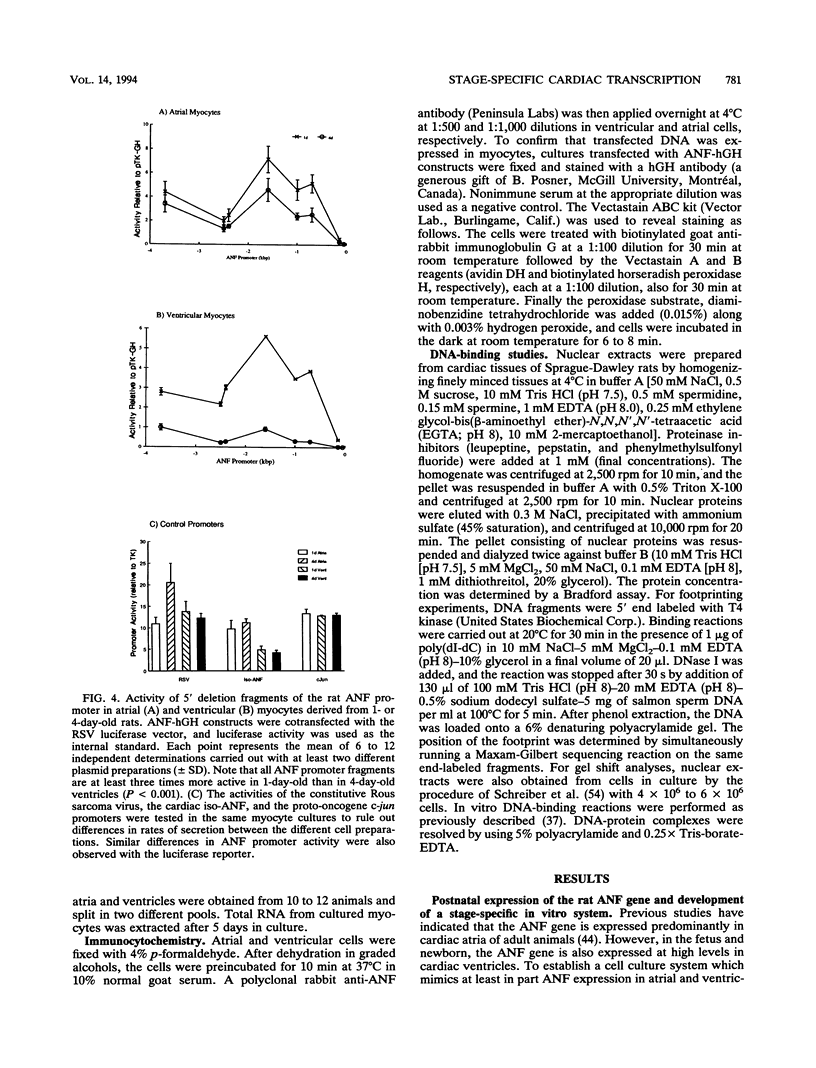
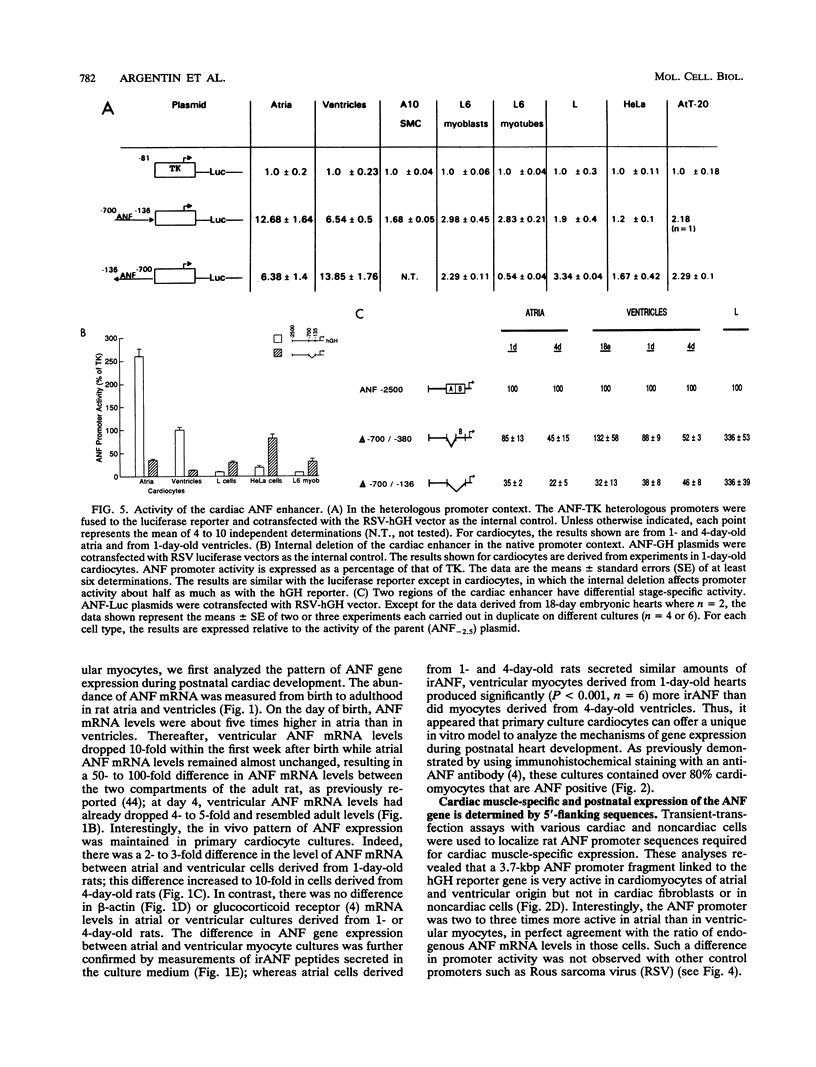
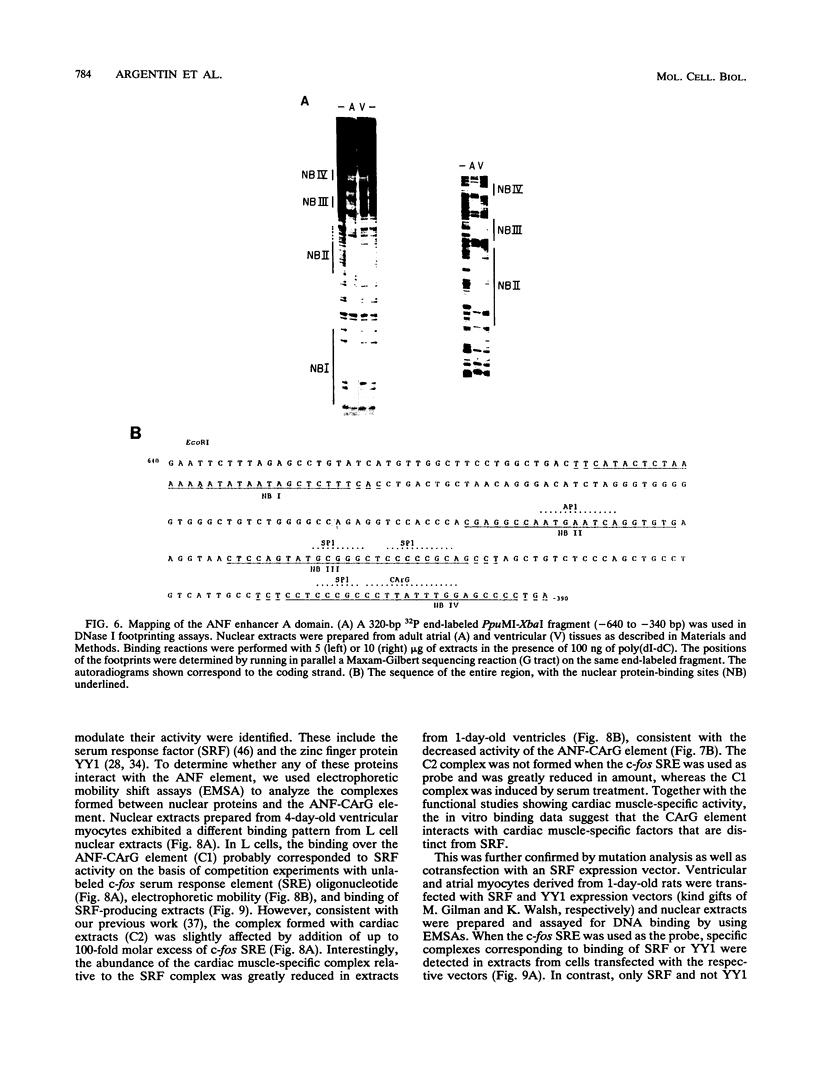
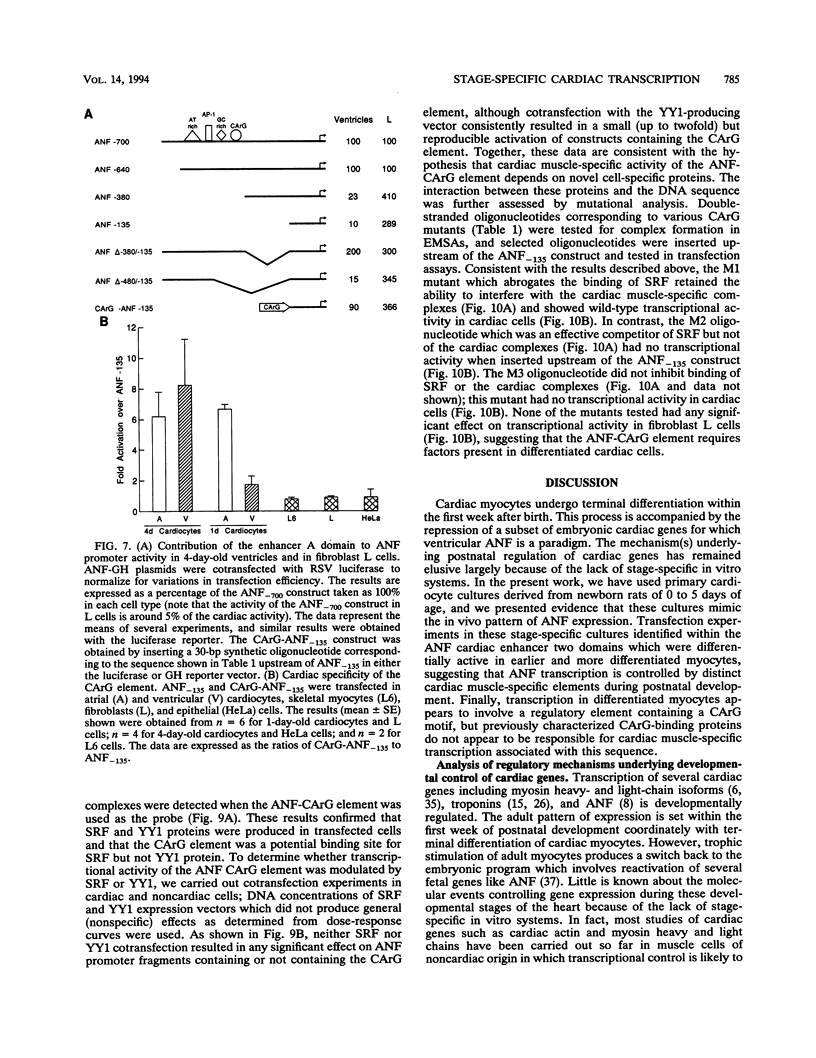
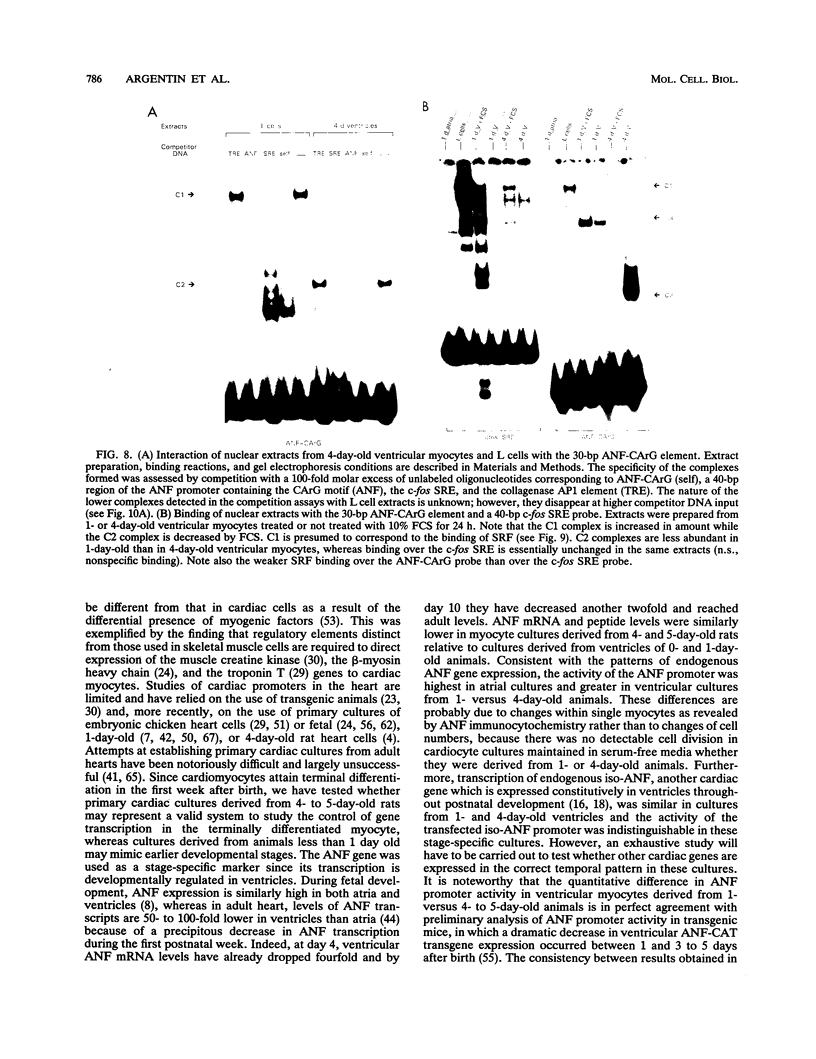
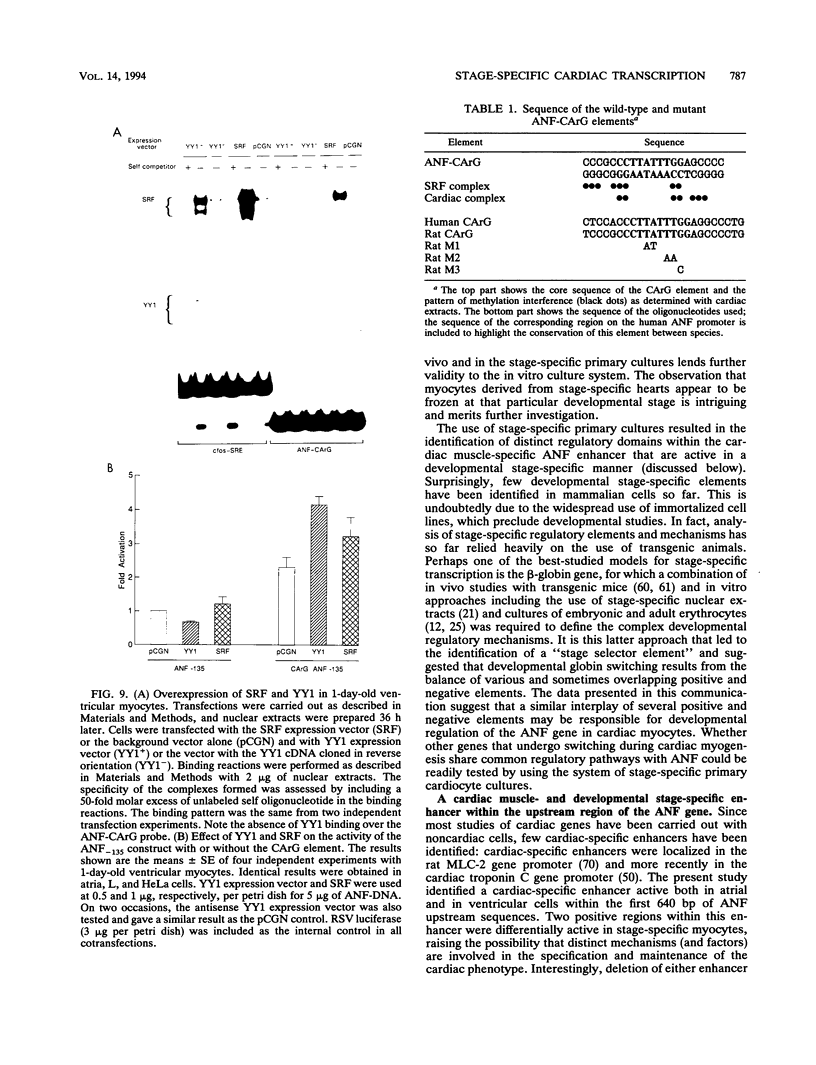
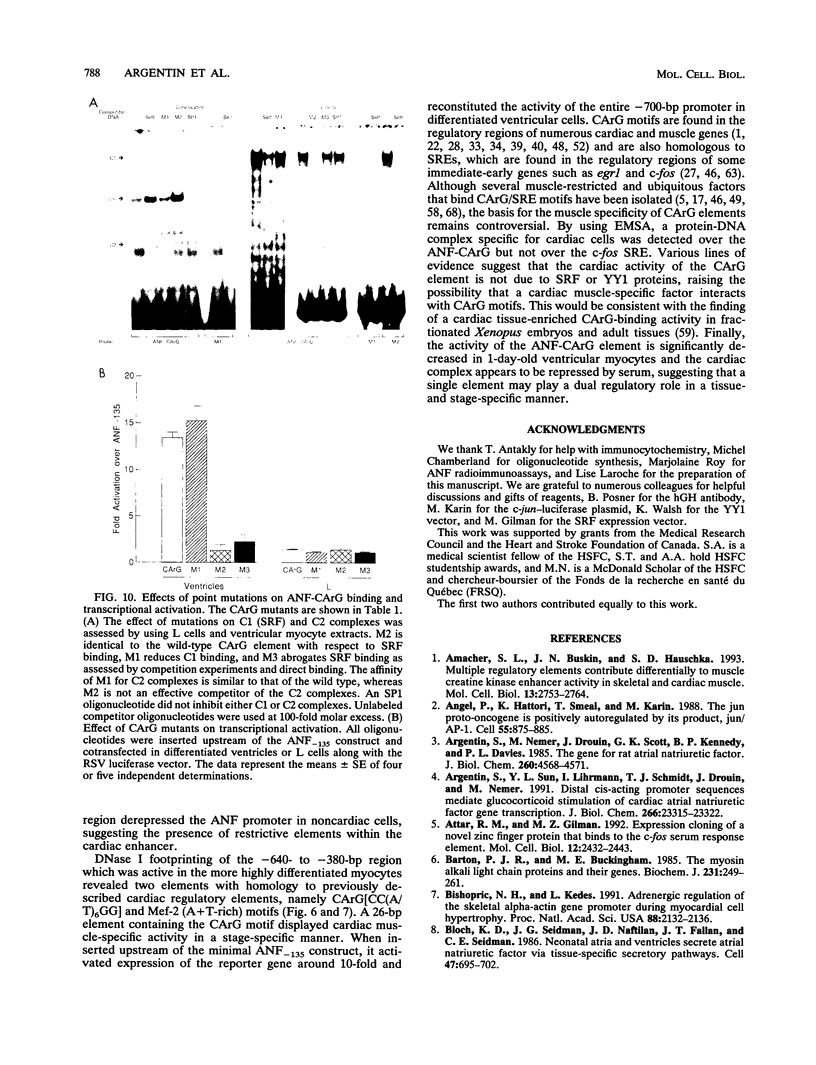
Abstract
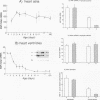
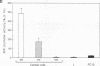
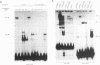
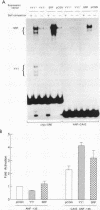
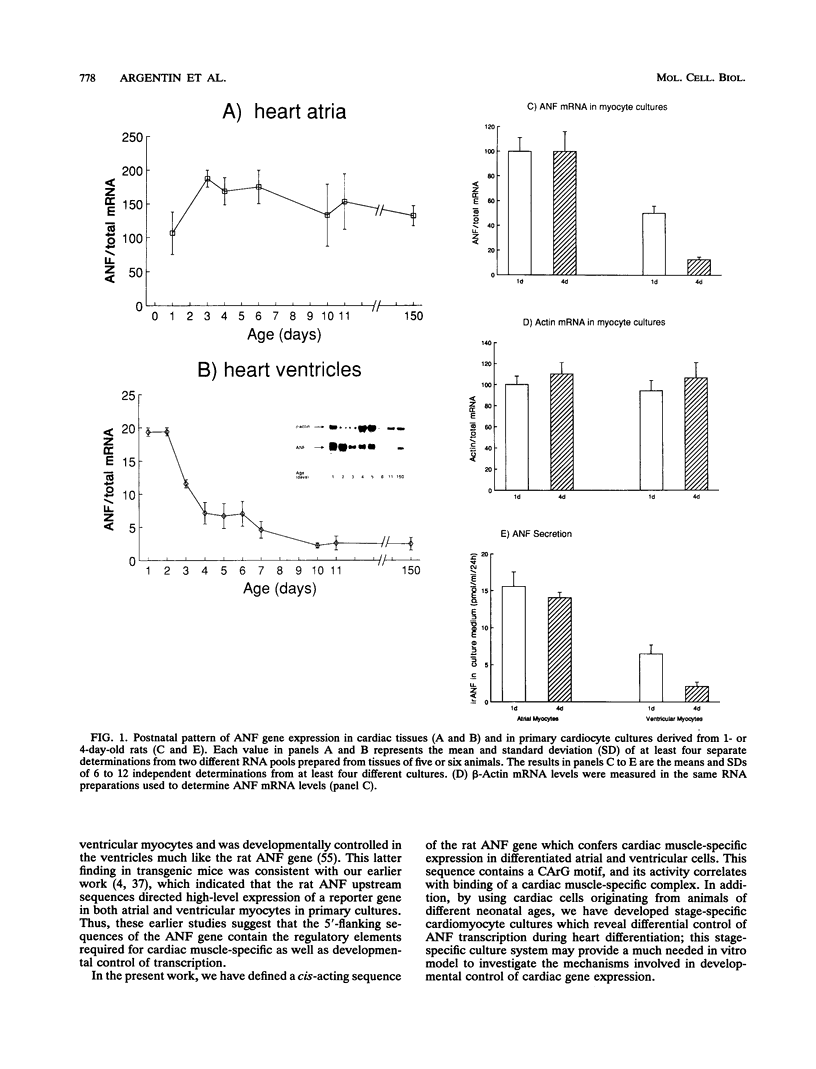
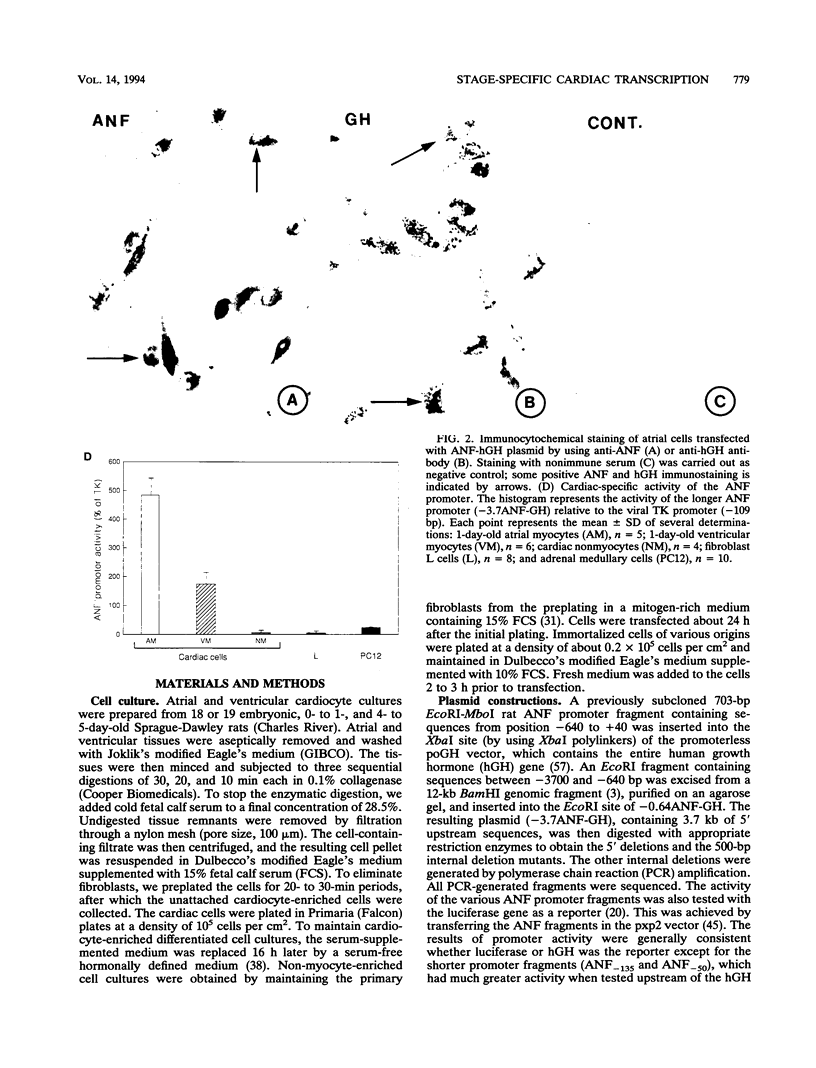
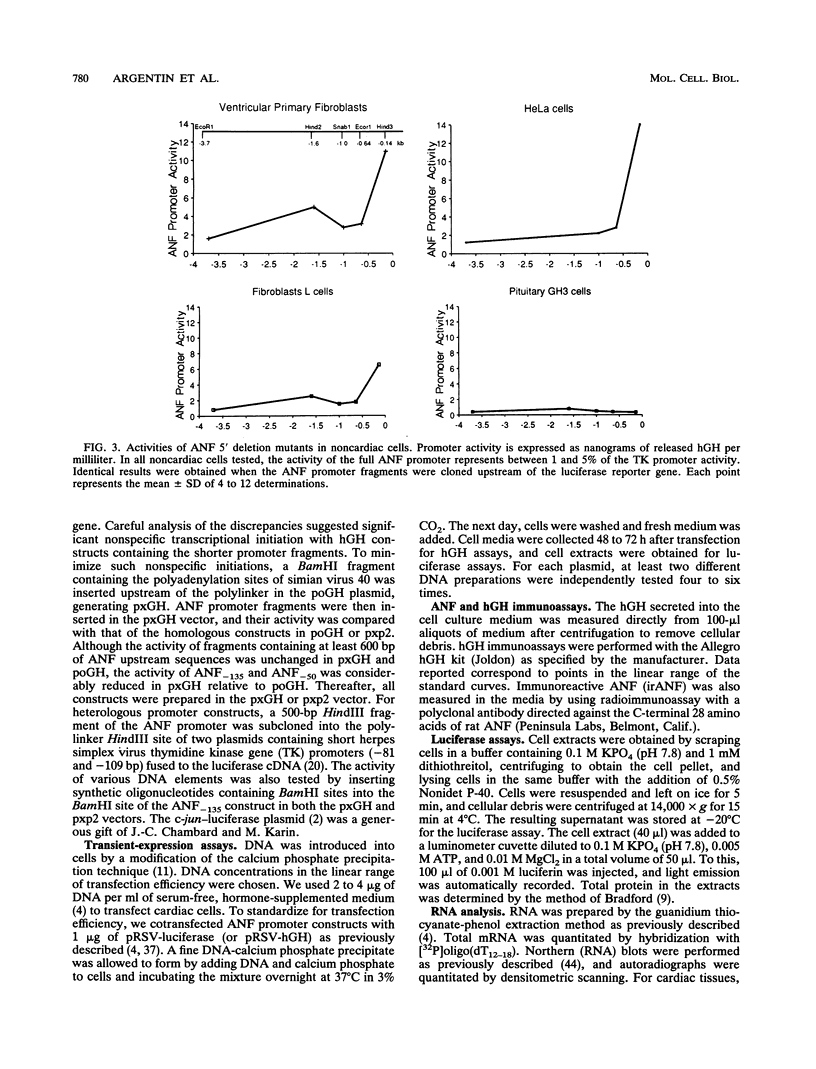
Cardiac myocytes undergo a major genetic switch within the first week of postnatal development, when cell division ceases terminally and many cardiac genes are either activated or silenced. We have developed stage-specific cardiocyte cultures to analyze transcriptional control of the rat atrial natriuretic factor (ANF) gene to identify the mechanisms underlying tissue-specific and developmental regulation of this gene in the heart. The first 700 bp of ANF flanking sequences was sufficient for cardiac muscle- and stage-specific expression in both atrial and ventricular myocytes, and a cardiac muscle-specific enhancer was localized between -136 and -700 bp. Deletion of this enhancer markedly reduced promoter activity in cardiac myocytes and derepressed ANF promoter activity in nonexpressing cells. Two distinct domains of the enhancer appeared to contribute differentially to cardiac specificity depending on the differentiation stage of the myocytes. DNase I footprinting of the enhancer domain active in differentiated cells revealed four putative regulatory elements including an A+T-rich region and a CArG element. Deletion mutagenesis and promoter reconstitution assays revealed an important role for the CArG-containing element exclusively in cardiac cells, where its activity was switched on in differentiated myocytes. Transcriptional activity