Abstract
Introduction
Pyruvate dehydrogenase kinase (PDK) is activated in right ventricular hypertrophy (RVH), causing an increase in glycolysis relative to glucose oxidation that impairs RV function. The stimulus for PDK upregulation, its isoform specificity and the long-term effects of PDK inhibition are unknown. We hypothesize that FOXO1-mediated PDK4 upregulation causes bioenergetic impairment and RV dysfunction, which can be reversed by dichloroacetate.
Methods
Adult male Fawn-Hooded rats (FHR) with pulmonary arterial hypertension (PAH) and RVH (age 6–12 months) were compared to age-matched controls. Cardiac glucose and fatty acid oxidation (GO, FAO) were measured at baseline and after acute dichloroacetate (1mM×40-minutes) in isolated working-hearts and in freshly dispersed RV myocytes. The effects of chronic dichloroacetate (0.75 g/L drinking water for 6 months) on cardiac output (CO) and exercise capacity were measured in vivo. Expression of PDK4 and its regulatory transcription factor, FOXO1, were also measured in FHR and RV specimens from PAH patients (n=10).
Results
Microarray analysis of 168 genes related to glucose or FA metabolism showed >4-fold upregulation of PDK4, aldolase B and acyl-coenzyme A oxidase. FOXO1 was increased, in FHR RV whereas HIF-1α was unaltered. PDK4 expression was increased and the inactivated form of FOXO1 decreased in human PAH RV (P<0.01). PDH inhibition in RVH increased proton production and reduced GO’s contribution to the TCA cycle. Acutely, dichloroacetate reduced RV proton production and increased GO’s contribution (relative to FAO) to the TCA cycle and ATP production in FHR (P<0.01). Chronically dichloroacetate decreased PDK4 and FOXO1, thereby activating PDH and increasing GO in FHR. These metabolic changes increased CO (84±14 vs. 69±14 ml/min, P<0.05) and treadmill-walking distance (239±20 vs. 171±22 m, P<0.05).
Conclusion
Chronic dichloroacetate inhibits FOXO1-induced PDK4 upregulation and restores GO, leading to improved bioenergetics and RV function in RVH.
Keywords: glycolysis, HIF-1α, Aldolase B, FOXO3, acyl-coenzyme A oxidase 2
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a lethal disease characterized by pulmonary vascular obstruction and a progressive increase in pulmonary arterial pressure[1]. Although the primary insult is in the pulmonary vasculature, the leading cause of death in PAH is right ventricular (RV) failure[2, 3]. Chronic RV pressure overload stimulates right ventricular hypertrophy (RVH) and can ultimately lead to RV failure. While regression of pulmonary vascular disease remains the ultimate therapeutic goal, recent animal data suggests that metabolic derangements in the RV contribute to RV dysfunction[4, 5]. Moreover, several of these metabolic abnormalities are amenable to therapeutic intervention.
A conserved abnormality in RVH is increased activity and expression of pyruvate dehydrogenase kinase (PDK). PDK activation inhibits pyruvate dehydrogenase (PDH), decreasing the contribution of glucose oxidation to acetyl CoA production by the tricarboxylic acid (TCA) cycle and causing a bioenergetically disadvantageous increase in glycolysis, relative to glucose oxidation[5]. Such metabolic changes occur in rodent models of pulmonary hypertension (induced by monocrotaline or chronic hypoxia[6, 7]) and in models of RV pressure overload, induced by pulmonary artery banding[4]. Interestingly a similar PDK-mediated increase in glycolysis relative to glucose oxidation (or “glycolytic shift”) occurs in the pulmonary vasculature in PAH[8, 9]. The glycolytic phenotype appears to be maladaptive since dichloroacetate, a small molecular inhibitor of all 4 PDK isoforms[10], improves RV function, regresses pulmonary vascular disease, and increases exercise capacity in these experimental models[2, 7].
However, many questions regarding RV metabolism remain unanswered, three of which are addressed in the current study. First, the isoform specificity of RV PDK activation is uncertain. There are four PDK isoforms[11] and PDK4 is the predominant isoform in heart and skeletal muscle[12]. There is circumstantial evidence that PDK4 is upregulated in the RV of humans with PAH[13], and that this is associated with a maladaptive glycolytic RV phenotype[14, 15]. The RV PDK isoform is relevant not only for development of specific pharmacological inhibitors, but also mechanistically, because the PDK isoforms have different transcriptional regulation. HIF-1α causes transcriptional activation of PDK1 by interaction with the hypoxia response element (HRE) in its promoter[16]. In contrast, the promoter of PDK4 has glucocorticoid receptor (GR) binding sites, as well as binding sites for the forkhead transcription factor (FOXO1) and the estrogen related receptor (ERRα). Interestingly, elegant evidence for FOXO-1-mediated PDK4 activation comes from recent studies of the left ventricle in diabetic cardiomyopathy[17]. This cardiomyopathy results in part from microvascular ischemia and capillary rarefaction, a pathogenic mechanism that has been suggested to occur in PAH-associated RVH[18]. While the stimulus for PDK activation in the lung in PAH is redox-mediated activation of HIF-1α[8, 19], the stimulus in the RV is unknown. Finally, although short-term PDK inhibition is beneficial in many RVH models, including the fawn hooded rat (FHR)[2], therapy has not been extended beyond 1 month[5].
We chose to investigate the isoform specificity of PDK changes in RVH and evaluate the role of FOXO1 in increasing PDK expression in the FHR, a mutant strain which is unique in spontaneously developing PAH and RVH with age[20, 21]. FHR are an appealing model because the disease develops gradually and is not the result of a toxic pharmacological stimulus (as is the case with monocrotaline-induced PAH). FHR have a mitochondrial-metabolic abnormality in the lung circulation characterized by fragmented mitochondria that are deficient in ETC complex 1 and have a glycolytic shift in metabolism[2, 8]; however, the role of altered metabolism in the function of the RV in FHR has not been assessed.
We use complementary, quantitative metabolic techniques in LV working hearts and isolated RV myocytes to measure global and RV energy metabolism, respectively. We demonstrate that depressed GO in the FHR RV is mediated by FOXO-1-associated increases in PDK4. The PDK inhibitor dichloroacetate restores PDH activity and enhances GO with beneficial molecular effects (downregulation of FOXO-1 and PDK4) and functional improvement (enhanced RV function and exercise capacity).
METHODS
Experimental protocols
The Institutional Animal Care and Use Committees at the University of Chicago and the University of Alberta approved the protocols performed at the respective institutions. In the acute dichloroacetate treatment experiments, in which dichloroacetate (1mM) was administered for 40 minutes in isolated working hearts, two groups of 10–20 month-old rats were used: female FHR versus age-matched female Sprague-Dawley rats (as Control). In the chronic dichloroacetate treatment experiments, in which oral dichloroacetate was administered in the drinking water for 6 months (0.75g/L), three groups of 1-year old male and female rats were used: Brown Norway rats (as Control), FHR drinking water, and FHR with chronic dichloroacetate treatment (FHR+Dichloroacetate).
