Abstract
N-acetyl-4-aminophenol (ACET) may impair musculoskeletal adaptations to progressive resistance exercise training (PRT) by inhibiting exercise-induced muscle protein synthesis and bone formation. To test the hypothesis that ACET would diminish training-induced increases in fat-free mass (FFM) and osteogenesis, untrained men (n = 26) aged ≥50 years participated in 16 weeks of high-intensity PRT and bone-loading exercises and were randomly assigned to take ACET (1,000 mg/day) or placebo (PLAC) 2 h before each exercise session. Total body FFM was measured by DXA at baseline and week 16. Serum bone-specific alkaline phosphatase (BAP) and C-terminal crosslinks of type-I collagen (CTX) were measured at baseline and week 16. Vastus lateralis muscle biopsies were performed at baseline and weeks 3 and 16 for prostanoid, anabolic, and catabolic gene expression by RT-PCR. In exercise-compliant men (ACET, n = 10; PLAC, n = 7), the increase in FFM was not different between groups (p = 0.91). The changes in serum BAP and CTX were not different between groups (p > 0.7). There were no significant changes in any of the target genes at week 3. After 16 weeks of PRT, the mRNA expressions of the anabolic marker p70S6K (p = 0.003) and catabolic marker muscle-atrophy F-box (MAFbx) (p = 0.03) were significantly reduced as compared to baseline in ACET. The mRNA expression of the prostanoids were unchanged (all p ≥ 0.40) in both groups. The administration of ACET (1,000 mg) prior to each exercise session did not impair PRT-induced increases in FFM or significantly alter bone formation markers in middle aged and older men.
Keywords: Resistance exercise, Acetaminophen, Muscle hypertrophy, Osteogenesis, Mechanotransduction, Lean tissue mass
Introduction
Progressive resistance exercise training (PRT) is recommended to older adults to forestall sarcopenia and osteoporosis (American College of Sports Medicine 1998). Musculoskeletal discomfort, including that imparted by exercise, is often treated with over-the-counter analgesics, including N-acetyl-4-aminophenol (ACET; paracetomol, acetaminophen). In contrast to non-steroidal anti-inflammatory drugs (NSAIDS), ACET has traditionally been thought to have only weak, if any, inhibitory effect on cyclooxygenase (COX) activity (Graham and Scott 2005).
COX activity is relevant to the musculoskeletal system because it controls the synthesis of prostaglandins (PGs), which play an integral role in mechanotransduction in skeletal muscle (Horsley and Pavlath 2003; Vandenburgh et al. 1995; Lai et al. 1996; Palmer 1990) and bone (Somjen et al. 1980; Tang et al. 1997). The mechanical strain imparted by exercise triggers an increase in PGE2 in bone (Thoresen et al. 1996; Murray and Rushton 1990) and PGE2 and PGF2α in muscle (Karamouzis et al. 2001; Trappe et al. 2001). Key evidence that PGs are essential in the osteogenic process came from in vivo mechanical loading experiments performed in the presence of an NSAID, which failed to generate the usual osteogenic response (Pead and Lanyon 1989). In young men, increases in skeletal muscle PGE2 and PGF2α in response to a single bout of exercise were attenuated by both the NSAID ibuprofen (IBUP; 1,200 mg/day) and ACET (4,000 mg/day) (Trappe et al. 2001) when compared with placebo. Both drugs also impaired the exercise-induced increase in fractional muscle protein synthesis (Trappe et al. 2002). The Akt (protein kinase B)/mammalian target of rapamycin (mTOR) pathway, a key regulator of muscle protein synthesis and degradation, is responsive to mechanotransduction imposed by resistance exercise (Fry et al. 2011) and, at least in animals, to PGs and ACET (Wu et al. 2009; Markworth and Cameron-Smith 2011). It is not known if PRT-induced Akt/mTOR signaling is altered by ACET in human skeletal muscle.
Accordingly, the primary aim of this study was to determine the effects of ACET on musculoskeletal adaptations to PRT in middle aged and older men. We hypothesized that PRT-induced increases in total body fat-free mass (FFM) and bone-specific alkaline phosphatase (BAP), a marker of bone formation, would be attenuated by the use of ACET compared with placebo. An exploratory aim was to compare the early and late exercise training effects on gene expression of Akt/mTOR signaling intermediates in skeletal muscle of men treated with ACET or placebo.
Methods
This was a randomized, double-blinded, placebo-controlled preliminary study of the effects of ACET on the musculoskeletal responses to PRT aimed at increasing fat-free mass and stimulating bone formation. Volunteers provided written informed consent to participate and the study was approved by the Colorado Multiple Institution Review Board.
Participants
The participants were men aged 50 years or older who used ACET or NSAIDs (including low-dose aspirin) <3 days per month. Volunteers were excluded if they were regular exercisers, defined as performing moderate to vigorous resistance or weight-bearing exercise on at least 3 days per week over the previous 6 months. The other main exclusion criteria included known allergy or intolerance to ACET, moderate or severe renal impairment, chronic hepatobiliary disease, and hyperkalemia (K+ >5 mmol/L); diabetes mellitus requiring pharmacologic therapy; congestive heart failure classes III or IV; uncontrolled hypertension (resting blood pressure >150/90 mmHg); unstable cardiovascular disease; thyroid dysfunction (thyroid stimulating hormone<0.5 or >5.0 mU/L); orthopedic problems that would limit the ability to perform vigorous exercise and increase the likelihood of pain medication use; allergy to lidocaine; and the use of drugs known to alter bone metabolism, oral corticosteroids in the 6 months prior to study entry, anticoagulants, and narcotics. Participants completed a treadmill exercise stress test with monitoring of the electrocardiogram and blood pressure. Eligible volunteers (n = 26) were randomized to acetaminophen (ACET) or placebo (PLAC) treatment arms. Participants started the 16-week supervised PRT program after randomization. Liver and renal function tests were repeated at 8 and 16 weeks of the intervention for safety monitoring.
Drug intervention
The drug intervention was ACET (1,000 mg in two capsules) or PLAC (2 capsules) taken only on days of prescribed exercise. The dose of ACET, 1,000 mg, is recommended for pain relief in adults. The maximum safe over the counter dose of ACET is 4,000 mg/day. The goal for the ACET treatment arm was to have elevated circulating levels of ACET during the anabolic stimulus of the exercise. Because the time to peak serum concentration is 1–2 h and the elimination half life is 2–3 h (Sanaka et al. 1999), participants were instructed to take study drug 2 h before exercising.
