Abstract
The present study determined the effects of voluntary ethanol drinking and deprivation on basal extracellular glutamate concentrations and clearance in the mesolimbic system and tested the hypothesis that chronic ethanol drinking would persistently increase basal glutamate neurotransmission. Three groups of alcohol preferring (P) rats were used: ‘water group (WG)’, ‘ethanol maintenance group (MG; 24-hr free choice water vs 15% ethanol)’ and ‘ethanol deprivation group (DG; 2 weeks of deprivation)’. Quantitative microdialysis and Western blots were conducted to measure basal extracellular glutamate concentrations, clearance, and proteins associated with glutamate clearance. Chronic alcohol drinking produced a 70-100% increase of basal extracellular glutamate concentrations in the posterior ventral tegmental area (4.0 vs 7.0 μM) and nucleus accumbens shell (3.0 vs 6.0 μM). Glutamate clearances were reduced by 30-40% in both regions of MG rats compared to WG rats. In addition, Western blots revealed a 40-45% decrease of excitatory amino transporter 1 (EAAT1) protein, but no significant changes in the levels of EAAT2 or cystine-glutamate antiporter in these regions of MG vs WG rats. The enhanced glutamate concentrations returned to control levels, accompanied by a recovery of glutamate clearance following deprivation. These results indicated that chronic alcohol drinking enhanced extracellular glutamate concentrations in the mesolimbic system, as a result, in part, of reduced clearance, suggesting that enhanced glutamate neurotransmission may contribute to the maintenance of alcohol drinking. However, since the increased glutamate levels returned to normal after deprivation, elevated glutamate neurotransmission may not contribute to the initiation of relapse drinking.
Keywords: alcohol drinking, alcohol deprivation, alcohol preferring (P) rats, glutamate neurotransmission, glutamate transporter, nucleus accumbens, ventral tegmental area
Introduction
Alterations of brain glutamate functions have been linked to the effects of alcohol (Gass & Olive 2008, Spanagel 2009). The ionotropic NMDA receptor is sensitive to acute inhibition by ethanol, whereas chronic ethanol exposure can up-regulate NMDA expression and increase the excitatory synaptic strength in the mesolimbic system (Lovinger et al 1989, Stuber et al 2008, Woodward 1999). Acute systemic administration of ethanol produced a bi-phasic alteration of extracellular glutamate levels within several brain regions, including the nucleus accumbens (NAC) and ventral tegmental area (VTA) (Ding et al 2012, Moghaddam & Bolinao 1994). Preclinical studies have also shown that pharmacological antagonism of glutamate transmission can reduce the reinforcing and rewarding effects of ethanol in various animal models (Gass & Olive 2008, Vengeliene et al 2008). In addition, the clinical efficacy of acamprosate, a FDA-approved drug for the prevention of alcohol relapse, has been linked to its anti-glutamatergic properties (Mann et al 2008). A recent magnetic resonance spectroscopy study further substantiated that acamprosate significantly reduced glutamate levels in the cingulate cortex of abstinent alcoholics (Umhau et al 2010).
Microdialysis studies have demonstrated that ethanol exposure, via various administration regimens, produced a general up-regulation of extracellular glutamate transmission in the brain. Ethanol dependence, induced by repeated intra-gastric ethanol administration or prolonged ethanol vapor exposure, is associated with increased extracellular glutamate levels in the striatum, hippocampus, and NAC shortly after the cessation of the ethanol treatment (Dahchour & De Witte 1999, Dahchour & De Witte 2000, Rossetti & Carboni 1995). With the aid of a quantitative microdialysis technique, several groups reported that repeated i.p. injections of ethanol significantly enhanced basal extracellular glutamate concentrations in the mesolimbic system (Ding et al 2012, Kapasova & Szumlinski 2008, Melendez et al 2005). These findings are in agreement with recent neuroimaging studies in human alcoholics or alcohol-dependent rats that exhibit significantly higher levels of glutamate in the medial frontal cortex and striatum (Hermann et al 2012, Zahr et al 2009). It should be noted that all these animal studies utilized forced ethanol administration, whereas the effects of voluntary ethanol drinking on basal extracellular glutamate transmission have not been studied.
Extracellular glutamate is regulated by various processes, among which glutamate clearance by glutamate transporters plays a pivotal role. The astroglial cells-derived excitatory amino acid transporter 1 and 2 (EAAT1 & EAAT2) are the two most abundant transporters responsible for the majority of glutamate clearance in most brain regions (Danbolt 2001, Tzingounis & Wadiche 2007). A number of quantitative microdialysis studies revealed reduced glutamate clearance following repeated systemic ethanol injections (Ding et al 2012, Kapasova & Szumlinski 2008, Melendez et al 2005). On the other hand, current evidence from in vitro studies is not as consistent. Some reported a dampened glutamate re-uptake activity following acute or repeated ethanol exposure (Melendez et al 2005, Othman et al 2002), whereas others pointed to an increased glutamate uptake activity (Smith 1997, Smith & Zsigo 1996). However, the molecular mechanisms underlying the altered clearance function were not adequately explored in these studies. Using in vitro preparations, ethanol has been shown to alter the protein expression of various glutamate transporters (Kim et al 2005, Zink et al 2004). In addition to glutamate transporters, the cysteine-glutamate antiporter (xCT) also contributes to the regulation of extracellular glutamate levels. The release of glutamate from xCTs can contribute up to 60% of extracellular glutamate in the NAC (Baker et al 2002). Ample evidence suggested an important role of the xCT in the development of drug abuse and addiction (Kalivas 2009). Therefore, it is important to examine the effects of chronic voluntary alcohol drinking on expression levels of these proteins.