In Vivo Measurements of Cardiac Structure and Function
RVH was measured as the ratio of RV/(LV+septum) weight. For histology, frozen sections of RV (10μm thick) were stained with hematoxylin and eosin and photographed using a Zeiss Axiophot and Leaf Microlumina digital camera (Thornwood, NY).
Echocardiography and cardiac catheterization
Echocardiography (to measure RV function) and cardiac catheterization (to measure cardiac output, RV systolic pressure (RVSP) and tricuspid annular plane systolic excursion (TAPSE) were performed as described previously[22] (also see online supplement).
Energy Metabolism Measurements in Isolated Working Hearts
The measurement of metabolism and cardiac function in an isolated LV working heart preparation as described previously[5]. Briefly, the heart was rapidly excised and subsequently mounted on an aortic cannula and perfused in Langendorff mode (60mmHg) for approximately 10 min. Next the left atrium was cannulated and heart was placed into working mode. The hearts were set to work against an 80mmHg aortic afterload and a 11.5mmHg preload. The left side of the heart accounts for the majority of the heart’s metabolism. Therefore, we used the LV working heart model to assess global cardiac metabolism. To measure glycolysis and GO, the heart was perfused with Krebs–Henseleit buffer containing 11mM [5-3H/U-14C] glucose, 0.8mM [9,10-3H/1-14C]palmitic acid, 3% albumin, and 100μU/ml insulin. The effects of dichloroacetate on GO and glycolysis were evaluated at 10 minute intervals after adding 1mM dichloroacetate to the perfusate by the measurement of 3H2O (glycolysis marker) and 14CO2 (GO marker) using a Tri-Carb 2800TR liquid scintillation analyzer (PerkinElmer, Waltham, MA). In separate experiments FAO was measured by perfusing hearts under identical conditions, except that [1-14C]palmitate was used instead of the radiolabelled glucose. The measurement of cardiac output and cardiac work in this ex vivo model are described in the Supplemental Material.
Myocardial Energetics
Non-oxidative glycolytic metabolism of pyruvate yields lactate and ATP. If the ATP is hydrolyzed, there is a net production of 2H+ per molecule of glucose. However, if glycolysis is coupled to GO, there is no net production of protons. Therefore, the difference between the rates of glycolysis and GO multiplied by 2 yields the overall rate of proton production from glucose utilization. Total ATP production was calculated from each of the metabolic rates for glycolysis, glucose and palmitate oxidation using the following values based on phosphorous/oxygen ratios: 2 mol ATP per mol glucose undergoing glycolysis, 31 mol ATP per mol of glucose oxidized and 105 mol ATP per mol palmitate oxidized. TCA cycle production of acetyl CoA was calculated from glucose and palmitate oxidation rates assuming oxidation of 1 mol glucose yields 2 mol acetyl CoA and 1 mol palmitate produces 8 mol acetyl CoA.
Metabolism in freshly dispersed RV myocytes
The steps for isolation of RV myocytes and metabolic measurements have been described previously[4] (see online supplement). Briefly, enzymatically dispersed RV myocytes were plated into 24-well plates (2 × 104/plate; Seahorse Bioscience, Billerica, MA) and incubated in XF assay medium at 37°C for 2 hours, with or without dichloroacetate (5 mM). The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), a measure of lactate production, were measured using the Seahorse XF24 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA). OCR reflects either GO, when conducted with 5 mM glucose as the substrate, or fatty acid oxidation (FAO), when conducted with 1.2 mM palmitic acid as the substrate. ECAR represents glycolysis with 5 mM glucose in the medium. Metabolic data were normalized to total protein.
Metabolic gene expression profiles of GO and FAO
RNA was isolated from RV tissues from control, FHR and FHR+Dichloroacetate groups using RNeasy® Mini Kit (Qiagen, Valencia, CA) and cDNA was reverse transcribed using RT2 First Strand Kit (Qiagen, Valencia, CA), following the manufacturer’s instructions. cDNA (25μl) was mixed with RT2 Master Mix (Qiagen, Valencia, CA) and loaded into 96-well PCR array plates (Qiagen, Valencia, CA) to investigate the glucose and fatty acid metabolic pathways (84 genes each).
PDH activity, quantitative RT-PCR, Immunostaining and Western blotting
These techniques were performed as previously described[5] and are outlined in the online supplement.
Human tissue microarrays
Immunostaining was done on RV cores (1 mm in diameter) from formalin-fixed, paraffin-embedded archival material of autopsied PAH patients (n=10) or age-matched non-PAH patients (n=8). Samples were prepared on a tissue microarray. For patient demographics, see on-line supplement (Supplemental Table 3). The institutional review board was notified of research on deceased individuals.
After antigen retrieval (95–100°C for 30 min in citrate buffer), human RV tissue microarrays were incubated overnight at 4°C with anti-PDK4-AR (1:200, Abcam; Cambridge, MA) or anti-phospho-FOXO1 Thr 24 (1:200, Cell Signaling, Danvers, MA). Anti-dystrophin (1:100, Abcam) was used to identify the myocytes plasma membrane.. Subsequent processing was the same as for rat RV. The images were captured on a Zeiss LSM META using 20X and 100x 1.46 NA objective.
Statistics
Values were expressed as mean ± standard error, except for the PCR array data which are expressed as mean ± standard deviation (using Qiagen PCR Array Online Software, http://sabiosciences.com/pcrarraydataanalysis.php). Inter-group differences were assessed by ANOVA with post hoc testing using Fisher’s least significant difference test. A paired Student’s t-test was used for assessing the acute effects of dichloroacetate at baseline versus 40 minutes of perfusion. An unpaired, two-tailed t-test was used to determine the differences between FHR and FHR with chronic dichloroacetate treatment. A P<0.05 was considered statistically significant.
RESULTS
General
FHR had PAH, evident from their shorter PAAT corrected for heart rate (FHR 0.08±0.01 vs. Control 0.11±0.01 ms/bpm, P<0.05. Supplemental Fig 1A) and RVH (RV/LV+septum ratio: FHR 0.30±0.02 vs. Control 0.23±0.01, P<0.05, Supplemental Fig 1B). FHR also had lower heart rates (FHR: 279±11 vs. Control: 313±19 bpm, P>0.05).
Acute effects of dichloroacetate treatment on the RV
In the isolated working hearts, GO was reduced in FHR vs. Control (431±73 vs. 1030±174 nmol/min/g dry weight, P<0.05, Fig 1A). Addition of dichloroacetate (1mM) increased GO in FHR and Control (to 1551±141 and 1729±67 nmol/min/g dry weight, respectively, P<0.01 for each, Fig 1A).