ACET and PLAC capsules (Belmar Pharmacy, Lakewood, CO, USA) contained either 500 mg of acetaminophen or inactive ingredients and were identical in appearance. Participants recorded the time of dosing when they arrived at the exercise facility. The University of Colorado Hospital Research Pharmacist managed the randomization process, maintained drug intervention records, and prepared and dispensed the study drug.
Exercise training intervention
All participants engaged in a 16-week supervised PRT program. They were asked to complete a minimum of three exercise sessions per week but encouraged to complete five sessions per week. The goal of the PRT was to stimulate muscle hypertrophy and bone formation using high-intensity upper- and lower-body resistance exercises, and weight-bearing movements that generated high-bone-loading forces (e.g., jumps, stair climbing/descending), similar to that of our previous study in young women (Kohrt et al. 2010). Each exercise session (Table 1) began with a warm-up treadmill walk or jog, followed by three sets of resistance exercises, two sets of jumps, and one set of stair climbing/descending performed in circuits, and concluded with a cool-down treadmill walk or jog. During the first four sessions, participants were familiarized with the equipment, proper exercise form, and record keeping. Thereafter, the first 1-repetition maximum strength test was completed and subsequent exercise was prescribed accordingly. The first set of resistance training was performed at 60–70 % of the one-repetition maximum (1-RM; 8–12 repetitions) and the remaining sets at 80 % of the 1-RM (5–8 repetitions). When a participant could complete more than eight repetitions of an exercise with proper form in sets 2 and 3, the weight was increased by approximately 5 and 10 % for upper and lower-body exercises, respectively. To facilitate the safe execution of resistance exercise on consecutive days, two plans of exercises were alternated (plan A: lateral pull down, bench press, hip abduction and adduction, biceps curls, seated row, and assisted chin ups; plan B: overhead press, leg press, triceps extension, knee extension and flexion, heel raise, and shoulder external rotation). The number of jumps and stair flights was increased progressively. For example, 10 repetitions of jumps were performed in week 1 and then increased by 2 repetitions every 2 weeks. The jump patterns comprised combinations of forward, backward, lateral, and diagonal movements to provide novel strain exposure to bone (Kohrt et al. 2004). Stair climbs began with eight flights and were increased by two flights every 2 weeks. Exercise sessions were superised by appropriately trained research assistants. Participants recorded their performance on a log page with the prescribed exercise for the corresponding exercise session. Upon completion of each session, the research assistant reviewed the log pages. Exercise training data were later entered into electronic databases equipped with error detection signals (e.g., entries outside a preset limit).
Table 1.
Exercise training protocol (3–5 days per week for 16 weeks)
| Order of components | |
| Warm-up treadmill walk/jog | 10 min, ≥3.0 mph, 0–5 % grade |
| Jump set #1 | ≥10 repetitions |
| Resistance set #1 (warm up)a | 65–70 % 1-RM, 8–12 repetitions |
| Stair climbing/descending | ≥4 flights |
| Resistance set #2a | 80 % 1-RM, 5–8 repetitions |
| Resistance set #3a | 80 % 1-RM, 5–8 repetitions |
| Jump set #2 | ≥10 repetitions |
| Cool-down treadmill walk/jog | 10 min, ≥3.0 mph, 0–5 % grade |
Resistance exercises were three sets of lateral pull down, bench press, hip abduction and adduction, biceps curls, seated row, and assisted chin ups (Plan A); or overhead press, leg press, triceps extension, knee extension and flexion, heel raise, and shoulder external rotation (Plan B). Plans A and B exercises were performed on alternate days (e.g., Plan A on Monday, Plan B on Tuesday and so on)
Body composition and BMD
At baseline and after 16 weeks of PRT, FFM and fat mass were measured by total body dual-energy X-ray absorptiometry (DXA) using a Hologic Discovery W instrument (version 12.6; Hologic, Inc., Bedford, MA, USA). In our laboratory, the coefficients of variation (CVs) for FFM and fat mass are (mean ± SD) 1.2 ± 0.8 and 1.8 ± 0.9 %, respectively. Calibration procedures included spine phantom scans daily, whole-body phantom scans three times per week, air scans once a week, and tissue bar scans once a month. Lumbar spine (L1–L4) and proximal femur (total, neck, trochanter, and shaft) scans were used to determine bone mineral density (BMD) T scores for screening purposes. Men with T scores ≤−2.5 were advised to consult their primary care provider before continuing with the study.
Bone markers
Serum BAP and C-terminal crosslinks of type-I collagen (CTX) were measured by ELISA (Quidel Corporation, San Jose, CA, USA and Immunodiagnostics Systems, Fountain Hills, AZ, USA, respectively). Fasted (at least 8 h) morning blood samples were obtained before training began and during week 16 of PRT. Samples were processed and then stored at −80 °C. The Clinical and Translational Research Center Core laboratory intra- and inter-assay CVs are 2.3 ± 0.1 and 8.0 ± 3.1 % for BAP, and 5.7 ± 3.8 and 5.6 ± 3.3 % for CTX. All samples for an individual were analyzed in batch.
Muscle strength
Maximal muscle strength was evaluated as the 1RM [the maximal weight that can be lifted only 1 time using correct lifting procedure through the full range of motion (American College of Sports Medicine 2010)] for several upper- and lower-body exercises. The initial 1RM testing took place after the 4th exercise session to ensure that participants were familiar with the equipment and movements. The 1RM tests were repeated during week 16.
Muscle biopsy
Percutaneous samples of the vastus lateralis muscle were obtained after an overnight fast at baseline and in weeks 3 and 16 of PRT. Participants were instructed to avoid exercise the morning of the biopsy procedure. In preparation for the biopsies at weeks 3 and 16, exercise sessions were completed on each of the two preceding days. The time elapsed between the last exercise bout and the biopsy was 12–24 h. The assessment in week 3 was similar to the approach used by Schulte and Yarasheski (2001) to demonstrate that short-term resistance exercise increases muscle protein synthesis in elderly adults. After cleansing the area, 1 % lidocaine (without epinephrine) was injected under the skin. A 3- to 5-mm incision was made in the skin and fascia over the belly of the vastus lateralis and ~100 mg of muscle tissue was removed under suction with a Bergstrom biopsy needle. The tissue specimens were blotted, immediately immersed in liquid nitrogen, and then stored at −80 °C.