The current study utilized alcohol-preferring (P) rats to examine the effects of voluntary alcohol drinking and deprivation on glutamate neurotransmission in the posterior VTA and NACsh. The P rat shows high alcohol preference and exhibits reliable high voluntary alcohol drinking and a robust alcohol deprivation effect following a prolonged period of abstinence (McBride & Li 1998, Murphy et al 2002). Using this model, neuro-adaptive changes have been identified within the mesolimbic system produced by chronic alcohol drinking, some of which persist following a period of abstinence (Rodd et al 2005, Thielen et al 2004) A quantitative no-net-flux microdialysis technique was employed to measure the extracellular glutamate concentrations and glutamate clearance. Western blots were conducted to measure the expression of EAAT1/2 and xCT proteins. The central hypothesis is that chronic voluntary alcohol drinking will produce a persistent increase of glutamate neurotransmission within the mesolimbic system.
Methods and Materials
Animals
Adult female alcohol preferring P rats (weight 225-250 at the beginning of the experiments, Indiana University) were housed in pairs in temperature- and humidity-controlled rooms maintained on a regular 12-hr light-dark cycle (light on at 7:00 a.m.) with food and water available ad libitum. Female P rats were used because they maintain their head size better than male P rats for more accurate stereotaxic placements and have been used in several studies requiring accurate placements (Rodd et al 2005, Thielen et al 2004). The estrous cycle was not monitored in the present study. However, counterbalanced experiments were conducted on different days so that any effect of a given phase of the estrous cycle was distributed across experimental conditions. All experimental procedures were conducted during the light phase. Protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. All experiments were conducted in accordance with principles outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Chemical agents
Ethanol (190 proof) was obtained from McCormick Distilling, Weston, MO. O-phthalaldehyde powder and diluents for glutamate derivatization were purchased from Pickering Laboratory, Inc, CA. The salts used in the aCSF and mobile phase were all HPLC grade and obtained from Sigma (St. Louis, MO, USA). Goat anti-EAAT1 and goat anti-EAAT2 antibodies were obtained from Santa Cruz, CA, and rabbit polyclonal antibody to xCT was from Abcam, MA. The mouse anti-β-actin antibody was purchased from Sigma-Aldrich (St. Louis, MO, USA). Enhanced chemiluminescence agents were obtained from Pierce (Thermo Scientific, IL, USA).
Ethanol drinking procedure
Ethanol exposure of rats followed the procedure as described previously (Rodd et al 2005, Thielen et al 2004). After the acclimation period, rats were transferred to metal hanging cages and were individually housed throughout the experiment. The rats were randomly assigned to one of three groups (6-10 / group). The ‘water group’ (WG) received only water for 10 weeks. The ‘ethanol maintenance group’ (MG) was given continuous water for the first 2 weeks and a combination of 15% ethanol and water under a two-bottle choice paradigm for additional 8 weeks. The ‘ethanol deprivation group’ (DG) was given 8 weeks of free-choice access to 15% ethanol and water, followed by a two-week deprivation period during which only water was available. For rats that drank ethanol, the positions of the ethanol and water bottles were randomly altered every week, and fluid intake was recorded to the nearest 0.1 g by weighing the water and ethanol bottles three times a week (Monday, Wednesday and Friday). Ethanol fluid intake measures were converted into grams of ethanol per kilogram of body weight (grams per kilogram per day). Body weights of the rats were recorded at the same time the ethanol and water bottles were weighed.
Stereotaxic surgery
Stereotaxic surgery was performed during the 9th week of drinking. Rats were stereotaxically implanted with one 18-gauge guide cannula (Plastics One, Inc., Roanoke, VA, USA) aimed at the posterior VTA (AP −5.6 mm, ML +2.1 mm, DV −9.0 mm) or NACsh (AP +1.7 mm, ML +2.3 mm, DV −8.5 mm) (Paxinos & Watson 1998). The posterior VTA and NACsh were examined based on previous findings indicating that these two regions are more sensitive to the stimulating and reinforcing effects of ethanol than the anterior VTA and NAC core (Ding et al 2009, Engleman et al 2009, Rodd et al 2004). The cannula was implanted at a 10° angle to the vertical. Rats were individually housed following surgery, and were allowed to recover from surgery for at least 5 days and received daily habituation and handling in the microdialysis chambers. Following the recovery, microdialysis probes (active membrane length 1.5 mm, inner diameter 200 μm, molecular weight cut-off: 13,000, Spectrum Laboratories, Inc, Rancho Dominguez, CA, USA) were constructed and inserted into the posterior VTA or NACsh approximately 16-18 hr before the microdialysis, as previously described (Ding et al 2009).
