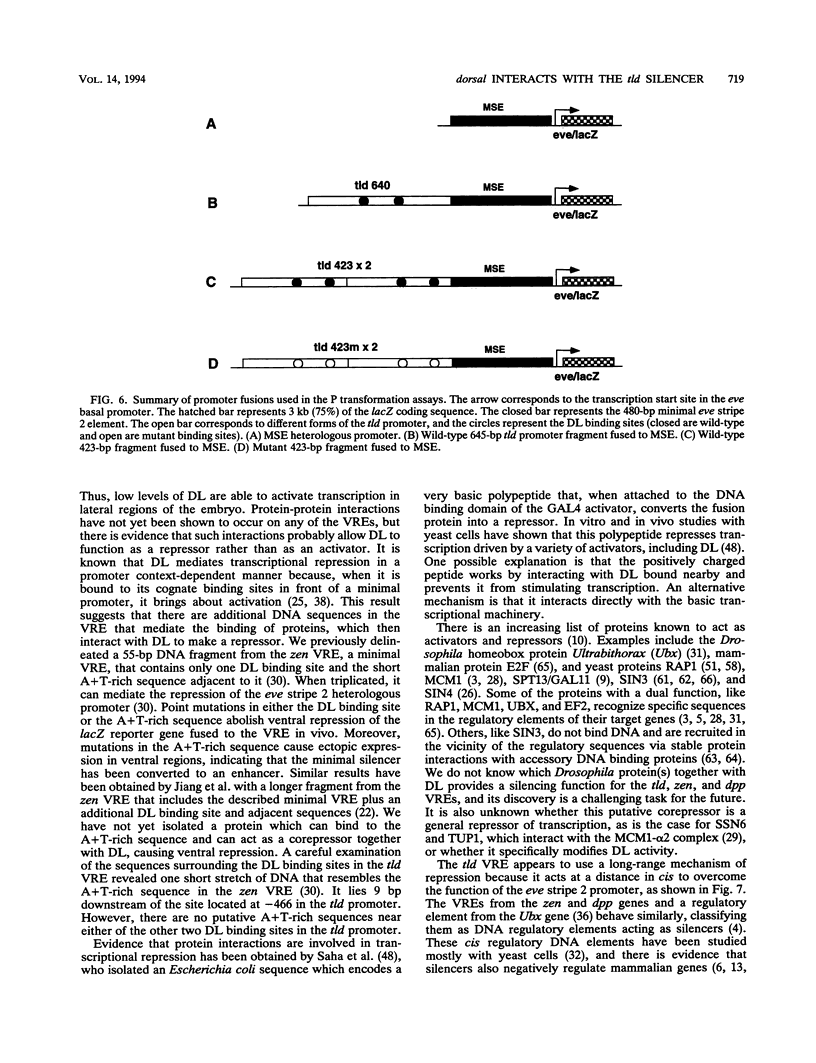



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender A., Sprague G. F., Jr MAT alpha 1 protein, a yeast transcription activator, binds synergistically with a second protein to a set of cell-type-specific genes. Cell. 1987 Aug 28;50(5):681–691. doi: 10.1016/0092-8674(87)90326-6. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Breeden L., Abraham J., Sternglanz R., Nasmyth K. Characterization of a "silencer" in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985 May;41(1):41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Buchman A. R., Kimmerly W. J., Rine J., Kornberg R. D. Two DNA-binding factors recognize specific sequences at silencers, upstream activating sequences, autonomously replicating sequences, and telomeres in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jan;8(1):210–225. doi: 10.1128/mcb.8.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S. X., Gutman P. D., Dave H. P., Schechter A. N. Identification of a transcriptional silencer in the 5'-flanking region of the human epsilon-globin gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5306–5309. doi: 10.1073/pnas.86.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle H. J., Kraut R., Levine M. Spatial regulation of zerknüllt: a dorsal-ventral patterning gene in Drosophila. Genes Dev. 1989 Oct;3(10):1518–1533. doi: 10.1101/gad.3.10.1518. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA bending and its relation to nucleosome positioning. J Mol Biol. 1985 Dec 20;186(4):773–790. doi: 10.1016/0022-2836(85)90396-1. [DOI] [PubMed] [Google Scholar]
- Fassler J. S., Winston F. The Saccharomyces cerevisiae SPT13/GAL11 gene has both positive and negative regulatory roles in transcription. Mol Cell Biol. 1989 Dec;9(12):5602–5609. doi: 10.1128/mcb.9.12.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes N. S., Sassone-Corsi P. More is better: activators and repressors from the same gene. Cell. 1992 Feb 7;68(3):411–414. doi: 10.1016/0092-8674(92)90178-f. [DOI] [PubMed] [Google Scholar]
- Govind S., Steward R. Dorsoventral pattern formation in Drosophila: signal transduction and nuclear targeting. Trends Genet. 1991 Apr;7(4):119–125. doi: 10.1016/0168-9525(91)90456-z. [DOI] [PubMed] [Google Scholar]
- Govind S., Whalen A. M., Steward R. In vivo self-association of the Drosophila rel-protein dorsal. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7861–7865. doi: 10.1073/pnas.89.17.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch M., Gerwin N., Taubert H., Jäckle H. Competition for overlapping sites in the regulatory region of the Drosophila gene Krüppel. Science. 1992 Apr 3;256(5053):94–97. doi: 10.1126/science.1348871. [DOI] [PubMed] [Google Scholar]
- Hofmann J. F., Laroche T., Brand A. H., Gasser S. M. RAP-1 factor is necessary for DNA loop formation in vitro at the silent mating type locus HML. Cell. 1989 Jun 2;57(5):725–737. doi: 10.1016/0092-8674(89)90788-5. [DOI] [PubMed] [Google Scholar]