Quantitative RT-PCR
Total RNA was extracted from powdered muscle specimens using the Qiagen RNeasy Fibrous Tissue Mini Kit (Qiagen Inc., Valencia, CA, USA). RNA concentration was determined using a Nanodrop 1000 Spectrophotometer system (Thermo Scientific, Wilmington, DE, USA; software version 3.2.1), and RNA integrity was verified with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Total RNA (1 μg) was reverse transcribed with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA), and quantitative PCR was performed using primer sets for genes of interest and reference genes (Table 1) and iQ SYBR Supermix (Bio-Rad) following manufacturer protocols. Reactions were run in duplicate on an iQ5 real-time PCR detection system (Bio-Rad) along with a no-template control for each gene. Validation experiments were performed to demonstrate that efficiencies of target and reference genes were approximately equal. The target genes were normalized to the geometric means of ribosomal proteins 28S and L13A (RPL13A) using the comparative Ct method (Vandesompele et al. 2002). The effects of the interventions on target gene expression are described as the fold-change from baseline to 3 weeks and baseline to 16 weeks.
The target genes were PGE2 and PGF2α (prostanoid activity), positive regulators of muscle hypertrophy under conditions of mechanical strain [phosphoinositide 3-kinase (PI3K), Akt, mTOR, Ras homolog enriched in brain (Rheb), phospholipase D-1 (PLD1)] (O’Neill et al. 2009), downstream effectors of mTOR [p70S6K, eukaryotic factor 4E-binding protein-1 (4EBP-1)], and markers of catabolic activity (muscle atrophy F box (MAFbx; atrogin-1) and muscle RING-finger protein-1 (MuRF-1).
Statistical analysis
This was a preliminary study of the effects of ACET on muscle and bone adaptations to PRT. Therefore, we focused on the participants who were compliant to exercise by using a per protocol (Tables 2, 3), rather than intent-to-treat, approach to the primary analysis to demonstrate proof of concept. Compliance was defined as attending at least 80 % of the prescribed exercise sessions (≥39 total sessions).
Table 2.
Primer sequences
| Primer sequence (5′–3′) |
||
|---|---|---|
| Forward | Reverse | |
| Target genes | ||
| PGE2 | ACCTCATCAGCAAGCGACTCAAGA | AAATCAGCGAGATTCGGCTTCTGG |
| PGF2α | ATCCAGCTCCTGGCGATAATGTGT | ATTCCATGTTGCCATTCGGAGAGC |
| Akt1 | AGCACGTGTACGAGAAGAAGCTCA | TTGGTCAGGTGGTGTGATGGTGAT |
| PI3K-p85a | GGAATGTTGGAAGCAGCAACCGAA | TTTACTTCGCCGTCCACCACTACA |
| PLD1 | AGAACACATGCAGAGCTCGAAGGA | TGCGGTCATTTATGTTGGCAGAGC |
| RheB | GGGAAAGCTTTGGCAGAATCTTGG | ATCACCGAGCATGAAGACTTGCCT |
| mTOR | TTACAGGCCTGGATGGCAACTACA | TTGTGTCCATCAGCCTCCAGTTCA |
| 4EBP1 | TGTCGGAACTCACCTGTGACCAAA | TCTCAAACTGTGACTCTTCACCGC |
| p70S6K | AATTATTGGCAGCCCACGAACAAC | TCCACAGGTGTCTGAGGATTTGCT |
| MAFbx-1 | AGTCTGTGCTGGTCGGGAACATTA | ACAAAGGCAGGTCAGTGAAGGTGA |
| MuRF-1 | TGCTGGTGGAGAACATCATCGACA | TTGTGGATCCCAAACACCTTGCAC |
| Reference genes | ||
| 28S ribosomal | ACTGAGAGTGGATCCGAAAGTGGT | TCCTTTGCAGGTCCCATCTGTGTA |
| RPL13A | CCTGGAGGAGAAGAGGAAAGAGA | TTGAGGACCTCTGTGTATTTGTCAA |
PI3K phosphoinositide 3-kinase, PLD-1 phospholipase D-1, Rheb ras homolog enriched in brain, mTOR mammalian target of rapamycin, 4EBP-1 eukaryotic factor 4 binding protein-1, PGE2, PGF2α prostaglandin-E2 and -F2α, MafBx muscle atrophy F-box, MuRF-1 muscle RING-finger protein-1, RPL13A ribosomal protein L13A
Table 3.
Enrollment and baseline characteristics of compliant participants
| Placebo | Acetaminophen | p | |
|---|---|---|---|
| Randomized (n) | 13 | 13 | |
| Withdrawals (n) | 2 | 1 | |
| Completed intervention (n) | 11 | 12 | |
| Compliant to exercise (n) | 7 | 10 | |
| Age (years) | 63.3 ± 10.3 | 64.0 ± 5.8 | 0.87 |
| BMI (kg/m2) | 28.4 ± 4.6 | 28.9 ± 6.2 | 0.84 |
| Height (cm) | 172.6 ± 9.2 | 182.8 ± 7.8 | 0.02 |
| Weight (kg) | 85.4 ± 21.5 | 95.7 ± 16.0 | 0.27 |
| Fat-free mass (kg) | 60.6 ± 12.1 | 66.2 ± 6.4 | 0.23 |
| Fat mass (kg) | 24.8 ± 10.1 | 29.5 ± 11.5 | 0.40 |
| Serum BAP (U/L) | 22.7 ± 2.3 | 25.3 ± 8.8 | 0.48 |
| Serum CTX (ng/mL) | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.99 |
Compliance was defined as attending at least 80 % of exercise sessions
BAP bone-specific alkaline phosphatase, CTX C-terminal cross links of type-I collagen
Exercise performance was quantified for weeks 5–6 (early) and 14–15 (late) of PRT. For each resistance exercise, the weight lifted was summed across sets and sessions and divided by the number of repetitions to yield the average resistance. A composite upper-body exercise intensity level was calculated by summing the exercise intensity for the upper-body lifts; a composite lower-body exercise intensity level was calculated in similar fashion.