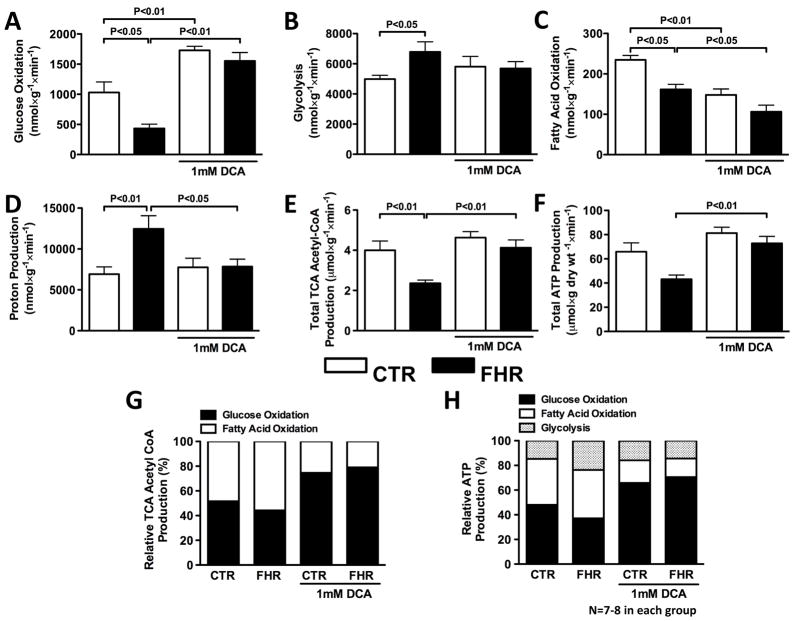
Fig 1. Acute dichloroacetate improves RV metabolism in FHR ex vivo.
In an isolated LV working heart preparation: A. GO is reduced in FHR versus CTR and infusion of dichloroacetate (DCA) (1mM for 40 minutes) increases GO in both CTR (age-matched Sprague-Dawley rats) and FHR. B. Glycolysis is increased in FHR and dichloroacetate tends to reduce this. C. FAO is reduced in FHR hearts. Dichloroacetate further decreases FAO in FHR and CTR. D. Proton production from uncoupled glucose metabolism, which is increased in FHR, is decreased by dichloroacetate. E&F. Total TCA cycle acetyl CoA production and ATP production are reduced in FHR hearts, and are increased by dichloroacetate. G. Dichloroacetate increases the TCA cycle acetyl CoA produced by GO in both CTR and FHR. H. ATP production from GO, which is reduced in FHR, is increased by dichloroacetate.
Glycolysis rates were increased in FHR vs. Control (to 6793±665 vs. 4987±248 nmol/min/g dry weight, P<0.05, Fig 1B). Dichloroacetate tended to reduce glycolysis in FHR, but this did not reach statistical significance (Fig 1B). The increase in glycolytic activity relative to GO resulted in an increased proton production in FHR vs. Control (to 12,452±1612 vs. 6,959±862 nmol/min/g dry weight, P<0.01, Fig 1D). Dichloroacetate reduced proton production in FHR (P<0.05) while having no effect in Control (Fig 1D).
FAO was reduced in FHR vs. Control (161±13 vs. 234±11 nmol/min/g dry weight, P<0.001, Fig 1C). Dichloroacetate further reduced FAO (to 106±17 nmol/min/g dry weight in FHR (P<0.01, Fig 1E) and 148±15 nmol/min/g dry weight in Control, P<0.01, Fig 1C).
Dichloroacetate normalized energetics in FHR. Total TCA cycle acetyl CoA production was reduced in FHR vs. Control (2.3±0.2 vs. 4.0±0.5 nmol/min/g dry weight, P<0.01, Fig 1E). Dichloroacetate did not alter TCA cycle acetyl CoA production in Control, but significantly increased TCA cycle acetyl CoA production in FHR (Fig 1E). Consistent with these findings, total ATP production was decreased in FHR vs. Control (43.1±3.6 vs. 66.6±7.4 nmol/min/g dry weight) and dichloroacetate treatment in FHR increased ATP production to 72.9±5.7 nmol/min/g dry weight (P<0.01, Fig 1F).
The contribution ratio of GO vs. FAO to TCA cycle acetyl CoA production was reduced from 52%/48% in Control to 44%/56% in FHR (Fig 1G). This ratio was increased by dichloroacetate to 75%/25% in Control and 79%/21% in FHR, and these changes were complemented by reductions in FAO contribution to TCA cycle acetyl CoA production (Fig 1G).
The contribution ratio of GO/FAO/glycolysis to total ATP production was 48%/37%/15% in Control whereas in FHR the ratios reflect a fall in the relative importance of GO versus glycolysis (37%/39%/24%). Dichloroacetate increased the contribution of GO to ATP production in Control (GO/FAO/glycolysis: 66%/18%/16%) and in FHR (GO/FAO/glycolysis: 70%/15%/15%) (Fig 1H). The dichloroacetate-induced increase in reliance on GO came at the expense of FAO, the contribution of which was significantly reduced (Fig 1H).
Theses results demonstrate that in RVH, there is a global increase in glycolysis and reduction in glucose oxidation. This indicates that, at least in FHR, the metabolic changes are not restricted to the RV (although subsequent data from dispersed RV myocytes directly confirm the involvement of the RV).
Chronic effects of dichloroacetate treatment on the RV in FHR
RVH and RV Failure
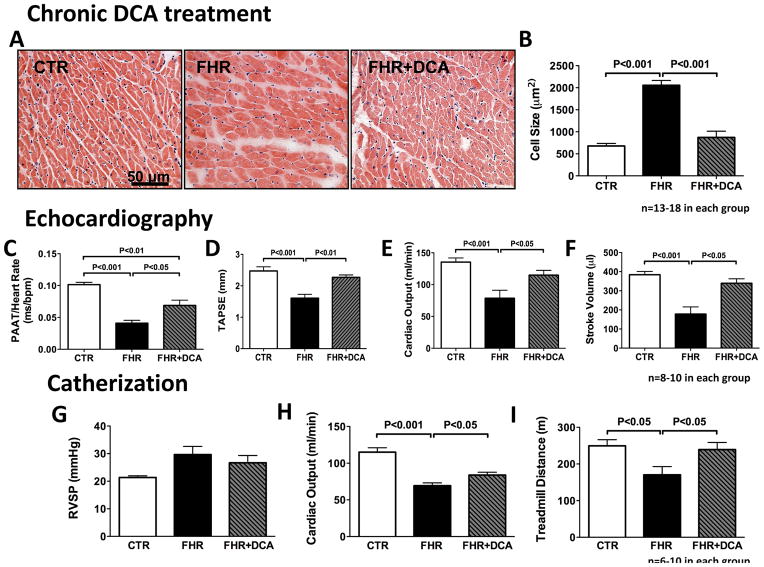
Histology showed RVH and RV myocyte hypertrophy in FHR versus Control (age-matched Brown Norway rats). Dichloroacetate treatment in FHR for 6 months reversed RVH (Fig 2A&B). Echocardiography confirmed that chronic dichloroacetate treatment reduced PH (Fig 2C) and improved cardiac function in FHR (Fig 2D-F). Dichloroacetate treatment increased PAAT corrected for heart rate in FHR (P<0.01, Fig 2C), consistent with a reduction of PH. The reduced TAPSE, a measure of RV function, was reduced in FHR and was increased by chronic dichloroacetate treatment (Fig 2D). Consistent with improved RV function, CO was increased from 79±13 (FHR) to 115±7.6 ml/min (FHR+Dichloroacetate), largely due to increased stroke volume (which increased from 178±37 to 339±23 μl in FHR, P<0.01, Fig 2E&F). There were no differences in heart rate between groups.
