Abstract
Varicose veins (VVs) are a common venous disease of the lower extremity characterized by incompetent valves, venous reflux, and dilated and tortuous veins. If untreated, VVs could lead to venous thrombosis, thrombophlebitis and chronic venous leg ulcers. Various genetic, hormonal and environmental factors may lead to structural changes in the vein valves and make them incompetent, leading to venous reflux, increased venous pressure and vein wall dilation. Prolonged increases in venous pressure and vein wall tension are thought to increase the expression/activity of matrix metalloproteinases (MMPs). Members of the MMPs family include collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs and others. MMPs are known to degrade various components of the extracellular matrix (ECM). MMPs may also affect the endothelium and vascular smooth muscle, causing changes in the vein relaxation and contraction mechanisms. ECs injury also triggers leukocyte infiltration, activation and inflammation, which lead to further vein wall damage. The vein wall dilation and valve dysfunction, and the MMP activation and superimposed inflammation and fibrosis would lead to progressive venous dilation and VVs formation. Surgical ablation is an effective treatment for VVs, but may be associated with high recurrence rate, and other less invasive approaches that target the cause of the disease are needed. MMP inhibitors including endogenous tissue inhibitors (TIMPs) and pharmacological inhibitors such as zinc chelators, doxycycline, batimastat and marimastat, have been used as diagnostic and therapeutic tools in cancer, autoimmune and cardiovascular disease. However, MMP inhibitors may have side effects especially on the musculoskeletal system. With the advent of new genetic and pharmacological tools, specific MMP inhibitors with fewer undesirable effects could be useful to retard the progression and prevent the recurrence of VVs.
Keywords: MMP, endothelium, vascular smooth muscle, extracellular matrix, chronic venous insufficiency disease, TIMP
INTRODUCTION
Chronic venous disease (CVD) is a common disorder of the lower extremity that depending on its severity could have different manifestations including varicose veins (VVs). VVs commonly affect the superficial veins of the lower extremity and manifest as abnormally dilated, twisted, and tortuous veins. If untreated VVs could lead to several complications including thrombophlebitis and chronic venous insufficiency (CVI) with venous leg ulcers.
VVs are characterized by incompetent valves, venous reflux and vein wall dilation. A primary valve dysfunction may cause significant reflux, increased venous hydrostatic pressure, chronic venous hypertension, and vein wall dilation. Also, a primary vein wall dilation to expand to neighboring valves causing valve distortion, dysfunction, and incompetence, and the resulting venous reflux leads to increased venous pressure and further vein wall dilation [1, 2].
Despite the major medical and socio-economical consequences of VVs, the pathophysiological mechanisms involved are not fully understood. Matrix metalloproteinases (MMPs) are proteolytic enzymes that have been identified in many tissues and organs including the venous system. MMPs play a major role in tissue remodeling and the continuous turnover of collagen, elastin and other proteins of the extracellular matrix (ECM), and have been implicated in cardiovascular remodeling and vascular disease. The last two decades have witnessed great advances in our understanding of the role of MMPs in the development and progression of VVs. Studies on venous tissue from experimental animals and human have shown marked changes in the expression/activity of various MMPs in association with vein wall remodeling. Also, studies on wound ulcer fluid environment have suggested possible correlation between the activity of MMPs and the development of skin lesions and venous leg ulcers. An imbalance between MMPs expression/activity and endogenous tissue inhibitors of MMPs (TIMPs) could cause pathological changes in the vein wall and valves and lead to CVD. However, the upstream mechanisms causing elevation of MMPs in VVs, and the downstream mechanism linking MMPs to vein wall dilation are not clearly understood. Several studies have shown that increased mechanical stretch or pressure in human tissues is associated with increased expression of MMPs [3–6], and increased venous hydrostatic pressure could be a primary cause of elevated MMPs levels in VVs. In addition to their proteolytic properties on ECM, MMPs may have early effects on other cellular components of the vein wall including ECs and vascular smooth muscle (VSM) [7–9]. Also, prolonged increases in venous hydrostatic pressure may cause EC injury and increase cell permeability, leading to leukocyte infiltration and vascular inflammation [5], which in turn lead to tissue fibrosis, wall resolution, valve degradation and irreversible vein damage characteristic of late stages of CVI.
In this review we will discuss reports published in the Pubmed database and experimental data from our laboratory to highlight the role of MMPs in VVs. The review will discuss VVs, the predisposing factors and the clinical and experimental evidence for a role of MMPs in VVs, thrombophlebitis and venous ulcers. We will describe how increases in venous pressure could lead to increased MMPs expression, and the potential transcription factors involved. We will also describe the effects of MMPs on ECM and the newly-discovered effects on ECs and VSM. The review will then discuss various strategies for management of VVs and their complications, and provide a perspective on new strategies and future directions to target the MMP pathway in order to retard the progression and prevent the recurrence of VVs.
Varicose Veins
VVs are a common health and socioeconomical problem affecting over 25 million of the adult population in the United States [10]. The first national screening program in the United States identified VVs in 32% of participants, and many participants were considered at risk of developing venous thromboembolism during abdominal or orthopedic surgery [11].
VVs are superficial lower extremity veins that are abnormally twisted, dilated and often associated with incompetent valves (Fig. 1). If untreated, VVs could cause complications such as thrombophlebitis, deep venous thrombosis and venous leg ulcer. According to CEAP (clinical-etiology-anatomy-pathophysiology) classification, CVD has seven clinical stages C0-6, with C0 indicating no visible sign of venous disease, C1 telangiectasies (spider veins), C2 VVs, C3 edema, C4a skin pigmentation or eczema, C4b lipodermatosclerosis or atrophie blanche, C5 healed ulcer, and C6 active ulcer. C4-6 are often designated as chronic venous insufficiency (CVI), reflecting the advanced stage of the disease [2, 12] (Table 1) (Fig. 2).
Fig. 1.
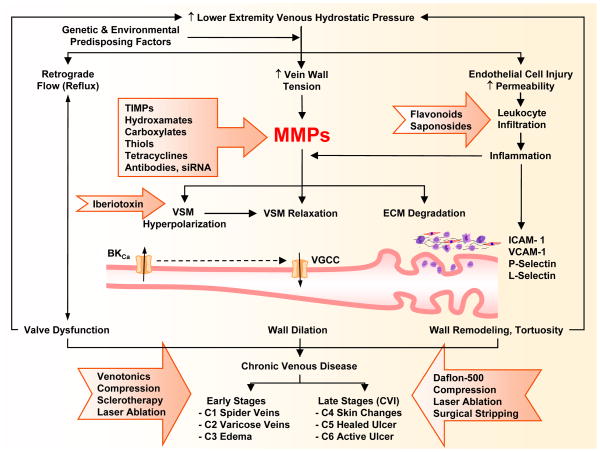
Parthophysiology and management of CVD. In the presence of genetic and environmental risk factors, increases in lower extremity venous hydrostatic pressure could cause valve dysfunction and venous reflux as well as increased wall tension, and increased expression/activity of MMPs. In addition to degradation of extracellular matrix (ECM) proteins, MMPs may cause venous smooth muscle (VSM) hyperpolarization and activation of K+ Channels, which in turn could cause inhibition of Ca2+ entry through voltage-gated Ca2+ channels (VGCC), VSM relaxation, vein wall dilation, and early manifestations of chronic venous disease (CVD). Persistent increases in venous pressure could cause ECs injury, leukocyte infiltration and inflammation of the vein wall, which could further increase MMPs expression/activity and lead to vein wall remodeling and tortuosity and late manifestations of chronic venous insufficiency (CVI). Several pharmacological and surgical approaches (presented in block arrows) are used for treatment of early and late manifestations of CVD. Different classes of MMP inhibitors may provide a new approach for management of CVD.
Table 1.
CEAP classification of clinical stages of CVD
| Stage | Clinical Signs |
|---|---|
| C0 | No visible or palpable signs of venous disease |
| C1 | Telangiectasia, reticular veins, or spider veins |
| C2 | Varicose veins. Distinguished from reticular veins by a diameter of ≥3mm |
| C3 | Edema |
| C4 | Changes in skin and subcutaneous tissue secondary to CVD |
| C4a | Skin pigmentation/eczema |
| C4b | Lipodermatosclerosis or atrophie blanche |
| C5 | Healed venous ulcer |
| C6 | Active venous ulcer |
Fig. 2.
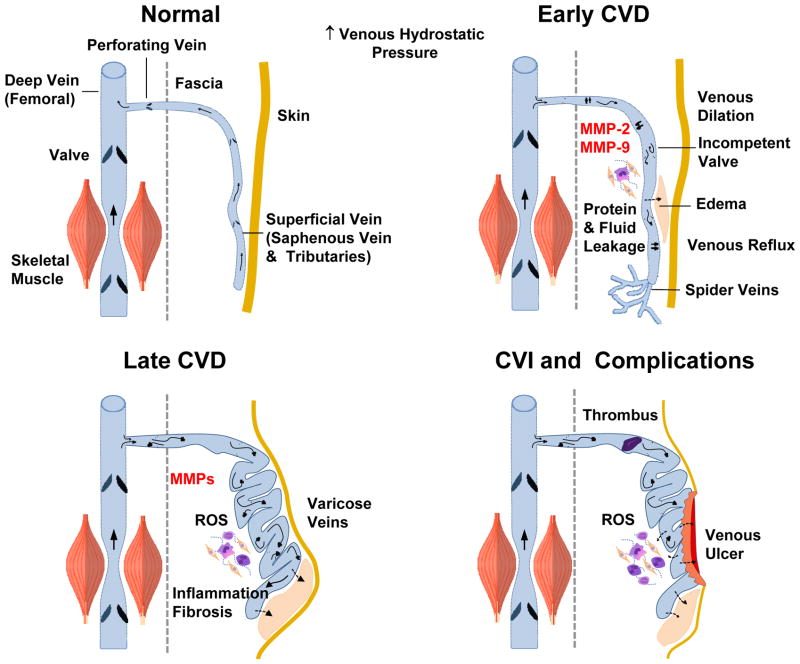
Early and late manifestations of CVD. In normal veins with intact valves, blood flows from superficial to deep femoral vein. Increases in lower extremity venous hydrostatic pressure and vein wall tension are associated with increased MMP expression/activity, venous dilation and venous reflux and early manifestations of CVD such as edema and spider veins. Late stages of CVD are associated with further increases in MMPs, varicose veins, edema, vein tissue remodeling, inflammation and fibrosis. CVI is complicated by thrombophlebitis, further increases in ROS, and venous wound leg ulcer.
Predisposing Factors for Varicose Veins
Several genetic and environmental factors have been associated with CVD including age, gender, pregnancy, estrogen therapy, obesity, family history of VVs, phlebitis and prior leg injury (Fig. 1). Environmental and behavioral factors associated with CVD include prolonged standing and sedentary lifestyle [13–15]. Family history and familial hereditary factors suggest a genetic component of VVs [16]. For instance, genetic mutations in iron metabolism genes may play a role in VVs. Prolonged venous reflux is associated with iron overload and dermal hemosiderin deposition that is directly correlated with clinical symptoms of CVI including skin changes and lipodermatosclerosis [17]. Iron deposition may induce the formation of free radical which could cause further tissue injury, and progression to advanced forms of CVI and leg ulcers [18, 19]. Also, Factor XIII is a cross-linking protein that plays a key role in ulcer healing [20]. Mutations in hemochromatosis C282Y (HFE) gene and Factor XIII V34L gene variants have been identified in patients with CVD and have been associated with increased risk of severe forms of CVI, skin changes and the size of venous ulcers [21, 22]. Interestingly, specific FXIII genotypes (L34 variant) have favorable ulcer healing rates, while HFE gene mutations may increase the risk of venous ulcer, but have no influence on healing time [23].
Some clinical conditions support a genetic component of VVs. Patients with Klippel-Trenaunay Syndrome have congenital venous anomalies in the form of atresia, agenesis of the deep venous system, valve insufficiency, venous aneurysms, and embryonic veins [24]. These patients have impaired venous muscle pump function and valve competence, and often present with VVs, limb hypertrophy, and dermal capillary hemangiomas (port wine stain) [25]. Primary lymphedema-distichiasis is a rare syndrome involving mutation in the FOXC2 gene, and one of its features is VVs at an early age, supporting a role of FOXC2 gene in the pathogenesis of VVs and a heritable element of the disease [26, 27]. A study of genealogical trees has shown that in nine families studied, VVs are linked to the candidate marker D16S520 on chromosome 16q24, which may account for the linkage to FOXC2 gene. Families of affected patients with the D16S520 marker have shown evidence of saphenofemoral junction reflux. The linkage to a candidate marker for the FOXC2 gene suggests that there is a functional variant within or in the vicinity of the gene that predisposes to VVs, and that CVI could be heritable in an autosomal dominant mode with incomplete penetrance [28]. Heterozygous mutation in the Notch3 gene has also been identified in the CASADIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) pedigree with VVs [29]. Microarray analysis of 3,063 human cDNAs from VVs and control veins have shown upregulation of 82 genes, particularly those regulating ECM, cytoskeletal proteins, and myofibroblasts production [30]. Ehlers-Danlos syndrome comprises more than ten connective tissue disorders with abnormal collagen synthesis, joint hypermobility, distensible skin, ocular disease, bone deformities and fragility, and cardiovascular disorders that render the blood vessels and visceral tissue walls fragile and susceptible to rupture. Patients with Ehlers-Danlos syndrome type IV are prone to vascular pathology and may present with VVs [31, 32]. Recently, single nucleotide polymorphisms in the promoter regions of MMP-9 genes have been identified among the Chinese population. In MMP-9, a 1562 C to T substitution has been associated with increased promoter activity and plasma levels of MMP-9, and polymorphisms in the promoter region of MMP-9 are associated with VVs in the Chinese population [33].
In support of a genetic component of CVD, the elasticity of the lower limb vein wall is reduced not only in patients with venous insufficiency, but also in individuals with a high risk of developing VVs and in children of patients with VVs [34, 35]. Also, VVs pathology may not be confined to lower extremity veins, and could affect other tissues and cells in a generalized fashion. In patients with VVs, arm veins also show abnormal increase in distensibility, suggesting a systemic disease of the vein wall [36]. Both cultured dermal fibroblasts from dermal biopsies of patients with chronic vascular disease and cultured VSMCs from VVs show increased synthesis of type I collagen and decreased synthesis of type III collagen despite normal gene transcription, suggesting that VVs patients may have a systemic abnormality in collagen production and post-translational inhibition of type III collagen synthesis in various tissues [37, 38]. Also, in both VSMCs and fibroblasts of patients with VVs there is an increase in hydroxyproline content, indicating increased collagen, as compared to control. However the proportion of collagen type III was reduced despite normal mRNA transcription. These data may explain the loss of distensibility in VVs, and suggest a generalized defect in collagen metabolism and a genetic component of VVs pathology [39].
Age and gender are important factors in the development of VVs, and CVD may be more prevalent in females [2]. The Framingham Study has shown that the incidence of VVs is 2.6% in women and 1.9% in men [40]. The Edinburgh Vein Study screened 1566 subjects 18–64 years old for CVD and found that women were more likely to report VVs-related leg symptoms [41]. However, in a follow-up study the age-adjusted prevalence of truncal VVs was 40% in males and 32% in females, and the prevalence of VVs and CVD increased with age [42]. Also, studies measuring vein reflux using duplex ultrasound found CVD in 9.4% of men and 6.6% of women, which rose with age (21.2% in men and 12.0% in women older than 50) [43]. Interestingly, α-adrenergic, AngII-, depolarization-induced, and [Ca2+]-dependent contraction are reduced in female compared with male rat inferior vena cava (IVC), possibly due to increased estrogen receptor expression/activity and enhanced endothelium-dependent relaxation pathways in females. These data suggested inherent sex differences in venous tissue function, whereby an enhanced estrogen receptor-mediated venous relaxation and decreased venous contraction would lead to more distended veins in females [44].
Pregnancy is associated with physiologic changes that could contribute to venous distension and the development of VVs. During pregnancy plasma levels of estrogen and progesterone increase [45]. Early in pregnancy there is an increase in blood volume and plasma volume expansion [46]. Also, fetal growth and weight gain increase intra-abdominal pressure and central venous return [47, 48], and the increased venous pressure could lead to vein valve failure and progression of varices. Studies have also shown that overweight and obese women are more likely to develop VVs [49]. Compared with non-overweight women, moderately overweight women (BMI = 25.0–29.9 kg/m2) were more likely to report VVs, and obese women (BMI ≥ 30.0 kg/m2) were three times more likely to report the presence of VVs. However, no relationship between BMI and venous disease was observed among males [50]. Importantly, overweight and obese women have greater plasma levels of total and bioavailable circulating estrogens than non-overweight women particularly after menopause [51], further supporting a relationship between estrogen levels and VVs formation.
The ergonomics and physical activity of an occupation may be a factor in the epidemiology of VVs. In a community-based study of VVs conducted in Jerusalem among men and women aged 20 to 64 years, the prevalence of VVs was higher among individuals who spent much of their work day standing. Women were more likely to report occupations requiring prolonged standing compared with men (31.4% vs. 13.6%). However, the prevalence ratio related to workplace posture (standing vs. sitting) was higher in men (1.88) than in women (1.53) [52].
MMPs and Varicose Veins
MMPs may play a role in the vascular remodeling associated with vascular disease including VVs [53]. MMPs are endopeptidases discovered in 1962 as a collagen proteolytic activity during the ECM protein degradation associated with resorption of the tadpole tail [54]. Since then, the MMP family has grown to include at least 28 members in vertebrates, 23 in humans, and 14 in blood vessels. MMPs, also called matrixins, are multidomain zinc (Zn2+) metalloproteinases that degrade various components of ECM and belong to the larger superfamily of proteases called metzincins, which also includes adamalysins, serralysins, and astacins. Typically MMPs consist of a propeptide of about 80 amino acids, a catalytic metalloproteinase domain of about 170 amino acids, a linker peptide (hinge region) of variable lengths and a hemopexin domain of about 200 amino acids [55, 56] (Fig. 3). Members of the MMP family have 3 distinguishing features: 1) Sequence homology with collagenase-1 (MMP-1); 2) Cysteine switch motif PRCGXPD in the prodomain that maintains MMPs in the proMMP zymogen form, and chelates the active Zn2+ site, except MMP-23 which lacks the cysteine switch motif; and 3) Zn2+-binding motif bound by 3 histidine molecules with the conserved sequence HEXGHXXGXXH located in the catalytic domain. MMPs are commonly classified based on domain organization and substrate preference into collagenases, gelatinases, stromelysins, matrilysins, membrane-type (MT)-MMPs and others [55, 57] (Table 2).
Fig. 3.
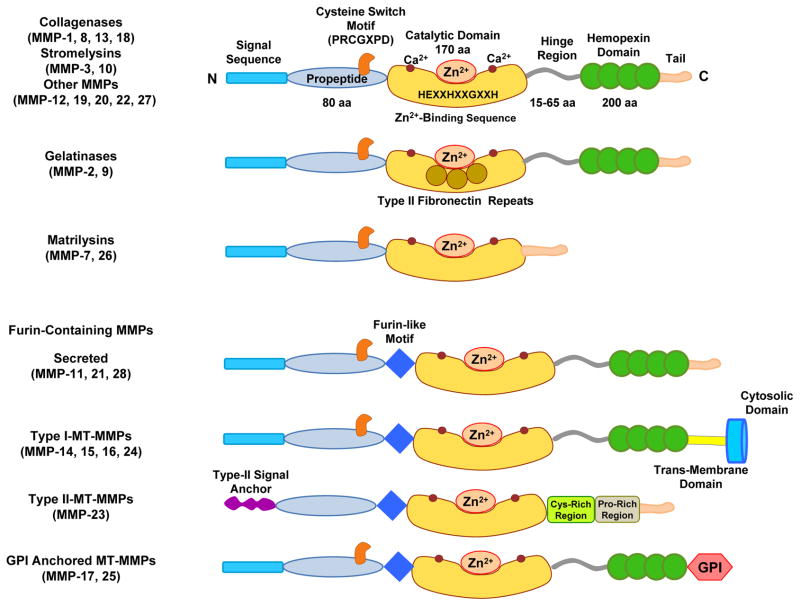
Major classes and structure of matrix metalloproteinase. Typically, MMPs consist of a propeptide of about 80 amino acids, a catalytic metalloproteinase domain of about 170 amino acids, a linker peptide (hinge region) of variable lengths and a hemopexin domain of about 200 amino acids. The catalytic domain contains the Zn2+ binding motif HEXXHXXGXXH. Matrilysins are exceptions as they lack the linker peptide and the hemopexin domain. Membrane-bound MMPs (MT-MMPs) have a furin-like proprotein convertase recognition sequence at the C-terminus of the propeptide and some of them have a glycosylphosphatidylinositol (GPI) anchor.
Table 2.
Members of the MMP family and their substrates
| MMP (Other Name) Chromosome Location | MW KDa Proform | MW KDa Active Form | Tissue Distribution | Collagen Substrates | Non-Collagen ECM Substrates | Non-Structural ECM Component Substrates |
|---|---|---|---|---|---|---|
|
Collagenases MMP-1 (Collagenase-1) 11q22.3 |
55 | 45 | Fibroblasts, interstitial, tissue collagenase | I, II, III, VII, VIII, X, and gelatin | Aggrecan, casein, nidogen, serpins, versican, perlecan, proteoglycan link protein, tenascin-C | α1-antichymotrypsin, α1-antitrypsin, α1-proteinase inhibitor, IGFBP-3, IGFBP-5, IL-1β, L-selectin, ovostatin, recombinant TNF-α peptide, SDF-1 |
| MMP-8 (Collagenase-2) 11q22.3 | 75 | 58 | Neutrophil, or PMNL collagenase | I, II, III, V, VII, VIII, X | Aggrecan, laminin, nidogen | α2-antiplasmin, pro-MMP-8 |
| MMP-13 (Collagenase-3) 11q22.3 | 60 | 48 | VVs, SMC, preeclampsia, breast cancer | I, II, III, IV | Aggrecan, fibronectin, laminin, perlecan, tenascin | Plasminogen activator 2, pro-MMP-9, pro-MMP-13, SDF-1 |
|
Gelatinases MMP-2 (Gelatinase-A) 16q13-q21 |
72 | 66 | Aortic aneurysm, VVs | I, II, III, IV, V, VII, X, XI | Aggrecan, elastin, fibronectin, laminin, nidogen, proteoglycan link protein, versican | Active MMP-9, active MMP-13, FGF R1, IGF-BP3, IGF-BP5, IL-1β, recombinant TNF-α peptide, TGF-β |
| MMP-9 (Gelatinase-B) 20q11.2-q13.1 | 92 | 86 | Aortic aneurysm, VVs | IV, V, VII, X, XIV | Fibronectin, laminin, nidogen, proteoglycan link protein, versican | CXCL5, IL-1β, IL2-R, plasminogen, pro-TNFα, SDF-1, TGF-β |
|
Stromelysins MMP-3 (Stromelysin-1) 11q22.3 |
57 | 45 | VSMC, coronary artery disease, hypertension, tumor invasion, synovial fibroblasts | II, III, IV, IX, X, XI | Aggrecan, casein, decorin, elastin, fibronectin, laminin, nidogen, perlecan, proteoglycan, proteoglycan link protein, versican | α1-antichymotrypsin, α1-proteinase inhibitor, antithrombin III, E-cadherin, fibrinogen, IGF-BP3, L-selectin, ovostatin, pro-HB-EGF, pro-IL-1β, pro-MMP-1, pro-MMP-8, pro-MMP-9, pro-TNFα, SDF-1 |
| MMP-10 (Stromelysin-2) 11q22.3 | 57 | 44 | Atherosclerosis, uterine, preeclampsia, arthritis, carcinoma cells | III, IV, V | Fibronectin, laminin, nidogen | Pro-MMP-1, pro-MMP-8, pro-MMP-10 |
|
Matrilysins MMP-7 (Matrilysin-1) 11q21-q22 |
28 | 19 | Uterine | IV, X | Aggrecan, casein, elastin, enactin, laminin, proteoglycan link protein | β4 integrin, decorin, defensin, E-cadherin, Fas-L, plasminogen, pro-MMP-2, pro-MMP-7, pro-MMP-8, pro-TNFα, transferrin, and syndecan α2-antiplasmin |
| MMP-26 (Matrilysin-2, endometase) 11p15 | 28 | 19 | Breast cancer cells, human endometrial tumor | IV, gelatin | Casein, fibrinogen, fibronectin | Fibrin, fibronectin Pro- MMP-2 β 1-proteinase inhibitor |
|
Membrane-Type MMP-14 (MT1-MMP) Chr: 14-14q11-q12 |
66 | 56 | Human fibroblasts, SMC, VSMC, uterine, angiogenesis | I, II, III | Aggrecan, dermatan proteoglycan, fibrin, fibronectin, laminin, nidogen, perlecan, tenascin, vitronectin | αvβ3 integrin, CD44, gC1qR, pro-MMP-2, pro-MMP-13, pro-TNFα, SDF-1, tissue transglutaminase |
| MMP-15 (MT2-MMP) 16q13 | 72 | 50 | Human fibroblasts, leukocytes, preeclampsia | I | Aggrecan, fibronectin, laminin, nidogen, perlecan, tenascin, vitronectin | Pro-MMP-2, pro-MMP-13, tissue transglutaminase |
| MMP-16 (MT3-MMP) 8q21.3 | 64 | 52 | Human leukocytes, angiogenesis | I | Aggrecan, casein, fibronectin, laminin, perlecan, vitronectin | Pro-MMP-2, pro-MMP-13 |
| MMP-24 (MT5-MMP) 20q11.2 | 57 | 53 | Leukocytes | None identified | Chondroitin sulfate, dermatin sulfate, fibronectin | Pro-MMP2, pro-MMP-13 |
|
Other MMPs MMP-11 (Stromelysin-3) 22q11.23 |
51 | 44 | Angiogenesis, uterine | Does not cleave | Laminin | α1-antitrypsin, α1-proteinase inhibitor, IGFBP-1 |
| MMP-12 (metalloelastase) 11q22.3 | 54 | 45–22 | Macrophages | IV | Elastin | Plasminogen |
| MMP-17 (MT4-MMP) 12q24.3 | 57 | 53 | Brain specific cerebellum, breast cancer | Gelatin | Fibrin | |
| MMP-18 (Xenopus Collagenase-4) 12q14 | 70 | 53 | Xenopus (amphibian) | I, gelatin | ||
| MMP-19 (RASI-1) Chr: 12-12q14 | 54 | 45 | Liver | I, IV, gelatin | Aggrecan, casein, fibronectin, laminin, nidogen, tenascin | |
| MMP-20 (Enamelysin) Chr: 11-11q22.3 | 54 | 22 | Tooth enamel | V | Aggrecan, amelogenin, cartilage oligomeric protein | |
| MMP-21 (X-MMP) 10q26.13 | 62 | 49 | Macrophages, fibroblasts, human placenta | α1-antitrypsin | ||
| MMP-22 (C-MMP) 1p36.3 | 51 | Chicken fibroblasts | Gelatin | |||
| MMP-23 (CA-MMP) | 28 | 19 | Ovary, testis, prostate | Gelatin | ||
| MMP-25 (Leukolysin, MT6-MMP) 16p13.3 | 34 | 28 | Leukocytes, anaplastic astrocytomas, glioblastomas | IV, gelatin | Fibrin, fibronectin, pro-MMP-2 | |
| MMP-28 (Epilysin) 17q21.1 | 56 | 45 | Skin keratinocytes | Casein |
Collagenases include MMP-1, -2 (neutrophil collagenase), -13 and -18 (Xenopus). These MMPs cleave fibrillar collagen type I, II and III into characteristic 3/4 and 1/4 fragments. They first unwind triple helical collagen then hydrolyze the peptide bonds. The MMPs hemopexin domain is essential for cleaving native fibrillar collagen while the catalytic domain is needed for cleaving noncollagen substrates [58, 59]. MMP-13 (collagenase 3) is overexpressed in cartilage tissues of osteoarthritis patients and is very efficient in degrading type II collagen [60].
Gelatinases include gelatinase A (MMP-2) and gelatinase B (MMP-9). Gelatinases digest denatured collagens (gelatins), and have 3 type II fibronectin repeats inserted in the catalytic domain, which bind to gelatin, collagens and laminin [61]. MMP-2 cleaves collagen in two phases, the first resembling that of the interstitial collagenases, followed by gelatinolysis, which is promoted by the fibronectin-like domain [62, 63]. The collagenolytic activity of MMP-2 is much weaker than collagenases. However, because proMMP-2 is recruited to the cell surface and activated by membrane-bound MT-MMPs, it may accumulate pericellularly and induce substantial localized collagenolytic activity [64].
Stromelysins 1, 2 and 3, also known as MMP-3, -10, and -11, respectively, have the same domain arrangement as collagenases, but do not cleave interstitial collagen. MMP-3 and -10 are similar in structure and substrate specificity, while MMP-11 is distantly related. Despite their similar substrate specificity, MMP-3 has higher proteolytic efficiency than MMP-10. MMP-3 and MMP-10 digest a number of ECM components and participate in proMMP activation, but MMP-11 has very weak activity toward ECM molecules. Also, MMP-3 and -10 are secreted from the cells as inactive proMMP, but MMP-11 is activated intracellularly by furin and secreted from the cells as an active enzyme [65].
Matrilysins, include matrilysin-1 (MMP-7) and matrilysin-2 (MMP-26, endometase), which lack the linker peptide or hinge region and the hemopexin domain [66, 67]. MMP-7 acts intracellularly in the intestine to process procryptidins to bactericidal forms. MMP-7 degrades ECM components, and cleaves cell surface molecules such as Fas–ligand, pro-tumor necrosis factor-α (TNF-α), syndecan 1 and E-cadherin to generate soluble forms [68]. MMP-26 is expressed in breast cancer cells [69].
Membrane-Type MMPs (MT-MMPs) include 4 transmembrane MMPs, MT1-, MT2-, MT3-and MT5-MMP (MMP-14, -15, -16 and -24, respectively), and the glycosylphosphatidylinositol-anchored proteins MT4- and MT6-MMP (MMP-17 and -25, respectively). MT-MMPs have a furin-like pro-protein convertase recognition sequence at the C-terminus of the propeptide. They are activated intracellularly and the active enzymes are expressed on the cell surface. All MT-MMPs except MT4-MMP (MMP-17) can activate proMMP-2 [70]. MT1-MMP digests collagen-I, -II and -III and other ECM proteins, and in the presence of TIMP-2 it activates proMMPs such as proMMP-13 on the cell surface [71, 72] (Table 2).
Other MMPs include MMP-12, -20 and -27 which have a domain arrangement and chromosome location similar to stromelysins. MMP-12 (metalloelastase) is expressed in macrophages and is essential for macrophage migration [73] and is also found in hypertrophic chondrocytes and osteoclasts [74, 75]. MMP-12 digests elastin and other ECM proteins. MMP-19 is a potent basement membrane-degrading enzyme that plays a role in tissue remodeling, wound healing and epithelial cell migration by cleaving laminin5-γ2 chain [76–79]. MMP-19 deficient mice develop diet-induced obesity due to adipocyte hypertrophy, but are less susceptible to skin cancers induced by chemical carcinogens [80].