Statistical power was estimated using the changes in FFM in response to PRT in older men in our lab (unpublished data) and our study of the effects of IBU on FFM in young women (Kohrt et al. 2010). The expected difference in FFM between groups was 1.8 ± 1.0 % with a predicted increase in FFM of 3.6 ± 1.0 % in PLAC. The study was designed to achieve 96 % power at the 0.05 level with 10 men per group. Homogeneity across groups at baseline was assessed by two group t tests. The effects of ACET on changes in FFM and BAP were tested using analysis of covariance (ANCOVA). For each measure, change from baseline to 16 weeks was regressed on the baseline measurement and an indicator for treatment. RT-PCR data were evaluated by two group t tests on the fold change from baseline to 3 weeks and baseline to 16 weeks. A two-sided alpha level of 0.05 was designated for statistical significance. SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses. Data are presented as mean ± standard deviation unless otherwise specified.
Results
Of the 26 men randomized to treatment, 3 withdrew due to time constraints and 17 met the exercise compliance criterion (Table 3). In this cohort, study drug compliance was 82 ± 19 %. At 8 and 16 weeks, liver function tests were within normal range for all participants (data not shown). Resistance training intensity was not significantly different between ACET and PLAC at weeks 5–6 or 14–15 of PRT (all p > 0.4; Fig. 1). There were no significant differences (all p > 0.3) in the jumping or stair climbing/descending activity per session between the PLAC and ACET groups early or late in exercise training. The number of stair flights climbed/descended by the PLAC group progressed from 6 ± 3 to 8 ± 7 flights/session between weeks 5 and 15 of PRT. In the ACET group, 7 ± 2 and 12 ± 5 flights/session were completed at weeks 5 and 15, respectively. The number of jumps per session performed by the PLAC group progressed from 40 ± 10 to 48 ± 32 between weeks 5 and 15, whereas in this time frame the ACET group progressed from 44 ± 17 to 53 ± 46 jumps/session.
Fig. 1.
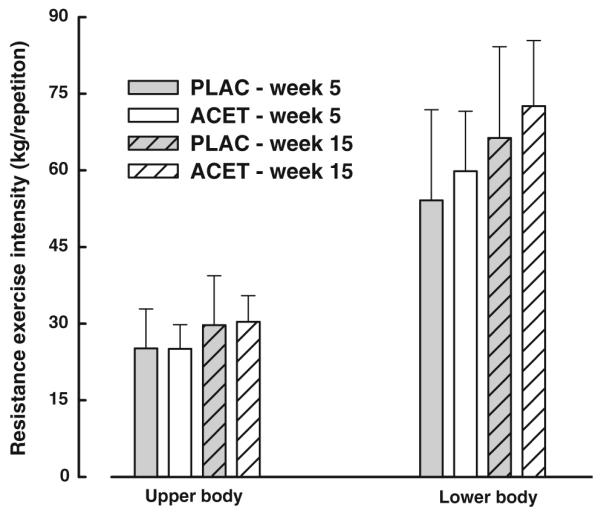
Training intensity (mean ± SD) of composite upper- and lower-body resistance exercises in the placebo (PLAC) and N-acetyl-4-aminophenol (ACET) groups in weeks 5–6 and 14–15 of the intervention
There were no significant differences in age, body composition (except for height), or serum bone markers between the groups at study entry (Table 3). Men in the ACET group had significantly greater hip abduction strength compared to PLAC at baseline but no other strength measures were significantly different between groups (Table 5). One participant in the ACET group was unable to complete all the baseline 1-RM tests due to an exacerbation of musculoskeletal pain.
Table 5.
Muscle strength (kg) before and after 16 weeks of resistance exercise in placebo and N-acetyl-4-aminophenol (ACET)-treated men
| Variable | Placebo (n = 7) |
ACET (n = 10) |
Group differencea | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 16 weeks | Differenceb | Baseline | 16 weeks | Differenceb | Difference (95 % CI) | |
| Bench press | 64.0 ± 18.0 | 77.4 ± 23.7 | 13.5 ± 10.2 | 56.6 ± 12.9 | 68.7 ± 14.4 | 12.9 ± 6.0 | 0.3 (−9.0, 9.5) |
| Overhead press | 44.5 ± 16.1 | 51.3 ± 18.1 | 6.8 ± 2.6 | 40.7 ± 11.9 | 45.7 ± 12.8 | 8.5 ± 3.4 | 2.8 (−0.1, 5.6) |
| Seated row | 56.2 ± 19.5 | 65.9 ± 15.5 | 9.7 ± 7.5 | 58.4 ± 10.6 | 69.5 ± 12.1 | 11.1 ± 6.4 | 1.8 (−5.1, 8.7) |
| Lateral pull down | 83.8 ± 23.9 | 104.2 ± 27.2 | 20.5 ± 5.6 | 85.7 ± 16.6 | 106.1 ± 17.9 | 20.5 ± 6.1 | −0.1 (−6.4, 6.1) |
| Knee flexion | 50.0 ± 18.6 | 57.1 ± 20.2 | 7.1 ± 2.0 | 56.8 ± 10.3 | 67.7 ± 12.1 | 11.1 ± 3.5 | 3.5 (0.3, 6.7) |
| Knee extension | 69.2 ± 14.9 | 86.4 ± 16.5 | 20.8 ± 7.2 | 70.7 ± 15.1 | 90.4 ± 16.5 | 19.7 ± 10.4 | −0.9 (−12.3, 10.4) |
| Leg press | 136.4 ± 21.3 | 169.8 ± 33.1 | 33.4 ± 19.0 | 138.9 ± 27.6 | 159.1 ± 37.3 | 20.2 ± 21.8 | −13.6 (−35.9, 8.6) |
| Hip abductionc | 66.2 ± 19.6 | 73.9 ± 20.4 | 7.6 ± 3.4 | 71.4 ± 10.1 | 82.0 ± 10.2 | 10.7 ± 9.8 | 3.6 (−5.0, 12.2) |
| Hip adduction | 68.2 ± 16.4 | 77.9 ± 16.1 | 9.7 ± 3.1 | 74.3 ± 9.6 | 87.5 ± 8.2 | 13.4 ± 5.2 | 4.3 (−0.6, 9.2) |
Group difference was estimated using ANCOVA, regressing 16 weeks change on baseline and group
All within-group differences were significant (p ≤ 0.02)
significantly different between groups at baseline (p = 0.04); mean ± SD
FFM increased and fat mass decreased in response to PRT (Table 4), but the changes in ACET were not significantly different from PLAC. The changes in BAP in response to PRT were in the expected opposing directions but not significantly different between groups (p = 0.72). There was no significant difference (p = 0.75) in the changes in serum CTX between PLAC and ACET (Table 4).