of the ANF-CArG box correlated with the presence of a cardiac- and stage-specific DNA-binding complex which was not recognized by the c-fos serum response element. Thus, the use of this in vitro model system representing stage-specific cardiac development unraveled the presence of different regulatory mechanisms for transcription of the ANF gene during cardiac differentiation and may be useful for studying the regulatory pathways of other genes that undergo switching during cardiac myogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amacher S. L., Buskin J. N., Hauschka S. D. Multiple regulatory elements contribute differentially to muscle creatine kinase enhancer activity in skeletal and cardiac muscle. Mol Cell Biol. 1993 May;13(5):2753–2764. doi: 10.1128/mcb.13.5.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Argentin S., Nemer M., Drouin J., Scott G. K., Kennedy B. P., Davies P. L. The gene for rat atrial natriuretic factor. J Biol Chem. 1985 Apr 25;260(8):4568–4571. [PubMed] [Google Scholar]
- Argentin S., Sun Y. L., Lihrmann I., Schmidt T. J., Drouin J., Nemer M. Distal cis-acting promoter sequences mediate glucocorticoid stimulation of cardiac atrial natriuretic factor gene transcription. J Biol Chem. 1991 Dec 5;266(34):23315–23322. [PubMed] [Google Scholar]
- Attar R. M., Gilman M. Z. Expression cloning of a novel zinc finger protein that binds to the c-fos serum response element. Mol Cell Biol. 1992 May;12(5):2432–2443. doi: 10.1128/mcb.12.5.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton P. J., Buckingham M. E. The myosin alkali light chain proteins and their genes. Biochem J. 1985 Oct 15;231(2):249–261. doi: 10.1042/bj2310249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopric N. H., Kedes L. Adrenergic regulation of the skeletal alpha-actin gene promoter during myocardial cell hypertrophy. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2132–2136. doi: 10.1073/pnas.88.