Enamelysin (MMP-20) is a tooth-specific MMP expressed in newly formed tooth enamel and digests amelogenin [81]. Amelogenin imperfecta, a genetic disorder with defective enamel formation involves mutation at MMP-20 cleavage sites [82]. MMP-21 is an MMP with measurable gelatinolytic activity expressed in various fetal and adult tissues, macrophages of granulomatous skin lesions, fibroblasts in dermatofibromas, and in basal and squamous cell carcinomas [83, 84]. MMP-22 was cloned first from chicken fibroblasts, and a human homologue was later identified, but its function and substrate are unclear [85].
MMP-23 is a type II membrane protein regulated by a single proteolytic cleavage for both its activation and secretion [86]. It harbors a transmembrane domain and a furin recognition motif (convertase cleavage site which activates MMP) in the propeptide, and is therefore cleaved in the Golgi and released as an active enzyme into the extracellular space [86]. MMP-23 is unique among the matrixins as it lacks the cysteine switch motif in the propeptide and the linker peptide or hinge region, and the hemopexin domain is substituted by cysteine-rich immunoglobulin-like domains immediately after the C-terminus of the catalytic domain [64, 71, 87, 88] (Fig. 3). MMP-23 is expressed predominantly in ovary, testis and prostate, suggesting a specialized role in reproduction [87]. MMP-27 is expressed in B-lymphocytes and is overexpressed in cultured human lymphocytes treated with anti-(IgG/IgM) [89]. Epilysin (MMP-28) is one of the latest MMPs to be identified [90]. Epilysin was first cloned from human keratinocyte and testis cDNA libraries, and is expressed in the lung, placenta, heart, gastrointestinal tract and testis [91, 92]. MMP-28 is increased in cartilage from patients with osteoarthritis and rheumatoid arthritis [93, 94].
Studies have shown increased MMPs in lower extremity venous blood of patients with VVs. The plasma and venous tissue levels of MMP-1, -2, -3, -9 and -13 are elevated in VVs [95–97]. In a study examining MMP-1, -3 and -13 in proximal and distal segments of VVs vs. control veins MMP-1 and -13 mRNA was not different in VVs vs. control veins or in proximal vs. distal segments of VVs. MMP-3 mRNA was not amplified in any of the vein segments studied. However, when protein levels were measured, MMP-1 was elevated in VVs compared to control veins, and MMP-1 and -13 was increased in proximal vs. distal VVs segments. These findings suggested that MMPs protein levels are increased in VVs, and that their post-transcriptional modification may explain their differential distribution in VVs [95]. Another study has linked the changes in the thickness of VVs to the balance between MMPs and TIMPs and showed greater expression of TIMP-2 and connective tissue accumulation in tunica media of VVs compared with arm and neck veins of control subjects. TIMP-2 and -3 expression was greater in hypertrophic than atrophic segments, and in the thicker proximal than distal segments of VVs [98]. Immunohistochemical studies have shown variable distribution of MMPs in the venous tissue intima, media and adventitia. VVs show increased distribution of MMP-1 in all layers, while in normal veins MMP-1 is localized in the endothelium and adventitia. MMP-9 is expressed throughout the vein wall in both control and VVs, with increased levels in the VSM layer in VVs. TIMPs were not detected in any of the veins examined [97, 99]. These findings suggest that MMPs may affect all layers of the vein wall including the ECM leading to vein wall degradation and VVs formation [97]. Other studies have localized MMP-9 immunostaining in VSMCs of VVs but not control veins [99]. While MMPs may contribute to the pathophysiology of VVs, the presence of MMPs in VVs does not imply causation, and the diverse location of different MMPs in various layers of the vein wall including ECM, ECs and VSM suggest that MMPs may have different effects at different stages of VVs.
MMPs in Thrombophlebitis
One of the complications of VVs is thrombophlebitis characterized by endothelial, vein wall and valve inflammation and leukocytes infiltration leading to disruption of vein function and venous thrombosis [2, 100, 101]. Thrombophlebitis may occur in the course of VVs or may be induced during treatment of VVs with sclerosing agents [101, 102]. Specimens of saphenous vein from patients with CVD have shown increased monocytes/macrophage infiltration in the vein wall and valves [100, 103]. Also, intercellular adhesion molecule-1 (ICAM-1) was elevated in CVD specimens, but other cytokines were not [101].
VVs with thrombophlebitis may have different MMPs expression compared with VVs. In a study examining MMP-1, -2, -3 and -9 in control veins, VVs and VVs complicated by thrombophlebitis, thrombophlebitic VVs showed an elevated content of MMPs in the vein wall, and increased MMP-1, -2 and -9 activity. VVs showed increased activity of MMP-2. These marked changes in MMPs content and activity in VVs especially those affected with thrombophlebitis could lead venous tissue remodeling and alterations in the mechanical properties of the vein wall [96]. Other studies have shown a higher count of mast cells, T cells and B cells in thrombotic compared with non-thrombotic VVs [104], further implicating inflammation in this stage of the disease.
MMPs in Lipodermatosclerotic Skin and venous Leg Ulcer
Some of the features of advanced stages of CVD are skin changes, lipodermatosclerotic skin and venous leg ulcer. Lipodermatosclerotic skin shows increased MMP activity and ECM turnover. Dermal biopsies from lipodermatosclerotic skin showed increased mRNA expression and protein levels of MMP-1 and -2 and TIMP-1, and increased activity of MMP-2 as compared to healthy skin. In addition, there was an increase in proMMP-1:TIMP-1 complex indicating the overexpression of proteinases and enhanced binding to TIMP [105]. Immunohistochemistry experiments revealed prominent expression of MMP-1 and -2 in the basal and suprabasal layers of the epidermis, perivascular region and reticular dermis, and reduced expression of TIMP-2 in the basement membrane of lipodermatosclerotic skin [105], supporting excessive and unrestrained MMP activity and ECM turnover.
Analysis of the components of venous leg ulcer microenvironment has shown dermal fibroblasts, keratinocytes, inflammatory cells, ECM, growth factors, cytokines and bacteria, and circulating ECs in the microcirculation. Also, the chronic venous ulcer wound fluid (VUWF) shows marked protease activity, with increased collagenase activity 116-fold over that in acute wound fluid, and decreased activity in venous ulcer that shows healing [106–108].
In both acute and chronic wounds, ECM provides a milieu for keratinocytes to migrate [109], and changes in protease activity could affect the ECM properties in wounds. Chronic VUWF has up to 10-fold increase in the levels of MMP-2 and -9 and increased MMP-1 and gelatinase activity as compared with acute wound fluid, suggesting increased ECM turnover [110, 111]. Inhibition studies with the MMPI doxycycline suggested that the source of collagenase and gelatinase activity in VUWF was from fibroblasts and mononuclear cells, but not neutrophils, which are involved in acute wound healing [111]. Studying the source of collagenase activity is important particularly because bacteria, a component of the venous ulcer microenvironment, produce collagenase. Human collagenase degrades collagen in a specific 3/4 and 1/4 fragments, whereas bacterial collagenase degrades collagen randomly in a non-specific manner. Collagenase from VUWF degrades collagen in the specific 3/4 and 1/4 fragments, supporting the presence of human collagenase activity [111]. MMP-1 is also found in migrating keratinocytes of the wound [112]. MMP-12 is abundant in fibroblasts in the ulcer bed, but not in the epidermis or in acute wounds. Increased expression of MMP-1, -3 and -13 with concomitant reduction in gene expression and immunoreactivity of TIMP-1 and -2 was also observed in the acute phase of skin ulcer with inflammation and dermatitis [113].
MMPs in wound ulcer could also cause abnormalities in tissue perfusion or affect angiogenesis and the microvasculature. When the effects of VUWF and acute wound fluid from donor skin graft sites were tested on ECs culture model of angiogenesis and tubule formation, VUWF caused marked reduction in the formation and length of tubules as compared with control fluid. Addition of the synthetic MMP-2/MMP-9 Inhibitor I to VUWF restored angiogenesis [114], suggesting that MMPs in VUWF have anti-angiogenic effects that disrupt the microcirculation in the perivascular regions and inhibit wound healing.
The regulation of MMP production in lipodermatosclerotic tissue and venous ulcer involves several factors. Dermal fibroblasts and leukocytes are major sources for MMPs especially MMP-2 [115]. MMP production in lipodermatosclerotic tissue and venous ulcer may involve mitogen-activated protein kinase (MAPK) which regulates MMP expression and proteolytic activity in dermal fibroblasts [116, 117]. In fibroblasts, TNF-α induces MMP-19 expression, which is inhibited by blocking the ERK1/2 pathway with PD98059 or p38 MAPK pathway with SB203580. Adenovirus-mediated induction of ERK1/2 and p38 MAPK in fibroblasts increases MMP-19 expression. Also, activation of c-JNK increases proMMP-19, highlighting the role of MAPK in regulating MMP expression and proteolytic activity in dermal fibroblasts, with potential implications in the pathogenesis of venous ulcer [116–118]. Post-translational modifications of MMPs are also essential for their activity and are regulated by TGF-β1 [115].
MMPs are thought to regulate not only the development of skin changes and venous ulcer but also wound healing. An important element in wound healing is Factor XIII (FXIII) which promotes collagen cross-linking, and thereby modulates the effects of MMPs. In vitro studies evaluated the effects of increasing concentrations of collagenase and FXIII on fibroblast survival. At high collagenase concentrations (2 mg/mL) 95% of fibroblasts were nonviable, and FXIII did not inhibit the effects of collagenase. However, at lower collagenase concentrations (0.5–1 mg/mL) FXIII abrogated the effects of collagenase and increased fibroblasts survival. Interestingly, topical application of FXIII improved venous ulcer healing [20]. In addition to FXIII, iron overlaod has been found in the serum and dermis of limbs of patients with venous ulcer compared with control subjects. Increased MMP-9 activity was also observed in venous ulcer. Iron overload can cause oxidative stress and increased production of reactive oxygen species (ROS). The iron deposits in the limbs could be released in the serum causing overload and activation of MMPs and ROS and lead to impaired ulcer healing [18].
MT1-MMP and extracellular MMP inducer (EMMPRIN, CD147) are important inducers/activators of MMPs [55, 56, 119]. Immunohistochemistry studies in venous ulcer biopsies have shown increased expression of MMP-2, MT1-MMP, MT2-MMP and EMMPRIN in venous ulcer dermis compared to control dermis. MMP-2 and EMMPRIN were overexpressed in the perivascular regions, which would favor unrestrained MMP activation and ECM turnover in the perivascular region of the venous ulcer [119]. Comparison of healing and non-healing venous ulcers has shown greater levels of PDGF AA in healing ulcers, but no difference in MMPs or EMMPRIN. Also, VUWF of healing ulcers had elevated levels of PDGF AA and TIMP-2 and low levels of MMP-2 as compared with non-healing ulcer, supporting that increased protease activity favors a non-healing environment [120]. Decreased MMPs levels favor enhanced ulcer wound healing, and compression treatment for 4 weeks was associated with reduced levels of MMP-1, -2 and -3 and greater rate of venous ulcer healing [121].
Wound healing involves an orderly process of inflammation, re-epithelisation, matrix deposition and tissue remodeling and the expression of MMPs and TIMPs varies in different CEAP stages of CVI [115]. Regulation of MMPs profile in CVI occurs at gene expression, transcriptional and post-transcriptional levels [115]. Biopsies from patients with stasis dermatitis (C4a) show upregulation of MMP-1, 2 and 13, but downregulation of TIMP-1 and TIMP-2 as compared with healthy controls [113]. The distribution of MMP/TIMP in acute wounds and chronic venous ulcer also varies. MMP-9 and MMP-13 are localized in the chronic venous ulcer bed [112], MMP-1 is localized in migrating cells in chronic venous ulcer [112], and TIMP-1 and TIMP-3 are localized in proliferating cells in the edges of acute wounds [122], suggesting that MMP-1, TIMP-1 and TIMP-3 are vital for re-epithelialization, while MMP-9 and MMP-13 may be involved in the remodeling of collagenous matrix in chronic wounds.
Although MMPs may be increased in VVs, the upstream mechanisms causing upregulation of MMPs and the downstream mechanisms linking the increased MMPs to the structural and functional changes in the various components of the vein wall are not clearly established and will be discussed in detail below.
Increased Venous Hydrostatic Pressure, MMPs Expression, and Vein Wall Dilation
Veins are capacitance vessels that play a major role in determining the venous return and preload. Although the vein wall is relatively thin, it has three layers; the intima with lining ECs, media with several layers of VSM and adventitia which contains fibroblasts embedded in ECM of various proteins including collagen and elastin. The lower extremity has an intricate network of superficial and deep veins connected by perforator veins that pass through fascial spaces [123] (Fig. 2). Blood flows from the superficial to deep veins, except in the foot where the flow is reversed. The superficial veins include small saphenous vein, which starts from the ankle, runs in the posterior of the leg and joins the popliteal vein at the saphenopopliteal junction. The great saphenous vein starts from the ankle in the medial side of the lower limb and joins the common femoral vein at the saphenofemoral junction. Deep leg veins include common femoral, deep femoral, femoral, popliteal and tibial veins. In the upright posture, the blood in the lower extremity veins travels against gravity and the fluctuating thoracoabdominal pressure towards the central circulation. The saphenous veins and their tributaries are subjected to high hydrostatic pressure. In all individuals in the standing position the column of blood in the lower extremity venous system reflects a venous pressure at the ankle of 90–100 mmHg [124, 125], causing marked increases in vein wall tension. To cope with the high hydrostatic pressure, the leg superficial and deep veins are equipped with bicuspid valves that maintain blood flow in the cephalic direction and prevent its return toward the feet (Fig. 2). Vein valves function in concert with muscles mainly in the calf but also in the foot and thigh, to pump blood against gravity towards the heart. Leg veins also cope with the high hydrostatic pressure by compensatory changes in the vein wall function/structure. Persistent increases in venous pressure could lead to alterations in the vein wall structure and venous tissue remodeling.
Studies have shown that increased mechanical stretch or pressure in human tissues is associated with increased expression of MMPs not only in fibroblasts but also in ECs and VSMCs [3–6]. These observations have suggested that increases in venous pressure or wall tension may increase MMPs expression/activity, and that MMPs may then affect different wall components and cell types including ECM, fibroblasts, VSMCs and ECs. In later stages of the disease, severe increases in venous hydrostatic pressure cause EC damage, leukocyte infiltration, and superimposed venous inflammation characteristic of advanced stages of CVI.
MMPs appear to be induced by postural changes in patients with VVs. Comparison of plasma from the brachial vein and lower extremity VVs in patients in the standing position following 30 minutes of stasis have shown an increase in proMMPs such as proMMP-9 in plasma from VVs compared to arm vein. The increased proteolytic activity was associated with increased plasma levels of endothelial and leukocyte activation markers including ICAM-1, vascular cell adhesion molecule-1 (VCAM-1), angiotensin converting enzyme, and L-selectin levels of suggesting EC and polymorphonuclear cell activation and enzymatic granule release in VVs during periods of postural blood stasis [126, 127]. Importantly, upregulation of these markers in VVs was inversely proportional to the PO2 of the blood sampled [127]. These observations support a role of MMPs as important proteolytic enzymes and in the interaction between leukocytes and ECs in VVs, and a potential link to blood PO2 level.
Studies in animal models have further examined the relationship between increased venous pressure and MMP expression in the vein wall [128]. In a rat model of acute venous hypertension produced by creating a femoral arterio-venous fistula and examined at three weeks, most rats had venous reflux and increased venous pressure in the ipsilateral compared to the contralateral control femoral vein. Also, the pressurized veins were dilated and the valve leaflets length and width were reduced. Long term, the pressurized veins demonstrated marked inflammatory changes and leukocyte infiltration with increased expression of P-selectin and ICAM-1. There were no differences in MMP-2 or MMP-9 at three weeks, and the number of apoptotic cells in the vein wall and valves was increased [129]. In a follow-up study evaluating the effects of chronic venous hypertension there was an increased pressure in the femoral vein and progressive reflux at 42 days post arterio-venous fistula induction. The valves distal to the fistula demonstrated increased diameter, decreased height, and valve fibrosis in the media and adventitia. Valve obliteration and elevated MMP-2 and -9 levels were observed after 21 and 42 days of venous hypertension [130]. The presence of MMP-2 and -9 in the vein wall with valve destruction supports a link between MMPs and VVs formation. However, in this model only proximal segments of veins were analyzed, and whether the venous changes are caused by venous hypertension, venous arterialization, or a combination needs to be examined in order to evaluate if these venous abnormalities are transmitted to distal vein segments as observed in human CVD. Also, an increase in apoptosis in this model is not a feature of human VVs, highlighting the differences between the pathological changes in VVs as compared to those resulting from adaptation to an AV fistula.
Increased venous hydrostatic pressure may induce ECs permeability/injury, leukocyte infiltration and attachment, and initiate inflammation [5] (Fig. 1). Leukocyte wall infiltration and inflammation in turn activate MMPs and lead to ECM degradation, vein wall weakening and wall/valve fibrosis. Persistent venous wall dilation and valve dysfunction lead to further increases in hydrostatic pressure and CVD. The early stages of CVD are contained within the vasculature leading to clinical sign of VVs, while more advanced CVD causes progression to CVI affecting surrounding tissues and leading to skin changes and venous leg ulcer.
Experiments on isolated rat inferior vena cava (IVC) have shown that prolonged increases in vein wall tension are associated with decreased vein contraction and increased amount of MMP-2 and -9. The decreases in vein contraction were prevented in veins pretreated with MMP inhibitors supporting a role of MMPs as a link between increased venous pressure/wall tension, decreased vein contraction and increased venous dilation [8].
The observations that increased mechanical stretch or pressure is associated with increases MMPs expression in fibroblasts, ECs, and smooth muscle cells [3–6], have suggested that increased venous hydrostatic pressure could be a primary cause of elevated MMPs levels in VVs. In search for the upstream mechanisms linking the increases in vein wall tension to the changes in MMP expression and reduction in venous contraction, studies have pointed to a potential role of the hypoxia inducible factors HIF-1α and HIF-2α. HIFs are nuclear transcriptional factors which regulate genes involved in oxygen homeostasis [131]. The role of HIF as a potential factor linking increased venous pressure and MMP expression in VVs has been supported by reports demonstrating upregulation of HIF-1α and HIF-2α transcription factors, and HIF target genes in VVs compared with non-VVs. Also, exposure of VVs and non-VVs to hypoxic conditions was associated with increased amount of HIF-1α and HIF-2α protein and HIF target genes. These findings suggest that the HIF pathway may be associated with several pathological changes in the VVs wall, and that hypoxia may contribute to the pathogenesis of VVs [132]. In addition to the regulation of HIF by oxygen tension, other factors such as hormones, cytokines and metallic ions as well as mechanical stretch may induce HIF expression [131, 133]. Increased expression of HIF in response to mechanical stretch has been demonstrated in several tissues and cell types including the myocardium [134], fibroblasts [135], VSMCs [136], and skeletal muscle fibres [4, 137] In support of transcriptional regulation of HIF-1α by mechanical stress, studies have shown upregulation of HIF-1α mRNA by ~ two-fold in VSMCs subjected to cyclic stretch for 4 hr [136]. Other studies have shown increased HIF-1α and HIF-2α mRNA and proteins in rat capillary ECs of skeletal muscle fibers exposed to prolonged mechanical stretch [137]. The protein amount and activity of HIF-1α and HIF-2α may also be regulated by mechanical stretch [136–138]. Studies have shown an increase of HIF-1α protein in response to increased mechanical stress of the left ventricular wall by induction of aortocaval shunt or intraventricular balloon expansion [134]. Similar increases in HIF-1α protein have been shown in fibroblasts that are cyclically stretched for 24 hr [135]. Other studies have shown that the expression and activity of MMP-2 and MMP-9 can be regulated by HIF [139, 140]. Also, prolonged increases in rat IVC wall tension are associated with reduced vein contraction, and the reduction in vein contraction was reversed by the HIF inhibitors U0126 and echinomycin, and enhanced in the presence of the HIF stabilizer dimethyloxallyl glycine (DMOG) [127]. Prolonged vein wall stretch was also associated with increased expression of HIF-1α and -2α and MMP-2 and -9, and the increased expression of MMP-2 and -9 was reversed by HIF inhibitors. These findings suggest that prolonged increases in vein wall tension are associated with overexpression of HIF-1α and -2α, increased MMP-2 and -9 expression, and reduced contraction in rat IVC. These data are consistent with the view that increased vein wall tension secondary to venous hypertension may induce HIF overexpression and cause an increase in MMP expression and reduction of venous contraction, leading to progressive venous dilation and VVs formation [127].
The mechanism of HIF regulation by mechanical stretch is unclear, but may involve PI3K and MAPK [133, 136, 137]. Cell membrane ion channels, integrins, and receptor tyrosine kinases are mechano-sensitive to stretch [141]. Mechanical stretch may stimulate PI3K by activating Ca2+ influx through transient receptor potential ion channels such as TRPV4 [142]. Also, integrins may transduce mechanical stretch to initiate signaling cascades and MAPK activation [143]. Receptor tyrosine kinases and G protein-coupled receptors are also stimulated by biomechanical stress with subsequent activation of MAPK [144]. Mechanical stretch may also increase the generation of ROS which in turn activate MAPK [145, 146]. Interestingly, the increased HIF-1α and HIF-2α mRNA expression and the reduction in venous contraction associated with prolonged vein wall stretch were reversed in veins treated with MAPK inhibitors, supporting a role of MAPK in the regulation of HIF by mechanical stretch. As MMPs are upregulated in response to increases in venous pressure and HIFs and in VVs, an important question is how MMPs are activated and what are their potential substrates.
MMP Cleavage, Activation and Potential Substrates
MMPs are highly homologous Zn2+-dependent endopeptidases that cleave most of the components of ECM. The basic MMP structure consists of a prodomain, catalytic domain, hinge region, and hemopexin domain [55, 56] (Fig. 3). The catalytic domain contains the Zn2+ binding motif HEXXHXXGXXH and a conserved methionine, forming a ‘Met-turn’ 8-residues downstream, which supports the active site cleft structure around the catalytic Zn2+ [147].
Matrixins are synthesized as pre-proenzymes and the signal peptide is removed during translation to generate proMMPs. ProMMPs have a ‘cysteine switch’ motif PRCGXPD in which the cysteine residue coordinates with the catalytic Zn2+ in the catalytic domain, keeping the proMMP in an inactive form [148]. The cysteine switch interacts with the Zn2+ active binding site, and thereby prevents MMP activation and substrate degradation. MMPs may function through one of three catalytic mechanisms. The first mechanism called the base-catalysis mechanism is carried out by the conserved glutamate residue and Zn2+ [149]. In the second mechanism, the catalytic action involves an interaction between a water molecule and Zn2+ during the acid-base catalysis [150]. In the third mechanism, a histidine from the HEXXHXXGXXH-motif participates in catalysis by dissociation of Zn2+ from it, thus allowing the Zn2+ ion to assume a quasi-penta coordinated state. In this state, the Zn2+ ion is coordinated with the two oxygen atoms from the catalytic glutamic acid, the substrate’s carbonyl oxygen atom, and the two histidine residues, and can polarize the glutamic acid’s oxygen atom, proximate the scissile bond, and induce it to act as reversible electron donor. This forms an oxy-anion transition state. At this stage, a water molecule acts on the dissociated scissile bond and completes the hydrolysis of the substrate [151]. Collectively, upon binding of the substrate, the Zn2+-bound water molecule attacks the substrate carbonyl carbon, and the transfer of protons through a conserved glutamine residue to the amide nitrogen of the scissile bond results in peptide cleavage [152, 153]. The hemopexin domain confers much of the substrate specificity for MMPs. For instance, the hemopexin domain of MMP-1 collagenase is essential for the specificity of the catalytic cleavage of collagen. Also, MMP-2 is localized to specific extracellular collagenous sites by its fibronectin domains and MT1-MMP (MMP-14) requires the hemopexin domain for cell surface clustering as part of its collagenolytic capacity and ability to activate proMMP-2. The hemopexin domain also determines MT1-MMP binding and shedding of CD44 [154]. Activation of MMPs involves cleavage of the cysteine switch and detachment of the hemopexin domain, and may require other MMPs or other proteinases. For example, MMP-3 activates proMMP-1 into fully active MMP-1 [155]. ProMMP-2 activation takes place on the cell surface by most MT-MMPs, but not MT4-MMP [156], a process that may require TIMP-2 [157, 158]. ProMMP-2 forms a complex with TIMP-2 via their C-terminal domains, thus permitting the N-terminal inhibitory domain of TIMP-2 to bind to MT1-MMP on the cell surface. The cell surface-bound proMMP-2 is then activated by an MT1-MMP that is free of TIMP-2. MT1-MMP inhibited by TIMP-2 can also act as a “receptor” for proMMP-2. The MT1-MMP-TIMP-2-proMMP-2 complex is then presented to an adjacent free MT1-MMP for activation [159]. Thus, TIMP-2 may determine the MT1-MMP choice between direct cleavage of its own substrates and activation of MMP-2 [160]. However, for a number of MMPs including membrane-bound MMP-11, -23, -28, activation occurs intracellularly via the endopeptidase furin, which selectively cleaves paired base residues [65, 87, 90, 161].
Oxidants generated by leukocytes or other cells can both activate (via oxidation of the prodomain thiol followed by autolytic cleavage) and inactivate MMPs (via modification of amino acids critical for catalytic activity), providing a mechanism to control bursts of proteolytic activity. Several proMMPs are activated by ROS in vitro [162–165]. Foam cell derived ROS can activate proMMP-2. Nitric oxide (NO) may also activate proMMP-9 during cerebral ischemia by reacting with the thiol group of the cysteine switch and forming an S-nitrosylated derivative [165]. Also, hypoxia may increase MMP-2 and -9 mRNA expression [166]. Other MMPs such as MMP-9 depend predominantly on plasmin for activation [167]. MMP-7 is activated both by MMP-3 and by hypochlorous acid, a product of myeloperoxidase found in plaque macrophages. MMP-7 can activate MMP-1 [162, 168]. Also, serine proteinases such as neutrophil elastase may favor matrix breakdown by inactivating TIMPs [169, 170].
MMPs can be activated by heat, low pH, thiol-modifying agents such as 4-aminophenylmercuric acetate, mercury chloride, and N-ethylmaleimide, oxidized glutathione, sodium dodecyl sulfate, and chaotropic agents by causing disturbance of the cysteine-Zn2+ interaction at the cysteine switch [171].
Collagen type I, II, III, IV, V, VI, VII, VIII, IX, X, and XIV are known substrates of MMPs, with different efficacies (Table 2). MMP substrates also include other ECM proteins such as fibronectin, vitronectin, laminin, entactin, tenascin, aggrecan, myelin basic protein. Certain forms of ECM proteins may require the cooperative effects of several MMPs to accomplish their complete degradation. MMP-1 and -8 degrade fibrillar helices into fragments and unfold their triple helix conformation. The so-formed single α-chain gelatins are then further degraded by the gelatinases MMP-2 and -9 into oligopeptides [63].
Casein and gelatin are the most common substrates used to study MMP activity. Gelatin is a valid substrate particularly for MMP-2 and -9. While casein is not a physiologically relevant MMP substrate, it is a generic proteinase substrate digested by a wide range of proteinases.
Biological Effects of MMPs
MMPs affect different receptors and pathways such that the overall effects of MMPs vary depending on the predominant receptors or pathways in the tissue examined. Also, individual MMPs vary in their proteolytic activity and tissue substrates, further contributing to the discrepancy in the effects of MMPs [172]. MMPs play a role in many biological processes including tissue remodeling and growth, wound healing and angiogenesis, cell proliferation, migration, differentiation and apoptosis, as well as tissue defense mechanisms and immune responses [173, 174]. Defensins, a family of polar antimicrobial peptides that contribute to the innate immune system of some animals, are synthesized in an inactive proform that are activated by the proteolytic removal of the pro-domain by MMP-7, thus allowing them to insert into the bacterial membrane and disrupt its integrity [175, 176]. MMP-3 and -7 can also cleave all IgG proteins, an important process that prevents the initiation of the complement cascade and helps in the removal of IgG from damaged or inflamed tissue [177]. Also, the receptor of the complement component C1q (C1qR) exists in both a membrane-bound form and a soluble form that inhibits the hemolytic activity of C1q. MT1-MMP releases the membrane bound C1qR, thus allows tumor cells to avoid targeted destruction by the complement system and thereby facilitates tumor-cell survival [178–180]. MMPs also modulate many bioactive molecules at the cell surface [181], and may regulate cell signaling by interaction with G-protein-coupled receptors [182]. Changes in MMP expression/activity may also be involved in vascular remodeling and placentation during pregnancy and in the vascular changes associated with preeclampsia [57]. Increased expression of MMPs has been observed during different stages of mammalian development, from embryonic implantation [183] to the morphogenesis of different tissues including lung, bone and mammary gland [184, 185].
MMPs and ECM Degradation
ECM is the extracellular component of mammalian tissue that provides support and anchorage for cells, segregating tissues from one another, regulating cell movement and intercellular communication, and providing a local depot for cellular growth factors. Formation of ECM is essential for biological processes involved in maintaining tissue integrity and regeneration including wound healing and fibrosis. ECM consists mainly of fibers, proteoglycans and polysaccharides. Fibers are mostly glycoproteins and include collagen and elastin. Collagen is the main extracellular protein while elastin, which is exceptionally unglycosylated, provides flexibility for the skin, arteries and lungs. Proteoglycans are glycoproteins containing more carbohydrate than protein. Proteoglycans attract water to keep the ECM environment hydrated and also bind and store growth factors. Proteoglycans include chondroitin sulfate which provides tensile strength to cartilage, ligaments and aortic wall, heparan sulfate which regulates biological activities such as angiogenesis and blood coagulation, and keratan sulfate in cartilage and bone. Syndecan-1 is a proteoglycan and integral transmembrane protein that binds chemotactic cytokines and plays a role in the inflammatory process. Other components of ECM include laminin in the basal lamina of epithelia, and fibronectin which binds cells to ECM, modulates the cell cytoskeleton and facilitates cell movement. ECM also contains polysaccharides such as hyaluronic acid, and proteolytic enzymes which cause continuous turnover of ECM proteins [186–188].