Fig 2. Chronic dichloroacetate treatment reverses RVH in FHR in vivo.
A&B. Histology and the statistical bar graph show that the RV myocytes hypertrophy in FHR is reduced by chronic dichloroacetate treatment (6 months of dichloroacetate 0.75g/l of drinking water). C. The reduced PAAT corrected for HR in FHR is increased by dichloroacetate treatment, reflecting a decrease in pulmonary hypertension. D–F. TAPSE, CO and stroke volume (SV), which are reduced in FHR, are increased by dichloroacetate. G. RVSP tends to increase in FHR and is unchanged by dichloroacetate. H. The reduced cardiac output measured by cardiac catheterization in FHR is increased in FHR+DCA. I. The reduced treadmill waking distance seen in FHR versus CTR is increased by DCA treatment.
Dichloroacetate also resulted in a statistically insignificant reduction in right ventricular systolic pressure (Fig 2G). Cardiac catheterization results were consistent with echocardiography measurements. The FHR had reduced CO (FHR: 69±14 vs. Control: 115±16 ml/min, P<0.05, Fig 2H) and treadmill capacity (FHR: 171±22 vs. Control: 249±17 m, P<0.05, Fig 2I), which were significantly increased in the FHR+Dichloroacetate group (to CO of 84±14 ml/min and treadmill capacity of 239±20 m, P<0.05 vs. FHR, Fig 2H&I).
Cardiomyocyte metabolism
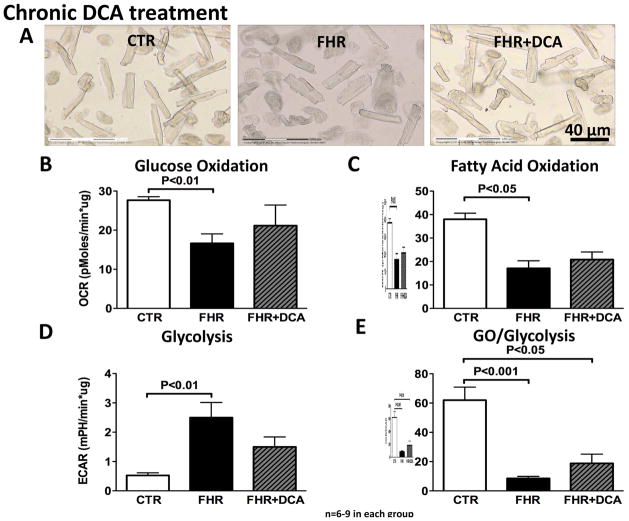
Using the Seahorse XF Extracellular Flux Analyzer to measure metabolism in freshly dispersed RV myocytes (Fig 3A), GO and FAO were reduced whereas glycolysis was increased in FHR (P<0.01, P<0.05 and P<0.01 vs. Control, Fig 3B–E). This confirms the results from the isolated working hearts and reflects the metabolic shift in metabolism in myocytes, rather than some other cardiac cell type. The GO/glycolysis ratio, which was reduced in FHR RV myocytes (P<0.001 vs. Control, Fig 3D), tended to increase with dichloroacetate, although these trends did not reach statistical significance (Fig 3B–E). FAO did not change significantly with dichloroacetate (Fig 3C).
Fig 3. Metabolic dysfunction in isolated RV myocytes in FHR.
A. Images of freshly isolated RV cardiomyocytes from CTR, FHR and FHR+DCA rats. B&C. GO and FAO is reduced in RV myocytes in FHR. D. Glycolysis is increased in FHR and dichloroacetate tends to reduce glycolysis in RV myocytes. E. GO/glycolysis is reduced in FHR and dichloroacetate tends to increase this ratio in isolated RV myocytes.
Metabolic Expression Profiles
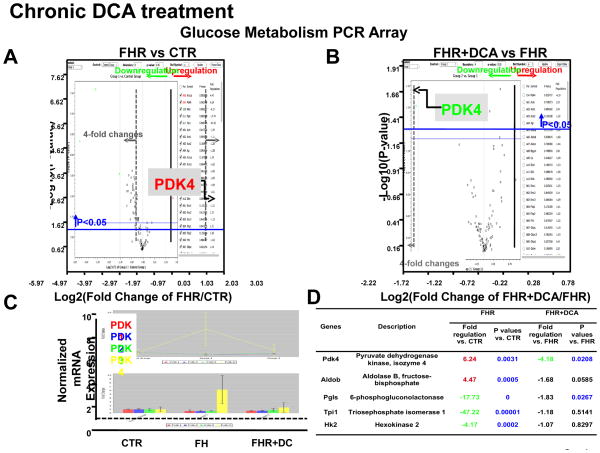
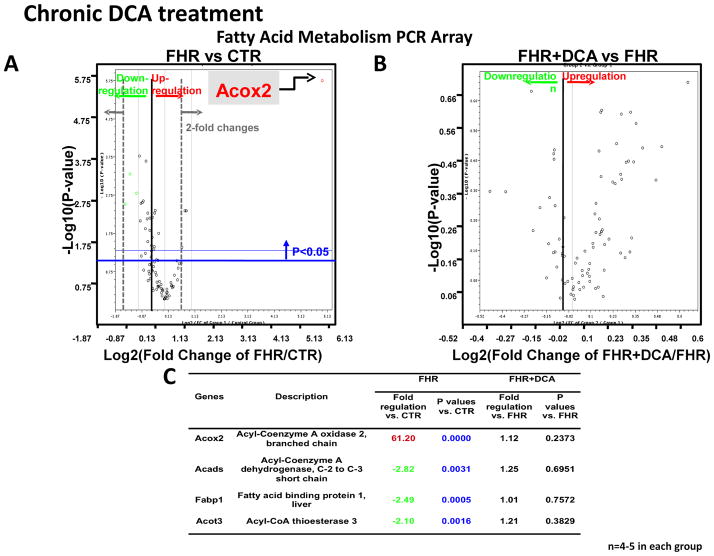
PCR array results revealed that among 84 glucose metabolism-related genes (Supplemental Table 1) the most upregulated genes were PDK4 (6.24-fold increase) (which phosphorylates and inhibits PDH), and aldolase B (4.47-fold increase), a key glycolytic enzyme required for conversion of 6-carbon, fructose-6-phosphate into 3-carbon, glyceraldehyde-3-phosphates (P<0.01, P<0.001, respectively for FHR vs Control RV, Fig 4A). Moreover, dichloroacetate treatment normalized expression of PDK4 (but not PDK1-3) and aldolase B mRNA (P<0.05, P=0.06, Fig 4B–D). Thirty-eight glucose metabolism-related genes were down-regulated and two other genes were up-regulated in the FHR RV (P<0.05); however, dichloroacetate did not normalize these genes (Supplemental Table 1). The expressions of the remaining 42 genes were unchanged in both FHR and FHR+Dichloroacetate groups.