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch K. D., Seidman J. G., Naftilan J. D., Fallon J. T., Seidman C. E. Neonatal atria and ventricles secrete atrial natriuretic factor via tissue-specific secretory pathways. Cell. 1986 Dec 5;47(5):695–702. doi: 10.1016/0092-8674(86)90512-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bucher E. A., Maisonpierre P. C., Konieczny S. F., Emerson C. P., Jr Expression of the troponin complex genes: transcriptional coactivation during myoblast differentiation and independent control in heart and skeletal muscles. Mol Cell Biol. 1988 Oct;8(10):4134–4142. doi: 10.1128/mcb.8.10.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. Developmental regulation of beta-globin gene switching. Cell. 1988 Oct 7;55(1):17–26. doi: 10.1016/0092-8674(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Claycomb W. C. Biochemical aspects of cardiac muscle differentiation. Deoxyribonucleic acid synthesis and nuclear and cytoplasmic deoxyribonucleic acid polymerase activity. J Biol Chem. 1975 May 10;250(9):3229–3235. [PubMed] [Google Scholar]
- Cooper T. A., Ordahl C. P. A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternate splicing. J Biol Chem. 1985 Sep 15;260(20):11140–11148. [PubMed] [Google Scholar]
- Cooper T. A., Ordahl C. P. A single troponin T gene regulated by different programs in cardiac and skeletal muscle development. Science. 1984 Nov 23;226(4677):979–982. doi: 10.1126/science.6095446. [DOI] [PubMed] [Google Scholar]
- Dagnino L., Drouin J., Nemer M. Differential expression of natriuretic peptide genes in cardiac and extracardiac tissues. Mol Endocrinol. 1991 Sep;5(9):1292–1300. doi: 10.1210/mend-5-9-1292. [DOI] [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Dave J. R., Eiden L. E., Karanian J. W., Eskay R. L. Ethanol exposure decreases pituitary corticotropin-releasing factor binding, adenylate cyclase activity, proopiomelanocortin biosynthesis, and plasma beta-endorphin levels in the rat. Endocrinology. 1986 Jan;118(1):280–286. doi: 10.1210/endo-118-1-280. [DOI] [PubMed] [Google Scholar]