ECM and its protein components provide the structural scaffolding necessary for blood vessel support, cell differentiation, signaling and function, as well as cell migration, epithelialization and wound repair. A key feature of the vein wall structure and integrity is its content of collagen and elastin, which are important MMP substrates. ECM contains other proteins and glycoproteins including fibronectin, vitronectin, aggrecan, entactin, proteoglycans, tenascin, fibrin, and laminin [189, 190]. The identification of MMPs in venous tissues undergoing remodeling supports their role in ECM turnover. MMPs are either secreted from the cell or anchored to the plasma membrane with heparin sulfate glycosaminoglycans. MMPs play an important role in the control of tissue architecture and in degradation of ECM and tissue remodeling [55]. MMPs degrade different components of ECM including collagen, casein and laminin. The collagenases MMP-1, -8, -13 and -14 efficiently degrade fibrillar collagens type I, II and III in their triple-helical domains [191]. Cleavage by these MMPs renders the collagen molecules thermally unstable so that they unwind to form gelatin, which is then degraded by other members of the MMP family including the major gelatinases MMP-2 and -9. MMP-2 localize at the cell surface by binding via its carboxyl terminus to integrin αvβ3 or the MMP-14-TIMP-2 complex [67]. When bound, the catalytic site of MMP-2 is exposed and can then be cleaved and activated. The α2 chains of collagen IV bind MMP-9 with a high affinity even when MMP-9 is inactive [192]. This juxtaposition of enzyme and substrate makes a pool of the enzyme that is rapidly available upon activation for any remodeling events.
In human saphenous vein grafts intended for bypass surgery, MMP production and ECM turnover have been implicated in intimal hyperplasia, a leading cause of graft stenosis and failure [193]. In a study evaluating intact versus endothelium-denuded human saphenous vein in culture for 14 days, veins with intact endothelium developed thicker neointima and increased production of MMP-9, that was mainly localized in the vicinity of internal elastic lamina. Inhibition of MMPs with doxycycline abrogated neoitima formation and MMP-9 expression [193]. Other studies examined the effects of perfusing saphenous vein segments ex vivo and demonstrated increased expression of MMP-2 and -9 when exposed to venous pressure, but a 50% decrease in the expression of gelatinases when veins were exposed to arterial pressure for up to 3 days. These data suggested an association between hemodynamic changes in venous pressure and shear stress, saphenous vein remodeling, and MMP expression [194]. Studies on saphenous vein grafts intended for coronary artery bypass surgery have also shown that the expression and activity of MMP-2 are higher in vein segments exhibiting increased pathologic venous remodeling and lower patency [195]. In addition to the role of MMPs in saphenous vein remodeling, MMPs have been implicated in the invasion of vena cava by metastatic pancreatic cancer. Specifically, liver metastasis is associated with vein invasion and overexpression of MMP-2 and -9 in vena cava [196].
Type III collagen is important for blood vessels elasticity and distensibility, and alterations in collagen synthesis and collagen type I to type III ratio may affect the vein wall architecture and lead to structural weakness, venous dilation and VVs formation. The levels of collagen I and III are co-regulated in fibroblasts, and addition of collagen III to cultured VSMCs from VVs decreases collagen I synthesis [37, 38]. Interestingly, among the various MMPs evaluated, proMMP-2 is increased in dermal fibroblasts cultured from patients with venous disease [197].
Both VVs and saphenous vein in the vicinity of varices show imbalance in ECM [198]. Decreased elastin has been implicated in the pathogenesis of VVs [199]. In contrast, studies have shown increased [198], decreased [200], or unchanged [201] collagen content in VVs wall. The net collagen content represents a balance between its biosynthesis and its degradation by MMPs [198]. In a study examining ECM proteins in 372 vein specimens from 17 patients and 36 control vein specimens from 6 patients, VVs showed marked increase in the ECM proteins collagen, laminin and tenascin, and borderline increase in fibronectin [202]. A study in 8 patients with VVs and 8 control subjects showed an increase in the elastic network, accumulation of collagen type I and fibrillin-1 and overproduction of MMP-1, -2 and -3 in the veins and skin of patients with VVs [203]. Importantly, normal appearing vein segments just inferior to VVs had the same ECM profile as the adjacent VVs [202].
The activity of MMPs in various tissues is tightly regulated by endogenous tissue inhibitors of MMPs (TIMPs) [55]. Alteration of the fine balance between MMPs and TIMPs contribute to the pathological changes in vascular diseases such as atherosclerosis, aneurysms and VVs [57]. MMPs have been proposed as biomarkers of certain pathological conditions including vascular disease, and are often assessed by measuring their plasma levels, tissue gene expression and protein amount, as well as proteolytic activity using gel zymography.
The venous dilation and tortuosity associated with VVs may be related to the effects of MMPs and their inhibitors TIMPs on ECM components and subsequent vein wall remodeling. The vein wall architecture markedly changes in VVs. In further defining the morphological ECM components and the role of a systemic effect of VVs in patients with CVD, the expression of matrix proteins collagen type I, fibrillin-1, and laminin, as well as MMPs and TIMPs were examined. The control group consisted of dermal biopsies and saphenous vein specimens from patients undergoing coronary revascularizaton. Both dermal biopsies and VVs specimens showed elevated matrix proteins, and MMP-1, -2 and -3 but not MMP-7 or -9 nor TIMP-1, -2 and -3, suggesting similar matrix protein production and degradation in VVs and adjacent skin [203]. These observations raise the possibility that a skin biopsy may be useful to identify patients at risk for the development of VVs before overt clinical manifestations. Other studies have shown an increase in collagen, a decrease in elastin and increased collagen:elastin ratio in both VVs and competent saphenous vein segments in proximity to varicosities compared to control saphenous vein, although gelatin zymography and elastase activity did not show any differences among the tissues examined. These findings suggested that in VVs and saphenous vein segments near the varices there is an imbalance in connective tissue matrix that may occur prior to valve insufficiency, and that increases in proteolytic activity and matrix degradation are not essential for VVs formation [198]. Also, evaluation of segments at the saphenofemoral junction from patients with VVs and bypass patients demonstrated that MMPs activity was unchanged in VVs compared with control veins, most MMPs located in the adventitia, and the amount of MMP-2 decreased while the amount of TIMP-1 increased. It was concluded that structural vein wall changes and VVs formation may occur despite a decrease in proteolytic activity [204]. Other studies have shown a three-fold increase in TIMP-1/MMP-2 ratio in VVs compared with control veins, suggesting that proteolytic inhibition and ECM accumulation may account for the pathogenesis of VVs [205]. These studies highlight several important points in VVs formation, namely imbalance in connective tissue matrix (collagen:elastin), imbalance of proteolytic tissue degradation, changes in vein wall connective tissue structure prior to valve insufficiency, and proteolytic activity and matrix degradation may not be necessary for VVs formation. These observations raise the question of what role do MMPs have in VVs formation if not degradation of ECM. In addition to their proteolytic effects on ECM in late stages of CVD, MMPs may affect other cell types including ECs and VSM particularly in early stages of VVs formation.
Varicose Veins, MMPs, and VSM Dysfunction
Studies have examined if the ECM changes observed in VVs are related to abnormalities in VSMCs. VSMCs cultured from VVs have decreased number of cells staining for collagen type III and fibronectin compared to VSMCs from control veins, although the transcriptional products of these two proteins were similar in VVs and control veins. The synthesis and deposition of collagen type III but not type I is markedly lower in VVs VSMC culture. Analysis of the supernatant of confluent cells showed no differences in the amount of MMP-1, -2 and -9 and TIMP-1 and -2 in cells from VVs vs. control veins. These data suggested that the regulation of collagen type III and fibronectin in VSMCs was altered at the post-transcriptional level [206]. Other studies have shown that treatment with non-selective MMP inhibitor marimastat (BB-2516) caused partial restoration of the production of collagen type III in VSMCs from VVs. Also, both gene transcription and protein amount of MMP-3, which degrades fibronectin, was elevated in cells from VVs. It was concluded that the degradation of collagen type III and fibronectin in VSMCs cultured from VVs likely involves the expression of MMP-3 [207]. Thus, cultured VSMCs from VVs show imbalance in collagen production with increased type I collagen but suppressed type III collagen production. Because of normal mRNA expression of type III collagen, the reduction in its production may be related to post-transcriptional events such as degradation/inhibition by MMP-3 and could be manifested as changes in the mechanical properties, elasticity and distensibility of the VVs wall. Recent studies suggested that desmuslin gene expression is required for the maintenance of VSMC phenotype, and decreased desmuslin expression may affect differentiation of VSMCs and contribute to the development of VVs [208].
Effects of MMPs on VSM Contraction
MMPs, via PI3K and ATP synthesis, may transactivate EGFR and mediate α-adrenergic receptor-induced maintenance of vascular tone. Inhibition of the expression of MMP-2 or -7 blunted the phosphorylation of Akt by PI3K and thus inhibited the response to phenylephrine (Phe) in rat mesenteric artery [209]. Other studies have shown that MMP-2 and -9 inhibit Phe-induced contraction of rat aorta [210]. The MMP-induced inhibition of aortic contraction is concentration- and time-dependent and reversible, suggesting that the actions of MMPs are not solely due to irreversible degradation of ECM. Also, the inhibitory effects of MMPs on VSM contraction are not likely due to degradation of Phe or α-adrenergic receptors because MMPs also inhibit prostaglandin F2α-induced contraction, suggesting that the effects of MMPs are not specific to a particular agonist/receptor, but likely involve direct effects on common VSM contraction pathway(s) downstream from receptor activation.
Although some studies suggest an increase in MMPs immunoreactivity in the adventitia, endothelium and VSM of the VVs wall [97], the question remains as of how MMPs cause venous dilation and VVs formation. The role of MMPs in VVs has largely been attributed to their proteolytic effects on ECM, degradation of the valve leaflets and weakening of vein wall structure [128, 211]. The localization of MMPs in the VVs wall adventitia and fibroblasts is consistent with a role in ECM degradation [97]. The proteolytic and degenerative effects of MMPs on ECM could play a role in late stages of VVs formation. On the other hand, the localization of MMPs in the vicinity of the venous endothelium and VSM raises the possibility of an additional effect of MMPs on these cell types [97, 212].
Recent evidence suggests that MMP-2 and -9 may affect aortic VSM function [210]. Also, in rat IVC MMP-2 caused acute relaxation of Phe-induced contraction [7]. VSM contraction is triggered by increases in Ca2+ release from the intracellular stores and Ca2+ entry from the extracellular space. MMPs do not inhibit Phe-induced contraction in Ca2+-free solution, suggesting that they do not inhibit the Ca2+ release mechanism. On the other hand, MMPs inhibit Phe-induced Ca2+ influx [210]. The mechanism by which MMPs inhibit Ca2+ entry could involve direct effects on Ca2+ channels. MMPs may also affect K+ channels [7]. MMP-2 induced IVC relaxation was abolished in 96 mmol/L KCl depolarizing solution, which prevents outward movement of K+ from the cell through K+ channels. To further define the K+ channels involved, it was found that MMP-2 caused further relaxation of IVC segments in the presence of cromakalim, activator of ATP-sensitive K+ channel (KATP), suggesting that MMP-2 was not working through KATP channel. In contrast, blockade of large conductance Ca2+-activated K+ channels (BKCa) using iberiotoxin inhibited the MMP-2 induced venous relaxation, suggesting that MMP-2 actions involve hyperpolarization and activation of BKCa. MMP-2 induced activation of K+ channels likely causes VSM hyperpolarization and leads to decreased Ca2+ influx through voltage-gated channels [7]. This is supported by reports that MMP-2 and -9 cause aortic relaxation by inhibiting Ca2+ influx into aortic VSM [210] and MMP-2 inhibits Ca2+-dependent contraction in rat IVC [9]. MMPs may also induce collagen degradation and produce Arg-Gly-Asp (RGD)-containing peptides, which could bind to αvβ3 integrin receptors and inhibit Ca2+ entry into VSM [213]. MMPs may also stimulate protease-activated receptors (PARs) and activate signaling pathways that could lead to blockade of VSM Ca2+ channels [214]. This is supported by reports that proteases such as thrombin activate PARs and promote endothelium-dependent VSM relaxation by inhibiting Ca2+ influx [215]. These data demonstrate novel effects of MMPs on venous function and suggest that protracted MMP-2 induced vein relaxation could lead to progressive wall dilatation and superimposed valve dysfunction, leading to VVs formation and CVD [57]. While MMPs may affect VSM contraction and ion channels, further studies are needed to define the role of integrins and PARs as possible molecular mechanisms via which MMPs could inhibit VSM contraction.
MMPs and VSMC Migration
MMPs play a role in VSMC migration. In rat aortic smooth muscle cells (RASMCs) cultured on collagen I gel to mimic ECM, exposure to flow enhanced cell motility. Upregulation of MMP-1 enhanced flow-induced motility, and the MMPI GM-6001 attenuated flow-induced migration. ERK1/2 phosphorylation and increased expression of AP-1 transcription factors c-Jun and c-Fos appear to be involved in MMP-mediated enhancement of flow-induced cell motility [216]. Young human aortic smooth muscle cells (HASMCs) produce active MMP-2 and have higher migratory capability than aged cells, likely due to increased MT1-MMP content and activation of proMMP-2 in young cells. In contrast, aged cells produce only proMMP-2, and its activation is prevented by upregulation of TIMPs, and treatment of young cells with TIMP-1 and -2 promotes aged HASMCs migratory behavior [217]. MMP-2 activity could also influence chemokine-induced chemotaxis of human VSMC monolayers [218]. Also, MMP-2 knockout decreases VSMC migration and intima formation in mouse model of carotid artery ligation [219, 220]. MMP-9 may also play a role in VSMC migration. Tanshinone IIA, a major constituent of Salvia miltiorrhiza bunge, inhibits TNF-α-induced HASMC migration, partly through inhibition of MMP-9 activity. Tanshinone IIA also inhibits TNF-α-induced ERK and c-jun phosphorylation, and NF-κB and AP-1 DNA-binding [221]. Suppression of MMP-9 expression by downregulation of NF-κB mediates the inhibitory effect of curcumin on migration of HASMCs [222]. Also, MMP-9 knockout is associated with reduced VSMC migration and intima formation in mouse model of filament loop injury [223] or carotid artery occlusion [224].
Disruption of the basement membrane is essential for VSMC migration [225]. MMPs, by degrading the basement membrane, can facilitate ECM integrin interactions leading to activation of focal adhesion kinases (FAK) and increased cell migration. MMPs also cause fragmentation of membrane components such as type I collagen, thus creating new integrin-binding sites. Growth factor receptors, cadherins and integrins mediate signaling pathways that play a role in reorganizing the cytoskeleton in preparation for migration [226, 227]. MMPs cleave E-cadherin in epithelial cells, VE-cadherin in ECs and N-cadherin in VSMCs [228, 229], thus dissolving adherence junctions and allowing the cells to migrate.
MMPs not only facilitate migration by promoting proteolysis of ECM, but may also directly enhance cell migration. MMP-1 promotes growth and invasion of cells by binding to and cleavage of PAR-1 which reveals a tethered ligand that initiates signaling via a G protein-coupled receptor and activates migration [230]. This mechanism allows the cells to sense a proteolytic environment and actively move towards an area of degraded matrix. Rearrangement and migration of SMCs into the intima may be seen in VVs [231–234]. SMCs in VVs appear disorganized and dedifferentiated from contractile to synthetic and proliferative phenotype, and demonstrate features such as vacuolization and phagocytosis [231, 232].
MMPIs have been used to demonstrate the effect of MMPs on VSMC migration. Gene transfer of TIMPs reduces VSMC migration in vitro and inhibits or delays intima thickening in vivo. TIMPs 1–4 delivered directly or by gene transfer inhibit migration of SMCs in vitro [235, 236] and reduce neointima formation in organ culture of human saphenous vein [237]. TIMP gene transfer also preserves medial basement membrane and inhibits VSMC migration to the intima. Synthetic MMPIs inhibit migration of VSMC from baboon arterial explant in culture [238], and early VSMC migration in the rat carotid balloon injury model [239]. Thus MMPs enhance VSMC migration, and MMPIs could reverse or prevent cell migration.
MMPs and VSMC Proliferation
In addition to facilitating VSMC migration, MMPs may regulate VSMC proliferation. Pretreatment of HASMCs with ethanol extract of Buddleja officinalis attenuates high-glucose-induced cell proliferation by suppressing MMP-9 activity [240]. Also, MMP-9 knockout is associated with inhibition of VSMC proliferation after filament loop carotid artery injury [223], but not after tying off the mouse carotid artery [224]. These inconsistent results suggest compensatory activation of other proteases [241]. When compared with SMCs derived from normal veins, SMCs derived from VVs demonstrate increased proliferation (2-fold), migration (3-fold), MMP-2 production (3-fold), and collagen synthesis (>2-fold), with decreased expression of phenotype-dependent markers [242].
MMPs can regulate VSMC proliferation by promoting permissive interactions between VSMC and components of ECM. Integrin-mediated pathways may be essential for stimulation of VSMC proliferation [243, 244]. Also, MMPs may free growth factors from attachment to ECM components or cell surface so that they can act on their receptors. ECM binds growth factors either directly or via growth-factor-binding proteins. MMPs stimulate the release of growth factors such as transforming growth factor β (TGF-β), fibroblast growth factor 1 (FGF-1), insulin-like growth factor 1 (IGF-1), TNFα and heparin-binding epidermal growth factor (HB-EGF) by cleaving either the growth-factor binding protein or the matrix molecule to which these proteins attach. HB-growth factors, in particular FGF-1 and FGF-2, are potent mitogens for VSMCs and are released by the action of MMPs on proteoglycan core proteins [55]. Although ADAMs are often implicated, MMPs could also be responsible for releasing cell surface HB-EGF and stimulation of VSMC proliferation [245, 246]. MMPs also activate TGF-h by cleaving off the latency-associated peptide [247]. MMPs can also liberate active IGF-1 by degrading its binding proteins. Together with signals from FAK, these processes upregulate and/or stabilize key regulators of the cell cycle. Also, MMP-induced cadherin shedding promotes dissolution of adherens junctions and translocation of h-catenin to the nucleus where it acts as a transcription factor to further promote cell proliferation [227, 229]. MMP-3 and -7 can cleave the adherens-junction protein E-cadherin [248–252]. MMP-3 can release a soluble form of the adhesion molecule L-selectin from leukocytes, and shed membrane-bound HB-EGF to exert signaling functions [248]. MMP-7 may release soluble Fas ligand which induces apoptosis [253]. Some growth factors are proteolytically inactivated by MMPs, including the chemokine connective tissue activating peptide III (CTAP-III), monocyte chemoattractant protein (MCP) and stromal cell-derived factor 1 [254, 255]. Growth factors and cytokines are also negatively regulated when MMPs cause shedding of their receptors from the cell membrane, as in the case of surface FGF receptor 1 [256].
MMPIs have been used to study the role of MMPs in VSMC proliferation. Some studies have shown excess neointima proliferation in rat carotid arteries subjected to balloon injury after treatment with the MMPI GM-6001 [257, 258]. Other studies have shown that synthetic MMPIs inhibit VSMC proliferation in vitro [229, 259]. Also, inhibition of MMPs is associated with decreased N-cadherin shedding, increased cell membrane N-cadherin levels, decreased h-catenin nuclear translocation and decreased proliferation of cultured human VSMCs. Dismantling of cadherin:catenin complex also occurs in balloon-injured rat carotid arteries in vivo leading to increased expression of the cell cycle gene cyclin D1 and VSMC proliferation [260]. Tetracycline-based MMPIs reduce VSMC migration and neointima formation after balloon injury of rat carotid artery [239, 261]. Thus, most of the experimental evidence points to a stimulatory effect of MMPs on VSMC proliferation, and inhibition of this effect by MMPIs.
Increases in wall tension may elicit a remodeling process in the vein wall similar to that during varicosis, and this biomechanical force may be sufficient to elevate the proteolytic and proliferative activity of VSMCs. Pressure-induced increase in MMP-2 expression, gelatinase, and proliferative activity is dependent on AP-1. An increase in venous filling pressure and circumferential wall tension is sufficient to activate AP-1, which in turn triggers varicose remodeling through fuelling MMP-2 activity and VSMC hyperplasia in the vein wall. Also, in both native and cultured VSMCs exposed to increased stretch inhibition of AP-1 decreased cell proliferation, MMP-2 expression and gelatinase activity by up to 80%. AP-1 also regulates the expression of many stress-response genes, including those associated with a proinflammatory phenotype of ECs and VSMCs [262, 263]. To test if AP-1 was a suitable target for therapeutic interference with varicosis, its activity was blocked in vivo and in vitro by a specific decoy ODN. These nucleic acid-based drugs have been introduced as potent inhibitors of maladaptive remodeling processes [264, 265]. Without the need for potentially harmful transfection reagents, the AP-1 decoy ODN-supplemented ointment readily penetrated the skin of the mouse auricle and locally inhibited the stretch-induced MCP-1 expression [266]. Also, application of the AP-1 decoy ODN virtually abolished outward venous remodeling and tortuosity in vivo, likely due to its inhibitory effects on EC and SMC proliferation, MMP-2 expression and gelatinase activity. In cardiac fibroblasts heterodimerization of the AP-1 subunits JunB-Fra1 and JunB-FosB may be crucial to control transcription of the MMP-2 gene [267]. This finding is in line with the observation that isolated perfused JunB-deficient mouse veins do not respond to increased hydrostatic pressure with an increase in MMP-2 expression. While taking into account its additional anti-inflammatory effects, therapeutic inhibition of stretch-induced MCP-1 expression attenuates the initiation and progression of VVs remodeling and may be effective in preventing recurrence. HMG-CoA reductase inhibitors — cholesterol synthesis inhibitors that inhibit AP-1 as part of their pleiotropic activity — may be potential drugs to treat VVs [268].
MMPs and VSMC Apoptosis
Apoptosis is a form of cell death that involves activation of the intracellular cysteine proteases, caspases. Apoptosis of VSMCs plays a role in attenuating intimal thickening and destabilizing atherosclerotic plaques [269, 270]. Several factors promote apoptosis including death signals originating from outside the cell and processes within the cell such as DNA damage, cell cycle status and levels of the tumor suppressor p53 [270]. MMP-7 is involved in the cleavage of N-cadherin and thus modulates VSMC apoptosis. In contrast, survival signals maintain VSMC viability even in the face of a pro-apoptotic environment. Survival pathways are closely linked to those involved in proliferation and therefore could be influenced by MMPs. Survival factors such as PDGF, HB-EGF and IGF-1 act via tyrosine kinase receptors to stimulate the PI3-K/Akt pathway. MMP-2, -7 and -9 cleave cell surface pro-HB-EGF and liberate the soluble active growth factor which binds to EGFR and promotes growth [245, 271]. Activation of PDGFR-β and ERK1/2 is involved in the production of MMP-1 in oxLDL- and 4-hydroxynonenal-stimulated human coronary VSMCs [272]. MMP-1, -2, -8 and -9 degrade members of the IGF binding protein family and thereby increase the bioavailability of IGF-1 and its anti-apoptotic effects [55].
Cell-matrix contacts promote VSMC survival, and their disruption leads to apoptosis and anoikis [273]. FAK activation triggered by ECM-integrin interactions induces p53, a survival signaling pathway [274, 275]. Regulated MMP production appears to favor FAK activation and hence survival signaling. Conversely, excessive MMP production could degrade ECM proteins or integrins and promote anoikis [276]. MMPs may also modulate apoptosis by cleaving death ligands such as TNF-α and Fas ligand and their receptors. MMP-1, -2, -9, -8 and -13 and the MT-MMPs MMPs 14–17 can cleave pro-TNF-α [55, 277]. Similarly, MMP-7 sheds Fas-L from the surface of several cell types [278, 279]. Caspase-mediated cleavage of the DNA repair enzyme poly-ADP ribosepolymerase is an important step in apoptosis, and in isolated cardiac myocytes, nuclear-localized MMP-2 can carry out this cleavage [280].
TIMP-3, but not TIMP-1 or -2, is an effective stimulator of apoptosis in many cells including VSMCs [236, 279], suggesting that an ADAM rather than an MMP is the target. TIMP-4 also stimulates VSMC apoptosis [281]. Thus, MMPs may regulate VSMC apoptosis via several pathways, and MMPIs could oppose the effects of MMPs on apoptosis. However, the role of VSMC apoptosis has not been clearly defined in VVs
MMPs and Endothelium-Dependent Vascular Relaxation
ECs control vascular tone by releasing relaxing factors including nitric oxide (NO), prostacyclin (PGI2), and also through hyperpolarization of the underlying VSMCs by endothelial-derived hyperpolarizing factor (EDHF) [282]. EDHF-mediated responses involve an increase in the intracellular Ca2+ concentration, the opening of Ca2+-activated K+ channels of small and intermediate conductance and hyperpolarization of ECs. EC hyperpolarization spreads via myoendothelial gap junctions and result in EDHF-mediated hyperpolarization and relaxation of VSMCs [283–285]. MMPs may have endothelium-dependent vasorelaxation effects. MMP-2 may mediate the bacterial LPS-induced vascular hyporeactivity to vasoconstrictors in rat aorta via an endothelium-dependent mechanism [286]. MMPs could increase PGI2 synthesis and activate PGI2-cAMP pathway, or increase EDHF release and enhance K+ efflux via K+ channels leading to venous tissue hyperpolarization and relaxation [287]. MMP-2 induced IVC relaxation is abolished in high KCl depolarizing solution, which prevents outward movement of K+ from the cell through K+ channels, and inhibited by the BKCa blocker iberiotoxin, suggesting a role of a hyperpolarization pathway [7]. Long-term, these novel effects of MMPs on venous relaxation pathways could lead to progressive venous dilatation and VVs formation.
Upregulation of MMPs may also be associated with impaired vasorelaxation. Upregulation of MMP-3 and downregulation of TIMP-1 mediate the impaired endothelium-dependent vasodilation, EC apoptosis and endothelial disruption exerted by FOXO3 in HUVECs [288]. Also, upregulation of MMP-2 and -9 may be responsible for nicotine-induced endothelial disruption and unresponsiveness of blood vessels to the vasodilator acetylcholine, and the MMPI doxycycline partially reversed this effect [289]. In renovascular rat model of hypertension, antioxidant treatment inhibited the decrease of endothelium-dependent vasorelaxation and attenuated the vascular dysfunction and remodeling by inhibiting oxidative stress-induced upregulation of MMP-2 [290].
Effects of MMPs on EC Integrity and Vascular Permeability
MMPs may regulate EC integrity and vascular permeability. MMP-1 mediates the activation of HUVECs into prothrombotic, proinflammatory, and cell-adhesive state by supernatants from cultured melanoma and colon cancer cells [291]. In mouse aorta, MMP-13 mediates the endothelial protective effect of NO by cleaving the pro-inflammatory ICAM-1 [292]. Infiltration of inflammatory cells and increased expression of surface markers including VCAM-1 and ICAM-1 is often observed in ECs of VVs [95, 231, 293]. MMPs may also increase vascular permeability and cause vascular disruption. Upregulation of MMP-2 and -9 mediate the increase in membrane permeability and vascular disruption induced by human immunodeficiency virus-1 envelope gp120 in rat brain [294]. Also, MMP-2 and -9 decrease the barrier integrity of primary porcine brain capillary ECs [295]. In support of these findings, the MMPI GM6001 prevents degradation of the tight junction protein occludin and reduces the intercellular gap formation and permeability in porcine cerebral microcapillary ECs [296]. Thus the expression of MMPs by SMCs and ECs may influence the relaxation/constriction properties of the vein wall and contribute to the development of VVs. Also, the loss of endothelial integrity may expose the components of the vein wall to degradation by MMPs.
Management of Varicose Veins
Management of VVs comprises compression therapy, pharmacological treatment, sclerotherapy and surgical intervention (Table 3). Graduated compression stockings relieves the edema and pain, controls progression to more severe forms of CVI with skin changes and ulceration, and prevents venous thromboembolism following surgery [297–301]. The benefits of compression garments in patients with CVI may not be related to improvement of the deep venous hemodynamics [302]. Instead, graduated compression stockings with an ankle pressure of 21 mmHg affect the residual venous volume fraction, which is linearly correlated to the amount of reflux and a measure of venous hypertension in the limb [303].
Table 3.
Management of varicose veins
| Treatment Strategy | Specific Treatment | Reference |
|---|---|---|
|
| ||
| Compression Therapy Pharmacological Treatment | Graduated compression stockings | [297] |
|
| ||
| Pharmacological Treatment | - α-benzopyrones (Coumarins) | - [447] |
| - γ-benzopyrones (Favonoids) | - [304] | |
| - Saponosides (Escin, horse chestnut seed extract) | - [306] | |
| - Plant extracts (Blueberry and grape seed, Ginkgo biloba) | - [311] | |
| - Daflon-500 | - [312] | |
| - Venoruton (Oxerutin) | - [309] | |
| - Others: Pentoxifylline, red vine leaves (AS-195), prostaglandin E1 | - [317, 319, 320] | |
|
| ||
| Sclerotherapy | Sodium tetradecyl sulfate (STS), sodium morrhuate with polidocanol | [321] |
|
| ||
| Surgical Intervention | - Endovenous Ablation (Radiofrequency or infrared laser) | - [325] |
| - Surgical stripping | - [329] | |
| - Phlebectomy (Stab phlebectomy, transilluminated power phlebectomy) | - [330] | |
Pharmacological treatment of VVs and CVD comprises naturally occurring plant extracts and glycosides including α-benzopyrones (coumarins), γ-benzopyrones (flavonoids), saponosides (escin, horse chestnut seed extract), and plant extracts (blueberry and grape seed, Ginkgo biloba) (Table 3). Although the precise mechanism of action of these venoactive drugs is not known, they appear to improve venous tone and capillary permeability, and induce venotonic stimulation and leukocyte modulation. Flavonoids may affect leukocytes and the endothelium and modify the degree of inflammation and reduce edema [304]. Saponosides may limit the distensibility and morphologic changes in the vein wall [305]. In rat aortic rings, escin (horse chestnut seed extract) protects the endothelium, causes Ca2+-dependent venous contraction [306, 307], and decreases contraction to venoconstrictors such as α-adrenergic and AngII (AT1R) receptor stimulation and membrane depolarization [307]. Diosmin may improve venous tone, microvascular permeability, lymphatic activity, and microcirculatory nutritive flow [308]. Also, rutosides enhance endothelial function in patients with CVI [309].
In patients with CVI there is elevated circulating ECs, a marker of venous injury and ischemia [310]. In a randomized clinical trial Ginkgo biloba extract, troxerutine, and heptaminol (Ginkor Fort) were tested for their venoprotective effects in patients with CVI. The treated patient had decreased circulating ECs, supporting venoprotective effects of these compounds against EC injury associated with CVD [311]. Daflon 500, a combination of the active venotonic diosmin and the inactive compound hesperidin, is formulated as a micronized purified flavonoid fraction for increased absorption. In 3 randomized clinical trials, involving 183 patients and equal number of controls, hemodynamic and clinical improvement of symptoms were observed in patients receiving Daflon vs. placebo [312]. Also, a trial of 231 patients with CVI showed that a combination of coumarin and the flavonoid troxerutin, with compression garments for 12 weeks resulted in less edema and pain compared with placebo [313]. Another trial evaluating the use of venoruton (oxerutin) vs. Daflon in patients with CVI for 8 weeks has shown that venoruton improved symptoms as assessed by a validated venous quality of life tool [309]. Also, a controlled trial in patients with venous ulcer demonstrated that using Daflon 500 in addition to compression increased the number and rate of healed venous ulcers and improved venous symptoms [314]. Horse chestnut seed extract is as effective as compression stockings in reducing the CVD symptoms of heaviness, itching, leg edema and pain in the short-term, but its long-term safety and efficacy have not been established [315, 316].