Fig 4. Expression profiling of genes related to glucose metabolism highlights the dysregulation of PDK4 in FHR.
A. Volcano plot from rat glucose metabolism PCR Array shows PDK4 mRNA is increased in FHR RV. B&C. Chronic dichloroacetate treatment normalizes PDK4 mRNA in FHR. D. The table shows genes that significantly changed (4-fold) in FHR.
Among 84 fatty acid metabolism-related genes, acyl-coenzyme A oxidase 2 and fatty acid binding protein 3 were increased to 61.2-fold and 1.56-fold (Fig 5A). Twenty-five genes were down-regulated and two other genes were up-regulated (P<0.05 vs. Control), whereas 57 genes were unchanged in the FHR RV (Supplemental Table 2). Dichloroacetate did not change the expression of any of the fatty acid metabolism-related genes including fatty acid binding proteins, fatty acid transporters Slc27 family, and carnitine palmitoyltransferase (CPT) family in FHR (Supplemental Table 2, Fig 5B–C, Supplemental Fig 2). Although acute DCA did decrease FAO in working heart setting, chronic DCA didn’t change FAO in RV myocytes. Moreover, chronic DCA didn’t alter the expressions of FAO-related genes such as FA binding proteins and FA transporters. Thus, the changes in response to chronic dichloroacetate in RVH appear largely related to genes controlling glucose metabolism.
Fig 5. Chronic dichloroacetate treatment does not change fatty acid metabolism gene expression profile.
Volcano plot for rat fatty acid metabolism shows: A. Increased acyl-coenzyme A oxidase 2 mRNA in FHR RV. B. Dichloroacetate did not change expression of fatty acid metabolism-related genes in FHR. D. The genes that were significantly changed (2-fold) in FHR are listed.
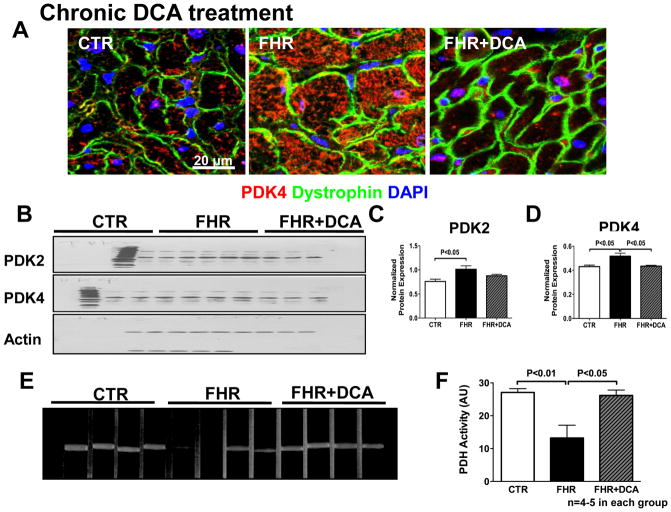
PDK expression and PDH activity
Immunostaining and Western blotting results demonstrated that PDK2 and PDK4 protein was upregulated in FHR RV myocytes. Dichloroacetate reduced the expression of PDK4 protein and tended to reduce PDK2 protein (Fig 6A-D). In contrast, PDK1 and PDK3 were not significantly up-regulated in RVH and remained unchanged by dichloroacetate (Supplemental Fig 3A–C). Expression of the glucose transporter, Glut1, was increased in RV in FHR and chronic dichloroacetate therapy reduced Glut1 expression (Supplemental Fig 3D–E). PDH activity, which was depressed in the FHR, was increased by chronic dichloroacetate treatment (P<0.05, Fig 6E&F). This result following 6 months of dichloroacetate therapy is consistent with the improved glucose oxidation observed with acute, ex vivo dichloroacetate treatment.
Fig 6. Pyruvate dehydrogenase activity is increased by chronic dichloroacetate treatment in FHR RV.
In rats treated with dichloroacetate or control water for 6 months: A. Immunostaining shows that the increased PDK4 protein expression (red) is reduced by dichloroacetate. Dystrophin (green) is used to mark the myocytes membrane and nuclei are stained with DAPI (blue). B&D. Representative immunoblot and mean data normalized to actin expression shows that both PDK2 and PDK4 are increased in the FHR RV. Dichloroacetate decreases PDK4 expression and tends to decrease PDK2 expression. E&F. Representative images and mean data showing reduced PDH activity (band density) in FHR RV. Dichloroacetate treatment increases PDH activity in the RV in FHR.
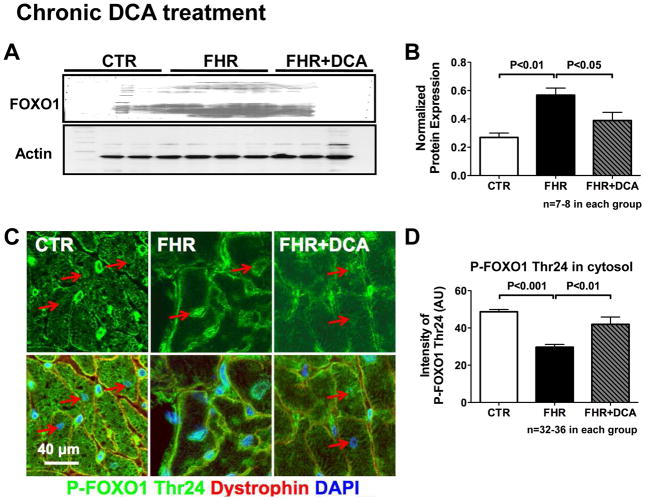
FOXO-1 expression and activity
We examined the basis for the upregulation of PDK4. mRNA level of FOXO1, the gene that increases PDK4 transcription[23], tended to be increased in FHR and dichloroacetate tended to reduced the expression of FOXO1. The expression of FOXO1 protein was significantly increased in FHR, and dichloroacetate normalized FOXO1 protein (Fig 7A&B). Although the increase in FOXO3 mRNA in FHR was reduced by dichloroacetate (Supplemental Fig 4B), the protein level of FOXO3 remained unchanged in the FHR RV (Supplemental Fig 4D–E). In addition, expression of the inactivated form of FOXO1, phospho-FOXO1 Thr24, was significantly reduced in FHR. Phosphorylation of FOXO1 at Thr24 causes export of the transcription factor to the cytosol (inactivation). In FHR RV myocytes cytosolic phospho-FOXO1 Thr24 was decreased (Fig 7C&D); however, with dichloroacetate treatment, cytosolic expression increased, consistent with FOXO1 inactivation (Fig 7C&D). The mRNA and protein expression of HIF1a remained unchanged in FHR and FHR+Dichloroacetate groups versus Control (Supplemental Fig 4C–D&F).