Microdialysis and glutamate analysis
On microdialysis day, ethanol or water bottles were removed at least 2-3 hours before the start of microdialysis to ensure that blood ethanol diminished to undetectable levels. General microdialysis followed the procedure described previously (Ding et al 2012, Ding et al 2009). Briefly, rats were placed into microdialysis chambers and connected to a Harvard pump with PE20 tubing (inner diameter 0.38 mm, Becton Dickinson & Co., MD, USA). Microdialysis started with a 90-min wash-out period during which artificial cerebrospinal fluid (aCSF: 140.0 mM NaCl, 3.0 mM KCl, 1.2 mM CaCl2, 2.0 mM Na2HPO4·7H2O, 1.0 mM MgCl2, pH 7.2-7.4) was perfused through probes, followed by the collection of baseline samples. Then, four different concentrations of glutamate (1, 5, 10 or 20 μM in aCSF) were perfused through the posterior VTA or the NACsh in random order. Each concentration of glutamate was perfused through the microdialysis probe for 25 min. After the perfusion of the last glutamate concentration, the perfusion medium was changed to aCSF and perfused for 20 min. Samples were collected every five minutes at a flow rate of 2.0 μl/min.
Glutamate was analyzed with a reversed-phase high performance liquid chromatography system with electrochemical detection, as described previously (Ding et al 2012). Briefly, precolumn derivatization of glutamate with o-phthalaldehyde was performed using an ESA Model 542 autosampler. The mobile phase consisted of 25% methanol (v/v), 100 mM Na2HPO4·7H2O, pH 6.75 and was delivered by an ESA 582 solvent delivery system. Dialysis samples were injected onto a reversed-phase column (BDS Hypersil C18 Pioneer, 150 × 2 mm) and glutamate was separated and detected with a BAS LC-4C amperometric detector with the oxidation potential set at +550 mV and the sensitivity set at 0.2 μA. The output from the detector was sent to a ChromPerfect chromatography data analysis system. The concentration of glutamate was quantified by comparing peak area with an external standard curve.
Western blot
Brain tissue from the NACsh and posterior VTA were micro-punched following the procedure described previously (McBride et al 2009). After micro-punching, tissue was transferred to a M-PER protein extraction buffer (Thermo Scientific, IL, USA) containing protease inhibitors (Roche Diagnostics, IN, USA), homogenized and centrifuged at 12,000 rpm at 4 °C for 20 min. The supernatant was stored at −20 °C until use. Western blots for EAAT1, EAAT2 and xCT followed the standard procedure previously described (Bailey & Lahiri 2010). Briefly, protein concentrations were estimated using the Bradford technique. Approximately 20 μg of tissue protein was loaded and separated on 10% polyacrylamide gels (Bio-Rad, Hercules, CA, USA), followed by transfer onto polyvinylidene difluoride membranes. Blots were probed with goat anti-EAAT1 (1:500, Santa Cruz, CA) (Melendez et al 2005), goat anti-EAAT2 antibodies (1:500, Santa Cruz, CA) or rabbit anti-xCT (1:1000, Abcam, MA) (Pampliega et al 2011), or mouse anti-β-actin (1:10,000, Sigma-Aldrich, MO) antibody. The blots were then probed with the appropriate peroxidase-conjugated secondary antibody and detected by enhanced chemiluminescence techniques (GE Healthcare, Piscataway, NJ, USA). Enhanced chemiluminescence films were then scanned and quantified using IMAGEJ software. Protein levels were normalized with the constitutively expressed protein β-actin.
Histology
At the end of each experiment, rats were euthanized with an overdose of CO2 inhalation, and 1% bromophenol blue was perfused through probes in the posterior VTA or NACsh. Brains were removed quickly and frozen immediately on dry ice and stored at −70 °C. Sections (40 μm) were sliced on a cryostat microtome and stained with cresyl violet for verification of the placements of probes with reference to the rat brain atlas of Paxinos & Watson (1998).
Statistical analysis
No-net-flux data were analyzed with multiple linear regressions, as described previously (Ding et al 2012, Thielen et al 2004). The glutamate concentrations perfused through the probes were defined as [GLU]in, and the glutamate concentrations obtained from microdialysis samples were defined as [GLU]out. The net gain or loss of glutamate was defined as [Glu]in-[Glu]out and was calculated for each sample. These values of [GLU]in - [GLU]out were plotted as ‘y’ axis against the values of [GLU]in as ‘x’ axis. The slope (the extraction fraction, Ed) and the x-intercept (the extracellular glutamate concentrations) for each group were determined by multiple linear regressions modeling and the effects of ethanol were analyzed with ANOVA using the SAS System for Windows, version 8.02. Western blot data were analyzed using a one-way ANOVA.