- Emerson B. M., Nickol J. M., Fong T. C. Erythroid-specific activation and derepression of the chick beta-globin promoter in vitro. Cell. 1989 Jun 30;57(7):1189–1200. doi: 10.1016/0092-8674(89)90056-1. [DOI] [PubMed] [Google Scholar]
- Ernst H., Walsh K., Harrison C. A., Rosenthal N. The myosin light chain enhancer and the skeletal actin promoter share a binding site for factors involved in muscle-specific gene expression. Mol Cell Biol. 1991 Jul;11(7):3735–3744. doi: 10.1128/mcb.11.7.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. J. Atrial natriuretic factor-SV40 T antigen transgenes produce tumors and cardiac arrhythmias in mice. Science. 1988 Feb 26;239(4843):1029–1033. doi: 10.1126/science.2964082. [DOI] [PubMed] [Google Scholar]
- Flink I. L., Edwards J. G., Bahl J. J., Liew C. C., Sole M., Morkin E. Characterization of a strong positive cis-acting element of the human beta-myosin heavy chain gene in fetal rat heart cells. J Biol Chem. 1992 May 15;267(14):9917–9924. [PubMed] [Google Scholar]
- Foley K. P., Engel J. D. Individual stage selector element mutations lead to reciprocal changes in beta- vs. epsilon-globin gene transcription: genetic confirmation of promoter competition during globin gene switching. Genes Dev. 1992 May;6(5):730–744. doi: 10.1101/gad.6.5.730. [DOI] [PubMed] [Google Scholar]
- Gahlmann R., Wade R., Gunning P., Kedes L. Differential expression of slow and fast skeletal muscle troponin C. Slow skeletal muscle troponin C is expressed in human fibroblasts. J Mol Biol. 1988 May 20;201(2):379–391. doi: 10.1016/0022-2836(88)90145-3. [DOI] [PubMed] [Google Scholar]