Other compounds have been tested in advanced CVI. In clinical trials pentoxifylline increased healed ulcers and healing rate over placebo, but its mechanism of action was unclear [317, 318]. Also, red vine leaves (AS 195) may increase microcirculatory blood flow and transcutaneous oxygen tensions and alleviate edema in CVD [319]. Prostaglandins that inhibit platelet aggregation, leukocyte activation and capillary permeability and promote venodilation and fibrinolysis can be useful. In a randomized trial, infusion of prostaglandin E1 vs. placebo for 20 days showed healed venous ulcers at 4 months, and a decrease in ulcer area at 80 days, suggesting beneficial effects in venous ulcer healing, possibly by improving the microcirculation and reducing the hypoxemia associated with venous ulcer [320].
Sclerotherapy is widely used for treatment of small telangiectasias (spider veins), reticular veins, and large VVs. For smaller diameter veins diluted forms of sclerosing agents are used to avoid tissue inflammation and necrosis. Concentrated sclerosing agents such as hypertonic saline, sodium morrhuate, and ethanolamine oleate are less commonly used because of their side effects. Sodium tetradecyl sulfate (STS) is a detergent that can be used as liquid or foam. Sodium morrhuate and STS are approved for treating varicosities in the United States. Polidocanol is superior to normal saline in obliterating incompetent VVs and improving venous hemodynamics [321]. Sclerotherapy with polidocanol foam under duplex ultrasound guidance is a standard treatment of intracutaneous telagectasies, subcutaneous VVs, transfascial perforating veins and venous malformations in Europe [322]. Polidocanol and STS are used in Europe and South America as a mixture of 1:4 liquid:air, which increases the surface area and contact with the vein wall. Because of some concerns such as air emboli, visual disturbances, cough and migraines, this form of therapy is not approved in the United States [323, 324].
Surgical treatment of CVI includes endovenous ablation, surgical stripping and phlebectomy of superficial veins. Endovenous ablation is performed with either radiofrequency or infrared laser at wavelengths 810 to 1320 nm, but could go up 1470 and 1550 nm. The resulting high intensity endoluminal thermal heat causes endothelial protein denaturation and vein occlusion [325]. Endovenous ablation has relatively good occlusion rates and long term benefits. Laser therapy has only 3–7% recurrence rates at 2–3 years follow up [326, 327], while radiofrequency therapy has 2% recanalization rate at 4 years follow up [328]. Surgical stripping of the saphenous vein with high ligation of the saphenofemoral junction is considered durable and the standard for many patients with CVD [329]. Large varicose clusters that communicate with the incompetent saphenous vein can be avulsed by “stab phlebectomy”. An alternative to open phlebectomy is the use of transilluminated power phlebectomy to remove clusters of varicosities using fewer incisions and thereby decrease the operation time [330]. In a clinical trial, 500 patients with incompetent superficial and deep venous systems and demonstrable CVI and venous ulcer were randomized to surgery only to the superficial veins plus compression vs. compression alone. Patients having surgery of the superficial veins demonstrated marked reduction in ulcer recurrence (12%) compared with compression alone (28%) at 12 months [331].
MMP Inhibitors in Varicose Veins
The current strategies for management of VVs have variable success rates, and recurrence of varicosities is observed in many cases. Also, most of the current therapies are used in overt and advanced cases of VVs, and few therapies are available for prevention or limiting the progression of early manifestations of CVD. The failure/recurrence of some of the current CVD treatment strategies is largely related to the fact that most of these techniques are designed to treat the manifestations rather than the cause of the disease. MMP inhibitors (MMPIs) have been used to investigate the role of MMPs in different physiologic processes and in the pathogenesis of specific diseases. Loss of certain cellular function following treatment with MMPIs has supported a role of MMP in the regulation of specific cellular functions. MMPIs have also been investigated as potential therapeutic tools for arthritis, cancer and vascular disease. The observations that MMPs expression/activity is modulated during the development of VVs and its progression to thrombophlebitis and wound ulcers have raised interest in the potential use of MMPIs in the management of CVD.
MMPIs can be macromolecules such as endogenous tissue inhibitors of MMPs (TIMPs) and monoclonal antibodies or small molecules (natural and synthetic products) (Table 4). Synthetic MMPIs include Zn2+ binding globulins (ZBG), non-ZBG, and mechanism-based inhibitors. Most MMPIs contain a Zn2+ binding group (e.g. hydroxamic acid, carboxylic acid, sulfhydryl group) that is either substituted with a peptide-like structure that mimics the MMP substrate that they cleave or appended to a smaller side chain substituent that interact with specific subsite or pocket (e.g., P1′, P2′, P3′) within the MMP active site [332]. The potency and specificity of an MMPI can be determined by the substituent groups. Substituent groups include α-substituents (increase the inhibitory activity against specific MMPs), P1′ substituents (major determinant of activity and selectivity), P2′ substituents (variable range of substituent groups; steric bulk proximity to amide provides oral bioavailability), and P3′ substituents (variable range of substituent groups; charged or polar groups affect biliary excretion) [333]. While a large number of MMPIs have been developed, many of them lack specificity. Novel pharmacological tools and genetic engineering have improved the specificity of MMPIs to target specific MMPs in vascular disease [53].
Table 4.
MMP inhibitors, their selectivity to MMPs (IC50 or Ki <1 nM to 10 μM), and their use in experimental trials.
| MMPI | MMP Specificity, IC50 or Ki | Experimental Trials | Ref. | ||||
|---|---|---|---|---|---|---|---|
| <1 nM | 1–10 nM | 11–100 nM | 0.1–1 μM | 1–10 μM | |||
| ZBG 1 Hydroxamates Batimastat, BB-94) | MMP-1, 2, 8, 9 | MMP-3 | [152] | ||||
| 2 Carboxylates, Prinomastat, AG3340 | MMP-2, 3, 9, 13, 14 | MMP-1 | MMP-7 | O2-induced retinal neovascularization, neuronal hypoxic injury, ventilator-induced lung injury, lung cancer, uveal melanoma, gliomas, prostate cancer | [448–453] | ||
| 3 Hydrazides | MMP-1 | [454] | |||||
| 4 Sulfonyl-hydrazides RS-104966 | MMP-13 | MMP-1 | [191] | ||||
| 5 | MMP-13 | MMP-3, 8 | MMP-2 | MMP-7, 9, 14 | Osteoarthritis | [455] | |
| 6 | MMP-11 | MMP-3, 12 | MMP-1, 9, 14 | Chronic obstructive pulmonary disease | [456] | ||
| 7 | MMP-2 | MMP-8, 9, 14 | MMP-1, 3 | MMP-7 | Stop tumor invasion | [359] | |
| 8 Mercaptosulfides | MMP-3 | MMP-2 | MMP-9 | Chronic non-healing wounds | [361] | ||
| 9 Carbamoyl phosphonates | MMP-2, 9 | MMP-1 | MMP-7 | MMP-3 | [382] | ||
| 10 | MMP-2, 3 | MMP-1 | [457] | ||||
| 12 | MMP-3 | [458] | |||||
| 16 | MMP-9 | MMP-2 | MMP-1, 7, 14 | MMP-3 | [383] | ||
| 17 | MMP-8 | MMP-2 | MMP-3 | [369] | |||
| 18 | MMP-8 | MMP-2, 9, 13, 14 | MMP-1, 3 | MMP-7 | Acute liver disease, multiple sclerosis, breast cancer | [459–461] | |
| 19 | MMP-11 | MMP-2, 9, 13 | [462] | ||||
| 20 | MMP-2 | Melanoma | [385] | ||||
| 21 | MMP-2 | Melanoma, prostate cancer | [384] | ||||
| 22 | MMP-11 | [387, 397] | |||||
| 23 Ro-28-2653 | MMP-2, 14 | MMP-8, 9 | MMP-3 | Anti-angiogenic and anti-invasive in tumor models | [389, 395] | ||
| 24 | MMP-13 | MMP-2, 9, 12 | Osteoarthritis | [388] | |||
| 25 Pyrone-Based AM-6 | MMP-3 | [401] | |||||
| 26 1,2 -HOPO-2 | MMP-8, 12 | MMP-2, 3 | MMP-13 | Heart ischemia and reperfusion | [402] | ||
| 27 | MMP-2, 9, 13 | MMP-3 | MMP-1 | Brain edema following ischemia–reperfusion | [402] | ||
| 28 | MMP-3, 9, 12 | MMP-8 | MMP-2, 13 | [463] | |||
| 29 | MMP-8, 12 | [402] | |||||
| 30 AM-2 | MMP-8, 12 | MMP-3 | MMP-2 | [402] | |||
| 31 | MMP-2, 8, 12, 13 | [402] | |||||
| Non-ZBG 34 | MMP-13 | Osteoarthritis | [410] | ||||
| 35 | MMP-12 | MMP-2, 8, 13 | MMP-3, 9 | [405] | |||
| 36 | MMP-2, 8, 13 | [405] | |||||
| 37 | MMP-13 | Osteoarthritis | [410] | ||||
| 38 | MMP-13 | [406] | |||||
| 39 | MMP-13 | [406] | |||||
| Mechanism-Based 40 SB-3CT | MMP-2 | MMP-9, 14 | MMP-3 | Inhibits bone metastasis in prostate cancer, liver metastasis in T-cell lymphoma | [413–415, 418] | ||
| 42 | MMP-2, 9 | MMP-14 | MMP-3 | [411] | |||
| 43 | MMP-2 | MMP-14 | MMP-9 | MMP-3 | [418] | ||
| 45 | MMP-9 | MMP-2 | MMP-3, 14 | [420] | |||
Endogenous MMP Inhibitors
TIMPs and α2-Macroglobulin are two major endogenous inhibitors of MMPs. TIMPs are endogenous naturally occurring MMPIs that bind MMPs in a 1:1 stoichiometry. To date, 4 homologous endogenous TIMPs, TIMP-1, -2, -3 and 4, have been identified. TIMP-1 and -3 are glycoproteins, while TIMP-2 and -4 do not contain carbohydrates. TIMPs have an N-terminal domain (125 aa) and C-terminal domain (65 aa), each containing 3 disulfide bonds. The N-terminal domain folds as a separate unit and is capable of inhibiting MMPs [334, 335]. The TIMP molecule wedges into the active-site cleft of MMP in a manner similar to that of the substrate. Cys1 is instrumental in chelating the active site Zn2+ with its N-terminal α-amino group and carbonyl group, thereby expelling the water molecule bound to the catalytic Zn2+. TIMPs have poor specificity for a given MMP, and each TIMP can inhibit multiple MMPs. An exception is the ability of TIMP-2 and -3 to inhibit MT1-MMP and MT2-MMP, while TIMP-1 has poor inhibitory effect on MT1-MMP, MT3-MMP, MT5-MMP and MMP-19 [336, 337]. TIMPs also inhibit a broader spectrum of metalloproteinases. ADAMs are members of the disintegrin and metalloproteinase family. In contrast with ADAMs, ADAMTS (ADAMs with a thrombospondin motif) are secreted and lack a membrane anchor. Both ADAM and ADAMTS have similar active and binding sites as MMPs, and hence are inhibited by broad spectrum MMPIs [333]. TIMP-1 inhibits ADAM-10 while TIMP-2 inhibits ADAM-12 [338] and TIMP-3 has a much broader inhibition profile including ADAM-10, -12 [339] and -17 [338] and ADAMTS-1, -2, -4 and -5 [340, 341]. Because of its broad inhibitory spectrum, TIMP-3 ablation in mice causes lung emphysema-like alveolar damage [342] and faster apoptosis of mammary epithelial cells after weaning [343], whereas TIMP-1-null mice and TIMP-2-null mice do not exhibit obvious abnormalities. Binding studies have shown that native TIMPs can distinguish between stromelysins. TIMP-1 and -2 binding to human MMP-10 (stromelysin-2) catalytic domain is 10-fold weaker than their binding to the MMP-3 (stromelysin-1) catalytic domain. The x-ray crystal structure of TIMP-1 bound to MMP-10 catalytic domain demonstrated substantial differences with the structure of the TIMP-1.MMP-3 catalytic domain at the binding interface. These structural differences may help in the design of more selective TIMP-based inhibitors toward members of the stromelysin family and increase their therapeutic applications [344]. TIMP-1 has a threonine 2 residue that interacts with the specific P1′ substituent of MMP-3. Substitutions at threonine 2 of TIMP-1 affect the stability of complexes with MMPs and influence the specificity for different MMPs. For example, a substitution of alanine for threonine 2 causes a 17-fold decrease in binding of TIMP-1 to MMP-1 relative to MMP-3 [345, 346]. A functional and intact catalytic domain and the ability for the histidine residues to bind zinc are necessary for the interaction and complex formation TIMP with MMP [347].
MMP activity is partly regulated by α2-macroglobulin and related proteins. Human α2-macroglobulin, a glycoprotein consisting of four identical subunits and found in blood and tissue fluids, acts as a general proteinase inhibitor. α2-Macroglobulin inhibits most endopepidases by entrapping the peptidase within the macroglobulin. The complex is then rapidly cleared by endocytosis via a low density lipoprotein receptor-related protein-1 [348].
Other proteins inhibit selected members of MMPs: a secreted form of β-amyloid precursor protein inhibits MMP-2 [349]; a C-terminal fragment of procollagen C-proteinase enhancer protein inhibits MMP-2 [350]; tissue factor pathway inhibitor-2, a serine proteinase inhibitor, inhibits MMP-1 and -2 [351], and RECK, a GPI-anchored glycoprotein, inhibits MMP-2, -9 and -14. However, the mechanism of action of these inhibitors is not well-described [352].
Exogenous (Synthetic) MMP Inhibitors
Because the mechanism by which MMPs cleave their substrates requires catalytic Zn2+ ion, the design of MMPI has traditionally utilized Zn2+ binding globulin (ZBG). ZBGs displace the Zn2+-bound water molecule and inactivate the enzyme [353]. ZBG also acts as an anchor to lock the MMPI in the active site and direct the backbone of the inhibitor into the target substrate-binding pockets [354] (Table 4).
Early MMPIs included hydroxamic acids (ZBG1), carboxylates (ZBG2), thiols, and phosphonic acids (phosphorus-based ZBGs) [152]. Of these MMPIs, hydroxamic acids are preferred due to their relative ease of synthesis and potent binding [355]. Hydroxamates have a collagen mimicking structure which facilitates MMP inhibition by bidentate chelation of the active site Zn2+ ion [356]. A contributing factor to the effectiveness of hydroxamates is the hydrogen bonding that results between the heteroatoms of the ZBG and neighboring amino acid residues that are conserved in all MMP active sites. Although hydroxamates are potent MMPIs and some of them may show selectivity among different MMPs [357–361], many of them have musculoskeletal side effects and poor oral bioavailability [362, 363].
Succinyl hydroxamates such as batimastat (BB-94) and marimastat (BB-2516) are broad spectrum compounds that inhibit a wide range of MMPs. Batimastat has low water solubility and limited use in treating human inflammatory and malignant conditions. Marimastat is soluble in aqueous conditions and is orally bioavailable. An in vitro study showed a decrease in type III collagen and upregulation of MMP-3 in SMCs isolated from human VVs, with partial reversal of these changes with marimastat [207]. A limiting factor of this class of MMPIs is the non-specific cross-reactivity with other MMPs besides MMP-2 and -9. Because gelatinases have been implicated in tumor invasion and rheumatoid arthritis, attempts have been made to increase the selectivity of hydroxamates by designing compounds with greater interaction with the active site and the fibronectin type II domain of MMP-2 [364, 365].
Sulfonamide hydroxamates inhibit a variety of MMPs [366], but inhibit other enzymes with an active site Zn2+ moiety such as carbonic anhydrases and tumor necrosis factor converting enzyme (TACE), a member of ADAMs [333, 367]. Sultam hydroxamates are a group sulfonamide hydroxamate MMPIs with increased specificity toward MMP-2, -9 and -13 and an IC50 for MMP-2 (1 nM) 1000 fold more selective over MMP-1 [357]. Sultam hydroxamates can also be chemically modified to become potent TACE inhibitors (IC50=3.7 nM) without any cross reactivity with MMP-1, -2, -9 and -13 [368]. Sulfonamide phosphonates are structural analogs of hydroxamates in which incorporation of a favorable structure to attain affinity at the Zn2+ binding group and an arylsulfonamino backbone leads to nM potency and selective inhibition of MMP-8 [369]. Addition of other substituent groups to such as a tetrahydroisoquinoline moiety to sulfonamide hydroxamates led to a compound that inhibits a large number of MMPs, except MMP-7 [370]. Phosphinamide hydroxamates are potent MMPIs with IC50 of 20.5 nM for MMP-1 and 24.4 nM for MMP-3, but the hydrolysis of the phosphinamide bond in acidic conditions leads to inadequate oral bioavailability and limit their clinical use [333, 371].
Other small molecule MMPIs include carboxylates and thiols. In contrast with hydroxamates which have great affinity for MMPs and their binding is pH independent, carboxylates binding and MMP inhibition is dependent on the pH [333]. The IC50 for MMP-1 inhibition by carboxylates is in the μM range, i.e. 1000 fold less potent than hydroxamates [333, 372]. Several carboxylate inhibitors have been developed from the parent compound N(α)substituted 2,3-diaminoproprionic acid to improve their MMP specificity. The 2-arylsulfonyl-1,2,3,4-tetrahydro-isoquinoline-3-carboxylates form MMP-8 inhibitor complex directed at the S1′ hydrophobic specificity region near the catalytic Zn2+ ion. The molecular scaffold for Zn2+ binding and the hydrophobic region lie in the 1,2,3,4-tetrahydro-isoquinoline and the aryl aromatic rings, respectively, and enhance MMP-8 hydrophobic specific binding [373]. Several carboxylates such as PGE-2909492, PGE-6292544 have specificity for MMP-13 [374]. Other carboxylates prepared by the reaction of arylsulfonyl isocyanates with a 5-dibenzo-suberenyl/suberyl moieties, followed by the conversion of the carboxy-o-methyl to the carboxylate moieties are powerful MMPIs with activities in the low nM range, and selectivity for the deep pocket MMP-2, -8 and -9 over the shallow pocket MMP-1 [375].
Thiols are another class of small molecule MMPIs which are highly dependent on the mercapto group and the substitution for the P1′ and P2′ amino groups on the MMP molecule. Thiols are potent MMPIs with IC50 in the nM IC50 range, and stereotactic substitutions at the thiol group can change their potency by 100 fold [376]. Thiol MMPIs such as 4-(4-phenoxphenylsulfonyl)butane-1,2-dithiol and 5-(4-phenoxphenylsulfonyl)pentane-1,2-dithiol show selectivity for MMP-2 and -9. Both inhibitors chelate the catalytic Zn2+ ion of MMP-2 by 2 sulfur atoms and lead to conformational changes at the catalytic Zn2+ binding group [377].
By improving the potency and selectivity of ZBGs, some of these MMPIs have been considered as potential therapies for certain degenerative and vascular disease. Site specific delivery is another approach that allowed the use of MMPIs with low potency. Using these approaches, a series of biphenyl sulfonamide carboxylate MMPIs with high selectivity for MMP-13 were designed for treatment of osteoarthritis [378]. The carboxylic acid scaffold of these MMPIs was also used to develop selective MMP-12 inhibitors for treatment of chronic obstructive pulmonary disease [379]. Also, a series of MMPIs with improved selectivity towards MMP-12 over MMP-13 were generated by using a fused ring system. Selective hydroxamate inhibitors of MMP-2 have also been developed as potent anti-angiogenic agents, and compund 7 is the most selective inhibitor of this series. Compound 7 (UK-370, 106) is also a selctive inhibitor of MMP-3 and has been developed for topical treatment of CVU and chronic non-healing wounds [361], but has not been tested in clinical trials. Systemic administration of compound 7 may also modify the MMP profile in CVU because when it is administered intravenous in rats, it clears rapidly from plasma but slowly from dermal tissue [380]. Another class of arylsulfonamido-based type A hydroxamic acid compounds substituted on their sulfonamido nitrogen with an oxyalkyl side chain, instead of the hydrogen atom of type B compounds, or an alkyl side chain of type C compounds produced potent and selective MMPIs of MMP-2 and -9 that block in vitro tumour cell invasion. The prototype showed a very good MMP inhibitory profile, with high potency towards MMP-2 (IC50 = 0.41 nM), MMP-9 (IC50 = 16 nM) and MMP-14 (IC50 = 7.7 nM), and blocked angiogenesis in the chemioinvasion model of HUVECs at submicromolar concentrations [359].
Derivatives of Early ZBGs
Hydrazide (ZBG3) and sulfonylhydrazide (ZBG4) analogs of the hydroxamate MMPI illomastat (GM-6001) have been developed [381] (Table 4). Sulfonylhydrazide 9 is a potent inhibitor of MMP-1, -2 and -9 [382]. Mercaptosulfide (ZBG8) inhibitors target MMP-14. MMPIs with phosphorus-based ZBGs have demonstrated improved selectivity, and compound 18 is a potent phosphonate inhibitor that exhibits selectivity for MMP-8 [383]. Other phosphorus-based ZBGs include the carbamoyl phosphonate ZBG9 [354].
The net negative charge on ZBGs prevents cell penetration and restricts these MMPIs to the extracellular space, and therefore lowers their toxicity [384]. Several MMPIs based on these ZBGs are selective for MMP-2 in both in vitro and in vivo models of tumor invasion and angiogenesis. Compound 20 (N-methylcarbamoylphosphonic3Acids) shows marked specificity towards MMP-2 with little inhibition of MMP-1, -3, -8 and -9. Chronic intraperitoneal administration of compound 20 in a murine model of melanoma markedly inhibits lung metastasis [385]. Compound 21 (cis-2-Aminocyclohexylcarbamoylphosphonic acid, cis-ACCP) was introduced as a carbamoyl phosphonate MMPI that targets MMP-2 and -9, but spares MMP-1, -3, -8, -12 and -13. Compound 21 dose-dependently inhibits cell invasion in a Matrigel assay and prevents tumor colonization in the murine melanoma model, and reduces tumor growth and metastasis in the more aggressive murine model developed by implantation of human prostate tumor cells in immunodeficient mice. These ZBGs have the advantage of being water soluble at physiological pH, show efficacy both orally and intraperitoneally, and are not acutely toxic at the concentrations used in the murine models [384].
Other ZBGs have been developed to improve selectivity, bioavailability, and pharmacokinetics, and include oxygen, nitrogen, and sulfur donor–atom ligands and monodentate, bidentate, and tridentate chelators.
Nitrogen-Based ZBGs
Nitrogen-based ZBGs (ZBG10–16) have binding preference to late transition metals and improved selectivity towards Zn2+-dependent enzymes [386, 387]. An example of these ZBGs is compound 22, a modest inhibitor of MMP-9 that does not inhibit MMP-1, -2 or -12. The most extensively studied nitrogen-based ZBGs are the pyrimidine-2,4,6-trione and dionethione compounds. Pyrimidinetrione-based inhibitors have 100-fold selectivity for MMP-13 over MMP-2, -8 and -12 [388]. The pyrimidine-2,4,6-trione group is a known constituent of several FDA-approved drugs such as barbiturates, and therefore its metabolic disposition and bioavailability have been well-studied [389]. The pyrimidine-2,4,6-trione MMPIs have been optimized for gelatinase specificity and as anticancer drugs [390] and also to inhibit MMP-13 as part of the development of anti-osteoarthritis drugs [391–393].
Other MMPIs with high selectivity for specific MMPs include compound 23 (Ro 28-2653) with high selectivity for MMP-2, MMP-9, and MT1-MMP [394]. Ro 28-2653 has been evaluated for its anti-invasive, anti-tumorogenic, and anti-angiogenic activity and has shown inhibition of chemoinvasion by 85% at concentrations as low as 10 nM and anti-cancer efficacy in several in vitro and in vivo models [395]. In a rat model of tumorogenesis, Ro 28-2653 showed marked reduction in tumor growth and spread, and angiogenesis, with very few side effects [395]. Another compound Ro 32-3555 (trocade) has broad specificity for the collagenases MMP-1, -8 and -13 with affinity and IC50 in the nM range and good oral bioavailability, making it a potential therapeutic drug for rheumatoid and osteoarthritis [396]. Ro 32-3555 also inhibits MMP-3, and the gelatinases MMP-2 and -9.
Heterocyclic Bidentate ZBGs
A series of heterocyclic bidentate chelators ZBG20–30 was developed as alternative ZBGs and MMPIs [397]. These ZBGs have some features in common with hydroxamic acids but with better biostability and tighter Zn2+ binding due to ligand rigidity and, in some cases, the incorporation of sulfur donor atoms [398, 399]. These ZBGs inhibited MMP-1, -2 and -3 with greater potency than acetohydroxamic acid [397], and low cell toxicity [400]. Compound 25 (AM-6) is a pyrone-based inhibitor with greater selectivity toward MMP-3 over MMP-1 and -2 [401]. Compound 26 (1,2 -HOPO-2) inhibits MMP-12 at low concentrations, and is a potent inhibitor of MMP-2, -3 and -8, but less effective against MMP-1, -7, -9 and -13 [402]. In an ex vivo rat heart model of ischemia and reperfusion, hearts treated with compound 26 recovered more than 80% of their contractile function compared with 50% of untreated hearts [402]. Compound 30 (AM-2) inhibits MMP-3 with an IC50 of 240 nM [401, 402], while the structural isomer compound 32 (hydroxypyrone 9b) shows weaker inhibition (~30%) at concentrations as high as 100 μM [403].
Non-Zinc-Binding MMPIs
Because the Zn2+ binding site is the most conserved feature in all MMPs, eliminating or minimizing the interaction of an MMPI with the catalytic Zn2+ could improve MMP selectivity [354, 404-409] (Table 4). Several non-Zn2+-binding MMPIs show a noncompetitive inhibition mechanism [408]. Nearly all non-Zn2+-binding MMPIs show high MMP-13 selectivity and effectiveness in the treatment of animal models of osteoarthritis [404, 410]. Compound 37 inhibits MMP-13 but does not appreciably inhibit MMP-1, -2, -3, -7, -8, -9, -12, -13, -14 or -17. Binding of these inhibitors may rigidify the MMP active site into a specific conformation that is less conducive for substrate binding. The flexibility of MMP-13, relative to other MMPs, may allow for a favorable conformation that is not accessible in other MMPs [406, 410].
While these non-Zn2+-binding MMPIs are potent and selective, their hydrophobicity is critical in maintaining their protein–inhibitor interaction and high potency, but also results in poor water solubility. To improve the solubility without affecting potency, derivatives were developed to modify the solvent-exposed portions of the molecule and improve the compound solubility while maintaining the hydrophobic core structures and drug properties [404, 407].
Preclinical studies with compound 37 have shown encouraging results in models of osteoarthritis. Compound 37 has an efficacy at doses as low as 0.1 mg/kg in MMP-13-induced rat model of cartilage knee joint damage. Also, oral administration of compound 37 twice daily at 30mg/kg resulted in a 68% reduction in the cartilage lesion area of tibial plateaus in rats with surgically induced cartilage knee damage. When the rat joints were subsequently examined for evidence of fibroplasias and expanded inner synovial lining, which are indicative of musculoskeletal syndrome, fibroplasias were absent in rats treated with compound 37, but present in rats treated with broad-spectrum MMPIs. These highly selective MMPIs may minimize the side effects associated with broad-spectrum MMPIs, although it is not clear if their selectivity is due to their non-ZBG properties or other factors [406, 410].
Mechanism-Based MMPIs
Compound 40 (SB-3CT) was introduced as the first mechanism-based inhibitor of MMPs. SB-3CT binds in the active site and forms a covalent bond with the substrate protein upon activation by Zn2+ coordination [354] (Table 4). The formation of the covalent bond impedes inhibitor dissociation as compared to the traditional chelating competitive inhibitors. This reduces the rate of catalytic turnover and decreases the amount of MMPI needed to saturate the enzyme active sites. SB-3CT is a selective inhibitor of MMP-2 and -9. The structure of SB-3CT is relatively simple, as reflected by its low molecular weight. The mechanism of inhibition of SB-3CT is similar to that of a “suicide substrate” in which a functional group is activated, leading to covalent modification of the enzyme active site [411]. SB-3CT exhibits slow-binding kinetics with MMP-2, -3 and -9, with a time scale for establishing equilibrium between the enzyme and inhibitor and the enzyme–inhibitor complex in the order of seconds to minutes. Although binding rate can vary in speed, slow-binding inhibition is characterized by slow dissociation rates [412]. Progress curves, which display the enzyme activity of MMP-2, -9 and -3 with SB-3CT over time, are non-linear [411]. The curves show that the initial enzyme rate is not maintained and is instead reduced to a new “steady-state rate” of MMP activity. This indicates a slow-binding mechanism of inhibition, characteristic of an interaction between an enzyme and an inhibitor that resists dissociation. The selectivity of SB-3CT towards MMP-2 and -9 may be related to its slow-binding mechanism nearly irreversible inhibition. Following SB-3CT induced 95% inhibition, MMP-2 regains 50% activity only after 3 days with dialysis, but this slight reversibility may distinguish it from a true suicide inhibitor, which operates strictly by an irreversible mechanism [411, 412].
In preclinical studies, SB-3CT has shown anti-cancer effects in T-cell lymphoma and prostate cancer models [413–415] and reduced the invasion ability of human prostate cancer cells in in vitro Matrigel tests [414]. In a mouse model of T-cell lymphoma, SB-3CT administered at 5–50 mg/kg/d causes dose-dependent reduction in the number of liver metastases [416]. At 50 mg/kg/d, SB-3CT inhibited liver metastases by 73% and reduced the colony size of the metastases, while the broad-spectrum inhibitor batimastat increased metastasis. SB-3CT also showed promising results in a bone metastasis model of prostate cancer demonstrating reduced tumor growth and angiogenesis [414]. Also, SB-3CT provides neuronal protection in a murine stroke model [417]. In mice treated with SB-3CT either prior to or 2 h following cerebral ischemia induced by right middle cerebral artery occlusion, the infarct volume is decreased to 30% of the control. Administration of SB-3CT is protective up to 6 h after the ischemic event in mice. Also, neurological behavioral scores evaluated 24 h after reperfusion show that SB-3CT-treated mice exhibit marked improvement as compared to control mice, and the improvement is correlated with the infarct volume.