Results
Fig. 1 shows, on the right hemisphere of the brain, the representative placements of probes in the posterior VTA and NACsh. The posterior VTA is defined at the levels from 5.3 mm to 6.0 mm posterior to bregma (Ding et al 2009, Rodd et al 2004). To be included, the probes should have at least 75% of the active membrane within the target area. Approximately 85% of animals fulfilled the criteria and were included in the analysis. The micro-punch samples for Western blots were conducted in both sides of the NACsh and posterior VTA, but are only shown on the left side of the brain.
Figure 1.

Representative placements of microdialysis probes and micro-punch samples in the nucleus accumbens shell (NACsh, A) and posterior ventral tegmental area (VTA, B). The posterior VTA was determined based on previous findings (Ding et al 2009, Rodd et al 2004). The lines represent the microdialysis membrane and circles represent micro-punch samples. Micro-punch samples were taken from both sides of the NACsh and posterior VTA, but are only indicated in the left side of the brain. Overlapping probes and micro-punch samples are not shown for clarity purposes.
Glutamate No-net-flux
Average daily ethanol intakes before surgery were 5.1 ± 0.4 g/kg/day for the MG group and 4.7 ± 0.3 g/kg/day for the DG group (t31 = 0.21, p = 0.83). There was no significant difference in alcohol intake before and after surgery in the MG group (t30 = 1.5, p = 0.15).
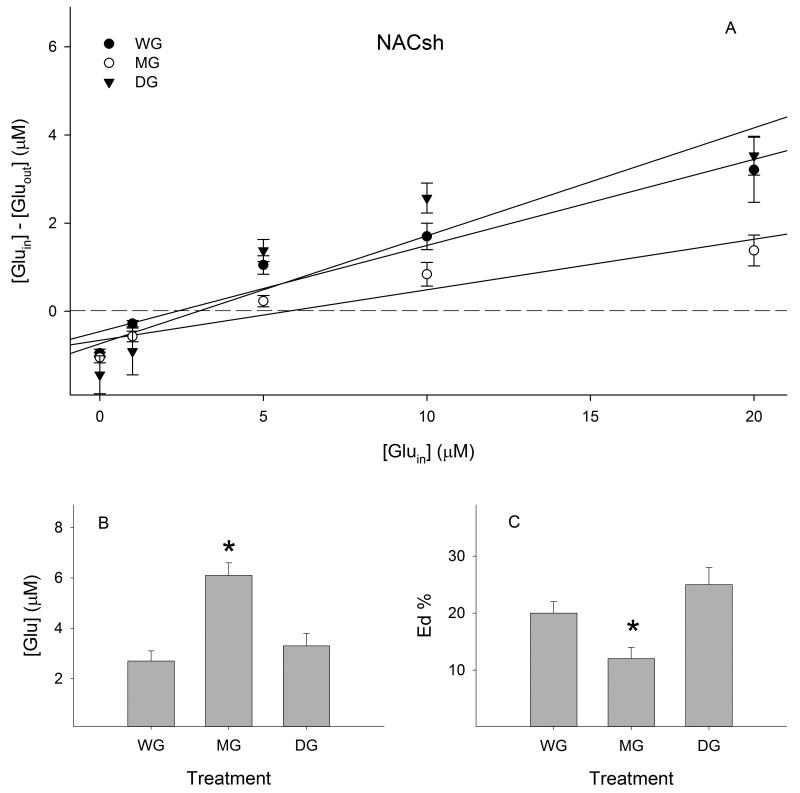
Extracellular glutamate concentrations were 2.7 ± 0.4 μM in the WG group, 6.1 ± 0.5 μM in the MG group, and 3.3 ± 0.5 μM in the DG group in the NACsh (Fig. 2B). Overall ANOVA revealed a significant effect of treatment (F (2, 404) = 4.90, p < 0.01). Post-hoc analysis indicated that glutamate concentrations in the MG group were significantly higher than those in both the WG group and DG group (p values < 0.05). No significant difference was observed between the DG group and WG group.
Figure 2.
Effects of ethanol maintenance (MG) and deprivation (DG) on extracellular glutamate concentrations and extraction fractions (Eds) in the nucleus accumbens shell (NACsh, n = 8-10 /group). A) Linear regression plots of glutamate for the water (WG), MG and DG groups; B) extracellular glutamate concentrations determined from points of no-net-flux in each group; C) Ed values of glutamate determined from the slope of the plots for the linear regression analysis in each group. * p < 0.05, significantly different from the WG group.
The extraction fractions (Eds) for each group were 20 ± 2% in the WG group, 12 ± 2% in the MG group, and 25 ± 3% in the DG group in the NACsh (Fig. 2C). A one-way ANOVA revealed a significant effect of treatment (F (2, 402) = 12.35, p < 0.001). Further analysis indicated that the Eds in the MG group were significantly lower than those in both the WG group and DG group (p values < 0.05), whereas the Eds in the DG group were not significantly different from those in the WG group.