Table 4.
Changes (mean ± SEM) in body composition and serum bone markers in men treated with placebo (PLAC) or N-acetyl-4-aminophenol (ACET) during resistance exercise training
| PLAC (n = 7) | ACET (n = 10) | Difference (PLAC–ACET) | p | |
|---|---|---|---|---|
| Body composition | ||||
| Fat-free mass (kg) | 1.4 ± 0.7 | 1.5 ± 0.6 | −0.1 ± 0.9 | 0.91 |
| Fat mass (kg) | −0.8 ± 0.7 | −1.8 ± 0.6 | 1.0 ± 1.0 | 0.34 |
| Serum BAP (U/L) | 0.16 ± 0.83 | −0.22 ± 0.67 | 0.38 ± 1.08 | 0.72 |
| Serum CTX (ng/mL) | 0.00 ± 0.05 | −0.02 ± 0.04 | 0.02 ± 0.07 | 0.75 |
Baseline to 16 weeks
BAP bone-specific alkaline phosphatase, CTX C-terminal cross links of type-I collagen
The ACET group had significantly greater increases in knee flexion (p = 0.03), with a tendency for greater strength development for the overhead press (p = 0.06) and hip adduction (p = 0.08) (Table 5; Fig. 2). One man in the PLAC group and 6 men in the ACET group were unable to complete all 1-RM tests at week 16.
Fig. 2.
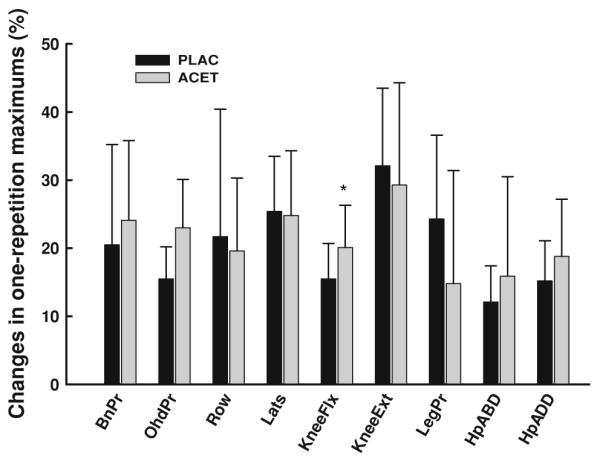
Percent changes in muscle strength (mean ± SD) during 16 weeks of progressive resistance exercise. For the between-group comparisons, *p < 0.05 BnPr bench press, OhdPr overhead press, Row seated row, Lats lateral pull-down, KneeFlx knee flexion, KneeExt knee extension, LegPr leg press, HpABD hip abduction, HpADD hip adduction
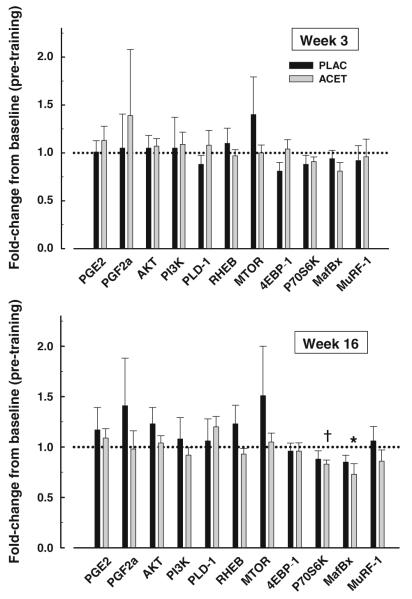
An exploratory aim was designed to measure changes in skeletal muscle gene expression in response to ACET in the early (week 3) and late (week 16) phases of the PRT. We evaluated the changes in the expression of select prostanoid, anabolic, and catabolic genes.
Expression of prostanoid genes
There were no significant changes from baseline in the expression of PGE2 and PGF2α within each treatment group or between groups at weeks 3 or 16 (Fig. 3).
Fig. 3.
Skeletal muscle expression (mean ± SEM) of target genes relative to baseline in the placebo (PLAC) and N-acetyl-4-aminophenol (ACET) groups early (week 3) and late (week 16) in the progressive resistance exercise intervention †p < 0.01 within ACET, *p < 0.05 within ACET. PGE2, PGF2α prostaglandin-E2 and -F2α, PI3K phosphoinositide 3-kinase, PLD-1 phospholipase D-1, Rheb ras homolog enriched in brain, mTOR mammalian target of rapamycin, 4EBP-1 eukaryotic factor 4 binding protein-1, MAFBx muscle atrophy F-box, MuRF-1 muscle RING-finger protein-1
Expression of anabolic genes
The expression of 4EBP-1 tended to be lower (a pro-anabolic direction) in PLAC and p70S6K lower in ACET at week 3 compared to baseline, but these changes were not significant (both p = 0.08). At week 16 in ACET, p70S6K expression was significantly (p = 0.003) lower than baseline. The expression of PLD-1 tended to increase in ACET at 16 weeks, but was not significant (p = 0.08). There was a trend for Rheb expression to be greater in PLAC compared with ACET at week 16 (p = 0.09). The increase in mTOR at weeks 3 and 16 in PLAC was not significant (p = 0.35 and p = 0.38, respectively).
Expression of catabolic genes
In ACET, MAFbx expression tended to be lower at week 3 (p = 0.06) and was significantly lower at week 16 (p = 0.03). Within PLAC, expression of MAFbx tended to be lower at week 16 (p = 0.09).
In summary, the exercise intensity was comparable in the ACET and PLAC groups. We found no evidence that ACET administered prior to each exercise session impaired the musculoskeletal responses to exercise training. The preliminary gene expression data suggest that ACET may have an effect on decreasing the skeletal muscle expression of the anabolic marker p70S6K and the catabolic marker MAFbx after 16 weeks of PRT.