Fig 7. Forkhead box protein O1 (FOXO1) is increased in FHR RV.
A&B. Representative Immunoblot and mean data normalized to actin shows that FOXO1 protein is increased in FHR RV. Dichloroacetate normalizes FOXO1. C&D. Immunostaining of Phospho-FOXO1 Thr24 (an inactivated form of FOXO1) shows that cytosolic expression is reduced in FHR and is restored by chronic dichloroacetate.
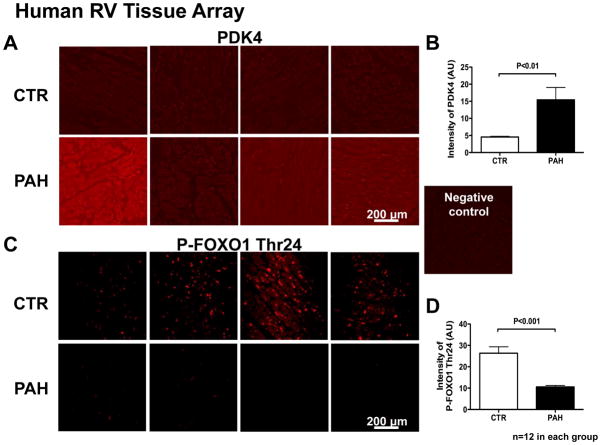
Human RV tissue microarray
The protein expression of PDK4 was upregulated in RV tissue microarray specimens in human PAH versus age-gender matched Control (Fig 8 A&B). As in the FHR, the inactive form of FOXO1, phospho-FOXO1 Thr24, was decreased in RV in PAH patients (Fig 8 C&D), suggesting an increased FOXO1 activity.
Fig 8.
PDK4 protein is increased and inactive FOXO1 is decreased in RV tissue in Human PAH.
Representative images (each panel is from 1 patient) and mean values from human RV microarray showing the upregulation of PDK4 (A&B) and downregulation of inactive FOXO1 (P-FOXO1 Thr24) (C&D) in human PAH RVs vs. age- and gender-match Control.
DISCUSSION
The study has five major findings. First, we show that impaired oxidative metabolism in RV myocytes in human and experimental RVH is caused by FOXO-1 mediated up-regulation of PDK4. Second, in experimental RVH we show that the resulting glycolytic metabolic shift is associated with RV dysfunction. Third, we show that humans with PAH-associated RVH has similar changes in FOXO1-PDK4 expression as do the FHR. Although prior studies have identified a role for PDK in pulmonary hypertension, neither the isoform specificity of the RV metabolic shift nor the role of FOXO-1 in upregulating PDK4 has been identified. Moreover, it is demonstrated that whereas FOXO1 is dysregulated in RVH there is no change in the other putative transcriptional regulator of PDK, HIF-1α or FOXO3 The fourth major finding is that dichloroacetate, an oral PDK inhibitor, results in sustained benefits to hemodynamics, RV function, and exercise capacity over a prolonged period (6 months). It is noteworthy that therapy was not begun until the FHR already manifested PAH and RVH (i.e. it regressed established disease). The fifth finding is that long-term dichloroacetate decreases the expression of FOXO1 and PDK4, correcting the pathologic metabolic pathway. This observation of therapeutic benefit builds on prior shorter studies of dichloroacetate in rodents with RVH (<1 month in duration) and clinical trials of dichloroacetate in children with inherited metabolic disorders and adults with glioblastoma multiforme[24, 25].
Compared to age-matched Control, FHR have marked increases in rates of glycolysis with a concomitant suppression of GO and FAO in the FHR RV (Fig 1 & Fig 3). One consequence of the FHR’s uncoupling of glycolysis from glucose oxidation is an excess proton production which promotes intracellular acidosis. As expected, the bioenergetic efficiency of the increased glycolytic RV metabolism was reduced in FHR, leading to reduction in total TCA cycle acetyl CoA production and ATP production (Fig 1). At the molecular level, the changes in metabolism can be attributed to increased expression of PDK4 (perhaps with some contribution from PDK2), and the resulting decrease in RV PDH activity (Fig 4&6). This metabolic profile, with excess proton production and reduced ATP availability, can impair cardiac function. Correction of the metabolic abnormalities, which is carefully documented in the current study, restores RV function, as measured by increased stroke volume and cardiac output and increases exercise capacity on a motorized treadmill (Fig 2). Importantly, we also found the upregulation of PDK4 in RV tissue microarray in PAH patients (Fig 8 A&B), which is consistent with our findings in FHR RVH.