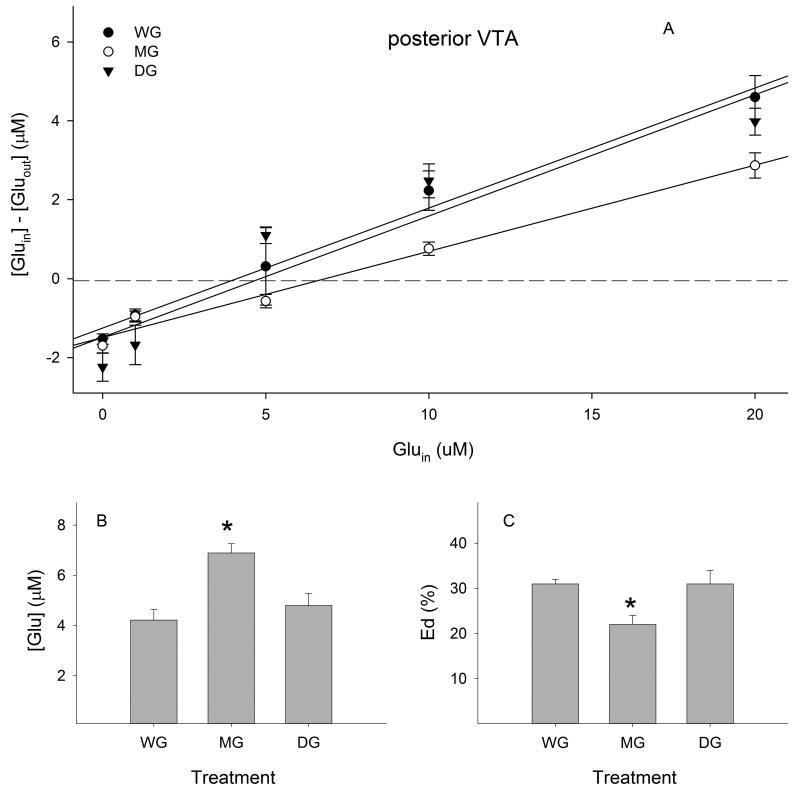
Extracellular glutamate concentrations were 4.2 ± 0.4 μM in the WG group, 6.9 ± 0.4 μM in the MG group, and 4.8 ± 0.5 μM in the DG group in the posterior VTA (Fig. 3B). A one-way ANOVA revealed a significant effect of treatment (F (2, 379) = 6.96, p < 0.01). Post-hoc analysis indicated that glutamate concentrations in the MG group were significantly higher than those in both the WG group and DG group (p values < 0.05), whereas glutamate concentrations in the DG group were not different than those in the WG group.
Figure 3.
Effects of ethanol maintenance (MG) and deprivation (DG) on extracellular glutamate concentrations and extraction fractions (Eds) in the posterior ventral tegmental area (VTA, n= 7-8 / group). A) Linear regression plots of glutamate for the water (WG), MG and DG groups; B) extracellular glutamate concentrations determined from points of no-net-flux in each group; C) Ed values of glutamate determined from the slope of the plots for the linear regression analysis in each group. * p < 0.05, significantly different from the WG group.
The extraction fractions (Eds) in each group were: 31 ± 1% in the WG group, 22 ± 2% in the MG group, and 31 ± 3% in the DG group in the posterior VTA (Fig. 3C). A one-way ANOVA revealed a significant effect of treatment (F (2, 377) = 7.32, p < 0.001). Further analysis indicated that the Ed value in the MG group was significantly lower than those in both the WG group and DG group (p values < 0.05), whereas the Ed value in the DG group was not different than the WG group.
Western blots
Average daily ethanol intakes across the 8-week drinking period were 5.8 ± 0.4 g/kg/day for the MG group and 5.4 ± 0.7 g/kg/day for the DG group. There was no significant difference between the two groups (t28 = 0.44, p = 0.63).
Relative expression levels of proteins in the NACsh are presented in Fig. 4. For EAAT1, there is a significant effect of ethanol drinking and deprivation history (n = 6 - 9 / group, F (2, 18) = 5.2, p < 0.05.) EAAT1 protein levels in both the MG and DG groups were significantly lower than those in the WG group (p < 0.05). There was no difference between the MG and DG groups. There were no significant differences in protein levels of EAAT2 (n = 6 - 7 / group), F (2, 17) = 0.8, p = 0.48) or xCT (n = 8 / group, F (2, 21) = 0.9, p > 0.05) across the three groups.
Figure 4.
Western blot analysis indicating effects of ethanol maintenance (MG) and deprivation (DG) on the protein level of excitatory amino acid transporter 1 & 2 (EAAT1 & 2), and the cystine-glutamate antiporter (xCT) in the nucleus accumbens shell (NACsh, n = 6-9 / group). * p < 0.05, significantly different from the WG group.
Similar to the NACsh, the expression levels of EAAT1 in the posterior VTA (Fig. 5) exhibited significant differences among the three groups (n = 6 - 9 / group, F (2, 18) = 4.5, p < 0.05). Post-hoc analysis indicated that protein levels in both the MG and DG groups were significantly lower than those in the WG group (p < 0.05). Although there was an apparent reduction (approximately 30%) of EAAT2 in the MG group than the WG group (Fig. 5B), a one-way ANOVA did not reveal significant changes among these groups (n = 8 / group, F (2, 21) = 1.2, p = 0.32). In addition, there did not appear to be any significant differences among the three groups in the expression of xCT (n = 7 - 8 / group, F (2, 20) = 0.1, p > 0.05).