- Gius D., Cao X. M., Rauscher F. J., 3rd, Cohen D. R., Curran T., Sukhatme V. P. Transcriptional activation and repression by Fos are independent functions: the C terminus represses immediate-early gene expression via CArG elements. Mol Cell Biol. 1990 Aug;10(8):4243–4255. doi: 10.1128/mcb.10.8.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto A., LePage D., Pons G., Mader S. L., Park K., Atchison M. L., Walsh K. Functional antagonism between YY1 and the serum response factor. Mol Cell Biol. 1992 Sep;12(9):4209–4214. doi: 10.1128/mcb.12.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello R. C., Mar J. H., Ordahl C. P. Characterization of a promoter element required for transcription in myocardial cells. J Biol Chem. 1991 Feb 15;266(5):3309–3316. [PubMed] [Google Scholar]
- Johnson J. E., Wold B. J., Hauschka S. D. Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Mol Cell Biol. 1989 Aug;9(8):3393–3399. doi: 10.1128/mcb.9.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtz D. S., Dische N. R., Inagami T., Goldman B. Growth and partial differentiation of presumptive human cardiac myoblasts in culture. J Cell Biol. 1989 Mar;108(3):1067–1078. doi: 10.1083/jcb.108.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe M. C., Wu J. P., Greenberg B., Gardner D. G. Upstream sequences confer atrial-specific expression on the human atrial natriuretic factor gene. J Biol Chem. 1988 Jul 5;263(19):9075–9078. [PubMed] [Google Scholar]
- Lee T. C., Chow K. L., Fang P., Schwartz R. J. Activation of skeletal alpha-actin gene transcription: the cooperative formation of serum response factor-binding complexes over positive cis-acting promoter serum response elements displaces a negative-acting nuclear factor enriched in replicating myoblasts and nonmyogenic cells. Mol Cell Biol. 1991 Oct;11(10):5090–5100. doi: 10.1128/mcb.11.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. C., Shi Y., Schwartz R. J. Displacement of BrdUrd-induced YY1 by serum response factor activates skeletal alpha-actin transcription in embryonic myoblasts. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9814–9818. doi: 10.1073/pnas.89.20.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- Lyons G. E., Schiaffino S., Sassoon D., Barton P., Buckingham M. Developmental regulation of myosin gene expression in mouse cardiac muscle. J Cell Biol. 1990 Dec;111(6 Pt 1):2427–2436. doi: 10.1083/jcb.111.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride K., Robitaille L., Tremblay S., Argentin S., Nemer M. fos/jun repression of cardiac-specific transcription in quiescent and growth-stimulated myocytes is targeted at a tissue-specific cis element. Mol Cell Biol. 1993 Jan;13(1):600–612. doi: 10.1128/mcb.13.1.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed S. N., Holmes R., Hartzell C. R. A serum-free, chemically-defined medium for function and growth of primary neonatal rat heart cell cultures. In Vitro. 1983 Jun;19(6):471–478. doi: 10.1007/BF02619594. [DOI] [PubMed] [Google Scholar]
- Mohun T. J., Taylor M. V., Garrett N., Gurdon J. B. The CArG promoter sequence is necessary for muscle-specific transcription of the cardiac actin gene in Xenopus embryos. EMBO J. 1989 Apr;8(4):1153–1161. doi: 10.1002/j.1460-2075.1989.tb03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T., Garrett N., Treisman R. Xenopus cytoskeletal actin and human c-fos gene promoters share a conserved protein-binding site. EMBO J. 1987 Mar;6(3):667–673. doi: 10.1002/j.1460-2075.1987.tb04806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag A. C., Cheng M. Biochemical evidence for cellular dedifferentiation in adult rat cardiac muscle cells in culture: expression of myosin isozymes. Biochem Biophys Res Commun. 1986 Jun 13;137(2):855–862. doi: 10.1016/0006-291x(86)91158-7. [DOI] [PubMed] [Google Scholar]