- Huang J. D., Schwyter D. H., Shirokawa J. M., Courey A. J. The interplay between multiple enhancer and silencer elements defines the pattern of decapentaplegic expression. Genes Dev. 1993 Apr;7(4):694–704. doi: 10.1101/gad.7.4.694. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Kraut R., Levine M., Rushlow C. A. The dorsal morphogen is a sequence-specific DNA-binding protein that interacts with a long-range repression element in Drosophila. Cell. 1991 Jan 25;64(2):439–446. doi: 10.1016/0092-8674(91)90651-e. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Park R. E., Kosman D., Bier E., Levine M. The dorsal gradient morphogen regulates stripes of rhomboid expression in the presumptive neuroectoderm of the Drosophila embryo. Genes Dev. 1992 Sep;6(9):1728–1739. doi: 10.1101/gad.6.9.1728. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Park R. E., Kosman D., Yazdanbakhsh K., Levine M. dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 1992 Aug;6(8):1518–1530. doi: 10.1101/gad.6.8.1518. [DOI] [PubMed] [Google Scholar]
- Isoda K., Roth S., Nüsslein-Volhard C. The functional domains of the Drosophila morphogen dorsal: evidence from the analysis of mutants. Genes Dev. 1992 Apr;6(4):619–630. doi: 10.1101/gad.6.4.619. [DOI] [PubMed] [Google Scholar]
- Jiang J., Cai H., Zhou Q., Levine M. Conversion of a dorsal-dependent silencer into an enhancer: evidence for dorsal corepressors. EMBO J. 1993 Aug;12(8):3201–3209. doi: 10.1002/j.1460-2075.1993.tb05989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Kosman D., Ip Y. T., Levine M. The dorsal morphogen gradient regulates the mesoderm determinant twist in early Drosophila embryos. Genes Dev. 1991 Oct;5(10):1881–1891. doi: 10.1101/gad.5.10.1881. [DOI] [PubMed] [Google Scholar]
- Jiang J., Levine M. Binding affinities and cooperative interactions with bHLH activators delimit threshold responses to the dorsal gradient morphogen. Cell. 1993 Mar 12;72(5):741–752. doi: 10.1016/0092-8674(93)90402-c. [DOI] [PubMed] [Google Scholar]
- Jiang Y. W., Stillman D. J. Involvement of the SIN4 global transcriptional regulator in the chromatin structure of Saccharomyces cerevisiae. Mol Cell Biol. 1992 Oct;12(10):4503–4514. doi: 10.1128/mcb.12.10.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Scheidereit C., Roeder R. G. Identification and purification of a human immunoglobulin-enhancer-binding protein (NF-kappa B) that activates transcription from a human immunodeficiency virus type 1 promoter in vitro. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4700–4704. doi: 10.1073/pnas.85.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleher C. A., Goutte C., Johnson A. D. The yeast cell-type-specific repressor alpha 2 acts cooperatively with a non-cell-type-specific protein. Cell. 1988 Jun 17;53(6):927–936. doi: 10.1016/s0092-8674(88)90449-7. [DOI] [PubMed] [Google Scholar]
- Keleher C. A., Redd M. J., Schultz J., Carlson M., Johnson A. D. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell. 1992 Feb 21;68(4):709–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- Kirov N., Zhelnin L., Shah J., Rushlow C. Conversion of a silencer into an enhancer: evidence for a co-repressor in dorsal-mediated repression in Drosophila. EMBO J. 1993 Aug;12(8):3193–3199. doi: 10.1002/j.1460-2075.1993.tb05988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow M. A., Saffman E. E., Kornfeld K., Hogness D. S. Transcriptional activation and repression by Ultrabithorax proteins in cultured Drosophila cells. Cell. 1989 Jun 16;57(6):1031–1043. doi: 10.1016/0092-8674(89)90341-3. [DOI] [PubMed] [Google Scholar]