Conserved Glycolytic Phenotypes in Models of RVH
By tracking the metabolic fate of radiolabeled glucose or palmitate in the RV working-heart model and by measuring myocyte O2-consumption and acid production, using the Seahorse XF24 Extracellular Flux Analyzer, we avoid reliance on the specificity of pharmacological inhibitors. These techniques directly confirm that RVH is associated with a switch from glucose oxidation to glycolysis, consistent with findings in other models of RVH, both with (monocrotaline) and without (PAB) PAH[4, 5]. In monocrotaline-RVH, GO is also impaired, and glycolysis, measured by the same 4H-glucose technique, is increased[5]. As in the current study of FHR, expression of Glut1 (a fetal isoform of the glucose transporter) is increased in monocrotaline-RVH, while PDH is phosphorylated and inactivated[5]. In both FHR and monocrotaline RVH, the increased Glut1 supports increased cytosolic influx of glucose required to support an energetically less efficient glycolytic metabolism (Supplemental Fig 3D–E). The reduction in PDH activity (also seen in FHR RV) inhibits GO and the resulting increase in lactate and proton production creates an acidosis that further impairs contractility. In FHR this acidosis is both calculated from isolated working heart data (as a proton excess, Fig 1D) and directly measured (from isolated FHR isolated myocytes, as an increased ECAR, Fig 3D). The potential clinical benefit of improving GO/glycolysis coupling has also been observed in RVH induced by PAB, with one important difference: rats with PAB-induced RVH have increased FAO (unlike the mild decrease in FAO in FHR) (Fig 1C& Fig 3C). In PAB-associated RVH suppression of FAO (using inhibitors of FAO, trimetazidine and ranolazine) caused a reciprocal increase in GO (a response called the “Randle Cycle”). This indirect means of increasing GO improves RV function[4, 5]. Consistent with the importance of the Randle Cycle in the therapy of RVH, the large decrease in FAO induced by acute dichloroacetate was accompanied by a large increase in GO and an improvement in RV function. Although FAO was depressed in FHR (unlike our prior PAB study where it was increased) it can be argued that it was not optimally depressed. Dichloroacetate can also inhibit FAO by blocking free fatty acid uptake through inhibitory effects on CPT1[26]. However, most of the effect of chronic dichloroacetate was on the glucose metabolic pathways and it had little effect on FAO or the expression of fatty acid metabolism-related genes (Fig 5 & Supplemental Fig 2). Fatty acid transporters and fatty acid binding proteins were unchanged in the FHR+Dichloroacetate group. Although acute DCA did decrease FAO in working heart setting, chronic DCA didn’t change FAO in FHR. Moreover, chronic DCA also didn’t alter the expressions of FAO-related genes such as FA binding proteins and FA transporters. Thus, the changes in response to chronic dichloroacetate in RVH appear largely related to genes controlling glucose metabolism.
Interestingly, we found that acyl-coenzyme A oxidase 2 is dramatically increased in RV in FHR group, although chronic dichloroacetate did not alter its expression (Fig 5). Although acyl-coenzyme A oxidase’s role in RVH is unclear, this enzyme is important in peroxisomal β-FAO. Treatment of mice with troglitazone, an antidiabetic drug, which activates peroxisome proliferator-activated receptors, causes hypertrophy and downregulates acyl-coenzyme A oxidase expression[27]. The effect of acyl-coenzyme A oxidase on cardiac metabolism and cardiac hypertrophy needs to be further determined.
Effects of Dichloroacetate Treatment on Mitochondrial Metabolism
Following acute dichloroacetate treatment in the isolated working hearts, GO is restored to rates comparable to that of control hearts, while FAO, which is reduced at baseline, is further decreased (Fig 1 & Supplemental Fig 1). The increase in GO is expected with dichloroacetate, reflecting the reactivation of PDH (demonstrated in Fig 6E–F). Multiple lines of evidence indicate that the metabolic effects of dichloroacetate are beneficial. First, proton production in FHR hearts is markedly lowered by dichloroacetate treatment. This is accompanied by increased TCA cycle acetyl-CoA production and ATP production (Fig 2), suggesting improved coupling between glycolysis and glucose oxidation. This is consistent with the reduction of Glut1 expression by dichloroacetate (Supplemental Fig 3D–E). We also observed similar beneficial effects (increased GO and ratio of GO/glycolysis) in the RV myocytes of FHR (Fig 3E) after 6 months of therapy. PDK4 mRNA and protein expression are normalized by dichloroacetate (Fig 4&6). This beneficial molecular profile after chronic dichloroacetate treatment is consistent with improved mitochondrial metabolism (Fig 6E–F), and translates to improve cardiac function in FHR in vivo (Fig 2).
Interestingly, dichloroacetate reduces the expression of aldolase B, a glycolytic enzyme (Fig 4B&D). Aldolase B catalyzes conversion of fructose 1,6-bisphosphate into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate, therefore regulating glycolysis[28]. Liu et al. have found that the expression of Glut5 (a fructose transporter) and aldolase B are increased in hypoxic aortic smooth muscle cells[29]. Aldolase B, hexokinase and phosphofructokinase are increased in fish ventricles subjected to hypoxia conditions[30]. The reduction in aldolase B by dichloroacetate may suggest the beneficial effect of dichloroacetate on GO reduce the demands on glycolytic metabolism.
PDK and Regulation of PDH
Not all PDK isoforms are perturbed in RVH. We note upregulation of predominant cardiac isoforms, PDK2 and PDK4 (Fig 4&6) while PDK1 and PDK3 are unchanged (Supplemental Fig 3). It is the upregulation of PDK2 and PDK4 that correlate with the observed extensive inhibition of cardiac PDH activity. This fingerprint (increased PDK4 but not PDK1) argues against a role for HIF-1α and favors activation of FOXO1. Consistent with this, the expression of HIF-1α, mRNA or protein, was not increased in FHR RV (Supplemental Fig 4C–D&F). In contrast, not only was the expression of FOXO1 increased in FHR RV, but also the inactivated form (phospho-FOXO1) was decreased (as judged by its cytosolic localization) in RV in both rat and PAH patients (Fig 7&8). FOXO1 increases expression of PDK4 by binding to its promoter[31]. In skeletal muscle, FOXO1 is activated within hours of starvation, leading to transcriptional upregulation of PDK4[31]. In human myocytes, FOXO1 has been shown to regulate PDK4 activity in response to dexamethasone-induced decreases in GO[23]. The inhibition of FOXO1 by dichloroacetate has also been reported to improve human skeletal muscle PDH activity after exercise[32]. These findings suggest the possibility of a feedback loop in RVH in which energy deficiency, perhaps stimulated by RV ischemia leads to activation of FOXO1 which, in turn, increases PDK4 (and PDK2) activity, thereby creating a glycolytic RV metabolic phenotype. Supporting the bi-directionality of this relationship between FOXO1 and PDK4 we report that the PDK inhibitor dichloroacetate reduces FOXO1 expression (Fig 7C&D). This feedback loop, reflecting the restoration of bioenergetics (TCA cycle acetyl CoA production and ATP production) by dichloroacetate, has not previously been identified to our knowledge.
Limitations
Although we provide evidence for increased expression of activated FOXO1 in the RV in a model of PAH, we did not directly inhibit FOXO1. The use of FOXO1 knockout mice, as recently published[17], may be helpful in future studies of regulation of PDK4 in RVH, with the caveat that murine models of PAH and RVH are not particularly robust.
Although we didn’t measure PVR, we did show PAAT in FHR is shortened and dichloroacetate normalized PAAT. Thus there are indirect beneficial effects of dichloroacetate on the lung vasculature. However, there are also direct beneficial effects (seen in the working heart model where dichloroacetate improved metabolism). Acutely DCA did not increase cardiac output and only tended to increase cardiac work. We suspect this indicates that the metabolic changes precede mechanical improvement in function, particularly as the acute dichloroacetate administration was only performed is an LV working heart model and improvements in RV function would likely only be see in an RV working heart model in this pulmonary hypertensive animal. However, the functional benefit of PDK inhibition is supported by the improvement in RV function that occurs with long-term therapy in vivo (Fig 2).