- Navankasattusas S., Zhu H., Garcia A. V., Evans S. M., Chien K. R. A ubiquitous factor (HF-1a) and a distinct muscle factor (HF-1b/MEF-2) form an E-box-independent pathway for cardiac muscle gene expression. Mol Cell Biol. 1992 Apr;12(4):1469–1479. doi: 10.1128/mcb.12.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemer M., Chamberland M., Sirois D., Argentin S., Drouin J., Dixon R. A., Zivin R. A., Condra J. H. Gene structure of human cardiac hormone precursor, pronatriodilatin. Nature. 1984 Dec 13;312(5995):654–656. doi: 10.1038/312654a0. [DOI] [PubMed] [Google Scholar]
- Nemer M., Lavigne J. P., Drouin J., Thibault G., Gannon M., Antakly T. Expression of atrial natriuretic factor gene in heart ventricular tissue. Peptides. 1986 Nov-Dec;7(6):1147–1152. doi: 10.1016/0196-9781(86)90145-2. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Norman C., Runswick M., Pollock R., Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988 Dec 23;55(6):989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- Park K., Atchison M. L. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3' enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmacek M. S., Vora A. J., Shen T., Barr E., Jung F., Leiden J. M. Identification and characterization of a cardiac-specific transcriptional regulatory element in the slow/cardiac troponin C gene. Mol Cell Biol. 1992 May;12(5):1967–1976. doi: 10.1128/mcb.12.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasba P., Lin E., Zhou M. D., Kumar A., Siddiqui M. A. A single transcription factor binds to two divergent sequence elements with a common function in cardiac myosin light chain-2 promoter. Mol Cell Biol. 1992 Mar;12(3):1107–1116. doi: 10.1128/mcb.12.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V., Webster K. A., Kedes L. Muscle-specific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 1990 Oct;4(10):1811–1822. doi: 10.1101/gad.4.10.1811. [DOI] [PubMed] [Google Scholar]