- Laurenson P., Rine J. Silencers, silencing, and heritable transcriptional states. Microbiol Rev. 1992 Dec;56(4):543–560. doi: 10.1128/mr.56.4.543-560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Kuang A., Gifford A., Baltimore D. NF-kappa B protein purification from bovine spleen: nucleotide stimulation and binding site specificity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8825–8829. doi: 10.1073/pnas.85.23.8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Müller J., Bienz M. Long range repression conferring boundaries of Ultrabithorax expression in the Drosophila embryo. EMBO J. 1991 Nov;10(11):3147–3155. doi: 10.1002/j.1460-2075.1991.tb04876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. J., Huang J. D., Courey A. J. Functional analysis of the Drosophila twist promoter reveals a dorsal-binding ventral activator region. Genes Dev. 1991 Oct;5(10):1892–1901. doi: 10.1101/gad.5.10.1892. [DOI] [PubMed] [Google Scholar]
- Pan D., Courey A. J. The same dorsal binding site mediates both activation and repression in a context-dependent manner. EMBO J. 1992 May;11(5):1837–1842. doi: 10.1002/j.1460-2075.1992.tb05235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., Gifford A. M., Baltimore D. Silencing of the expression of the immunoglobulin kappa gene in non-B cells. Mol Cell Biol. 1991 Mar;11(3):1431–1437. doi: 10.1128/mcb.11.3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R. P., Arora K., Nüsslein-Volhard C., Gelbart W. M. The control of cell fate along the dorsal-ventral axis of the Drosophila embryo. Development. 1991 Sep;113(1):35–54. doi: 10.1242/dev.113.1.35. [DOI] [PubMed] [Google Scholar]
- Roth S., Stein D., Nüsslein-Volhard C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell. 1989 Dec 22;59(6):1189–1202. doi: 10.1016/0092-8674(89)90774-5. [DOI] [PubMed] [Google Scholar]
- Rushlow C. A., Han K., Manley J. L., Levine M. The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell. 1989 Dec 22;59(6):1165–1177. doi: 10.1016/0092-8674(89)90772-1. [DOI] [PubMed] [Google Scholar]
- Rushlow C., Arora K. Dorsal ventral polarity and pattern formation in the Drosophila embryo. Semin Cell Biol. 1990 Jun;1(3):137–149. [PubMed] [Google Scholar]
- Rushlow C., Doyle H., Hoey T., Levine M. Molecular characterization of the zerknüllt region of the Antennapedia gene complex in Drosophila. Genes Dev. 1987 Dec;1(10):1268–1279. doi: 10.1101/gad.1.10.1268. [DOI] [PubMed] [Google Scholar]
- Rushlow C., Frasch M., Doyle H., Levine M. Maternal regulation of zerknüllt: a homoeobox gene controlling differentiation of dorsal tissues in Drosophila. Nature. 1987 Dec 10;330(6148):583–586. doi: 10.1038/330583a0. [DOI] [PubMed] [Google Scholar]
- Rushlow C., Warrior R. The rel family of proteins. Bioessays. 1992 Feb;14(2):89–95. doi: 10.1002/bies.950140204. [DOI] [PubMed] [Google Scholar]
- Saha S., Brickman J. M., Lehming N., Ptashne M. New eukaryotic transcriptional repressors. Nature. 1993 Jun 17;363(6430):648–652. doi: 10.1038/363648a0. [DOI] [PubMed] [Google Scholar]
- Saksela K., Baltimore D. Negative regulation of immunoglobulin kappa light-chain gene transcription by a short sequence homologous to the murine B1 repetitive element. Mol Cell Biol. 1993 Jun;13(6):3698–3705. doi: 10.1128/mcb.13.6.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimell M. J., Ferguson E. L., Childs S. R., O'Connor M. B. The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell. 1991 Nov 1;67(3):469–481. doi: 10.1016/0092-8674(91)90522-z. [DOI] [PubMed] [Google Scholar]