Although SB-3CT has marked in vivo activity, it undergoes rapid metabolism in mice [418], suggesting that a metabolite of the parent compound may be responsible for the in vivo activity [419]. Analysis of the different MMPI metabolites has led to the design of derivatives that have better in vivo stability and longer systemic effects [420]. Compound 43 is a more potent inhibitor of MMP-2, -3, -7, -9 and -14 than SB-3CT, and demonstrates slow-binding kinetics with MMP-2, -9 and -14 [420]. Compound 45 is a slow-binding and more potent inhibitor of MMP-9 than MMP-2, but a competitive inhibitor of other MMPs. Metabolites of compound 45 are 75% more stable than those of SB-3CT, resulting in prolonged systemic effects [420].
Mechanism-based slow-binding inhibitors such as SB-3CT and its successors have shown clinical promise. Other types of covalent modification in the active site may help in the design of more selective MMPIs [354]. However, even with the marked improvements in the design of MMPIs, therapeutic inhibition of MMPs is still a challenge and the antibiotic doxycycline remains the only FDA-approved MMPI [362, 363, 421, 422]. One limitation of many MMPIs is that they may cause musculoskeletal side effects including joint stiffness, pain, inflammation and tendinitis [354, 363, 423, 424].
Other MMP Inhibitors
Tetracyclines such as doxcycline and monocyclines are known antibiotics that also inhibit MMPs. Tetracyclines bind the divalent Zn2+ and Ca2+ ions and have an effect on MMP gene transcription [333]. Clinical trials have evaluated the tetracycline analog COL-3, a potent inhibitor of MMP-2 and -9, in patients with advanced metastatic soft tissue sarcoma, but minimal changes in tumor progression or survival have been observed [425].
In VVs organ cultures, statins such as simvastatin and pravastatin suppress the production and activity of MMP-9, but not MMP-2, in a dose-dependent manner. However, the doses of simvastatin and pravastatin required to influence MMP-9 are much higher than the peak plasma concentrations achieved after a typical oral dose [426].
Studies have evaluated the effects of glycosaminoglycan sulodexide, an antithrombotic/profibrinolytic drug, on the activity and release of MMPs in human blood. In a prospective non-randomized study, zymography and ELISA were used to analyze the in vitro effects of sulodexide on pro-enzyme, complexed, and active MMP forms in plasma and serum from 60 healthy donors, and in U-937 leukemia cell line [427]. Pro- and complexed forms of MMP-9 were markedly affected by sulodexide treatment, with marked decrease in MMP-9 secretion from white blood cells in a dose-dependent manner, without any displacement of MMP prodomains. The mechanism of action of sulodexide likely involves direct inhibition of the active Zn2+ binding site of the proMMP-9 molecule [428]. The levels and zymographic profile of MMP-2 did not show marked differences among samples and with sulodexide treatment. The reduced release of MMP-9 forms from leukocytes and inhibition of proteolytic activity by sulodexide treatment support that inhibitors of MMP-9 activity may provide a therapeutic tool for the underlying pathological changes in ECM, and offer novel pharmacologic approaches for chronic inflammatory vascular diseases associated with enhanced MMP activation in blood and limbs, including VVs and CVD [427].
Topical products have been developed to modify the MMP/TIMP balance and improve wound healing. Hydroactive wound dressings containing polyacylate superabsorber particles inhibit MMPs, including MMP-2 and -9, by competing for Zn2+ and Ca2+ [429]. Also, in a randomized controlled trial, oxidized regenerated cellulose/collagen matrix (ORC/collagen matrix) accelerate the healing rate in venous leg ulcer [430]. ORC/collagen matrix modifies wound microenvironments by binding and inactivating excess proteases. Exudates obtained from CVU treated with ORC/collagen matrix showed marked reduction in elastase, plasmin and gelatinase activity as compared with those treated with hydrocolloid dressings [431]. Although few clinical trials have been conducted, a 12-week multi-centred randomized trial of 117 patients comparing the efficiency of nanooligosaccharide factor (NOSF) and ORC in the local management of CVU demonstrated that NOSF was superior wound healing [432]. A case report has shown that combining topical tacrolimus and oral doxycyline successfully treated 3 resistant venous ulcers [433].
Monoclonal antibodies target specific MMPs with high affinity. The monoclonal antibodies REGA-3G12 and REGA-2D9 are specific for MMP-9 and do not cross-react with MMP-2. MMP inhibition by REGA-3G12 does not involve the Zn2+ binding domain or the fibronectin region, but instead binds to the catalytic domain [434]. REGA-1G8 is not as specific, and cross-reacts with serum albumin. In addition to inhibiting MMPs, monoclonal antibodies are useful to detect MMPs in the body fluids and tissues of patients with inflammation or cancer [434–436].
Small interfering RNA (siRNA) inhibits the transcriptional product of specific MMPs with efficient inhibition of the target molecule. Research in cancer therapy using siRNA is evolving. Adenovirus-mediated siRNA 21 base-pair for MMP-9 caused a dose-dependent cell cycle arrest of medulloblastoma cells and inhibited tumor growth [437]. Other tumor cell lines such as human chondrosarcoma have shown decreased tumor invasion of type I collagen matrix in vitro by siRNA directed inhibition of MMP-1 [438]. Gene silencing using siRNA targeted for MT1-MMP to reduce activation of proMMP-2 in bone marrow derived stromal cells attenuated the induction of cell necrosis [439]. The use of siRNA allows for precise and specific gene knockdown and minimizes the potential for non-specific inhibition by longer double stranded antisense RNAs. Because siRNA require transfection into the cell, the efficiency of transfection and duration of siRNA inhibition may vary, and may result in only transient gene knockdown. Also, siRNA may cause nonspecific activation of innate immune responses or off-targeting of MMP genes and inadvertent downregulation of other genes [440].
Conclusions and Perspectives
CVD including VVs is not a minor disease with only cosmetic and social implications. If untreated, VVs could lead to thrombophlebitis and CVI with skin changes and chronic leg ulcer. The pathogenesis of VVs is a complex multifactorial process involving both valve insufficiency and vein wall changes. VVs can form in any vein segment of the lower extremity, and tributaries that connect to axial veins may show varicosities without any reflux in the communicating vein. Also, normal vein segments adjacent to VVs have the same biochemical properties as the VVs wall, supporting the role of vein wall changes in the pathogenesis of VVs. VVs pathology may affect not only the lower extremity veins, but also other tissues, suggesting that VVs may be a localized manifestation of a systemic venous disease.
Most of the current therapies aim at enhancing the cosmetic appearance and comfort of the patient’s symptoms, and interventional and surgical approaches in advanced cases of CVD. Because current treatment strategies are directed towards the manifestations or complications rather than the cause of CVD, they often show variable success and high recurrence rates. MMPs play an important role in ECM metabolism and other physiological processes, and increased MMP expression/activity has been associated with connective tissue remodeling in autoimmune disease. MMPs and their naturally occurring TIMPs have also been implicated in several degenerative and vascular disease including VVs. The abundance of MMPs in venous tissue supports a role in VVs, and MMPs may have distinct roles at different stages of CVD. In the early stages of CVD, MMPs may cause venous dilation by inducing hyperpolarization of the vein wall, and inhibiting balanced production of collagen subtypes. In later stages of CVD, MMPs may alter the vein wall matrix composition leading to further venous dilation and tortuosity.
The role of MMPs is often examined by measuring their plasma levels, and tissue expression and activity. Molecular imaging of MMP function in vivo is an emerging area of research. In murine tumors a near infrared fluorescent MMP substrate detects the activity of MMPs in vivo [441]. Similar approaches have identified active cathepsin B in atherosclerotic mice and increased MMP-2 and -9 activities after murine myocardial infarction [442]. Although this technology presents challenges such as limited tissue penetration and autofluorescence of elastin in arteries, initial results have provided proof of principle for proteinase imaging in vivo.
Several MMPIs have been used as investigative tools of the role of MMPs in physiological and pathological processes. MMPIs have also been examined as potential therapeutic tools in cancer, arthritis, autoimmune and vascular disease. Although several MMPIs have been developed, their limited specificity and oral bioavailability as well as potential cytotoxicity prevented their further development for clinical use. In animal models, MMPIs prevent the development and progression of early disease, but show little effect in advanced disease. Also, clinical trials using synthetic MMPIs in cancer patients have shown little success, mainly due to the lack of efficacy and untoward side effects including musculoskeletal pain and tendonitis [443]. In many studies MMPIs was used as single agent therapy in patients with advanced disease, which may explain the poor performance of MMPIs in clinical trials [423].
Several approaches have been suggested to overcome the drawbacks of classic MMPIs. Specific antibody fragments could target the MMP active site in a more specific way than chemical inhibitors and could identify sites on the MMP molecule that determine their substrate specificity and extracellular location. Understanding of the nature of these interactions will allow the development of specific inhibitors of MMP-substrate binding or fragment antibodies that target these interactions. The availability of specific MMPIs may provide a new tool to limit the progression and complications of CVD and reduce the recurrence of VVs
Several other strategies may potentially downregulate MMPs. Both the intracellular signaling pathways and the downstream transcription factors which induce gene expression are being studied. Blockade of MAPK pathways, NF-κB or AP-1 have shown some efficacy in vitro or in animal models of arthritis [444]. The use of biological reagents to block inflammatory cytokines also reduces MMP expression in many tissues. Tetracyclines are weak inhibitors of MMP, but they could influence MMP synthesis and have been tested successfully in rheumatoid arthritis [445]. Gene therapy has been successful in animal models, and overexpression of TIMPs may have future application after overcoming the problems of safe and efficient delivery of genes into target tissues [446].
Certain gene mutations and variants may play a role in CVD. Identifying the genetic basis of VVs will not only help elucidate the molecular abnormalities involved, but also identify individuals at risk and the most appropriate prevention and treatment strategies. Also, while significant progress has been made in identifying the role of MMPs in VVs, their upstream regulation and downstream signaling at the different stages of CVD need to be examined. Understanding of the molecular basis of VVs formation and MMPs-induced changes in ECs and VSM function and vein wall remodeling will provide valuable information on the mechanisms involved in CVD development and progression. Also, the interaction of MMPs with other proteolytic enzymes such as ADAM and ADAMTS in the pathogenesis of VVs and stretch induced increase in AP-1, which triggers varicose remodeling through MMPs and VSMC hyperplasia in the vein wall need to be examined. Further research of the molecular mechanisms of VVs and CVD would help identify possible targets for genetic manipulation and more efficient pharmacologic interventions of the disease.
Acknowledgments
This work was partly supported by grants from National Heart, Lung, and Blood Institute (HL-65998 and HL-98724).
List of abbreviations
- AP-1
activator protein-1
- CVD
chronic venous disease
- CVI
chronic venous insufficiency
- EC
endothelial cell
- ECM
extracellular matrix
- EMMPRIN
extracellular MMP inducer
- HUVECs
human umbilical vein endothelial cells
- IVC
inferior vena cava
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- MMPI
matix metalloproteinase inhibitor
- Phe
phenylephrine
- ROS
reactive oxygen species
- TIMP
tissue inhibitor of matrix metalloproteinase
- TNF-α
tumor necrosis factor-α
- VSMC
vascular smooth muscle cell
- VUWF
venous ulcer wound fluid
- VVs
varicose veins
References
- 1.Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23(2):85–98. doi: 10.1258/phleb.2007.007027. [DOI] [PubMed] [Google Scholar]
- 2.Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355(5):488–98. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 3.Zhou D, Lee HS, Villarreal F, et al. Differential MMP-2 activity of ligament cells under mechanical stretch injury: an in vitro study on human ACL and MCL fibroblasts. J Orthop Res. 2005;23(4):949–57. doi: 10.1016/j.orthres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Milkiewicz M, Haas TL. Effect of mechanical stretch on HIF-1{alpha} and MMP-2 expression in capillaries isolated from overloaded skeletal muscles: laser capture microdissection study. Am J Physiol Heart Circ Physiol. 2005;289(3):H1315–20. doi: 10.1152/ajpheart.00284.2005. [DOI] [PubMed] [Google Scholar]
- 5.Schmid-Schonbein GW, Takase S, Bergan JJ. New advances in the understanding of the pathophysiology of chronic venous insufficiency. Angiology. 2001;52 (Suppl 1):S27–34. doi: 10.1177/0003319701052001S04. [DOI] [PubMed] [Google Scholar]
- 6.Asanuma K, Magid R, Johnson C, Nerem RM, Galis ZS. Uniaxial strain upregulates matrix-degrading enzymes produced by human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;284(5):H1778–84. doi: 10.1152/ajpheart.00494.2002. [DOI] [PubMed] [Google Scholar]
- 7.Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg. 2007;45(2):373–80. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raffetto JD, Qiao X, Koledova VV, Khalil RA. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: Potential implications in varicose veins. J Vasc Surg. 2008;48(2):447–56. doi: 10.1016/j.jvs.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raffetto JD, Barros YV, Wells AK, Khalil RA. MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res. 2010;159(2):755–64. doi: 10.1016/j.jss.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15(3):175–84. doi: 10.1016/j.annepidem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 11.McLafferty RB, Lohr JM, Caprini JA, et al. Results of the national pilot screening program for venous disease by the American Venous Forum. J Vasc Surg. 2007;45(1):142–8. doi: 10.1016/j.jvs.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 12.Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40(6):1248–52. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Scott TE, LaMorte WW, Gorin DR, Menzoian JO. Risk factors for chronic venous insufficiency: a dual case-control study. J Vasc Surg. 1995;22(5):622–8. doi: 10.1016/s0741-5214(95)70050-1. [DOI] [PubMed] [Google Scholar]
- 14.Jawien A. The influence of environmental factors in chronic venous insufficiency. Angiology. 2003;54 (Suppl 1):S19–31. doi: 10.1177/0003319703054001S04. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix P, Aboyans V, Preux PM, Houles MB, Laskar M. Epidemiology of venous insufficiency in an occupational population. Int Angiol. 2003;22(2):172–6. [PubMed] [Google Scholar]
- 16.Lee AJ, Evans CJ, Allan PL, Ruckley CV, Fowkes FG. Lifestyle factors and the risk of varicose veins: Edinburgh Vein Study. J Clin Epidemiol. 2003;56(2):171–9. doi: 10.1016/s0895-4356(02)00518-8. [DOI] [PubMed] [Google Scholar]
- 17.Christopoulos D, Nicolaides AN, Szendro G. Venous reflux: quantification and correlation with the clinical severity of chronic venous disease. Br J Surg. 1988;75(4):352–6. doi: 10.1002/bjs.1800750419. [DOI] [PubMed] [Google Scholar]
- 18.Zamboni P, Scapoli G, Lanzara V, et al. Serum iron and matrix metalloproteinase-9 variations in limbs affected by chronic venous disease and venous leg ulcers. Dermatol Surg. 2005;31(6):644–9. doi: 10.1111/j.1524-4725.2005.31611. discussion 9. [DOI] [PubMed] [Google Scholar]
- 19.Zamboni P, Izzo M, Tognazzo S, et al. The overlapping of local iron overload and HFE mutation in venous leg ulcer pathogenesis. Free Radic Biol Med. 2006;40(10):1869–73. doi: 10.1016/j.freeradbiomed.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Zamboni P, De Mattei M, Ongaro A, et al. Factor XIII contrasts the effects of metalloproteinases in human dermal fibroblast cultured cells. Vasc Endovascular Surg. 2004;38(5):431–8. doi: 10.1177/153857440403800506. [DOI] [PubMed] [Google Scholar]
- 21.Zamboni P, Tognazzo S, Izzo M, et al. Hemochromatosis C282Y gene mutation increases the risk of venous leg ulceration. J Vasc Surg. 2005;42(2):309–14. doi: 10.1016/j.jvs.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Tognazzo S, Gemmati D, Palazzo A, et al. Prognostic role of factor XIII gene variants in nonhealing venous leg ulcers. J Vasc Surg. 2006;44(4):815–9. doi: 10.1016/j.jvs.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Gemmati D, Tognazzo S, Catozzi L, et al. Influence of gene polymorphisms in ulcer healing process after superficial venous surgery. J Vasc Surg. 2006;44(3):554–62. doi: 10.1016/j.jvs.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Noel AA, Gloviczki P, Cherry KJ, Jr, Rooke TW, Stanson AW, Driscoll DJ. Surgical treatment of venous malformations in Klippel-Trenaunay syndrome. J Vasc Surg. 2000;32(5):840–7. doi: 10.1067/mva.2000.110343. [DOI] [PubMed] [Google Scholar]
- 25.Delis KT, Gloviczki P, Wennberg PW, Rooke TW, Driscoll DJ. Hemodynamic impairment, venous segmental disease, and clinical severity scoring in limbs with Klippel-Trenaunay syndrome. J Vasc Surg. 2007;45(3):561–7. doi: 10.1016/j.jvs.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Ng MY, Andrew T, Spector TD, Jeffery S. Linkage to the FOXC2 region of chromosome 16 for varicose veins in otherwise healthy, unselected sibling pairs. J Med Genet. 2005;42(3):235–9. doi: 10.1136/jmg.2004.024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brice G, Mansour S, Bell R, et al. Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J Med Genet. 2002;39(7):478–83. doi: 10.1136/jmg.39.7.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serra R, Buffone G, de Franciscis A, et al. A genetic study of chronic venous insufficiency. Ann Vasc Surg. 2012;26(5):636–42. doi: 10.1016/j.avsg.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Saiki S, Sakai K, Saiki M, et al. Varicose veins associated with CADASIL result from a novel mutation in the Notch3 gene. Neurology. 2006;67(2):337–9. doi: 10.1212/01.wnl.0000224758.52970.19. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Lee W, Choe Y, et al. Gene expression profiles in varicose veins using complementary DNA microarray. Dermatol Surg. 2005;31(4):391–5. doi: 10.1111/j.1524-4725.2005.31103. [DOI] [PubMed] [Google Scholar]
- 31.Dalal A, Phadke SR. Hemihyperplasia with Ehlers-Danlos syndrome like skin changes. Clin Dysmorphol. 2005;14(4):207–8. [PubMed] [Google Scholar]
- 32.Badauy CM, Gomes SS, Sant’Ana Filho M, Chies JA. Ehlers-Danlos syndrome (EDS) type IV: review of the literature. Clin Oral Investig. 2007;11(3):183–7. doi: 10.1007/s00784-006-0092-x. [DOI] [PubMed] [Google Scholar]
- 33.Xu HM, Zhao Y, Zhang XM, Zhu T, Fu WG. Polymorphisms in MMP-9 and TIMP-2 in Chinese patients with varicose veins. J Surg Res. 2011;168(1):e143–8. doi: 10.1016/j.jss.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Clarke GH, Vasdekis SN, Hobbs JT, Nicolaides AN. Venous wall function in the pathogenesis of varicose veins. Surgery. 1992;111(4):402–8. [PubMed] [Google Scholar]
- 35.Reagan B, Folse R. Lower limb venous dynamics in normal persons and children of patients with varicose veins. Surg Gynecol Obstet. 1971;132(1):15–8. [PubMed] [Google Scholar]
- 36.Zsoter T, Cronin RF. Venous distensibility in patients with varicose veins. Can Med Assoc J. 1966;94(25):1293–7. [PMC free article] [PubMed] [Google Scholar]
- 37.Sansilvestri-Morel P, Rupin A, Badier-Commander C, et al. Imbalance in the synthesis of collagen type I and collagen type III in smooth muscle cells derived from human varicose veins. J Vasc Res. 2001;38(6):560–8. doi: 10.1159/000051092. [DOI] [PubMed] [Google Scholar]
- 38.Miskulin M, Dalgleish R, Kluve-Beckerman B, et al. Human type III collagen gene expression is coordinately modulated with the type I collagen genes during fibroblast growth. Biochemistry. 1986;25(6):1408–13. doi: 10.1021/bi00354a033. [DOI] [PubMed] [Google Scholar]
- 39.Sansilvestri-Morel P, Rupin A, Badier-Commander C, Fabiani JN, Verbeuren TJ. Chronic venous insufficiency: dysregulation of collagen synthesis. Angiology. 2003;54 (Suppl 1):S13–8. doi: 10.1177/0003319703054001S03. [DOI] [PubMed] [Google Scholar]
- 40.Brand FN, Dannenberg AL, Abbott RD, Kannel WB. The epidemiology of varicose veins: the Framingham Study. Am J Prev Med. 1988;4(2):96–101. [PubMed] [Google Scholar]
- 41.Bradbury A, Evans C, Allan P, Lee A, Ruckley CV, Fowkes FG. What are the symptoms of varicose veins? Edinburgh vein study cross sectional population survey. BMJ. 1999;318(7180):353–6. doi: 10.1136/bmj.318.7180.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans CJ, Fowkes FG, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999;53(3):149–53. doi: 10.1136/jech.53.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruckley CV, Evans CJ, Allan PL, Lee AJ, Fowkes FG. Chronic venous insufficiency: clinical and duplex correlations. The Edinburgh Vein Study of venous disorders in the general population. J Vasc Surg. 2002;36(3):520–5. doi: 10.1067/mva.2002.126547. [DOI] [PubMed] [Google Scholar]
- 44.Raffetto JD, Qiao X, Beauregard KG, Khalil RA. Estrogen receptor-mediated enhancement of venous relaxation in female rat: implications in sex-related differences in varicose veins. J Vasc Surg. 2010;51(4):972–81. doi: 10.1016/j.jvs.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21(5):514–50. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 46.Bernstein IM, Ziegler W, Badger GJ. Plasma volume expansion in early pregnancy. Obstet Gynecol. 2001;97(5 Pt 1):669–72. doi: 10.1016/s0029-7844(00)01222-9. [DOI] [PubMed] [Google Scholar]
- 47.Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54(6):2056–63. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 48.Stansby G. Women, pregnancy, and varicose veins. Lancet. 2000;355(9210):1117–8. doi: 10.1016/S0140-6736(00)02057-2. [DOI] [PubMed] [Google Scholar]
- 49.Mekky S, Schilling RS, Walford J. Varicose veins in women cotton workers. An epidemiological study in England and Egypt. Br Med J. 1969;2(5657):591–5. doi: 10.1136/bmj.2.5657.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seidell JC, Bakx KC, Deurenberg P, van den Hoogen HJ, Hautvast JG, Stijnen T. Overweight and chronic illness--a retrospective cohort study, with a follow-up of 6–17 years, in men and women of initially 20–50 years of age. J Chronic Dis. 1986;39(8):585–93. doi: 10.1016/0021-9681(86)90183-9. [DOI] [PubMed] [Google Scholar]
- 51.Kaye SA, Folsom AR, Soler JT, Prineas RJ, Potter JD. Associations of body mass and fat distribution with sex hormone concentrations in postmenopausal women. Int J Epidemiol. 1991;20(1):151–6. doi: 10.1093/ije/20.1.151. [DOI] [PubMed] [Google Scholar]
- 52.Abramson JH, Hopp C, Epstein LM. The epidemiology of varicose veins. A survey in western Jerusalem. J Epidemiol Community Health. 1981;35(3):213–7. doi: 10.1136/jech.35.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. EXS. 2012;103:209–79. doi: 10.1007/978-3-0348-0364-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962;48:1014–22. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 56.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90(3):251–62. [PubMed] [Google Scholar]
- 57.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–59. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagase H, Fushimi K. Elucidating the function of non catalytic domains of collagenases and aggrecanases. Connect Tissue Res. 2008;49(3):169–74. doi: 10.1080/03008200802151698. [DOI] [PubMed] [Google Scholar]
- 59.Chung L, Dinakarpandian D, Yoshida N, et al. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004;23(15):3020–30. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalvie D, Cosker T, Boyden T, Zhou S, Schroeder C, Potchoiba MJ. Metabolism distribution and excretion of a matrix metalloproteinase-13 inhibitor, 4-[4-(4-fluorophenoxy)-benzenesulfonylamino]tetrahydropyran-4-carboxylic acid hydroxyamide (CP-544439), in rats and dogs: assessment of the metabolic profile of CP-544439 in plasma and urine of humans. Drug Metab Dispos. 2008;36(9):1869–83. doi: 10.1124/dmd.108.022566. [DOI] [PubMed] [Google Scholar]
- 61.Allan JA, Docherty AJ, Barker PJ, Huskisson NS, Reynolds JJ, Murphy G. Binding of gelatinases A and B to type-I collagen and other matrix components. Biochem J. 1995;309 (Pt 1):299–306. doi: 10.1042/bj3090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270(11):5872–6. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 63.Patterson ML, Atkinson SJ, Knauper V, Murphy G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001;503(2–3):158–62. doi: 10.1016/s0014-5793(01)02723-5. [DOI] [PubMed] [Google Scholar]
- 64.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375(6528):244–7. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- 66.Uria JA, Lopez-Otin C. Matrilysin-2, a new matrix metalloproteinase expressed in human tumors and showing the minimal domain organization required for secretion, latency, and activity. Cancer Res. 2000;60(17):4745–51. [PubMed] [Google Scholar]
- 67.Park HI, Ni J, Gerkema FE, Liu D, Belozerov VE, Sang QX. Identification and characterization of human endometase (Matrix metalloproteinase-26) from endometrial tumor. J Biol Chem. 2000;275(27):20540–4. doi: 10.1074/jbc.M002349200. [DOI] [PubMed] [Google Scholar]
- 68.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 69.Marchenko ND, Marchenko GN, Weinreb RN, et al. Beta-catenin regulates the gene of MMP-26, a novel metalloproteinase expressed both in carcinomas and normal epithelial cells. Int J Biochem Cell Biol. 2004;36(5):942–56. doi: 10.1016/j.biocel.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 70.English WR, Holtz B, Vogt G, Knauper V, Murphy G. Characterization of the role of the “MT-loop”: an eight-amino acid insertion specific to progelatinase A (MMP2) activating membrane-type matrix metalloproteinases. J Biol Chem. 2001;276(45):42018–26. doi: 10.1074/jbc.M107783200. [DOI] [PubMed] [Google Scholar]
- 71.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272(4):2446–51. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 72.Knauper V, Will H, Lopez-Otin C, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271(29):17124–31. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- 73.Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci U S A. 1996;93(9):3942–6. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kerkela E, Bohling T, Herva R, Uria JA, Saarialho-Kere U. Human macrophage metalloelastase (MMP-12) expression is induced in chondrocytes during fetal development and malignant transformation. Bone. 2001;29(5):487–93. doi: 10.1016/s8756-3282(01)00595-6. [DOI] [PubMed] [Google Scholar]
- 75.Hou P, Troen T, Ovejero MC, et al. Matrix metalloproteinase-12 (MMP-12) in osteoclasts: new lesson on the involvement of MMPs in bone resorption. Bone. 2004;34(1):37–47. doi: 10.1016/j.bone.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 76.Stracke JO, Fosang AJ, Last K, et al. Matrix metalloproteinases 19 and 20 cleave aggrecan and cartilage oligomeric matrix protein (COMP) FEBS Lett. 2000;478(1–2):52–6. doi: 10.1016/s0014-5793(00)01819-6. [DOI] [PubMed] [Google Scholar]
- 77.Sadowski T, Dietrich S, Muller M, et al. Matrix metalloproteinase-19 expression in normal and diseased skin: dysregulation by epidermal proliferation. J Invest Dermatol. 2003;121(5):989–96. doi: 10.1046/j.1523-1747.2003.12526.x. [DOI] [PubMed] [Google Scholar]
- 78.Sadowski T, Dietrich S, Koschinsky F, Sedlacek R. Matrix metalloproteinase 19 regulates insulin-like growth factor-mediated proliferation, migration, and adhesion in human keratinocytes through proteolysis of insulin-like growth factor binding protein-3. Mol Biol Cell. 2003;14(11):4569–80. doi: 10.1091/mbc.E03-01-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sadowski T, Dietrich S, Koschinsky F, et al. Matrix metalloproteinase 19 processes the laminin 5 gamma 2 chain and induces epithelial cell migration. Cell Mol Life Sci. 2005;62(7–8):870–80. doi: 10.1007/s00018-005-4478-8. [DOI] [PubMed] [Google Scholar]
- 80.Pendas AM, Folgueras AR, Llano E, et al. Diet-induced obesity and reduced skin cancer susceptibility in matrix metalloproteinase 19-deficient mice. Mol Cell Biol. 2004;24(12):5304–13. doi: 10.1128/MCB.24.12.5304-5313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ryu OH, Fincham AG, Hu CC, et al. Characterization of recombinant pig enamelysin activity and cleavage of recombinant pig and mouse amelogenins. J Dent Res. 1999;78(3):743–50. doi: 10.1177/00220345990780030601. [DOI] [PubMed] [Google Scholar]