Discussion
We hypothesized that an over-the-counter dose of ACET (1,000 mg) taken by middle aged and older men prior to each bout of resistance exercise would diminish the musculoskeletal adaptations to PRT when compared with adaptations in placebo-treated men. This hypothesis was based on the observations by Trappe et al. that ACET (4,000 mg/day) blunted the increases in fractional muscle protein synthesis in response to an acute bout of exercise, possibly by inhibiting an exercise-induced increase in muscle PGE2 and PGF2α (Trappe et al. 2001, 2002). In contrast to our hypothesis, we found that the increase in FFM during PRT was similar in the ACET and PLAC groups. The only other study of ACET in conjunction with exercise training was that of Trappe et al. (2011). They found that quadriceps muscle volume increased more in older women and men treated with ACET (~12 %) than in those on PLAC (~8 %) after 12 weeks of knee extension training. Trappe et al. (2011) acknowledged that the augmentation of muscle adaptations to exercise training by ACET was unexpected given their previous finding that ACET blunted the exercise-induced increase in fractional muscle protein synthesis in response to acute exercise. However, because PGs are involved in both muscle protein synthesis and breakdown (Rodemann and Goldberg 1982), it is possible that the suppression of PGs by ACET has a relatively greater effect on suppressing catabolism than anabolism during PRT, resulting in a positive muscle protein balance and increased muscle mass.
In the present study, the muscle strength responses to training were similar in the ACET and PLAC groups, with the exception of knee flexion strength which increased significantly more in ACET. Trappe et al. (2011) found greater knee extension strength increases in ACET-than PLAC-treated exercisers, although their training paradigm was targeted to the quadriceps as compared to the total body approach of the present study.
There were several differences between the current study and that of Trappe et al. (2011) that may explain why we did not find an augmentation of the training response in FFM by ACET. The dose, frequency, and timing of ACET administration differed between the two studies. We used a single 1,000 mg dose of ACET, whereas Trappe et al. used a dose of 4,000 mg/day. In the current study, drug was taken only on exercise days (average of 3 days per week), as opposed to every day, and was taken 2 h before the exercise sessions so that circulating N-acetyl-4-aminophenol would be increased during the anabolic stimulus of the exercise. This dosing regime was adapted from studies in rats, in which the administration of non-steroidal anti-inflammatory agents before (0.5–3.0 h) (Chow and Chambers 1994; Li et al. 1997) but not after, bone loading attenuated bone formation rates. It is possible that the hypertrophic effects of ACET on skeletal muscle during exercise training, as observed by Trappe et al. (2011), were related to persistent muscle turnover adaptations in response to daily ACET administration. Distinct acute and chronic PG-mediated mechanisms of bone for mation have been proposed in animal models using very high-bone loading forces in the presence of indomethacin (Chow and Chambers 1994). Finally, the current study did not include women. Sexual dimorphism in skeletal muscle protein turnover is a controversial issue. However, if older women have greater rates of muscle protein breakdown compared to men (Henderson et al. 2009) and ACET acts primarily to suppress catabolism, then gains in FFM in response to PRT may be greater in women than in men taking ACET. Further studies will be needed to determine if ACET has sex-specific effects on exercise-stimulated skeletal muscle protein turnover. Additional investigations of a dose–response and the timing of ACET dosing relative to exercise are needed to discern the independent and combined effects of these interventions on muscle size and strength in older adults.
Because 16 weeks of PRT is an insufficient timeframe for measuring changes in BMD, we measured serum BAP and CTX as surrogates of the potential effects of boneloading exercise. We found that the bone formation marker BAP increased non-significantly in the PLAC group and decreased (also non-significantly) in the ACET group, suggesting that ACET blunted some of the osteogenic effects of PRT. The changes in CTX were negligible in both groups. In a study of young women (Kohrt et al. 2010), we found that hip and spine BMD tended to decrease slightly (−0.2 to −0.4 %) over a 9-month intervention of weight-bearing endurance and resistance exercise when ibuprofen (400 mg) was taken before exercise sessions. However, when women took ibuprofen immediately after each exercise session, BMD increased by 1–2 %. One proposed mechanism by which ACET modulates bone remodeling is through the inhibition of COX activity and downstream suppression of PG synthesis (Graham and Scott 2005). Administration of exogenous PGE2 had anabolic effects on bone (Ito et al. 1993) and appeared to amplify the osteogenic effects of mechanical loading (Tang et al. 1997) in animal models. Blockade of COX using non-selective or COX-2 selective agents administered before exposure to mechanical strain attenuated bone formation responses in adult rats (Chow and Chambers 1994; Li et al. 1997) When taken immediately after exercise, ACET may enhance osteogenesis by reducing pro-inflammatory cytokines (e.g., interleukin-6) which increase several-fold after vigorous exercise (Moldoveanu et al. 2001) and have strong resorptive effects on bone (Mundy 2007). It remains to be seen if ACET taken after exercise enhances the osteogenic effects of PRT on BMD in young or older adults.
An exploratory aim of the study was to determine the changes in the expression of prostanoid, anabolic, and catabolic genes in skeletal muscle in response to PRT with and without ACET. We found in the ACET group that the expressions of the anabolic gene p70S6K and the catabolic gene MAFbx were significantly reduced at week 16 of PRT. Given that the increases in FFM in response to PRT were not significantly different between the groups, it is possible that the suppression of catabolic signaling was sufficient to offset reductions in anabolic signaling in the ACET group. However, these gene expression results should be interpreted with caution; future confirmatory protein and phospho-protein expression studies are needed to better elucidate the muscle-specific signaling mechanisms associated with ACET.