Figure 5.
Western blot analysis indicating effects of ethanol maintenance (MG) and deprivation (DG) on the protein level of excitatory amino acid transporter 1 & 2 (EAAT1 & 2), and the cysteine-glutamate antiporter (xCT) in the posterior ventral tegmental area (n = 6-9 / group). * p < 0.05, significantly different from the WG group.
Discussion
The results of the current study indicate that chronic voluntary ethanol drinking in P rats reduced glutamate clearance and increased extracellular glutamate concentrations in both the posterior VTA and NACsh. These changes were accompanied by reduced protein levels of EAAT1, but not EAAT2 or xCT. These results suggest that enhanced extracellular glutamate concentrations in the mesolimbic system, mediated by reduced EAAT1 levels, may contribute to maintaining alcohol drinking. However, following two weeks of deprivation, glutamate clearance and concentrations returned to control levels despite the persistent reduction of EAAT1 levels, suggesting that other factors (e.g., post-translational changes) may have developed during the deprivation period to counter the lower levels of EAAT1. These latter results also suggest that elevated extracellular glutamate concentrations may not be involved in the initiation of relapse alcohol drinking.
One major advantage of the current study is the utilization of P rats as a voluntary drinking model, which has been shown to produce pharmacologically relevant blood alcohol levels (60-90 mg%) with the current continuous drinking paradigm (McBride & Li 1998, Murphy et al 2002). After chronic drinking, these rats displayed a 60-100% increase in basal extracellular glutamate concentrations in both regions. These results are in line with previous microdialysis studies in animals receiving forced exposure to ethanol (Dahchour & De Witte 2000, Ding et al 2012, Kapasova & Szumlinski 2008, Melendez et al 2005). These findings suggest that the pharmacological effects of ethanol are mainly responsible for the observed changes. In addition to the mesolimbic system, the increased extracellular glutamate levels were seen in other brain regions following ethanol exposure, including the striatum and hippocampus in rodents (Dahchour & De Witte 1999, Rossetti & Carboni 1995). Recent neuroimaging studies also reported increased glutamate levels in both human alcoholics (in the anterior cingulate cortex) and animal models (in the medial prefrontal cortex and striatum) upon acute withdrawal from alcohol exposure (Hermann et al 2012, Zahr et al 2009). These findings suggest that ethanol may produce a general increase of extracellular glutamate levels across the brain. Chronic alcohol drinking in P rats produced a 30-40% reduction of extracellular glutamate clearance in the posterior VTA and NACsh. These results are in line with previous quantitative microdialyis studies in animals receiving repeated i.p. injections of ethanol (Ding et al 2012, Kapasova & Szumlinski 2008, Melendez et al 2005). Further measurements of proteins that contribute to the regulation of extracellular glutamate levels and clearance revealed a significant reduction of EAAT1 protein (about 50%), but not EAAT2 or xCT proteins, in both regions. A previous study by Melendez and colleagues (2005) also examined the protein expression of EAAT1 and EAAT2 bud did not find significant changes in their study. It is possible that the ethanol exposure regimen these authors used (daily repeated i.p. injections for 7 days) may not be sufficient to induce such changes.
These results suggest that the reduced EAAT1 protein may be one underlying mechanism responsible for the decreased glutamate clearance and increased extracellular glutamate concentrations during the maintenance of alcohol drinking. However, it should be noted that Western blot measures only protein levels but not function. Therefore, functional studies may need to be conducted to validate the reduced glutamate re-uptake activity following chronic ethanol exposure. The mechanisms responsible for the reduction of EAAT1 protein remain unclear. Possible explanations may be (a) ethanol-induced reduction of EAAT1 mRNA transcription; (b) ethanol-altered epigenetic regulation of the EAAT1 gene; and / or (c) ethanol-induced increase of EAAT1 protein degradation. It also remains unknown whether alterations of EAAT1 may have occurred within the cell membrane or intracellular components. Future studies using other techniques, such as cell surface biotinylation or cross-linking coupled with immunoblot analysis (Boudreau & Wolf 2005, Xu et al 2003), may help answer such questions.
On the other hand, chronic alcohol drinking did not appear to significantly change the levels of EAAT2 or xCT in the mesolimbic system. In the posterior VTA, EAAT2 levels were reduced by approximately 30%, but high variability in the WG group prevented observing statistical significances. Future studies may need to be undertaken to re-assess the effects of ethanol on EAAT2 expression and function. These data suggest that EAAT2 or xCT protein levels may not be involved in the altered glutamate clearance and concentrations revealed with microdialysis. However, cautions are needed to interpret the results given that Western blots don’t directly measure function. Therefore, it should not be excluded that ethanol may produce changes that alter the activities of EAAT2 or xCT through post-translational modifications.