- 82.Li W, Gibson CW, Abrams WR, Andrews DW, DenBesten PK. Reduced hydrolysis of amelogenin may result in X-linked amelogenesis imperfecta. Matrix Biol. 2001;19(8):755–60. doi: 10.1016/s0945-053x(00)00121-9. [DOI] [PubMed] [Google Scholar]
- 83.Skoog T, Ahokas K, Orsmark C, Jeskanen L, Isaka K, Saarialho-Kere U. MMP-21 is expressed by macrophages and fibroblasts in vivo and in culture. Exp Dermatol. 2006;15(10):775–83. doi: 10.1111/j.1600-0625.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 84.Ahokas K, Lohi J, Illman SA, et al. Matrix metalloproteinase-21 is expressed epithelially during development and in cancer and is up-regulated by transforming growth factor-beta1 in keratinocytes. Lab Invest. 2003;83(12):1887–99. doi: 10.1097/01.lab.0000106721.86126.39. [DOI] [PubMed] [Google Scholar]
- 85.Yang M, Kurkinen M. Cloning and characterization of a novel matrix metalloproteinase (MMP), CMMP, from chicken embryo fibroblasts. CMMP, Xenopus XMMP, and human MMP19 have a conserved unique cysteine in the catalytic domain. J Biol Chem. 1998;273(28):17893–900. doi: 10.1074/jbc.273.28.17893. [DOI] [PubMed] [Google Scholar]
- 86.Pei D, Kang T, Qi H. Cysteine array matrix metalloproteinase (CA-MMP)/MMP-23 is a type II transmembrane matrix metalloproteinase regulated by a single cleavage for both secretion and activation. J Biol Chem. 2000;275(43):33988–97. doi: 10.1074/jbc.M006493200. [DOI] [PubMed] [Google Scholar]
- 87.Velasco G, Pendas AM, Fueyo A, Knauper V, Murphy G, Lopez-Otin C. Cloning and characterization of human MMP-23, a new matrix metalloproteinase predominantly expressed in reproductive tissues and lacking conserved domains in other family members. J Biol Chem. 1999;274(8):4570–6. doi: 10.1074/jbc.274.8.4570. [DOI] [PubMed] [Google Scholar]
- 88.Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42(3):113–85. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 89.Bar-Or A, Nuttall RK, Duddy M, et al. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126(Pt 12):2738–49. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]
- 90.Marchenko GN, Strongin AY. MMP-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene. 2001;265(1–2):87–93. doi: 10.1016/s0378-1119(01)00360-2. [DOI] [PubMed] [Google Scholar]
- 91.Lohi J, Wilson CL, Roby JD, Parks WC. Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J Biol Chem. 2001;276(13):10134–44. doi: 10.1074/jbc.M001599200. [DOI] [PubMed] [Google Scholar]
- 92.Saarialho-Kere U, Kerkela E, Jahkola T, Suomela S, Keski-Oja J, Lohi J. Epilysin (MMP-28) expression is associated with cell proliferation during epithelial repair. J Invest Dermatol. 2002;119(1):14–21. doi: 10.1046/j.1523-1747.2002.01790.x. [DOI] [PubMed] [Google Scholar]
- 93.Kevorkian L, Young DA, Darrah C, et al. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50(1):131–41. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- 94.Momohara S, Okamoto H, Komiya K, et al. Matrix metalloproteinase 28/epilysin expression in cartilage from patients with rheumatoid arthritis and osteoarthritis: comment on the article by Kevorkian et al. Arthritis Rheum. 2004;50(12):4074–5. doi: 10.1002/art.20799. author reply 5. [DOI] [PubMed] [Google Scholar]
- 95.Gillespie DL, Patel A, Fileta B, et al. Varicose veins possess greater quantities of MMP-1 than normal veins and demonstrate regional variation in MMP-1 and MMP-13. J Surg Res. 2002;106(2):233–8. doi: 10.1006/jsre.2002.6455. [DOI] [PubMed] [Google Scholar]
- 96.Kowalewski R, Sobolewski K, Wolanska M, Gacko M. Matrix metalloproteinases in the vein wall. Int Angiol. 2004;23(2):164–9. [PubMed] [Google Scholar]
- 97.Woodside KJ, Hu M, Burke A, et al. Morphologic characteristics of varicose veins: possible role of metalloproteinases. J Vasc Surg. 2003;38(1):162–9. doi: 10.1016/s0741-5214(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 98.Aravind B, Saunders B, Navin T, et al. Inhibitory effect of TIMP influences the morphology of varicose veins. Eur J Vasc Endovasc Surg. 2010;40(6):754–65. doi: 10.1016/j.ejvs.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 99.Kosugi I, Urayama H, Kasashima F, Ohtake H, Watanabe Y. Matrix metalloproteinase-9 and urokinase-type plasminogen activator in varicose veins. Ann Vasc Surg. 2003;17(3):234–8. doi: 10.1007/s10016-003-0005-2. [DOI] [PubMed] [Google Scholar]
- 100.Sayer GL, Smith PD. Immunocytochemical characterisation of the inflammatory cell infiltrate of varicose veins. Eur J Vasc Endovasc Surg. 2004;28(5):479–83. doi: 10.1016/j.ejvs.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 101.Takase S, Bergan JJ, Schmid-Schonbein G. Expression of adhesion molecules and cytokines on saphenous veins in chronic venous insufficiency. Ann Vasc Surg. 2000;14(5):427–35. doi: 10.1007/s100169910092. [DOI] [PubMed] [Google Scholar]
- 102.Myers KA, Jolley D, Clough A, Kirwan J. Outcome of ultrasound-guided sclerotherapy for varicose veins: medium-term results assessed by ultrasound surveillance. Eur J Vasc Endovasc Surg. 2007;33(1):116–21. doi: 10.1016/j.ejvs.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 103.Ono T, Bergan JJ, Schmid-Schonbein GW, Takase S. Monocyte infiltration into venous valves. J Vasc Surg. 1998;27(1):158–66. doi: 10.1016/s0741-5214(98)70303-9. [DOI] [PubMed] [Google Scholar]
- 104.Chu HB, Yan F, Zhao JH, Xu YB, Wang T, Guo WJ. Assessment of the Infiltration of Inflammatory Cells in the Walls of Thrombotic Varicose Veins. Angiology. 2012 doi: 10.1177/0003319711435147. [DOI] [PubMed] [Google Scholar]
- 105.Herouy Y, May AE, Pornschlegel G, et al. Lipodermatosclerosis is characterized by elevated expression and activation of matrix metalloproteinases: implications for venous ulcer formation. J Invest Dermatol. 1998;111(5):822–7. doi: 10.1046/j.1523-1747.1998.00369.x. [DOI] [PubMed] [Google Scholar]
- 106.Mendez MV, Raffetto JD, Phillips T, Menzoian JO, Park HY. The proliferative capacity of neonatal skin fibroblasts is reduced after exposure to venous ulcer wound fluid: A potential mechanism for senescence in venous ulcers. J Vasc Surg. 1999;30(4):734–43. doi: 10.1016/s0741-5214(99)70113-8. [DOI] [PubMed] [Google Scholar]
- 107.Raffetto JD, Mendez MV, Marien BJ, et al. Changes in cellular motility and cytoskeletal actin in fibroblasts from patients with chronic venous insufficiency and in neonatal fibroblasts in the presence of chronic wound fluid. J Vasc Surg. 2001;33(6):1233–41. doi: 10.1067/mva.2001.113297. [DOI] [PubMed] [Google Scholar]
- 108.Harris IR, Yee KC, Walters CE, et al. Cytokine and protease levels in healing and non-healing chronic venous leg ulcers. Exp Dermatol. 1995;4(6):342–9. doi: 10.1111/j.1600-0625.1995.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 109.Ongenae KC, Phillips TJ, Park HY. Level of fibronectin mRNA is markedly increased in human chronic wounds. Dermatol Surg. 2000;26(5):447–51. doi: 10.1046/j.1524-4725.2000.99281.x. [DOI] [PubMed] [Google Scholar]
- 110.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101(1):64–8. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 111.Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. J Invest Dermatol. 1996;106(5):1119–24. doi: 10.1111/1523-1747.ep12340167. [DOI] [PubMed] [Google Scholar]
- 112.Vaalamo M, Mattila L, Johansson N, et al. Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol. 1997;109(1):96–101. doi: 10.1111/1523-1747.ep12276722. [DOI] [PubMed] [Google Scholar]
- 113.Herouy Y, Mellios P, Bandemir E, et al. Inflammation in stasis dermatitis upregulates MMP-1, MMP-2 and MMP-13 expression. J Dermatol Sci. 2001;25(3):198–205. doi: 10.1016/s0923-1811(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 114.Ulrich D, Lichtenegger F, Unglaub F, Smeets R, Pallua N. Effect of chronic wound exudates and MMP-2/-9 inhibitor on angiogenesis in vitro. Plast Reconstr Surg. 2005;116(2):539–45. doi: 10.1097/01.prs.0000173447.81513.7a. [DOI] [PubMed] [Google Scholar]
- 115.Saito S, Trovato MJ, You R, et al. Role of matrix metalloproteinases 1, 2, and 9 and tissue inhibitor of matrix metalloproteinase-1 in chronic venous insufficiency. J Vasc Surg. 2001;34(5):930–8. doi: 10.1067/mva.2001.119503. [DOI] [PubMed] [Google Scholar]
- 116.Raffetto JD, Vasquez R, Goodwin DG, Menzoian JO. Mitogen-activated protein kinase pathway regulates cell proliferation in venous ulcer fibroblasts. Vasc Endovascular Surg. 2006;40(1):59–66. doi: 10.1177/153857440604000108. [DOI] [PubMed] [Google Scholar]
- 117.Seah CC, Phillips TJ, Howard CE, et al. Chronic wound fluid suppresses proliferation of dermal fibroblasts through a Ras-mediated signaling pathway. J Invest Dermatol. 2005;124(2):466–74. doi: 10.1111/j.0022-202X.2004.23557.x. [DOI] [PubMed] [Google Scholar]
- 118.Hieta N, Impola U, Lopez-Otin C, Saarialho-Kere U, Kahari VM. Matrix metalloproteinase-19 expression in dermal wounds and by fibroblasts in culture. J Invest Dermatol. 2003;121(5):997–1004. doi: 10.1046/j.1523-1747.2003.12533.x. [DOI] [PubMed] [Google Scholar]
- 119.Norgauer J, Hildenbrand T, Idzko M, et al. Elevated expression of extracellular matrix metalloproteinase inducer (CD147) and membrane-type matrix metalloproteinases in venous leg ulcers. Br J Dermatol. 2002;147(6):1180–6. doi: 10.1046/j.1365-2133.2002.05025.x. [DOI] [PubMed] [Google Scholar]
- 120.Mwaura B, Mahendran B, Hynes N, et al. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase-2, tissue inhibitor of matrix metalloproteinase-2 and PDGF-AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg. 2006;31(3):306–10. doi: 10.1016/j.ejvs.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 121.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen. 2008;16(5):642–8. doi: 10.1111/j.1524-475X.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 122.Vaalamo M, Leivo T, Saarialho-Kere U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum Pathol. 1999;30(7):795–802. doi: 10.1016/s0046-8177(99)90140-5. [DOI] [PubMed] [Google Scholar]
- 123.Caggiati A, Bergan JJ, Gloviczki P, Jantet G, Wendell-Smith CP, Partsch H. Nomenclature of the veins of the lower limbs: an international interdisciplinary consensus statement. J Vasc Surg. 2002;36(2):416–22. doi: 10.1067/mva.2002.125847. [DOI] [PubMed] [Google Scholar]
- 124.Pollack AA, Wood EH. Venous pressure in the saphenous vein at the ankle in man during exercise and changes in posture. J Appl Physiol. 1949;1(9):649–62. doi: 10.1152/jappl.1949.1.9.649. [DOI] [PubMed] [Google Scholar]
- 125.Recek C. Conception of the venous hemodynamics in the lower extremity. Angiology. 2006;57(5):556–63. doi: 10.1177/0003319706293117. [DOI] [PubMed] [Google Scholar]
- 126.Jacob MP, Cazaubon M, Scemama A, et al. Plasma matrix metalloproteinase-9 as a marker of blood stasis in varicose veins. Circulation. 2002;106(5):535–8. doi: 10.1161/01.cir.0000027521.83518.4c. [DOI] [PubMed] [Google Scholar]
- 127.Lim CS, Qiao X, Reslan OM, et al. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. J Vasc Surg. 2011;53(3):764–73. doi: 10.1016/j.jvs.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pascarella L, Penn A, Schmid-Schonbein GW. Venous hypertension and the inflammatory cascade: major manifestations and trigger mechanisms. Angiology. 2005;56 (Suppl 1):S3–10. doi: 10.1177/00033197050560i102. [DOI] [PubMed] [Google Scholar]
- 129.Takase S, Pascarella L, Bergan JJ, Schmid-Schonbein GW. Hypertension-induced venous valve remodeling. J Vasc Surg. 2004;39(6):1329–34. doi: 10.1016/j.jvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 130.Pascarella L, Schmid-Schonbein GW, Bergan J. An animal model of venous hypertension: the role of inflammation in venous valve failure. J Vasc Surg. 2005;41(2):303–11. doi: 10.1016/j.jvs.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 131.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 132.Lim CS, Kiriakidis S, Paleolog EM, Davies AH. Increased activation of the hypoxia-inducible factor pathway in varicose veins. J Vasc Surg. 2012;55(5):1427–39. doi: 10.1016/j.jvs.2011.10.111. [DOI] [PubMed] [Google Scholar]
- 133.Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann Rheum Dis. 2005;64(7):971–80. doi: 10.1136/ard.2004.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1alpha in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res. 2002;90(2):E25–33. doi: 10.1161/hh0202.104923. [DOI] [PubMed] [Google Scholar]
- 135.Petersen W, Varoga D, Zantop T, Hassenpflug J, Mentlein R, Pufe T. Cyclic strain influences the expression of the vascular endothelial growth factor (VEGF) and the hypoxia inducible factor 1 alpha (HIF-1alpha) in tendon fibroblasts. J Orthop Res. 2004;22(4):847–53. doi: 10.1016/j.orthres.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 136.Chang H, Shyu KG, Wang BW, Kuan P. Regulation of hypoxia-inducible factor-1alpha by cyclical mechanical stretch in rat vascular smooth muscle cells. Clin Sci (Lond) 2003;105(4):447–56. doi: 10.1042/CS20030088. [DOI] [PubMed] [Google Scholar]
- 137.Milkiewicz M, Doyle JL, Fudalewski T, Ispanovic E, Aghasi M, Haas TL. HIF-1alpha and HIF-2alpha play a central role in stretch-induced but not shear-stress-induced angiogenesis in rat skeletal muscle. J Physiol. 2007;583(Pt 2):753–66. doi: 10.1113/jphysiol.2007.136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70(5):1469–80. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 139.Fujiwara S, Nakagawa K, Harada H, et al. Silencing hypoxia-inducible factor-1alpha inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int J Oncol. 2007;30(4):793–802. [PubMed] [Google Scholar]
- 140.Misra S, Fu AA, Rajan DK, et al. Expression of hypoxia inducible factor-1 alpha, macrophage migration inhibition factor, matrix metalloproteinase-2 and -9, and their inhibitors in hemodialysis grafts and arteriovenous fistulas. J Vasc Interv Radiol. 2008;19(2 Pt 1):252–9. doi: 10.1016/j.jvir.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 141.Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40(5):947–60. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 142.Thodeti CK, Matthews B, Ravi A, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104(9):1123–30. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oktay M, Wary KK, Dans M, Birge RB, Giancotti FG. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J Cell Biol. 1999;145(7):1461–9. doi: 10.1083/jcb.145.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li C, Xu Q. Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell Signal. 2000;12(7):435–45. doi: 10.1016/s0898-6568(00)00096-6. [DOI] [PubMed] [Google Scholar]
- 145.Hishikawa K, Oemar BS, Yang Z, Luscher TF. Pulsatile stretch stimulates superoxide production and activates nuclear factor-kappa B in human coronary smooth muscle. Circ Res. 1997;81(5):797–803. doi: 10.1161/01.res.81.5.797. [DOI] [PubMed] [Google Scholar]
- 146.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108(10):1253–8. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- 147.Bode W, Gomis-Ruth FX, Stockler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins’. FEBS Lett. 1993;331(1–2):134–40. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- 148.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990;87(14):5578–82. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Browner MF, Smith WW, Castelhano AL. Matrilysin-inhibitor complexes: common themes among metalloproteases. Biochemistry. 1995;34(20):6602–10. doi: 10.1021/bi00020a004. [DOI] [PubMed] [Google Scholar]
- 150.Kester WR, Matthews BW. Crystallographic study of the binding of dipeptide inhibitors to thermolysin: implications for the mechanism of catalysis. Biochemistry. 1977;16(11):2506–16. doi: 10.1021/bi00630a030. [DOI] [PubMed] [Google Scholar]
- 151.Manzetti S, McCulloch DR, Herington AC, van der Spoel D. Modeling of enzyme-substrate complexes for the metalloproteases MMP-3, ADAM-9 and ADAM-10. J Comput Aided Mol Des. 2003;17(9):551–65. doi: 10.1023/b:jcam.0000005765.13637.38. [DOI] [PubMed] [Google Scholar]
- 152.Skiles JW, Gonnella NC, Jeng AY. The design, structure, and therapeutic application of matrix metalloproteinase inhibitors. Curr Med Chem. 2001;8(4):425–74. doi: 10.2174/0929867013373417. [DOI] [PubMed] [Google Scholar]
- 153.Lovejoy B, Hassell AM, Luther MA, Weigl D, Jordan SR. Crystal structures of recombinant 19-kDa human fibroblast collagenase complexed to itself. Biochemistry. 1994;33(27):8207–17. doi: 10.1021/bi00193a006. [DOI] [PubMed] [Google Scholar]
- 154.Suenaga N, Mori H, Itoh Y, Seiki M. CD44 binding through the hemopexin-like domain is critical for its shedding by membrane-type 1 matrix metalloproteinase. Oncogene. 2005;24(5):859–68. doi: 10.1038/sj.onc.1208258. [DOI] [PubMed] [Google Scholar]
- 155.Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin) Biochemistry. 1990;29(44):10261–70. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- 156.Mulvany MJ, Baumbach GL, Aalkjaer C, et al. Vascular remodeling. Hypertension. 1996;28(3):505–6. [PubMed] [Google Scholar]
- 157.Butler GS, Butler MJ, Atkinson SJ, et al. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998;273(2):871–80. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- 158.Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275(34):26411–5. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Itoh Y, Takamura A, Ito N, et al. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001;20(17):4782–93. doi: 10.1093/emboj/20.17.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kudo T, Takino T, Miyamori H, Thompson EW, Sato H. Substrate choice of membrane-type 1 matrix metalloproteinase is dictated by tissue inhibitor of metalloproteinase-2 levels. Cancer Sci. 2007;98(4):563–8. doi: 10.1111/j.1349-7006.2007.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van de Ven WJ, Voorberg J, Fontijn R, et al. Furin is a subtilisin-like proprotein processing enzyme in higher eukaryotes. Mol Biol Rep. 1990;14(4):265–75. doi: 10.1007/BF00429896. [DOI] [PubMed] [Google Scholar]
- 162.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276(44):41279–87. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 163.Fu X, Kao JL, Bergt C, et al. Oxidative cross-linking of tryptophan to glycine restrains matrix metalloproteinase activity: specific structural motifs control protein oxidation. J Biol Chem. 2004;279(8):6209–12. doi: 10.1074/jbc.C300506200. [DOI] [PubMed] [Google Scholar]
- 164.Okamoto T, Akaike T, Nagano T, et al. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch Biochem Biophys. 1997;342(2):261–74. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 165.Gu Z, Kaul M, Yan B, et al. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297(5584):1186–90. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 166.Oh C, Dong Y, Liu H, Thompson LP. Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts. Am J Obstet Gynecol. 2008;199(1):78, e1–6. doi: 10.1016/j.ajog.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 167.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267(6):3581–4. [PubMed] [Google Scholar]
- 168.Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006;69(3):625–35. doi: 10.1016/j.cardiores.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 169.Liu Z, Zhou X, Shapiro SD, et al. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 2000;102(5):647–55. doi: 10.1016/s0092-8674(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 170.Desrochers PE, Mookhtiar K, Van Wart HE, Hasty KA, Weiss SJ. Proteolytic inactivation of alpha 1-proteinase inhibitor and alpha 1-antichymotrypsin by oxidatively activated human neutrophil metalloproteinases. J Biol Chem. 1992;267(7):5005–12. [PubMed] [Google Scholar]
- 171.Chen LC, Noelken ME, Nagase H. Disruption of the cysteine-75 and zinc ion coordination is not sufficient to activate the precursor of human matrix metalloproteinase 3 (stromelysin 1) Biochemistry. 1993;32(39):10289–95. doi: 10.1021/bi00090a003. [DOI] [PubMed] [Google Scholar]
- 172.Iyer RP, Patterson NL, Fields GB, Lindsey ML. The history of matrix metalloproteinases: milestones, myths, and misperceptions. Am J Physiol Heart Circ Physiol. 2012;303(8):H919–30. doi: 10.1152/ajpheart.00577.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 174.Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair (review) Int J Mol Med. 2000;6(4):391–407. [PubMed] [Google Scholar]
- 175.Ganz T. Defensins and host defense. Science. 1999;286(5439):420–1. doi: 10.1126/science.286.5439.420. [DOI] [PubMed] [Google Scholar]
- 176.Wilson CL, Ouellette AJ, Satchell DP, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286(5437):113–7. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 177.Gearing AJ, Thorpe SJ, Miller K, et al. Selective cleavage of human IgG by the matrix metalloproteinases, matrilysin and stromelysin. Immunol Lett. 2002;81(1):41–8. doi: 10.1016/s0165-2478(01)00333-9. [DOI] [PubMed] [Google Scholar]
- 178.Ruiz S, Henschen-Edman AH, Nagase H, Tenner AJ. Digestion of C1q collagen-like domain with MMPs-1,-2,-3, and -9 further defines the sequence involved in the stimulation of neutrophil superoxide production. J Leukoc Biol. 1999;66(3):416–22. doi: 10.1002/jlb.66.3.416. [DOI] [PubMed] [Google Scholar]
- 179.Feng X, Tonnesen MG, Peerschke EI, Ghebrehiwet B. Cooperation of C1q receptors and integrins in C1q-mediated endothelial cell adhesion and spreading. J Immunol. 2002;168(5):2441–8. doi: 10.4049/jimmunol.168.5.2441. [DOI] [PubMed] [Google Scholar]
- 180.Rozanov DV, Ghebrehiwet B, Postnova TI, Eichinger A, Deryugina EI, Strongin AY. The hemopexin-like C-terminal domain of membrane type 1 matrix metalloproteinase regulates proteolysis of a multifunctional protein, gC1qR. J Biol Chem. 2002;277(11):9318–25. doi: 10.1074/jbc.M110711200. [DOI] [PubMed] [Google Scholar]
- 181.Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood. 2006;108(5):1441–50. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- 182.Eck SM, Blackburn JS, Schmucker AC, Burrage PS, Brinckerhoff CE. Matrix metalloproteinase and G protein coupled receptors: co-conspirators in the pathogenesis of autoimmune disease and cancer. J Autoimmun. 2009;33(3–4):214–21. doi: 10.1016/j.jaut.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Harvey MB, Leco KJ, Arcellana-Panlilio MY, Zhang X, Edwards DR, Schultz GA. Proteinase expression in early mouse embryos is regulated by leukaemia inhibitory factor and epidermal growth factor. Development. 1995;121(4):1005–14. doi: 10.1242/dev.121.4.1005. [DOI] [PubMed] [Google Scholar]
- 184.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14(17):2123–33. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 185.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200(4):423–8. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 187.Teti A. Regulation of cellular functions by extracellular matrix. J Am Soc Nephrol. 1992;2(10 Suppl):S83–7. doi: 10.1681/ASN.V210s83. [DOI] [PubMed] [Google Scholar]
- 188.Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57(5–6):195–202. doi: 10.1016/s0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 189.Midwood KS, Mao Y, Hsia HC, Valenick LV, Schwarzbauer JE. Modulation of cell-fibronectin matrix interactions during tissue repair. J Investig Dermatol Symp Proc. 2006;11(1):73–8. doi: 10.1038/sj.jidsymp.5650005. [DOI] [PubMed] [Google Scholar]
- 190.Geutjes PJ, Daamen WF, Buma P, Feitz WF, Faraj KA, van Kuppevelt TH. From molecules to matrix: construction and evaluation of molecularly defined bioscaffolds. Adv Exp Med Biol. 2006;585:279–95. doi: 10.1007/978-0-387-34133-0_19. [DOI] [PubMed] [Google Scholar]
- 191.Lovejoy B, Welch AR, Carr S, et al. Crystal structures of MMP-1 and -13 reveal the structural basis for selectivity of collagenase inhibitors. Nat Struct Biol. 1999;6(3):217–21. doi: 10.1038/6657. [DOI] [PubMed] [Google Scholar]
- 192.Olson MW, Toth M, Gervasi DC, Sado Y, Ninomiya Y, Fridman R. High affinity binding of latent matrix metalloproteinase-9 to the alpha2(IV) chain of collagen IV. J Biol Chem. 1998;273(17):10672–81. doi: 10.1074/jbc.273.17.10672. [DOI] [PubMed] [Google Scholar]
- 193.Porter KE, Thompson MM, Loftus IM, et al. Production and inhibition of the gelatinolytic matrix metalloproteinases in a human model of vein graft stenosis. Eur J Vasc Endovasc Surg. 1999;17(5):404–12. doi: 10.1053/ejvs.1998.0761. [DOI] [PubMed] [Google Scholar]
- 194.Mavromatis K, Fukai T, Tate M, Chesler N, Ku DN, Galis ZS. Early effects of arterial hemodynamic conditions on human saphenous veins perfused ex vivo. Arterioscler Thromb Vasc Biol. 2000;20(8):1889–95. doi: 10.1161/01.atv.20.8.1889. [DOI] [PubMed] [Google Scholar]
- 195.Anstadt MP, Franga DL, Portik-Dobos V, et al. Native matrix metalloproteinase characteristics may influence early stenosis of venous versus arterial coronary artery bypass grafting conduits. Chest. 2004;125(5):1853–8. doi: 10.1378/chest.125.5.1853. [DOI] [PubMed] [Google Scholar]
- 196.Nagakawa Y, Aoki T, Kasuya K, Tsuchida A, Koyanagi Y. Histologic features of venous invasion, expression of vascular endothelial growth factor and matrix metalloproteinase-2 and matrix metalloproteinase-9, and the relation with liver metastasis in pancreatic cancer. Pancreas. 2002;24(2):169–78. doi: 10.1097/00006676-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 197.Sansilvestri-Morel P, Rupin A, Jaisson S, Fabiani JN, Verbeuren TJ, Vanhoutte PM. Synthesis of collagen is dysregulated in cultured fibroblasts derived from skin of subjects with varicose veins as it is in venous smooth muscle cells. Circulation. 2002;106(4):479–83. doi: 10.1161/01.cir.0000022846.22923.46. [DOI] [PubMed] [Google Scholar]
- 198.Gandhi RH, Irizarry E, Nackman GB, Halpern VJ, Mulcare RJ, Tilson MD. Analysis of the connective tissue matrix and proteolytic activity of primary varicose veins. J Vasc Surg. 1993;18(5):814–20. [PubMed] [Google Scholar]
- 199.Venturi M, Bonavina L, Annoni F, et al. Biochemical assay of collagen and elastin in the normal and varicose vein wall. J Surg Res. 1996;60(1):245–8. doi: 10.1006/jsre.1996.0038. [DOI] [PubMed] [Google Scholar]
- 200.Haviarova Z, Weismann P, Stvrtinova V, Benuska J. The determination of the collagen and elastin amount in the human varicose vein by the computer morphometric method. Gen Physiol Biophys. 1999;18 (Suppl 1):30–3. [PubMed] [Google Scholar]
- 201.Kockx MM, Knaapen MW, Bortier HE, Cromheeke KM, Boutherin-Falson O, Finet M. Vascular remodeling in varicose veins. Angiology. 1998;49(11):871–7. doi: 10.1177/000331979804901101. [DOI] [PubMed] [Google Scholar]
- 202.Kirsch D, Dienes HP, Kuchle R, et al. Changes in the extracellular matrix of the vein wall--the cause of primary varicosis? Vasa. 2000;29(3):173–7. doi: 10.1024/0301-1526.29.3.173. [DOI] [PubMed] [Google Scholar]
- 203.Sansilvestri-Morel P, Fioretti F, Rupin A, et al. Comparison of extracellular matrix in skin and saphenous veins from patients with varicose veins: does the skin reflect venous matrix changes? Clin Sci (Lond) 2007;112(4):229–39. doi: 10.1042/CS20060170. [DOI] [PubMed] [Google Scholar]
- 204.Parra JR, Cambria RA, Hower CD, et al. Tissue inhibitor of metalloproteinase-1 is increased in the saphenofemoral junction of patients with varices in the leg. J Vasc Surg. 1998;28(4):669–75. doi: 10.1016/s0741-5214(98)70093-x. [DOI] [PubMed] [Google Scholar]
- 205.Badier-Commander C, Verbeuren T, Lebard C, Michel JB, Jacob MP. Increased TIMP/MMP ratio in varicose veins: a possible explanation for extracellular matrix accumulation. J Pathol. 2000;192(1):105–12. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH670>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 206.Sansilvestri-Morel P, Nonotte I, Fournet-Bourguignon MP, et al. Abnormal deposition of extracellular matrix proteins by cultured smooth muscle cells from human varicose veins. J Vasc Res. 1998;35(2):115–23. doi: 10.1159/000025573. [DOI] [PubMed] [Google Scholar]
- 207.Sansilvestri-Morel P, Rupin A, Jullien ND, et al. Decreased production of collagen Type III in cultured smooth muscle cells from varicose vein patients is due to a degradation by MMPs: possible implication of MMP-3. J Vasc Res. 2005;42(5):388–98. doi: 10.1159/000087314. [DOI] [PubMed] [Google Scholar]
- 208.Xiao Y, Huang Z, Yin H, Zhang H, Wang S. Desmuslin gene knockdown causes altered expression of phenotype markers and differentiation of saphenous vein smooth muscle cells. J Vasc Surg. 2010;52(3):684–90. doi: 10.1016/j.jvs.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 209.Nagareddy PR, Chow FL, Hao L, et al. Maintenance of adrenergic vascular tone by MMP transactivation of the EGFR requires PI3K and mitochondrial ATP synthesis. Cardiovasc Res. 2009;84(3):368–77. doi: 10.1093/cvr/cvp230. [DOI] [PubMed] [Google Scholar]
- 210.Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J Vasc Surg. 2004;40(5):1001–10. doi: 10.1016/j.jvs.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 211.Herouy Y, Nockowski P, Schopf E, Norgauer J. Lipodermatosclerosis and the significance of proteolytic remodeling in the pathogenesis of venous ulceration (Review) Int J Mol Med. 1999;3(5):511–5. doi: 10.3892/ijmm.3.5.511. [DOI] [PubMed] [Google Scholar]
- 212.Thulesius O. The venous wall and valvular function in chronic venous insufficiency. Int Angiol. 1996;15(2):114–8. [PubMed] [Google Scholar]