There is increasing evidence that PGs play an important role in adaptive muscle remodeling via mTOR-dependent mechanisms. In response to PGF2α exposure, myotube diameter has been shown to increase in an mTOR-dependent, Akt-independent, manner via a type-F prostanoid receptor (Markworth and Cameron-Smith 2011). PGF2α induced a rapid and transient phosphorylation of p70S6K and eIF4G in vitro; suppression of protein activation occurred when PGF2α synthesis was blocked (Markworth and Cameron-Smith 2011). Other potential PG-mediated mechanisms of muscle remodeling include promotion of myofiber growth and recruitment of myonuclei during the reloading of atrophied muscle (Bondesen et al. 2006). Although we and others have focused on prostaglandinmediated mechanisms associated with ACET, alternative mechanisms have been proposed. For example, Wu et al (2009) found that ACET normalized Akt hyperphosphorylation by reducing nitric oxide synthase (iNOS) and n-nitrosylated Akt in very old rats. In that study, ACET administration for 6 months was associated with increases in contractile protein and myocyte size and decreased myocyte apoptosis. Whether this iNOS-centered mechanism translates to human skeletal muscle and how, if at all, it interacts with mechanical strain-induced signaling (e.g., mTOR, extracellular receptor kinase 1/2) in human skeletal muscle is unknown.
This preliminary, proof-of-concept study had several limitations. The sample size was small thus limiting the ability to detect significant changes in some of the out-comes. We did not measure serum concentrations of ACET during the exercise sessions, but planned the timing of ACET administration using published pharmacokinetic profiles. Furthermore, abnormal ACET metabolism would not be expected given that we included in the study individuals with normal renal and hepatic function who did not use ACET or NSAIDs regularly. Our use of total body FFM rather than a more direct measure of skeletal muscle hypertrophy, such as muscle cross-sectional area, poses some limitation but was appropriate considering the total body training approach. The exercise training program was designed to stimulate muscle and bone anabolism but the relatively short duration of training (16 weeks) may have contributed to the variability in some outcomes (e.g., bone markers, gene expression). Dietary intake was not standardized prior to the collection of blood and tissue samples except that participants had fasted for at least 8 h. The timing of the muscle biopsies at 12–24 h after the last bout of resistance exercise may have caused us to miss the peak expression of target genes. Investigations that include protein and phospho-protein expression for prostaglandin, anabolic, and catabolic signaling pathways will be needed to elucidate the signaling mechanisms underlying the influence of ACET on skeletal muscle.
Conclusion
In summary, ACET (1,000 mg) did not attenuate the increase in FFM during 16 weeks of progressive resistance exercise training, as hypothesized. Similarly, bone turnover was not adversely affected by ACET but its effects on bone mineral density should be determined after a longer intervention. To the best of our knowledge, only the current study and Trappe et al. (2011) have evaluated the effects of ACET on musculoskeletal adaptations to exercise training and both studies involved middle aged and older adults. The administration of ACET in the recommended dose for pain relief prior to each exercise bout in the present study did not attenuate or augment the effects of PRT on FFM in middle aged men.
Acknowledgments
We sincerely thank the men who participated in this study. We greatly appreciate the technical expertise of Dr. Erin Giles and Ms. Rachel Jansen for the gene expression studies. Supported by the National Institutes of Health R21 AG027809, R01 AG018857, P30 DK048520 and Colorado Clinical Translational Science Institute UL1 TR000154. Contents are the sole responsibility of the authors and do not necessarily represent the official National Institutes of Health views. The authors report no conflicts of interest in this work.
Abbreviations
- 1RM
1-Repetition maximum
- 4EBP-1
1-Eukaryotic factor 4E-binding protein-1
- ACET
N-acetyl-4-aminophenol (paracetomol, acetaminophen)
- ANCOVA
Analysis of covariance BAP Bone-specific alkaline phosphatase
- BMD
Bone mineral density
- COX
Cyclooxygenase
- CTX
C-terminal crosslinks of type-I collagen
- CV
Coefficient of variation
- DXA
Dual-energy X-ray absorptiometry
- FFM
Fat-free mass
- IBUP
Ibuprofeni
- NOS
Nitric oxide synthase
- MAFbx
Muscle atrophy F-box mTOR Mammalian target of rapamycin
- MuRF-1
Muscle RING-finger protein-1 NSAIDS Non-steroidal anti-inflammatory drugs
- PGs
Prostaglandins
- PGE2
Prostaglandin E2
- PGF2α
Prostaglandin F2α
- PI3K
Phosphoinositide 3-kinase
- PLAC
Placebo
- PLD1
Phospholipase D-1
- PRT
Progressive resistance exercise training
- Rheb
Ras homolog enriched in brain
- RPL-13A
Ribosomal protein L13A
Footnotes
Communicated by Arnold de Haan.
Contributor Information
Catherine M. Jankowski, College of Nursing, University of Colorado Anschutz Medical Campus, Mail Stop C288-19, 13120 East 19th Avenue, Aurora, CO 80045-2527, USA; Division of Geriatric Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA
Wendolyn S. Gozansky, Division of Geriatric Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA
Paul S. MacLean, Division of Endocrinology, Metabolism and Diabetes, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA
Benjamin Shulman, Department of Preventive Medicine and Biostatistics, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA.
Pamela Wolfe, Department of Preventive Medicine and Biostatistics, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA.