Conclusions
In conclusion, there is a maladaptive decrease in GO in the RV of FHR that impairs cardiac function. This reflects FOXO1-mediated activation of PDK4. Inhibiting PDK improves RV function and exercise capacity, suggesting a potential therapeutic role for PDK inhibitors in right ventricular hypertrophy/right ventricular failure.
Supplementary Material
Acknowledgments
This work is supported by NIH-RO1-HL071115 and 1RC1HL099462-01.
Footnotes
Disclosures: None.
References
- 1.Bichel T, Spahr-Schopfer I, Berner M, Jaeggi E, Velkovski Y, Friedli B, Kalangos A, Faidutti B, Rouge JC. Successful weaning from cardiopulmonary bypass after cardiac surgery using inhaled nitric oxide. Paediatric anaesthesia. 1997;7:335–339. doi: 10.1046/j.1460-9592.1997.d01-82.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 3.Pelouch V, Kolar F, Ost’adal B, Milerova M, Cihak R, Widimsky J. Regression of chronic hypoxia-induced pulmonary hypertension, right ventricular hypertrophy, and fibrosis: effect of enalapril. Cardiovasc Drugs Ther. 1997;11:177–185. doi: 10.1023/a:1007788915732. [DOI] [PubMed] [Google Scholar]
- 4.Fang YH, Piao L, Hong Z, Toth PT, Marsboom G, Bache-Wiig P, Rehman J, Archer SL. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle’s cycle. J Mol Med (Berl) 2012;90:31–43. doi: 10.1007/s00109-011-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, Lopaschuk GD, Archer SL. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med. 2010;88:47–60. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer SL, Wu XC, Thebaud B, Nsair A, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res. 2004;95:308–318. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- 7.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 8.Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, Morrow E, Thenappan T, Bache-Wiig P, Piao L, Paul J, Chen CT, Archer SL. Lung (1)(8)F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:670–679. doi: 10.1164/rccm.201108-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, Erzurum SC. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci U S A. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stacpoole PW. The pharmacology of dichloroacetate. Metabolism. 1989;38:1124–1144. doi: 10.1016/0026-0495(89)90051-6. [DOI] [PubMed] [Google Scholar]
- 11.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284:E855–862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- 12.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329 (Pt 1):191–196. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rich S, Pogoriler J, Husain AN, Toth PT, Gomberg-Maitland M, Archer SL. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest. 2010;138:1234–1239. doi: 10.1378/chest.09-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, Takahashi T, Nawata J, Ido T, Watanabe J, Shirato K. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol. 2005;45:1849–1855. doi: 10.1016/j.jacc.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 15.Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, Lopaschuk GD, Archer SL. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med (Berl) 2010;88:47–60. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 19.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan J, Bloch K, Archer SL. Rodent models of pulmonary hypertension: harmonisation with the world health organisation’s categorisation of human PH. International journal of clinical practice. 2011;65(Suppl 172):15–34. doi: 10.1111/j.1742-1241.2011.02710.x. [DOI] [PubMed] [Google Scholar]
- 21.Rehman J, Archer SL. A proposed mitochondrial-metabolic mechanism for initiation and maintenance of pulmonary arterial hypertension in fawn-hooded rats: the Warburg model of pulmonary arterial hypertension. Adv Exp Med Biol. 2010;661:171–185. doi: 10.1007/978-1-60761-500-2_11. [DOI] [PubMed] [Google Scholar]
- 22.Urboniene D, Haber I, Fang YH, Thenappan T, Archer SL. Validation of high-resolution echocardiography and magnetic resonance imaging vs. high-fidelity catheterization in experimental pulmonary hypertension. American journal of physiology Lung cellular and molecular physiology. 2010;299:L401–412. doi: 10.1152/ajplung.00114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puthanveetil P, Wang Y, Wang F, Kim MS, Abrahani A, Rodrigues B. The increase in cardiac pyruvate dehydrogenase kinase-4 after short-term dexamethasone is controlled by an Akt-p38-forkhead box other factor-1 signaling axis. Endocrinology. 2010;151:2306–2318. doi: 10.1210/en.2009-1072. [DOI] [PubMed] [Google Scholar]
- 24.Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, Felitsyn NM, Gilmore RL, Greer M, Henderson GN, Hutson AD, Neiberger RE, O’Brien RG, Perkins LA, Quisling RG, Shroads AL, Shuster JJ, Silverstein JH, Theriaque DW, Valenstein E. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117:1519–1531. doi: 10.1542/peds.2005-1226. [DOI] [PubMed] [Google Scholar]
- 25.Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2011;2:31ra34. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 26.Stanley WC, Hernandez LA, Spires D, Bringas J, Wallace S, McCormack JG. Pyruvate dehydrogenase activity and malonyl CoA levels in normal and ischemic swine myocardium: effects of dichloroacetate. J Mol Cell Cardiol. 1996;28:905–914. doi: 10.1006/jmcc.1996.0085. [DOI] [PubMed] [Google Scholar]
- 27.Cabrero A, Jove M, Planavila A, Merlos M, Laguna JC, Vazquez-Carrera M. Down-regulation of acyl-CoA oxidase gene expression in heart of troglitazone-treated mice through a mechanism involving chicken ovalbumin upstream promoter transcription factor II. Mol Pharmacol. 2003;64:764–772. doi: 10.1124/mol.64.3.764. [DOI] [PubMed] [Google Scholar]
- 28.Millo H, Werman MJ. Hepatic fructose-metabolizing enzymes and related metabolites: role of dietary copper and gender. J Nutr Biochem. 2000;11:374–381. doi: 10.1016/s0955-2863(00)00093-0. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Wang R, Desai K, Wu L. Upregulation of aldolase B and overproduction of methylglyoxal in vascular tissues from rats with metabolic syndrome. Cardiovasc Res. 2011;92:494–503. doi: 10.1093/cvr/cvr239. [DOI] [PubMed] [Google Scholar]
- 30.Treberg JR, MacCormack TJ, Lewis JM, Almeida-Val VM, Val AL, Driedzic WR. Intracellular glucose and binding of hexokinase and phosphofructokinase to particulate fractions increase under hypoxia in heart of the amazonian armored catfish (Liposarcus pardalis) Physiol Biochem Zool. 2007;80:542–550. doi: 10.1086/520129. [DOI] [PubMed] [Google Scholar]
- 31.Furuyama T, Kitayama K, Yamashita H, Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem J. 2003;375:365–371. doi: 10.1042/BJ20030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Constantin-Teodosiu D, Constantin D, Stephens F, Laithwaite D, Greenhaff PL. The Role of FOXO and PPAR Transcription Factors in Diet-Mediated Inhibition of PDC Activation and Carbohydrate Oxidation During Exercise in Humans and the Role of Pharmacological Activation of PDC in Overriding These Changes. Diabetes. 2012;61:1017–1024. doi: 10.2337/db11-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.