- 213.Waitkus-Edwards KR, Martinez-Lemus LA, Wu X, et al. alpha(4)beta(1) Integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circ Res. 2002;90(4):473–80. doi: 10.1161/hh0402.105899. [DOI] [PubMed] [Google Scholar]
- 214.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53(2):245–82. [PubMed] [Google Scholar]
- 215.Hamilton JR, Nguyen PB, Cocks TM. Atypical protease-activated receptor mediates endothelium-dependent relaxation of human coronary arteries. Circ Res. 1998;82(12):1306–11. doi: 10.1161/01.res.82.12.1306. [DOI] [PubMed] [Google Scholar]
- 216.Shi ZD, Ji XY, Berardi DE, Qazi H, Tarbell JM. Interstitial flow induces MMP-1 expression and vascular SMC migration in collagen I gels via an ERK1/2-dependent and c-Jun-mediated mechanism. Am J Physiol Heart Circ Physiol. 2010;298(1):H127–35. doi: 10.1152/ajpheart.00732.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vigetti D, Moretto P, Viola M, et al. Aortic smooth muscle cells migration and the role of metalloproteinases and hyaluronan. Connect Tissue Res. 2008;49(3):189–92. doi: 10.1080/03008200802143141. [DOI] [PubMed] [Google Scholar]
- 218.Haque NS, Fallon JT, Pan JJ, Taubman MB, Harpel PC. Chemokine receptor-8 (CCR8) mediates human vascular smooth muscle cell chemotaxis and metalloproteinase-2 secretion. Blood. 2004;103(4):1296–304. doi: 10.1182/blood-2002-05-1480. [DOI] [PubMed] [Google Scholar]
- 219.Cheng XW, Kuzuya M, Sasaki T, et al. Increased expression of elastolytic cysteine proteases, cathepsins S and K, in the neointima of balloon-injured rat carotid arteries. Am J Pathol. 2004;164(1):243–51. doi: 10.1016/S0002-9440(10)63114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004;24(1):54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- 221.Jin UH, Suh SJ, Chang HW, et al. Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J Cell Biochem. 2008;104(1):15–26. doi: 10.1002/jcb.21599. [DOI] [PubMed] [Google Scholar]
- 222.Yu YM, Lin HC. Curcumin prevents human aortic smooth muscle cells migration by inhibiting of MMP-9 expression. Nutr Metab Cardiovasc Dis. 2010;20(2):125–32. doi: 10.1016/j.numecd.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 223.Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res. 2002;91(9):845–51. doi: 10.1161/01.res.0000040420.17366.2e. [DOI] [PubMed] [Google Scholar]
- 224.Galis ZS, Johnson C, Godin D, et al. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91(9):852–9. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 225.Aguilera CM, George SJ, Johnson JL, Newby AC. Relationship between type IV collagen degradation, metalloproteinase activity and smooth muscle cell migration and proliferation in cultured human saphenous vein. Cardiovasc Res. 2003;58(3):679–88. doi: 10.1016/s0008-6363(03)00256-6. [DOI] [PubMed] [Google Scholar]
- 226.Carragher NO, Frame MC. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14(5):241–9. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 227.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Savani RC, Wang C, Yang B, et al. Migration of bovine aortic smooth muscle cells after wounding injury. The role of hyaluronan and RHAMM. J Clin Invest. 1995;95(3):1158–68. doi: 10.1172/JCI117764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Uglow EB, Slater S, Sala-Newby GB, et al. Dismantling of cadherin-mediated cell-cell contacts modulates smooth muscle cell proliferation. Circ Res. 2003;92(12):1314–21. doi: 10.1161/01.RES.0000079027.44309.53. [DOI] [PubMed] [Google Scholar]
- 230.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120(3):303–13. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 231.Somers P, Knaapen M. The histopathology of varicose vein disease. Angiology. 2006;57(5):546–55. doi: 10.1177/0003319706293115. [DOI] [PubMed] [Google Scholar]
- 232.Wali MA, Eid RA. Smooth muscle changes in varicose veins: an ultrastructural study. J Smooth Muscle Res. 2001;37(5–6):123–35. doi: 10.1540/jsmr.37.123. [DOI] [PubMed] [Google Scholar]
- 233.Wali MA, Eid RA. Intimal changes in varicose veins: an ultrastructural study. J Smooth Muscle Res. 2002;38(3):63–74. doi: 10.1540/jsmr.38.63. [DOI] [PubMed] [Google Scholar]
- 234.Elsharawy MA, Naim MM, Abdelmaguid EM, Al-Mulhim AA. Role of saphenous vein wall in the pathogenesis of primary varicose veins. Interact Cardiovasc Thorac Surg. 2007;6(2):219–24. doi: 10.1510/icvts.2006.136937. [DOI] [PubMed] [Google Scholar]
- 235.Forough R, Koyama N, Hasenstab D, et al. Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ Res. 1996;79(4):812–20. doi: 10.1161/01.res.79.4.812. [DOI] [PubMed] [Google Scholar]
- 236.Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101(6):1478–87. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.George SJ, Lloyd CT, Angelini GD, Newby AC, Baker AH. Inhibition of late vein graft neointima formation in human and porcine models by adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-3. Circulation. 2000;101(3):296–304. doi: 10.1161/01.cir.101.3.296. [DOI] [PubMed] [Google Scholar]
- 238.Kenagy RD, Vergel S, Mattsson E, Bendeck M, Reidy MA, Clowes AW. The role of plasminogen, plasminogen activators, and matrix metalloproteinases in primate arterial smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 1996;16(11):1373–82. doi: 10.1161/01.atv.16.11.1373. [DOI] [PubMed] [Google Scholar]
- 239.Islam MM, Franco CD, Courtman DW, Bendeck MP. A nonantibiotic chemically modified tetracycline (CMT-3) inhibits intimal thickening. Am J Pathol. 2003;163(4):1557–66. doi: 10.1016/S0002-9440(10)63512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lee YJ, Kim JS, Kang DG, Lee HS. Buddleja officinalis suppresses high glucose-induced vascular smooth muscle cell proliferation: role of mitogen-activated protein kinases, nuclear factor-kappaB and matrix metalloproteinases. Exp Biol Med (Maywood) 2010;235(2):247–55. doi: 10.1258/ebm.2009.009222. [DOI] [PubMed] [Google Scholar]
- 241.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85(1):1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 242.Xiao Y, Huang Z, Yin H, Lin Y, Wang S. In vitro differences between smooth muscle cells derived from varicose veins and normal veins. J Vasc Surg. 2009;50(5):1149–54. doi: 10.1016/j.jvs.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 243.Walker HA, Whitelock JM, Garl PJ, Nemenoff RA, Stenmark KR, Weiser-Evans MC. Perlecan up-regulation of FRNK suppresses smooth muscle cell proliferation via inhibition of FAK signaling. Mol Biol Cell. 2003;14(5):1941–52. doi: 10.1091/mbc.E02-08-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Morla AO, Mogford JE. Control of smooth muscle cell proliferation and phenotype by integrin signaling through focal adhesion kinase. Biochem Biophys Res Commun. 2000;272(1):298–302. doi: 10.1006/bbrc.2000.2769. [DOI] [PubMed] [Google Scholar]
- 245.Lucchesi PA, Sabri A, Belmadani S, Matrougui K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004;110(23):3587–93. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- 246.Hollenbeck ST, Sakakibara K, Faries PL, Workhu B, Liu B, Kent KC. Stem cell factor and c-kit are expressed by and may affect vascular SMCs through an autocrine pathway. J Surg Res. 2004;120(2):288–94. doi: 10.1016/j.jss.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 247.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116(Pt 2):217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 248.Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997;272(50):31730–7. doi: 10.1074/jbc.272.50.31730. [DOI] [PubMed] [Google Scholar]
- 249.Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem J. 1997;322 (Pt 3):809–14. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Manes S, Mira E, Barbacid MM, et al. Identification of insulin-like growth factor-binding protein-1 as a potential physiological substrate for human stromelysin-3. J Biol Chem. 1997;272(41):25706–12. doi: 10.1074/jbc.272.41.25706. [DOI] [PubMed] [Google Scholar]
- 251.Noe V, Fingleton B, Jacobs K, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114(Pt 1):111–8. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 252.Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K, Huber O. Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem. 2001;276(7):4972–80. doi: 10.1074/jbc.M006102200. [DOI] [PubMed] [Google Scholar]
- 253.Asamoto M, Hokaiwado N, Cho YM, et al. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res. 2001;61(12):4693–700. [PubMed] [Google Scholar]
- 254.Fujiwara K, Matsukawa A, Ohkawara S, Takagi K, Yoshinaga M. Functional distinction between CXC chemokines, interleukin-8 (IL-8), and growth related oncogene (GRO)alpha in neutrophil infiltration. Lab Invest. 2002;82(1):15–23. doi: 10.1038/labinvest.3780391. [DOI] [PubMed] [Google Scholar]
- 255.McQuibban GA, Butler GS, Gong JH, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276(47):43503–8. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 256.Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci U S A. 1996;93(14):7069–74. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bendeck MP, Irvin C, Reidy MA. Inhibition of matrix metalloproteinase activity inhibits smooth muscle cell migration but not neointimal thickening after arterial injury. Circ Res. 1996;78(1):38–43. doi: 10.1161/01.res.78.1.38. [DOI] [PubMed] [Google Scholar]
- 258.Zempo N, Koyama N, Kenagy RD, Lea HJ, Clowes AW. Regulation of vascular smooth muscle cell migration and proliferation in vitro and in injured rat arteries by a synthetic matrix metalloproteinase inhibitor. Arterioscler Thromb Vasc Biol. 1996;16(1):28–33. doi: 10.1161/01.atv.16.1.28. [DOI] [PubMed] [Google Scholar]
- 259.Lovdahl C, Thyberg J, Hultgardh-Nilsson A. The synthetic metalloproteinase inhibitor batimastat suppresses injury-induced phosphorylation of MAP kinase ERK1/ERK2 and phenotypic modification of arterial smooth muscle cells in vitro. J Vasc Res. 2000;37(5):345–54. doi: 10.1159/000025750. [DOI] [PubMed] [Google Scholar]
- 260.Slater SC, Koutsouki E, Jackson CL, et al. R-cadherin:beta-catenin complex and its association with vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 2004;24(7):1204–10. doi: 10.1161/01.ATV.0000130464.24599.e0. [DOI] [PubMed] [Google Scholar]
- 261.Bendeck MP, Conte M, Zhang M, Nili N, Strauss BH, Farwell SM. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. Am J Pathol. 2002;160(3):1089–95. doi: 10.1016/S0002-9440(10)64929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, Wang DL. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res. 1997;81(1):1–7. doi: 10.1161/01.res.81.1.1. [DOI] [PubMed] [Google Scholar]
- 263.Wu X, Shen H, Yu L, Peng M, Lai WS, Ding YL. Corticotropin-releasing hormone activates connexin 43 via activator protein-1 transcription factor in human myometrial smooth muscle cells. Am J Physiol Endocrinol Metab. 2007;293(6):E1789–94. doi: 10.1152/ajpendo.00249.2007. [DOI] [PubMed] [Google Scholar]
- 264.Buchwald AB, Wagner AH, Webel C, Hecker M. Decoy oligodeoxynucleotide against activator protein-1 reduces neointimal proliferation after coronary angioplasty in hypercholesterolemic minipigs. J Am Coll Cardiol. 2002;39(4):732–8. doi: 10.1016/s0735-1097(01)01797-1. [DOI] [PubMed] [Google Scholar]
- 265.Mann MJ, Dzau VJ. Therapeutic applications of transcription factor decoy oligonucleotides. J Clin Invest. 2000;106(9):1071–5. doi: 10.1172/JCI11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Demicheva E, Hecker M, Korff T. Stretch-induced activation of the transcription factor activator protein-1 controls monocyte chemoattractant protein-1 expression during arteriogenesis. Circ Res. 2008;103(5):477–84. doi: 10.1161/CIRCRESAHA.108.177782. [DOI] [PubMed] [Google Scholar]
- 267.Bergman MR, Cheng S, Honbo N, Piacentini L, Karliner JS, Lovett DH. A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers. Biochem J. 2003;369(Pt 3):485–96. doi: 10.1042/BJ20020707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Feldner A, Otto H, Rewerk S, Hecker M, Korff T. Experimental hypertension triggers varicosis-like maladaptive venous remodeling through activator protein-1. FASEB J. 2011;25(10):3613–21. doi: 10.1096/fj.11-185975. [DOI] [PubMed] [Google Scholar]
- 269.Geng YJ, Libby P. Progression of atheroma: a struggle between death and procreation. Arterioscler Thromb Vasc Biol. 2002;22(9):1370–80. doi: 10.1161/01.atv.0000031341.84618.a4. [DOI] [PubMed] [Google Scholar]
- 270.Stoneman VE, Bennett MR. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin Sci (Lond) 2004;107(4):343–54. doi: 10.1042/CS20040086. [DOI] [PubMed] [Google Scholar]
- 271.Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94(1):68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 272.Akiba S, Kumazawa S, Yamaguchi H, et al. Acceleration of matrix metalloproteinase-1 production and activation of platelet-derived growth factor receptor beta in human coronary smooth muscle cells by oxidized LDL and 4-hydroxynonenal. Biochim Biophys Acta. 2006;1763(8):797–804. doi: 10.1016/j.bbamcr.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 273.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 274.Almeida EA, Ilic D, Han Q, et al. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149(3):741–54. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ilic D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143(2):547–60. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Levkau B, Kenagy RD, Karsan A, et al. Activation of metalloproteinases and their association with integrins: an auxiliary apoptotic pathway in human endothelial cells. Cell Death Differ. 2002;9(12):1360–7. doi: 10.1038/sj.cdd.4401106. [DOI] [PubMed] [Google Scholar]
- 277.Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: old dogs with new tricks. Genome Biol. 2003;4(6):216. doi: 10.1186/gb-2003-4-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Mannello F, Luchetti F, Falcieri E, Papa S. Multiple roles of matrix metalloproteinases during apoptosis. Apoptosis. 2005;10(1):19–24. doi: 10.1007/s10495-005-6058-7. [DOI] [PubMed] [Google Scholar]
- 279.Bond M, Murphy G, Bennett MR, et al. Localization of the death domain of tissue inhibitor of metalloproteinase-3 to the N terminus. Metalloproteinase inhibition is associated with proapoptotic activity. J Biol Chem. 2000;275(52):41358–63. doi: 10.1074/jbc.M007929200. [DOI] [PubMed] [Google Scholar]
- 280.Kwan JA, Schulze CJ, Wang W, et al. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18(6):690–2. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 281.Guo YH, Gao W, Li Q, Li PF, Yao PY, Chen K. Tissue inhibitor of metalloproteinases-4 suppresses vascular smooth muscle cell migration and induces cell apoptosis. Life Sci. 2004;75(20):2483–93. doi: 10.1016/j.lfs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 282.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26(6):1215–25. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 283.Cai WJ, Kocsis E, Luo X, Schaper W, Schaper J. Expression of endothelial nitric oxide synthase in the vascular wall during arteriogenesis. Mol Cell Biochem. 2004;264(1–2):193–200. doi: 10.1023/b:mcbi.0000044388.27953.a0. [DOI] [PubMed] [Google Scholar]
- 284.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol. 2004;31(9):641–9. doi: 10.1111/j.1440-1681.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 285.Coleman HA, Tare M, Parkington HC. K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. J Physiol. 2001;531(Pt 2):359–73. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Cena J, Lalu MM, Rosenfelt C, Schulz R. Endothelial dependence of matrix metalloproteinase-mediated vascular hyporeactivity caused by lipopolysaccharide. Eur J Pharmacol. 2008;582(1–3):116–22. doi: 10.1016/j.ejphar.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 287.Cohen RA. The endothelium-derived hyperpolarizing factor puzzle: a mechanism without a mediator? Circulation. 2005;111(6):724–7. doi: 10.1161/01.CIR.0000156405.75257.62. [DOI] [PubMed] [Google Scholar]
- 288.Lee HY, You HJ, Won JY, et al. Forkhead factor, FOXO3a, induces apoptosis of endothelial cells through activation of matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2008;28(2):302–8. doi: 10.1161/ATVBAHA.107.150664. [DOI] [PubMed] [Google Scholar]
- 289.Jacob-Ferreira AL, Palei AC, Cau SB, et al. Evidence for the involvement of matrix metalloproteinases in the cardiovascular effects produced by nicotine. Eur J Pharmacol. 2010;627(1–3):216–22. doi: 10.1016/j.ejphar.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 290.Castro MM, Rizzi E, Rodrigues GJ, et al. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic Biol Med. 2009;46(9):1298–307. doi: 10.1016/j.freeradbiomed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 291.Goerge T, Barg A, Schnaeker EM, et al. Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res. 2006;66(15):7766–74. doi: 10.1158/0008-5472.CAN-05-3897. [DOI] [PubMed] [Google Scholar]
- 292.Tarin C, Gomez M, Calvo E, Lopez JA, Zaragoza C. Endothelial nitric oxide deficiency reduces MMP-13-mediated cleavage of ICAM-1 in vascular endothelium: a role in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(1):27–32. doi: 10.1161/ATVBAHA.108.169623. [DOI] [PubMed] [Google Scholar]
- 293.Aunapuu M, Arend A. Histopathological changes and expression of adhesion molecules and laminin in varicose veins. Vasa. 2005;34(3):170–5. doi: 10.1024/0301-1526.34.3.170. [DOI] [PubMed] [Google Scholar]
- 294.Louboutin JP, Agrawal L, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. J Neuropathol Exp Neurol. 2010;69(8):801–16. doi: 10.1097/NEN.0b013e3181e8c96f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Thanabalasundaram G, Pieper C, Lischper M, Galla HJ. Regulation of the blood-brain barrier integrity by pericytes via matrix metalloproteinases mediated activation of vascular endothelial growth factor in vitro. Brain Res. 2010;1347:1–10. doi: 10.1016/j.brainres.2010.05.096. [DOI] [PubMed] [Google Scholar]
- 296.Lischper M, Beuck S, Thanabalasundaram G, Pieper C, Galla HJ. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 2010;1326:114–27. doi: 10.1016/j.brainres.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 297.Horner J, Fernandes J, Fernandes E, Nicolaides AN. Value of graduated compression stockings in deep venous insufficiency. Br Med J. 1980;280(6217):820–1. doi: 10.1136/bmj.280.6217.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wells PS, Lensing AW, Hirsh J. Graduated compression stockings in the prevention of postoperative venous thromboembolism. A meta-analysis. Arch Intern Med. 1994;154(1):67–72. [PubMed] [Google Scholar]
- 299.Motykie GD, Caprini JA, Arcelus JI, Reyna JJ, Overom E, Mokhtee D. Evaluation of therapeutic compression stockings in the treatment of chronic venous insufficiency. Dermatol Surg. 1999;25(2):116–20. doi: 10.1046/j.1524-4725.1999.08095.x. [DOI] [PubMed] [Google Scholar]
- 300.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 301.Amaragiri SV, Lees TA. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev. 2000;(3):CD001484. doi: 10.1002/14651858.CD001484. [DOI] [PubMed] [Google Scholar]
- 302.Mayberry JC, Moneta GL, DeFrang RD, Porter JM. The influence of elastic compression stockings on deep venous hemodynamics. J Vasc Surg. 1991;13(1):91–9. doi: 10.1067/mva.1991.25386. discussion 9–100. [DOI] [PubMed] [Google Scholar]
- 303.Ibegbuna V, Delis KT, Nicolaides AN, Aina O. Effect of elastic compression stockings on venous hemodynamics during walking. J Vasc Surg. 2003;37(2):420–5. doi: 10.1067/mva.2003.104. [DOI] [PubMed] [Google Scholar]
- 304.Bergan JJ. Chronic venous insufficiency and the therapeutic effects of Daflon 500 mg. Angiology. 2005;56 (Suppl 1):S21–4. doi: 10.1177/00033197050560i104. [DOI] [PubMed] [Google Scholar]
- 305.Boisseau MR. Pharmacological targets of drugs employed in chronic venous and lymphatic insufficiency. Int Angiol. 2002;21(2 Suppl 1):33–9. [PubMed] [Google Scholar]
- 306.Carrasco OF, Vidrio H. Endothelium protectant and contractile effects of the antivaricose principle escin in rat aorta. Vascul Pharmacol. 2007;47(1):68–73. doi: 10.1016/j.vph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 307.Raffetto JD, Khalil RA. Ca(2+)-dependent contraction by the saponoside escin in rat vena cava: implications in venotonic treatment of varicose veins. J Vasc Surg. 2011;54(2):489–96. doi: 10.1016/j.jvs.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Le Devehat C, Khodabandehlou T, Vimeux M, Kempf C. Evaluation of haemorheological and microcirculatory disturbances in chronic venous insufficiency: activity of Daflon 500 mg. Int J Microcirc Clin Exp. 1997;17 (Suppl 1):27–33. doi: 10.1159/000179264. [DOI] [PubMed] [Google Scholar]
- 309.Cesarone MR, Belcaro G, Pellegrini L, et al. Venoruton vs Daflon: evaluation of effects on quality of life in chronic venous insufficiency. Angiology. 2006;57(2):131–8. doi: 10.1177/000331970605700201. [DOI] [PubMed] [Google Scholar]
- 310.Cesarone MR, Belcaro G, Pellegrini L, et al. Circulating endothelial cells in venous blood as a marker of endothelial damage in chronic venous insufficiency: improvement with venoruton. J Cardiovasc Pharmacol Ther. 2006;11(1):93–8. doi: 10.1177/107424840601100109. [DOI] [PubMed] [Google Scholar]
- 311.Janssens D, Michiels C, Guillaume G, Cuisinier B, Louagie Y, Remacle J. Increase in circulating endothelial cells in patients with primary chronic venous insufficiency: protective effect of Ginkor Fort in a randomized double-blind, placebo-controlled clinical trial. J Cardiovasc Pharmacol. 1999;33(1):7–11. doi: 10.1097/00005344-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 312.Geroulakos G, Nicolaides AN. Controlled studies of Daflon 500 mg in chronic venous insufficiency. Angiology. 1994;45(6 Pt 2):549–53. [PubMed] [Google Scholar]
- 313.Vanscheidt W, Rabe E, Naser-Hijazi B, et al. The efficacy and safety of a coumarin-/troxerutin-combination (SB-LOT) in patients with chronic venous insufficiency: a double blind placebo-controlled randomised study. Vasa. 2002;31(3):185–90. doi: 10.1024/0301-1526.31.3.185. [DOI] [PubMed] [Google Scholar]
- 314.Roztocil K, Stvrtinova V, Strejcek J. Efficacy of a 6-month treatment with Daflon 500 mg in patients with venous leg ulcers associated with chronic venous insufficiency. Int Angiol. 2003;22(1):24–31. [PubMed] [Google Scholar]
- 315.Siebert U, Brach M, Sroczynski G, Berla K. Efficacy, routine effectiveness, and safety of horsechestnut seed extract in the treatment of chronic venous insufficiency. A meta-analysis of randomized controlled trials and large observational studies. Int Angiol. 2002;21(4):305–15. [PubMed] [Google Scholar]
- 316.Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev. 2006;(1):CD003230. doi: 10.1002/14651858.CD003230.pub3. [DOI] [PubMed] [Google Scholar]
- 317.Falanga V, Fujitani RM, Diaz C, et al. Systemic treatment of venous leg ulcers with high doses of pentoxifylline: efficacy in a randomized, placebo-controlled trial. Wound Repair Regen. 1999;7(4):208–13. doi: 10.1046/j.1524-475x.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- 318.Belcaro G, Cesarone MR, Nicolaides AN, De Sanctis MT, Incandela L, Geroulakos G. Treatment of venous ulcers with pentoxifylline: a 6-month randomized, double-blind, placebo controlled trial. Angiology. 2002;53 (Suppl 1):S45–7. [PubMed] [Google Scholar]
- 319.Kalus U, Koscielny J, Grigorov A, Schaefer E, Peil H, Kiesewetter H. Improvement of cutaneous microcirculation and oxygen supply in patients with chronic venous insufficiency by orally administered extract of red vine leaves AS 195: a randomised, double-blind, placebo-controlled, crossover study. Drugs R D. 2004;5(2):63–71. doi: 10.2165/00126839-200405020-00001. [DOI] [PubMed] [Google Scholar]
- 320.Milio G, Mina C, Cospite V, Almasio PL, Novo S. Efficacy of the treatment with prostaglandin E-1 in venous ulcers of the lower limbs. J Vasc Surg. 2005;42(2):304–8. doi: 10.1016/j.jvs.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 321.Kahle B, Leng K. Efficacy of sclerotherapy in varicose veins-- prospective, blinded, placebo-controlled study. Dermatol Surg. 2004;30(5):723–8. doi: 10.1111/j.1524-4725.2004.30207.x. discussion 8. [DOI] [PubMed] [Google Scholar]
- 322.Breu FX, Guggenbichler S. European Consensus Meeting on Foam Sclerotherapy, April, 4–6, 2003, Tegernsee, Germany. Dermatol Surg. 2004;30(5):709–17. doi: 10.1111/j.1524-4725.2004.30209.x. discussion 17. [DOI] [PubMed] [Google Scholar]
- 323.Frullini A, Cavezzi A. Sclerosing foam in the treatment of varicose veins and telangiectases: history and analysis of safety and complications. Dermatol Surg. 2002;28(1):11–5. doi: 10.1046/j.1524-4725.2002.01182.x. [DOI] [PubMed] [Google Scholar]
- 324.Wollmann JC. The history of sclerosing foams. Dermatol Surg. 2004;30(5):694–703. doi: 10.1111/j.1524-4725.2004.30208.x. discussion. [DOI] [PubMed] [Google Scholar]
- 325.Proebstle TM, Lehr HA, Kargl A, et al. Endovenous treatment of the greater saphenous vein with a 940-nm diode laser: thrombotic occlusion after endoluminal thermal damage by laser-generated steam bubbles. J Vasc Surg. 2002;35(4):729–36. doi: 10.1067/mva.2002.121132. [DOI] [PubMed] [Google Scholar]
- 326.Agus GB, Mancini S, Magi G. The first 1000 cases of Italian Endovenous-laser Working Group (IEWG). Rationale, and long-term outcomes for the 1999–2003 period. Int Angiol. 2006;25(2):209–15. [PubMed] [Google Scholar]
- 327.Min RJ, Khilnani N, Zimmet SE. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol. 2003;14(8):991–6. doi: 10.1097/01.rvi.0000082864.05622.e4. [DOI] [PubMed] [Google Scholar]
- 328.Merchant RF, Pichot O, Myers KA. Four-year follow-up on endovascular radiofrequency obliteration of great saphenous reflux. Dermatol Surg. 2005;31(2):129–34. doi: 10.1111/j.1524-4725.2005.31031. [DOI] [PubMed] [Google Scholar]
- 329.Sarin S, Scurr JH, Coleridge Smith PD. Stripping of the long saphenous vein in the treatment of primary varicose veins. Br J Surg. 1994;81(10):1455–8. doi: 10.1002/bjs.1800811017. [DOI] [PubMed] [Google Scholar]
- 330.Aremu MA, Mahendran B, Butcher W, et al. Prospective randomized controlled trial: conventional versus powered phlebectomy. J Vasc Surg. 2004;39(1):88–94. doi: 10.1016/j.jvs.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 331.Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet. 2004;363(9424):1854–9. doi: 10.1016/S0140-6736(04)16353-8. [DOI] [PubMed] [Google Scholar]
- 332.Kontogiorgis CA, Papaioannou P, Hadjipavlou-Litina DJ. Matrix metalloproteinase inhibitors: a review on pharmacophore mapping and (Q)SARs results. Curr Med Chem. 2005;12(3):339–55. doi: 10.2174/0929867053363243. [DOI] [PubMed] [Google Scholar]
- 333.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6):480–98. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 334.Murphy G, Houbrechts A, Cockett MI, Williamson RA, O’Shea M, Docherty AJ. The N-terminal domain of tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry. 1991;30(33):8097–102. doi: 10.1021/bi00247a001. [DOI] [PubMed] [Google Scholar]
- 335.Williamson RA, Marston FA, Angal S, et al. Disulphide bond assignment in human tissue inhibitor of metalloproteinases (TIMP) Biochem J. 1990;268(2):267–74. doi: 10.1042/bj2680267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115(Pt 19):3719–27. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 337.Will H, Atkinson SJ, Butler GS, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996;271(29):17119–23. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- 338.Amour A, Knight CG, Webster A, et al. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 2000;473(3):275–9. doi: 10.1016/s0014-5793(00)01528-3. [DOI] [PubMed] [Google Scholar]
- 339.Jacobsen J, Visse R, Sorensen HP, et al. Catalytic properties of ADAM12 and its domain deletion mutants. Biochemistry. 2008;47(2):537–47. doi: 10.1021/bi701629c. [DOI] [PubMed] [Google Scholar]
- 340.Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276(16):12501–4. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- 341.Rodriguez-Manzaneque JC, Westling J, Thai SN, et al. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293(1):501–8. doi: 10.1016/S0006-291X(02)00254-1. [DOI] [PubMed] [Google Scholar]
- 342.Leco KJ, Waterhouse P, Sanchez OH, et al. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3) J Clin Invest. 2001;108(6):817–29. doi: 10.1172/JCI12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Fata JE, Leco KJ, Voura EB, et al. Accelerated apoptosis in the Timp-3-deficient mammary gland. J Clin Invest. 2001;108(6):831–41. doi: 10.1172/JCI13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Batra J, Robinson J, Soares AS, Fields AP, Radisky DC, Radisky ES. Matrix metalloproteinase-10 (MMP-10) interaction with tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2: binding studies and crystal structure. J Biol Chem. 2012;287(19):15935–46. doi: 10.1074/jbc.M112.341156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Meng Q, Malinovskii V, Huang W, et al. Residue 2 of TIMP-1 is a major determinant of affinity and specificity for matrix metalloproteinases but effects of substitutions do not correlate with those of the corresponding P1′ residue of substrate. J Biol Chem. 1999;274(15):10184–9. doi: 10.1074/jbc.274.15.10184. [DOI] [PubMed] [Google Scholar]
- 346.Iyer S, Wei S, Brew K, Acharya KR. Crystal structure of the catalytic domain of matrix metalloproteinase-1 in complex with the inhibitory domain of tissue inhibitor of metalloproteinase-1. J Biol Chem. 2007;282(1):364–71. doi: 10.1074/jbc.M607625200. [DOI] [PubMed] [Google Scholar]