Robert S. Schwartz, Division of Geriatric Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA
Wendy M. Kohrt, Division of Geriatric Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA
References
- American College of Sports Medicine Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;30:992–1008. [PubMed] [Google Scholar]
- American College of Sports Medicine . ACSM’s Guidelines for Exercise Testing and Prescription. 8th edn Lippincott Williams & Wilkins; Philadelphia: 2010. [Google Scholar]
- Bondesen BA, Mills ST, Pavlath GK. The COX-2 pathway regulates growth of atrophied muscles via multiple mechanisms. Am J Physiol Cell Physiol. 2006;290:C1651–C1659. doi: 10.1152/ajpcell.00518.2005. [DOI] [PubMed] [Google Scholar]
- Chow JW, Chambers TJ. Indomethacin has distinct early and late actions on bone formation induced by mechanical stimulation. Am J Physiol. 1994;267:E287–E292. doi: 10.1152/ajpendo.1994.267.2.E287. [DOI] [PubMed] [Google Scholar]
- Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gunderman DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1:1–11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham GG, Scott KF. Mechanism of action of paracetamol. Am J Ther. 2005;12:46–55. doi: 10.1097/00045391-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA, Jensen MD, Khosla S, O’Brien P, Nair KS. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J. 2009;23:631–641. doi: 10.1096/fj.08-117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Pavlath GK. Prostaglandin F2(alpha) stimulates growth of skeletal muscle cells via an NFATC2-dependent pathway. J Cell Biol. 2003;161:111–118. doi: 10.1083/jcb.200208085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Ke HZ, Jee WS, Sakou T. Anabolic responses of an adult cancellous bone site to prostaglandin E2 in the rat. Bone Miner. 1993;21:219–236. doi: 10.1016/s0169-6009(08)80232-2. [DOI] [PubMed] [Google Scholar]
- Karamouzis M, Karamouzis I, Vamvakoudis E, Ampatzidis G, Christoulas K, Angelopoulou N, Mandroukas K. The response of muscle interstitial prostaglandin E2 (PGE2), prosta-cycline I2 (PGI2) and thromboxane A2 (TXA2) levels during incremental dynamic exercise in humans determined by in vivo microdialysis. Prostaglandins Leukot Essent Fatty Acids. 2001;64:259–263. doi: 10.1054/plef.2001.0269. [DOI] [PubMed] [Google Scholar]
- Kohrt WM, Barry DW, Van Pelt RE, Jankowski, Wolfe P, Schwartz RS. Timing of ibuprofen use and bone mineral density adaptations to exercise training. J Bone Miner Res. 2010;25:1415–1422. doi: 10.1002/jbmr.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling YR, American College of Sports Medicine American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985–1996. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- Lai J, Jin H, Yang R, Winer J, Li W, Yen R, King KL, Zeigler F, Ko A, Cheng J, Bunting S, Paoni NF. Prostaglandin F2 alpha induces cardiac myocyte hypertrophy in vitro and cardiac growth in vivo. Am J Physiol. 1996;271:H2197–H2208. doi: 10.1152/ajpheart.1996.271.6.H2197. [DOI] [PubMed] [Google Scholar]
- Li J, Burr DB, Turner CH. Suppression of prostaglandin synthesis with NS-398 has different effects on endocortical and periosteal bone formation induced by mechanical loading. Calcif Tissue Int. 1997;70:320–329. doi: 10.1007/s00223-001-1025-y. [DOI] [PubMed] [Google Scholar]
- Markworth JF, Cameron-Smith D. Prostaglandin F2a stimulates PI3K/ERK/mTOR signaling and skeletal myotube hypertrophy. Am J Physiol Cell Physiol. 2011;300:C671–C682. doi: 10.1152/ajpcell.00549.2009. [DOI] [PubMed] [Google Scholar]
- Moldoveanu AI, Shephard RJ, Shek PN. The cytokine response to physical activity and training. Sports Med. 2001;31:115–144. doi: 10.2165/00007256-200131020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–S151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Murray DW, Rushton N. The effect of strain on bone cell prostaglandin E2 release: a new experimental method. Calcif Tissue Int. 1990;47:35–39. doi: 10.1007/BF02555863. [DOI] [PubMed] [Google Scholar]
- O’Neill TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contraction. J Physiol. 2009;587:3691–3701. doi: 10.1113/jphysiol.2009.173609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RM. Prostaglandins and the control of muscle protein synthesis and degradation. Prostaglandins Leukot Essent Fatty Acids. 1990;39:95–104. doi: 10.1016/0952-3278(90)90017-f. [DOI] [PubMed] [Google Scholar]
- Pead MJ, Lanyon LE. Indomethacin modulation of load-related stimulation of new bone formation in vivo. Calcif Tissue Int. 1989;45:34–40. doi: 10.1007/BF02556658. [DOI] [PubMed] [Google Scholar]
- Rodemann HP, Goldberg AL. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982;257:1632–1638. [PubMed] [Google Scholar]
- Sanaka M, Kuyama Y, Mineshita S, Qi J, Hanada Y, Enatsu I, Tanaka H, Makino H, Yamanaka M. Pharmacokinetic interaction between acetaminophen and lansoprazole. J Clin Gastroenterol. 1999;29:56–58. doi: 10.1097/00004836-199907000-00014. [DOI] [PubMed] [Google Scholar]
- Schulte JN, Yarasheski KE. Effects of resistance training on the rate of muscle protein synthesis in frail elderly people. Int J Sport Nutr Exerc Metab. 2001;11:S111–S118. doi: 10.1123/ijsnem.11.s1.s111. [DOI] [PubMed] [Google Scholar]
- Somjen D, Binderman I, Berger E, Harell A. Bone remodelling induced by physical stress is prostaglandin E2 mediated. Biochim Biophys Acta. 1980;627:91–100. doi: 10.1016/0304-4165(80)90126-9. [DOI] [PubMed] [Google Scholar]
- Tang LY, Cullen DM, Yee JA, Jee WS, Kimmel DB. Prostaglandin E2 increases the skeletal response to mechanical loading. J Bone Miner Res. 1997;12:276–278. doi: 10.1359/jbmr.1997.12.2.276. [DOI] [PubMed] [Google Scholar]
- Thoresen K, Kristoffersson AO, Lerner UH, Lorentzon RP. In situ microdialysis in bone tissue. Stimulation of prostaglandin E2 release by weight-bearing mechanical loading. J Clin Invest. 1996;98:2446–2449. doi: 10.1172/JCI119061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol. 2011;300:R655–R662. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe TA, Fluckey JD, White F, Lambert CP, Evans EM. Skeletal muscle PGF(2)(alpha) and PGE(2) in response to eccentric resistance exercise: influence of ibuprofen and acetaminophen. J Clin Endocrinol Metab. 2001;86:5067–5070. doi: 10.1210/jcem.86.10.7928. [DOI] [PubMed] [Google Scholar]
- Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans EM. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Endocrinol Metab. 2002;282:E551–E556. doi: 10.1152/ajpendo.00352.2001. [DOI] [PubMed] [Google Scholar]
- Vandenburgh HH, Shansky J, Solerssi R, Chromiak J. Mechanical stimulation of skeletal muscle increases prostaglandin F2 alpha production, cyclooxygenase activity, and cell growth by a pertussis toxin sensitive mechanism. J Cell Biol. 1995;163:285–294. doi: 10.1002/jcp.1041630209. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034.1–research0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Katta A, Gadde MK, Liu H, Kakarla SK, Fannin J, Paturi S, Arvapalli RK, Rice KM, Wang Y, Blough ER. Aging-associated dysfunction of Akt/protein kinase B: S-nitrosylation and acetaminophen intervention. PLoS ONE. 2009;4:e6430. doi: 10.1371/journal.pone.0006430. [DOI] [PMC free article] [PubMed] [Google Scholar]