Nonetheless, the current results suggest a possible role of reduced EAAT1 protein in the maintenance of alcohol drinking. Reduced brain EAAT1 mRNA and protein levels have been associated with increased alcohol intake in mice with mutant clock gene Per2 (Spanagel et al 2005). This is consistent with the findings that increasing extracellular glutamate levels with a glutamate transporter inhibitor DL-threo-β-benzyl-oxyaspartic acid (TBOA) facilitated ethanol consumption in mice (Kapasova & Szumlinski 2008). In contrast, a recent study (Karlsson et al 2012) reported reduced alcohol intake and dampened ethanol-induced conditioned place preference in EAAT1 knock-out mice. However, these authors also point out that no difference in extracellular glutamate levels were found between wild-type and knock-out mice, suggesting significant compensatory mechanisms to overcome the deletion of EAAT1 (Karlsson et al 2012). Therefore, more research is warranted in the future to delineate the role of EAAT1 in alcohol drinking.
The origin of glutamate measured in the current study was not examined. However, previous studies indicated that a significant portion of glutamate (20-30%) sampled with microdialysis in the mesolimbic system may derive from putative neuronal sources (Baker et al 2002, Ding et al 2012). It also remains unknown how the ethanol-induced increase of extracellular glutamate concentrations alters post-synaptic glutamate signaling. Electrophysiological studies demonstrated that increasing glutamate levels with TBOA enhanced ionotropic glutamate receptor-mediated currents (Tzingounis & Wadiche 2007). This indirect evidence suggests that increased extracellular glutamate concentrations in the current study may facilitate post-synaptic neuronal glutamate signaling, leading to an increase of glutamate neurotransmission, which may contribute to maintaining alcohol drinking.
Following two weeks of deprivation, the basal glutamate concentrations and clearance returned to control levels in both regions, a finding consistent with the report by Melendez et al. (2005). Recent neuroimaging studies also demonstrated that enhanced glutamate levels in human alcoholics and alcohol-dependent rats returned to control levels several weeks after the cessation of alcohol treatment (Hermann et al 2012). In addition, no significant differences in glutamate levels in the anterior cingulate and insula were found in young alcoholics abstinent for at least two weeks compared to healthy controls (Lee et al 2007). These results suggest that chronic ethanol drinking does not produce a persistent increase of basal extracellular glutamate concentrations following the prolonged absence of ethanol. Thus, the alcohol deprivation and later relapse-like drinking may not be associated with enhanced mesolimbic extracellular glutamate levels.
Interestingly, the EAAT1 levels remained reduced in both regions following deprivation. This appears to be contrary to the microdialysis findings. The reason for this inconsistency remains unknown. It is possible that there was a compensatory recovery of the functional activity of EAAT1 (or other transporters) to cope with the lower protein levels. Possible mechanisms may include altered post-translational modifications of EAAT1 protein, such as phosphorylation, etc. Another possibility is that alcohol deprivation may increase the activity of other glutamate transporters (e.g., EAAT2 or 3).
The elevated basal glutamate neurotransmission may have significant functional implications. Glutamate provides the major excitatory inputs to the mesolimbic dopamine systems (Kalivas & Volkow 2005) The enhanced basal glutamate transmission may contribute to the increased NAC dopamine neurotransmission and sensitized responses of the mesolimbic system to the reinforcing and stimulating effects of ethanol (Ding et al 2009, Kapasova & Szumlinski 2008, Rodd et al 2005, Thielen et al 2004), leading to progressive escalation of ethanol drinking and eventually alcohol abuse and dependence.
Glutamate neurotransmission has long been proposed to mediate the effects of other drugs of abuse, including cocaine (Gass & Olive 2008, Kalivas 2009). Microdialysis studies show that chronic cocaine self-administration reduced basal extracellular glutamate levels in the NAC (Miguens et al 2008, Suto et al 2010). In addition, these reduced glutamate levels persisted following a prolonged abstinence from cocaine administration, presumably resulting from down-regulation of xCT function (Baker et al 2003, Kalivas 2009). Taken together, these findings suggest that glutamate neurotransmission may be differentially involved in the effects of different drugs of abuse, such as alcohol and cocaine.
In summary, the current study demonstrated that chronic ethanol drinking by P rats dampened glutamate clearance and increased basal extracellular glutamate concentrations in the mesolimbic system. These changes may be mediated, in part, by reduced EAAT1 protein expression. The enhanced glutamate neurotransmission may contribute to the maintenance of alcohol drinking. However, because extracellular glutamate concentrations returned to control levels following deprivation, the initiation of relapse-like drinking may not be associated with increased glutamate neurotransmission in the mesolimbic system.
Acknowledgements
This study was supported, in part, by research grants AA07611, AA12262, and AA10721. We thank Amanda Moran, Sarah R. Hall and Erin Larrabe for their technical help. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
Footnotes
Author contribution
WJM, ZMD, ZAR, and DKL were responsible for concept development and research design. ZMD, EAE, and JAB participated in performing experiments and acquiring data. ZMD, JAB and WJM performed data analysis. ZMD, WJM and ZAR contributed to the writing of the manuscript.