- Shore D., Nasmyth K. Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell. 1987 Dec 4;51(5):721–732. doi: 10.1016/0092-8674(87)90095-x. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Small S., Blair A., Levine M. Regulation of even-skipped stripe 2 in the Drosophila embryo. EMBO J. 1992 Nov;11(11):4047–4057. doi: 10.1002/j.1460-2075.1992.tb05498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston R. D., Gelbart W. M. Decapentaplegic transcripts are localized along the dorsal-ventral axis of the Drosophila embryo. EMBO J. 1987 Sep;6(9):2785–2791. doi: 10.1002/j.1460-2075.1987.tb02574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanojević D., Hoey T., Levine M. Sequence-specific DNA-binding activities of the gap proteins encoded by hunchback and Krüppel in Drosophila. Nature. 1989 Sep 28;341(6240):331–335. doi: 10.1038/341331a0. [DOI] [PubMed] [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987 Oct 30;238(4827):692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- Steward R. Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell. 1989 Dec 22;59(6):1179–1188. doi: 10.1016/0092-8674(89)90773-3. [DOI] [PubMed] [Google Scholar]
- Sussel L., Shore D. Separation of transcriptional activation and silencing functions of the RAP1-encoded repressor/activator protein 1: isolation of viable mutants affecting both silencing and telomere length. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7749–7753. doi: 10.1073/pnas.88.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C., Perrin-Schmitt F., Stoetzel C., Thisse B. Sequence-specific transactivation of the Drosophila twist gene by the dorsal gene product. Cell. 1991 Jun 28;65(7):1191–1201. doi: 10.1016/0092-8674(91)90014-p. [DOI] [PubMed] [Google Scholar]
- Thummel C. S., Boulet A. M., Lipshitz H. D. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988 Dec 30;74(2):445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Vidal M., Strich R., Esposito R. E., Gaber R. F. RPD1 (SIN3/UME4) is required for maximal activation and repression of diverse yeast genes. Mol Cell Biol. 1991 Dec;11(12):6306–6316. doi: 10.1128/mcb.11.12.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Clark I., Nicholson P. R., Herskowitz I., Stillman D. J. The Saccharomyces cerevisiae SIN3 gene, a negative regulator of HO, contains four paired amphipathic helix motifs. Mol Cell Biol. 1990 Nov;10(11):5927–5936. doi: 10.1128/mcb.10.11.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Stillman D. J. In vitro regulation of a SIN3-dependent DNA-binding activity by stimulatory and inhibitory factors. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9761–9765. doi: 10.1073/pnas.87.24.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Stillman D. J. Transcriptional repression in Saccharomyces cerevisiae by a SIN3-LexA fusion protein. Mol Cell Biol. 1993 Mar;13(3):1805–1814. doi: 10.1128/mcb.13.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S. J., Prater C. A., Dean D. C. Retinoblastoma protein switches the E2F site from positive to negative element. Nature. 1992 Jul 16;358(6383):259–261. doi: 10.1038/358259a0. [DOI] [PubMed] [Google Scholar]
- Yoshimoto H., Ohmae M., Yamashita I. The Saccharomyces cerevisiae GAM2/SIN3 protein plays a role in both activation and repression of transcription. Mol Gen Genet. 1992 May;233(1-2):327–330. doi: 10.1007/BF00587597. [DOI] [PubMed] [Google Scholar]