- 347.Vallon R, Muller R, Moosmayer D, Gerlach E, Angel P. The catalytic domain of activated collagenase I (MMP-1) is absolutely required for interaction with its specific inhibitor, tissue inhibitor of metalloproteinases-1 (TIMP-1) Eur J Biochem. 1997;244(1):81–8. doi: 10.1111/j.1432-1033.1997.00081.x. [DOI] [PubMed] [Google Scholar]
- 348.Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265(29):17401–4. [PubMed] [Google Scholar]
- 349.Higashi S, Miyazaki K. Novel processing of beta-amyloid precursor protein catalyzed by membrane type 1 matrix metalloproteinase releases a fragment lacking the inhibitor domain against gelatinase A. Biochemistry. 2003;42(21):6514–26. doi: 10.1021/bi020643m. [DOI] [PubMed] [Google Scholar]
- 350.Mott JD, Thomas CL, Rosenbach MT, Takahara K, Greenspan DS, Banda MJ. Post-translational proteolytic processing of procollagen C-terminal proteinase enhancer releases a metalloproteinase inhibitor. J Biol Chem. 2000;275(2):1384–90. doi: 10.1074/jbc.275.2.1384. [DOI] [PubMed] [Google Scholar]
- 351.Herman MP, Sukhova GK, Kisiel W, et al. Tissue factor pathway inhibitor-2 is a novel inhibitor of matrix metalloproteinases with implications for atherosclerosis. J Clin Invest. 2001;107(9):1117–26. doi: 10.1172/JCI10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29(5):290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Rao BG. Recent developments in the design of specific Matrix Metalloproteinase inhibitors aided by structural and computational studies. Curr Pharm Des. 2005;11(3):295–322. doi: 10.2174/1381612053382115. [DOI] [PubMed] [Google Scholar]
- 354.Jacobsen JA, Major Jourden JL, Miller MT, Cohen SM. To bind zinc or not to bind zinc: an examination of innovative approaches to improved metalloproteinase inhibition. Biochim Biophys Acta. 2010;1803(1):72–94. doi: 10.1016/j.bbamcr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 355.Brown S, Meroueh SO, Fridman R, Mobashery S. Quest for selectivity in inhibition of matrix metalloproteinases. Curr Top Med Chem. 2004;4(12):1227–38. doi: 10.2174/1568026043387854. [DOI] [PubMed] [Google Scholar]
- 356.Wojtowicz-Praga SM, Dickson RB, Hawkins MJ. Matrix metalloproteinase inhibitors. Invest New Drugs. 1997;15(1):61–75. doi: 10.1023/a:1005722729132. [DOI] [PubMed] [Google Scholar]
- 357.Cherney RJ, Mo R, Meyer DT, et al. Sultam hydroxamates as novel matrix metalloproteinase inhibitors. J Med Chem. 2004;47(12):2981–3. doi: 10.1021/jm049833g. [DOI] [PubMed] [Google Scholar]
- 358.Nakatani S, Ikura M, Yamamoto S, et al. Design and synthesis of novel metalloproteinase inhibitors. Bioorg Med Chem. 2006;14(15):5402–22. doi: 10.1016/j.bmc.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 359.Rossello A, Nuti E, Catalani MP, et al. A new development of matrix metalloproteinase inhibitors: twin hydroxamic acids as potent inhibitors of MMPs. Bioorg Med Chem Lett. 2005;15(9):2311–4. doi: 10.1016/j.bmcl.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 360.Subramaniam R, Haldar MK, Tobwala S, Ganguly B, Srivastava DK, Mallik S. Novel bis-(arylsulfonamide) hydroxamate-based selective MMP inhibitors. Bioorg Med Chem Lett. 2008;18(11):3333–7. doi: 10.1016/j.bmcl.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Whitlock GA, Dack KN, Dickinson RP, Lewis ML. A novel series of highly selective inhibitors of MMP-3. Bioorg Med Chem Lett. 2007;17(24):6750–3. doi: 10.1016/j.bmcl.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 362.Vihinen P, Ala-aho R, Kahari VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets. 2005;5(3):203–20. doi: 10.2174/1568009053765799. [DOI] [PubMed] [Google Scholar]
- 363.Fingleton B. MMPs as therapeutic targets--still a viable option? Semin Cell Dev Biol. 2008;19(1):61–8. doi: 10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Jani M, Tordai H, Trexler M, Banyai L, Patthy L. Hydroxamate-based peptide inhibitors of matrix metalloprotease 2. Biochimie. 2005;87(3–4):385–92. doi: 10.1016/j.biochi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 365.Trexler M, Briknarova K, Gehrmann M, Llinas M, Patthy L. Peptide ligands for the fibronectin type II modules of matrix metalloproteinase 2 (MMP-2) J Biol Chem. 2003;278(14):12241–6. doi: 10.1074/jbc.M210116200. [DOI] [PubMed] [Google Scholar]
- 366.Scozzafava A, Supuran CT. Carbonic anhydrase and matrix metalloproteinase inhibitors: sulfonylated amino acid hydroxamates with MMP inhibitory properties act as efficient inhibitors of CA isozymes I, II, and IV, and N-hydroxysulfonamides inhibit both these zinc enzymes. J Med Chem. 2000;43(20):3677–87. doi: 10.1021/jm000027t. [DOI] [PubMed] [Google Scholar]
- 367.Levin JI, Chen JM, Cheung K, et al. Acetylenic TACE inhibitors. Part 1. SAR of the acyclic sulfonamide hydroxamates. Bioorg Med Chem Lett. 2003;13(16):2799–803. doi: 10.1016/s0960-894x(03)00514-6. [DOI] [PubMed] [Google Scholar]
- 368.Cherney RJ, King BW, Gilmore JL, et al. Conversion of potent MMP inhibitors into selective TACE inhibitors. Bioorg Med Chem Lett. 2006;16(4):1028–31. doi: 10.1016/j.bmcl.2005.10.078. [DOI] [PubMed] [Google Scholar]
- 369.Pochetti G, Gavuzzo E, Campestre C, et al. Structural insight into the stereoselective inhibition of MMP-8 by enantiomeric sulfonamide phosphonates. J Med Chem. 2006;49(3):923–31. doi: 10.1021/jm050787+. [DOI] [PubMed] [Google Scholar]
- 370.Ma D, Wu W, Yang G, Li J, Ye Q. Tetrahydroisoquinoline based sulfonamide hydroxamates as potent matrix metalloproteinase inhibitors. Bioorg Med Chem Lett. 2004;14(1):47–50. doi: 10.1016/j.bmcl.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 371.Pikul S, McDow Dunham KL, Almstead NG, et al. Design and synthesis of phosphinamide-based hydroxamic acids as inhibitors of matrix metalloproteinases. J Med Chem. 1999;42(1):87–94. doi: 10.1021/jm980142s. [DOI] [PubMed] [Google Scholar]
- 372.Fujisawa T, Katakura S, Odake S, et al. Design and synthesis of carboxylate inhibitors for matrix metalloproteinases. Chem Pharm Bull (Tokyo) 2001;49(10):1272–9. doi: 10.1248/cpb.49.1272. [DOI] [PubMed] [Google Scholar]
- 373.Matter H, Schudok M, Schwab W, et al. Tetrahydroisoquinoline-3-carboxylate based matrix-metalloproteinase inhibitors: design, synthesis and structure-activity relationship. Bioorg Med Chem. 2002;10(11):3529–44. doi: 10.1016/s0968-0896(02)00215-8. [DOI] [PubMed] [Google Scholar]
- 374.Janusz MJ, Hookfin EB, Brown KK, et al. Comparison of the pharmacology of hydroxamate- and carboxylate-based matrix metalloproteinase inhibitors (MMPIs) for the treatment of osteoarthritis. Inflamm Res. 2006;55(2):60–5. doi: 10.1007/s00011-005-0014-4. [DOI] [PubMed] [Google Scholar]
- 375.Ilies M, Banciu MD, Scozzafava A, Ilies MA, Caproiu MT, Supuran CT. Protease inhibitors: synthesis of bacterial collagenase and matrix metalloproteinase inhibitors incorporating arylsulfonylureido and 5-dibenzo-suberenyl/suberyl moieties. Bioorg Med Chem. 2003;11(10):2227–39. doi: 10.1016/s0968-0896(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 376.Fujisawa T, Odake S, Ogawa Y, Yasuda J, Morita Y, Morikawa T. Design and synthesis of sulfur based inhibitors of matrix metalloproteinase-1. Chem Pharm Bull (Tokyo) 2002;50(2):239–52. doi: 10.1248/cpb.50.239. [DOI] [PubMed] [Google Scholar]
- 377.Rosenblum G, Meroueh SO, Kleifeld O, et al. Structural basis for potent slow binding inhibition of human matrix metalloproteinase-2 (MMP-2) J Biol Chem. 2003;278(29):27009–15. doi: 10.1074/jbc.M301139200. [DOI] [PubMed] [Google Scholar]
- 378.Li J, Rush TS, 3rd, Li W, et al. Synthesis and SAR of highly selective MMP-13 inhibitors. Bioorg Med Chem Lett. 2005;15(22):4961–6. doi: 10.1016/j.bmcl.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 379.Churg A, Wang RD, Tai H, et al. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am J Respir Crit Care Med. 2003;167(8):1083–9. doi: 10.1164/rccm.200212-1396OC. [DOI] [PubMed] [Google Scholar]
- 380.Fray MJ, Dickinson RP, Huggins JP, Occleston NL. A potent, selective inhibitor of matrix metalloproteinase-3 for the topical treatment of chronic dermal ulcers. J Med Chem. 2003;46(16):3514–25. doi: 10.1021/jm0308038. [DOI] [PubMed] [Google Scholar]
- 381.Auge F, Hornebeck W, Decarme M, Laronze JY. Improved gelatinase a selectivity by novel zinc binding groups containing galardin derivatives. Bioorg Med Chem Lett. 2003;13(10):1783–6. doi: 10.1016/s0960-894x(03)00214-2. [DOI] [PubMed] [Google Scholar]
- 382.Ledour G, Moroy G, Rouffet M, et al. Introduction of the 4-(4-bromophenyl)benzenesulfonyl group to hydrazide analogs of Ilomastat leads to potent gelatinase B (MMP-9) inhibitors with improved selectivity. Bioorg Med Chem. 2008;16(18):8745–59. doi: 10.1016/j.bmc.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 383.Hurst DR, Schwartz MA, Jin Y, et al. Inhibition of enzyme activity of and cell-mediated substrate cleavage by membrane type 1 matrix metalloproteinase by newly developed mercaptosulphide inhibitors. Biochem J. 2005;392(Pt 3):527–36. doi: 10.1042/BJ20050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Hoffman A, Qadri B, Frant J, et al. Carbamoylphosphonate matrix metalloproteinase inhibitors 6: cis-2-aminocyclohexylcarbamoylphosphonic acid, a novel orally active antimetastatic matrix metalloproteinase-2 selective inhibitor--synthesis and pharmacodynamic and pharmacokinetic analysis. J Med Chem. 2008;51(5):1406–14. doi: 10.1021/jm701087n. [DOI] [PubMed] [Google Scholar]
- 385.Breuer E, Salomon CJ, Katz Y, et al. Carbamoylphosphonates, a new class of in vivo active matrix metalloproteinase inhibitors. 1. Alkyl- and cycloalkylcarbamoylphosphonic acids. J Med Chem. 2004;47(11):2826–32. doi: 10.1021/jm030386z. [DOI] [PubMed] [Google Scholar]
- 386.Jacobsen FE, Lewis JA, Cohen SM. A new role for old ligands: discerning chelators for zinc metalloproteinases. J Am Chem Soc. 2006;128(10):3156–7. doi: 10.1021/ja057957s. [DOI] [PubMed] [Google Scholar]
- 387.Cook GR, Manivannan E, Underdahl T, Lukacova V, Zhang Y, Balaz S. Synthesis and evaluation of novel oxazoline MMP inhibitors. Bioorg Med Chem Lett. 2004;14(19):4935–9. doi: 10.1016/j.bmcl.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 388.Reiter LA, Freeman-Cook KD, Jones CS, et al. Potent, selective pyrimidinetrione-based inhibitors of MMP-13. Bioorg Med Chem Lett. 2006;16(22):5822–6. doi: 10.1016/j.bmcl.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 389.Grams F, Brandstetter H, D’Alo S, et al. Pyrimidine-2,4,6-Triones: a new effective and selective class of matrix metalloproteinase inhibitors. Biol Chem. 2001;382(8):1277–85. doi: 10.1515/BC.2001.159. [DOI] [PubMed] [Google Scholar]
- 390.Foley LH, Palermo R, Dunten P, Wang P. Novel 5,5-disubstitutedpyrimidine-2,4,6-triones as selective MMP inhibitors. Bioorg Med Chem Lett. 2001;11(8):969–72. doi: 10.1016/s0960-894x(01)00104-4. [DOI] [PubMed] [Google Scholar]
- 391.Kim SH, Pudzianowski AT, Leavitt KJ, et al. Structure-based design of potent and selective inhibitors of collagenase-3 (MMP-13) Bioorg Med Chem Lett. 2005;15(4):1101–6. doi: 10.1016/j.bmcl.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 392.Blagg JA, Noe MC, Wolf-Gouveia LA, et al. Potent pyrimidinetrione-based inhibitors of MMP-13 with enhanced selectivity over MMP-14. Bioorg Med Chem Lett. 2005;15(7):1807–10. doi: 10.1016/j.bmcl.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 393.Freeman-Cook KD, Reiter LA, Noe MC, et al. Potent, selective spiropyrrolidine pyrimidinetrione inhibitors of MMP-13. Bioorg Med Chem Lett. 2007;17(23):6529–34. doi: 10.1016/j.bmcl.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 394.Lein M, Jung K, Ortel B, et al. The new synthetic matrix metalloproteinase inhibitor (Roche 28–2653) reduces tumor growth and prolongs survival in a prostate cancer standard rat model. Oncogene. 2002;21(13):2089–96. doi: 10.1038/sj.onc.1205267. [DOI] [PubMed] [Google Scholar]
- 395.Maquoi E, Sounni NE, Devy L, et al. Anti-invasive, antitumoral, and antiangiogenic efficacy of a pyrimidine-2,4,6-trione derivative, an orally active and selective matrix metalloproteinases inhibitor. Clin Cancer Res. 2004;10(12 Pt 1):4038–47. doi: 10.1158/1078-0432.CCR-04-0125. [DOI] [PubMed] [Google Scholar]
- 396.Lewis EJ, Bishop J, Bottomley KM, et al. Ro 32–3555, an orally active collagenase inhibitor, prevents cartilage breakdown in vitro and in vivo. Br J Pharmacol. 1997;121(3):540–6. doi: 10.1038/sj.bjp.0701150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Puerta DT, Lewis JA, Cohen SM. New beginnings for matrix metalloproteinase inhibitors: identification of high-affinity zinc-binding groups. J Am Chem Soc. 2004;126(27):8388–9. doi: 10.1021/ja0485513. [DOI] [PubMed] [Google Scholar]
- 398.Puerta DT, Cohen SM. Examination of novel zinc-binding groups for use in matrix metalloproteinase inhibitors. Inorg Chem. 2003;42(11):3423–30. doi: 10.1021/ic026029g. [DOI] [PubMed] [Google Scholar]
- 399.Jacobsen FE, Lewis JA, Cohen SM. The design of inhibitors for medicinally relevant metalloproteins. ChemMedChem. 2007;2(2):152–71. doi: 10.1002/cmdc.200600204. [DOI] [PubMed] [Google Scholar]
- 400.Puerta DT, Griffin MO, Lewis JA, et al. Heterocyclic zinc-binding groups for use in next-generation matrix metalloproteinase inhibitors: potency, toxicity, and reactivity. J Biol Inorg Chem. 2006;11(2):131–8. doi: 10.1007/s00775-005-0053-x. [DOI] [PubMed] [Google Scholar]
- 401.Puerta DT, Mongan J, Tran BL, McCammon JA, Cohen SM. Potent, selective pyrone-based inhibitors of stromelysin-1. J Am Chem Soc. 2005;127(41):14148–9. doi: 10.1021/ja054558o. [DOI] [PubMed] [Google Scholar]
- 402.Agrawal A, Romero-Perez D, Jacobsen JA, Villarreal FJ, Cohen SM. Zinc-binding groups modulate selective inhibition of MMPs. ChemMedChem. 2008;3(5):812–20. doi: 10.1002/cmdc.200700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Yan YL, Cohen SM. Efficient synthesis of 5-amido-3-hydroxy-4-pyrones as inhibitors of matrix metalloproteinases. Org Lett. 2007;9(13):2517–20. doi: 10.1021/ol0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Li JJ, Nahra J, Johnson AR, et al. Quinazolinones and pyrido[3,4-d]pyrimidin-4-ones as orally active and specific matrix metalloproteinase-13 inhibitors for the treatment of osteoarthritis. J Med Chem. 2008;51(4):835–41. doi: 10.1021/jm701274v. [DOI] [PubMed] [Google Scholar]
- 405.Morales R, Perrier S, Florent JM, et al. Crystal structures of novel non-peptidic, non-zinc chelating inhibitors bound to MMP-12. J Mol Biol. 2004;341(4):1063–76. doi: 10.1016/j.jmb.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 406.Engel CK, Pirard B, Schimanski S, et al. Structural basis for the highly selective inhibition of MMP-13. Chem Biol. 2005;12(2):181–9. doi: 10.1016/j.chembiol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 407.Dublanchet AC, Ducrot P, Andrianjara C, et al. Structure-based design and synthesis of novel non-zinc chelating MMP-12 inhibitors. Bioorg Med Chem Lett. 2005;15(16):3787–90. doi: 10.1016/j.bmcl.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 408.Gooljarsingh LT, Lakdawala A, Coppo F, et al. Characterization of an exosite binding inhibitor of matrix metalloproteinase 13. Protein Sci. 2008;17(1):66–71. doi: 10.1110/ps.073130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Pochetti G, Montanari R, Gege C, Chevrier C, Taveras AG, Mazza F. Extra binding region induced by non-zinc chelating inhibitors into the S1′ subsite of matrix metalloproteinase 8 (MMP-8) J Med Chem. 2009;52(4):1040–9. doi: 10.1021/jm801166j. [DOI] [PubMed] [Google Scholar]
- 410.Johnson AR, Pavlovsky AG, Ortwine DF, et al. Discovery and characterization of a novel inhibitor of matrix metalloprotease-13 that reduces cartilage damage in vivo without joint fibroplasia side effects. J Biol Chem. 2007;282(38):27781–91. doi: 10.1074/jbc.M703286200. [DOI] [PubMed] [Google Scholar]
- 411.Bernardo MM, Brown S, Li ZH, Fridman R, Mobashery S. Design, synthesis, and characterization of potent, slow-binding inhibitors that are selective for gelatinases. J Biol Chem. 2002;277(13):11201–7. doi: 10.1074/jbc.M111021200. [DOI] [PubMed] [Google Scholar]
- 412.Morrison JF, Walsh CT. The behavior and significance of slow-binding enzyme inhibitors. Adv Enzymol Relat Areas Mol Biol. 1988;61:201–301. doi: 10.1002/9780470123072.ch5. [DOI] [PubMed] [Google Scholar]
- 413.Kruger A, Arlt MJ, Gerg M, et al. Antimetastatic activity of a novel mechanism-based gelatinase inhibitor. Cancer Res. 2005;65(9):3523–6. doi: 10.1158/0008-5472.CAN-04-3570. [DOI] [PubMed] [Google Scholar]
- 414.Bonfil RD, Sabbota A, Nabha S, et al. Inhibition of human prostate cancer growth, osteolysis and angiogenesis in a bone metastasis model by a novel mechanism-based selective gelatinase inhibitor. Int J Cancer. 2006;118(11):2721–6. doi: 10.1002/ijc.21645. [DOI] [PubMed] [Google Scholar]
- 415.Bonfil RD, Dong Z, Trindade Filho JC, et al. Prostate cancer-associated membrane type 1-matrix metalloproteinase: a pivotal role in bone response and intraosseous tumor growth. Am J Pathol. 2007;170(6):2100–11. doi: 10.2353/ajpath.2007.060720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Banke IJ, Arlt MJ, Mueller MM, et al. Effective inhibition of experimental metastasis and prolongation of survival in mice by a potent factor Xa-specific synthetic serine protease inhibitor with weak anticoagulant activity. Thromb Haemost. 2005;94(5):1084–93. doi: 10.1160/TH05-04-0249. [DOI] [PubMed] [Google Scholar]
- 417.Gu Z, Cui J, Brown S, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25(27):6401–8. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Lee M, Villegas-Estrada A, Celenza G, et al. Metabolism of a highly selective gelatinase inhibitor generates active metabolite. Chem Biol Drug Des. 2007;70(5):371–82. doi: 10.1111/j.1747-0285.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 419.Celenza G, Villegas-Estrada A, Lee M, et al. Metabolism of (4-phenoxyphenylsulfonyl) methylthiirane, a selective gelatinase inhibitor. Chem Biol Drug Des. 2008;71(3):187–96. doi: 10.1111/j.1747-0285.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 420.Lee M, Celenza G, Boggess B, et al. A potent gelatinase inhibitor with anti-tumor-invasive activity and its metabolic disposition. Chem Biol Drug Des. 2009;73(2):189–202. doi: 10.1111/j.1747-0285.2008.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Nuti E, Tuccinardi T, Rossello A. Matrix metalloproteinase inhibitors: new challenges in the era of post broad-spectrum inhibitors. Curr Pharm Des. 2007;13(20):2087–100. doi: 10.2174/138161207781039706. [DOI] [PubMed] [Google Scholar]
- 422.Georgiadis D, Yiotakis A. Specific targeting of metzincin family members with small-molecule inhibitors: progress toward a multifarious challenge. Bioorg Med Chem. 2008;16(19):8781–94. doi: 10.1016/j.bmc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 423.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 424.Renkiewicz R, Qiu L, Lesch C, et al. Broad-spectrum matrix metalloproteinase inhibitor marimastat-induced musculoskeletal side effects in rats. Arthritis Rheum. 2003;48(6):1742–9. doi: 10.1002/art.11030. [DOI] [PubMed] [Google Scholar]
- 425.Chu QS, Forouzesh B, Syed S, et al. A phase II and pharmacological study of the matrix metalloproteinase inhibitor (MMPI) COL-3 in patients with advanced soft tissue sarcomas. Invest New Drugs. 2007;25(4):359–67. doi: 10.1007/s10637-006-9031-6. [DOI] [PubMed] [Google Scholar]
- 426.Nomura S, Yoshimura K, Akiyama N, et al. HMG-CoA reductase inhibitors reduce matrix metalloproteinase-9 activity in human varicose veins. Eur Surg Res. 2005;37(6):370–8. doi: 10.1159/000090339. [DOI] [PubMed] [Google Scholar]
- 427.Mannello F, Medda V, Ligi D, Raffetto JD. Glycosaminoglycan Sulodexide Inhibition of MMP-9 Gelatinase Secretion and Activity: Possible Pharmacological Role Against Collagen Degradation in Vascular Chronic Diseases. Curr Vasc Pharmacol. 2012 doi: 10.2174/1570161111311030010. [DOI] [PubMed] [Google Scholar]
- 428.Mannello F, Raffetto JD. Matrix metalloproteinase activity and glycosaminoglycans in chronic venous disease: the linkage among cell biology, pathology and translational research. Am J Transl Res. 2011;3(2):149–58. [PMC free article] [PubMed] [Google Scholar]
- 429.Eming S, Smola H, Hartmann B, et al. The inhibition of matrix metalloproteinase activity in chronic wounds by a polyacrylate superabsorber. Biomaterials. 2008;29(19):2932–40. doi: 10.1016/j.biomaterials.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 430.Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. J Wound Care. 2002;11(9):335–41. doi: 10.12968/jowc.2002.11.9.26438. [DOI] [PubMed] [Google Scholar]
- 431.Smeets R, Ulrich D, Unglaub F, Woltje M, Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J. 2008;5(2):195–203. doi: 10.1111/j.1742-481X.2007.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Schmutz JL, Meaume S, Fays S, et al. Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers: results of a randomised, controlled trial. Int Wound J. 2008;5(2):172–82. doi: 10.1111/j.1742-481X.2008.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Mackelfresh J, Soon S, Arbiser JL. Combination therapy of doxycycline and topical tacrolimus for venous ulcers. Arch Dermatol. 2005;141(11):1476–7. doi: 10.1001/archderm.141.11.1476. [DOI] [PubMed] [Google Scholar]
- 434.Martens E, Leyssen A, Van Aelst I, et al. A monoclonal antibody inhibits gelatinase B/MMP-9 by selective binding to part of the catalytic domain and not to the fibronectin or zinc binding domains. Biochim Biophys Acta. 2007;1770(2):178–86. doi: 10.1016/j.bbagen.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 435.Paemen L, Martens E, Masure S, Opdenakker G. Monoclonal antibodies specific for natural human neutrophil gelatinase B used for affinity purification, quantitation by two-site ELISA and inhibition of enzymatic activity. Eur J Biochem. 1995;234(3):759–65. doi: 10.1111/j.1432-1033.1995.759_a.x. [DOI] [PubMed] [Google Scholar]
- 436.Hu J, Van den Steen PE, Houde M, Ilenchuk TT, Opdenakker G. Inhibitors of gelatinase B/matrix metalloproteinase-9 activity comparison of a peptidomimetic and polyhistidine with single-chain derivatives of a neutralizing monoclonal antibody. Biochem Pharmacol. 2004;67(5):1001–9. doi: 10.1016/j.bcp.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 437.Rao JS, Bhoopathi P, Chetty C, Gujrati M, Lakka SS. MMP-9 short interfering RNA induced senescence resulting in inhibition of medulloblastoma growth via p16(INK4a) and mitogen-activated protein kinase pathway. Cancer Res. 2007;67(10):4956–64. doi: 10.1158/0008-5472.CAN-07-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Jiang X, Dutton CM, Qi WN, Block JA, Garamszegi N, Scully SP. siRNA mediated inhibition of MMP-1 reduces invasive potential of a human chondrosarcoma cell line. J Cell Physiol. 2005;202(3):723–30. doi: 10.1002/jcp.20162. [DOI] [PubMed] [Google Scholar]
- 439.Currie JC, Fortier S, Sina A, Galipeau J, Cao J, Annabi B. MT1-MMP down-regulates the glucose 6-phosphate transporter expression in marrow stromal cells: a molecular link between pro-MMP-2 activation, chemotaxis, and cell survival. J Biol Chem. 2007;282(11):8142–9. doi: 10.1074/jbc.M610894200. [DOI] [PubMed] [Google Scholar]
- 440.Lakka SS, Gondi CS, Dinh DH, et al. Specific interference of urokinase-type plasminogen activator receptor and matrix metalloproteinase-9 gene expression induced by double-stranded RNA results in decreased invasion, tumor growth, and angiogenesis in gliomas. J Biol Chem. 2005;280(23):21882–92. doi: 10.1074/jbc.M408520200. [DOI] [PubMed] [Google Scholar]
- 441.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7(6):743–8. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 442.Chen J, Tung CH, Mahmood U, et al. In vivo imaging of proteolytic activity in atherosclerosis. Circulation. 2002;105(23):2766–71. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- 443.Milner JM, Cawston TE. Matrix metalloproteinase knockout studies and the potential use of matrix metalloproteinase inhibitors in the rheumatic diseases. Curr Drug Targets Inflamm Allergy. 2005;4(3):363–75. doi: 10.2174/1568010054022141. [DOI] [PubMed] [Google Scholar]
- 444.Mix KS, Coon CI, Rosen ED, Suh N, Sporn MB, Brinckerhoff CE. Peroxisome proliferator-activated receptor-gamma-independent repression of collagenase gene expression by 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid and prostaglandin 15-deoxy-delta(12,14) J2: a role for Smad signaling. Mol Pharmacol. 2004;65(2):309–18. doi: 10.1124/mol.65.2.309. [DOI] [PubMed] [Google Scholar]
- 445.Voils SA, Evans ME, Lane MT, Schosser RH, Rapp RP. Use of macrolides and tetracyclines for chronic inflammatory diseases. Ann Pharmacother. 2005;39(1):86–94. doi: 10.1345/aph.1E282. [DOI] [PubMed] [Google Scholar]
- 446.van der Laan WH, Quax PH, Seemayer CA, et al. Cartilage degradation and invasion by rheumatoid synovial fibroblasts is inhibited by gene transfer of TIMP-1 and TIMP-3. Gene Ther. 2003;10(3):234–42. doi: 10.1038/sj.gt.3301871. [DOI] [PubMed] [Google Scholar]
- 447.Krajnovic P. Effect of a benzopyrone preparation in venous diseases during pregnancy. Med Monatsschr. 1977;31(2):86–8. [PubMed] [Google Scholar]
- 448.Shalinsky DR, Brekken J, Zou H, et al. Broad antitumor and antiangiogenic activities of AG3340, a potent and selective MMP inhibitor undergoing advanced oncology clinical trials. Ann N Y Acad Sci. 1999;878:236–70. doi: 10.1111/j.1749-6632.1999.tb07689.x. [DOI] [PubMed] [Google Scholar]
- 449.El-Bradey MH, Cheng L, Bartsch DU, Niessman M, El-Musharaf A, Freeman WR. The effect of prinomastat (AG3340), a potent inhibitor of matrix metalloproteinase, on a new animal model of epiretinal membrane. Retina. 2004;24(5):783–9. doi: 10.1097/00006982-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 450.Garcia C, Bartsch DU, Rivero ME, et al. Efficacy of Prinomastat) (AG3340), a matrix metalloprotease inhibitor, in treatment of retinal neovascularization. Curr Eye Res. 2002;24(1):33–8. doi: 10.1076/ceyr.24.1.33.5429. [DOI] [PubMed] [Google Scholar]
- 451.Ozerdem U, Mach-Hofacre B, Varki N, et al. The effect of prinomastat (AG3340), a synthetic inhibitor of matrix metalloproteinases, on uveal melanoma rabbit model. Curr Eye Res. 2002;24(2):86–91. doi: 10.1076/ceyr.24.2.86.8159. [DOI] [PubMed] [Google Scholar]
- 452.Foda HD, Rollo EE, Drews M, et al. Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340) Am J Respir Cell Mol Biol. 2001;25(6):717–24. doi: 10.1165/ajrcmb.25.6.4558f. [DOI] [PubMed] [Google Scholar]
- 453.Price A, Shi Q, Morris D, et al. Marked inhibition of tumor growth in a malignant glioma tumor model by a novel synthetic matrix metalloproteinase inhibitor AG3340. Clin Cancer Res. 1999;5(4):845–54. [PubMed] [Google Scholar]
- 454.Borkakoti N, Winkler FK, Williams DH, et al. Structure of the catalytic domain of human fibroblast collagenase complexed with an inhibitor. Nat Struct Biol. 1994;1(2):106–10. doi: 10.1038/nsb0294-106. [DOI] [PubMed] [Google Scholar]
- 455.Hu Y, Xiang JS, DiGrandi MJ, et al. Potent, selective, and orally bioavailable matrix metalloproteinase-13 inhibitors for the treatment of osteoarthritis. Bioorg Med Chem. 2005;13(24):6629–44. doi: 10.1016/j.bmc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 456.Li W, Li J, Wu Y, et al. A selective matrix metalloprotease 12 inhibitor for potential treatment of chronic obstructive pulmonary disease (COPD): discovery of (S)-2-(8-(methoxycarbonylamino)dibenzo[b,d]furan-3-sulfonamido)-3-methylbu tanoic acid (MMP408) J Med Chem. 2009;52(7):1799–802. doi: 10.1021/jm900093d. [DOI] [PubMed] [Google Scholar]
- 457.Michaelides MR, Dellaria JF, Gong J, et al. Biaryl ether retrohydroxamates as potent, long-lived, orally bioavailable MMP inhibitors. Bioorg Med Chem Lett. 2001;11(12):1553–6. doi: 10.1016/s0960-894x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 458.Campestre C, Agamennone M, Tortorella P, et al. N-Hydroxyurea as zinc binding group in matrix metalloproteinase inhibition: mode of binding in a complex with MMP-8. Bioorg Med Chem Lett. 2006;16(1):20–4. doi: 10.1016/j.bmcl.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 459.Van Lint P, Wielockx B, Puimege L, Noel A, Lopez-Otin C, Libert C. Resistance of collagenase-2 (matrix metalloproteinase-8)-deficient mice to TNF-induced lethal hepatitis. J Immunol. 2005;175(11):7642–9. doi: 10.4049/jimmunol.175.11.7642. [DOI] [PubMed] [Google Scholar]
- 460.Biasone A, Tortorella P, Campestre C, et al. alpha-Biphenylsulfonylamino 2-methylpropyl phosphonates: enantioselective synthesis and selective inhibition of MMPs. Bioorg Med Chem. 2007;15(2):791–9. doi: 10.1016/j.bmc.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 461.Folgueras AR, Fueyo A, Garcia-Suarez O, et al. Collagenase-2 deficiency or inhibition impairs experimental autoimmune encephalomyelitis in mice. J Biol Chem. 2008;283(14):9465–74. doi: 10.1074/jbc.M709522200. [DOI] [PubMed] [Google Scholar]
- 462.Matziari M, Beau F, Cuniasse P, Dive V, Yiotakis A. Evaluation of P1′-diversified phosphinic peptides leads to the development of highly selective inhibitors of MMP-11. J Med Chem. 2004;47(2):325–36. doi: 10.1021/jm0308491. [DOI] [PubMed] [Google Scholar]
- 463.Romero-Perez D, Fricovsky E, Yamasaki KG, et al. Cardiac uptake of minocycline and mechanisms for in vivo cardioprotection. J Am Coll Cardiol. 2008;52(13):1086–94. doi: 10.1016/j.jacc.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]