References
- Bailey JA, Lahiri DK. A novel effect of rivastigmine on pre-synaptic proteins and neuronal viability in a neurodegeneration model of fetal rat primary cortical cultures and its implication in Alzheimer’s disease. J Neurochem. 2010;112:843–53. doi: 10.1111/j.1471-4159.2009.06490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–49. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–51. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effect of repeated ethanol withdrawal on glutamate microdialysis in the hippocampus. Alcohol Clin Exp Res. 1999;23:1698–703. doi: 10.1111/j.1530-0277.1999.tb04063.x. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Taurine blocks the glutamate increases in the nucleus accumbens microdialysate of ethanol-dependent rats. Pharmacol Biochem Behav. 2000;65:345–50. doi: 10.1016/s0091-3057(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Engleman EA, Rodd ZA, McBride WJ. Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats. Alcohol Clin Exp Res. 2012;36:633–40. doi: 10.1111/j.1530-0277.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009;33:1571–81. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, et al. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res. 2009;33:2162–71. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–65. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, et al. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry. 2012;71:1015–21. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–31. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Adermark L, Molander A, Perreau-Lenz S, Singley E, et al. Reduced alcohol intake and reward associated with impaired endocannabinoid signaling in mice with a deletion of the glutamate transporter GLAST. Neuropharmacology. 2012;63:181–89. doi: 10.1016/j.neuropharm.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Do SH, Kim YL, Zuo Z. Effects of chronic exposure to ethanol on glutamate transporter EAAT3 expressed in Xenopus oocytes: evidence for protein kinase C involvement. Alcohol Clin Exp Res. 2005;29:2046–52. doi: 10.1097/01.alc.0000187594.92476.07. [DOI] [PubMed] [Google Scholar]
- Lee E, Jang DP, Kim JJ, An SK, Park S, et al. Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport. 2007;18:1511–14. doi: 10.1097/WNR.0b013e3282ef7625. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–24. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Spanagel R, Littleton J. Acamprosate: recent findings and future research directions. Alcohol Clin Exp Res. 2008;32:105–10. doi: 10.1111/j.1530-0277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li T-K. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–69. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Schultz JA, Kimpel MW, McClintick JN, Wang M, et al. Differential effects of ethanol in the nucleus accumbens shell of alcohol-preferring (P), alcohol-non-preferring (NP) and Wistar rats: A proteomics study. Pharmacol Biochem Behav. 2009;92:304–13. doi: 10.1016/j.pbb.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–33. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology. 2008;196:303–13. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci Lett. 1994;178:99–102. doi: 10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–88. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Othman T, Sinclair CJ, Haughey N, Geiger JD, Parkinson FE. Ethanol alters glutamate but not adenosine uptake in rat astrocytes: evidence for protein kinase C involvement. Neurochem Res. 2002;27:289–96. doi: 10.1023/a:1014955111742. [DOI] [PubMed] [Google Scholar]
- Pampliega O, Domercq M, Soria FN, Villoslada P, Rodriguez-Antiguedad A, Matute C. Increased expression of cystine/glutamate antiporter in multiple sclerosis. J Neuroinflammation. 2011;8:63. doi: 10.1186/1742-2094-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, et al. Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J Pharmacol Exp Ther. 2005;315:648–57. doi: 10.1124/jpet.105.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, et al. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–57. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283:177–83. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Smith TL. Regulation of glutamate uptake in astrocytes continuously exposed to ethanol. Life Sci. 1997;61:2499–505. doi: 10.1016/s0024-3205(97)00985-5. [DOI] [PubMed] [Google Scholar]
- Smith TL, Zsigo A. Increased Na(+)-dependent high affinity uptake of glutamate in astrocytes chronically exposed to ethanol. Neurosci Lett. 1996;218:142–4. doi: 10.1016/s0304-3940(96)13123-2. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behaviora. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zqhoul T, Sanchis-Sequra C, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature medicine. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32:1714–20. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Ecke LF, You Z, Wise RA. Extracellular fluctuation of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology. 2010;211:267–75. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, et al. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther. 2004;309:216–25. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–47. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, et al. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry. 2010;67:1069–77. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ. Ionotropic glutamate receptors as sites of action of ethanol in the brain. Neurochem Int. 1999;35:107–13. [PubMed] [Google Scholar]
- Xu NJ, Bao L, Fan HP, Bao GB, Pu L, et al. Morphine withdrawal increases glutamate uptake and surface expression of glutamate transporter GLT1 at hippocampal synapses. J Neurosci. 2003;23:4775–84. doi: 10.1523/JNEUROSCI.23-11-04775.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Vinco S, Orduna J, Luong R, et al. In vivo evidence for alcohol-induced neurochemical changes in rat brain without protracted withdrawal, pronounced thiamine deficiency, or severe liver damage. Neuropsychopharmacology. 2009;34:1427–42. doi: 10.1038/npp.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink M, Schmitt A, Vengeliene V, Henn FA, Spanagel R. Ethanol induces expression of the glutamate transporters EAAT1 and EAAT2 in organotypic cortical slice cultures. Alcohol Clin Exp Res. 2004;28:1752–7. doi: 10.1097/01.alc.0000145810.12545.b3. [DOI] [PubMed] [Google Scholar]