- Sassoon D., Lyons G., Wright W. E., Lin V., Lassar A., Weintraub H., Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989 Sep 28;341(6240):303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman C. E., Schmidt E. V., Seidman J. G. cis-dominance of rat atrial natriuretic factor gene regulatory sequences in transgenic mice. Can J Physiol Pharmacol. 1991 Oct;69(10):1486–1492. doi: 10.1139/y91-223. [DOI] [PubMed] [Google Scholar]
- Seidman C. E., Wong D. W., Jarcho J. A., Bloch K. D., Seidman J. G. Cis-acting sequences that modulate atrial natriuretic factor gene expression. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4104–4108. doi: 10.1073/pnas.85.11.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Taylor M. V. A family of muscle gene promoter element (CArG) binding activities in Xenopus embryos: CArG/SRE discrimination and distribution during myogenesis. Nucleic Acids Res. 1991 May 25;19(10):2669–2675. doi: 10.1093/nar/19.10.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel M., Costantini F. A 3' enhancer contributes to the stage-specific expression of the human beta-globin gene. Genes Dev. 1987 Nov;1(9):954–961. doi: 10.1101/gad.1.9.954. [DOI] [PubMed] [Google Scholar]
- Trudel M., Magram J., Bruckner L., Costantini F. Upstream G gamma-globin and downstream beta-globin sequences required for stage-specific expression in transgenic mice. Mol Cell Biol. 1987 Nov;7(11):4024–4029. doi: 10.1128/mcb.7.11.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsika R. W., Bahl J. J., Leinwand L. A., Morkin E. Thyroid hormone regulates expression of a transfected human alpha-myosin heavy-chain fusion gene in fetal rat heart cells. Proc Natl Acad Sci U S A. 1990 Jan;87(1):379–383. doi: 10.1073/pnas.87.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuil D., Clergue N., Montarras D., Pinset C., Kahn A., Phan-Dinh-Tuy F. CC Ar GG boxes, cis-acting elements with a dual specificity. Muscle-specific transcriptional activation and serum responsiveness. J Mol Biol. 1990 Jun 20;213(4):677–686. doi: 10.1016/S0022-2836(05)80255-4. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Bugaisky G., Buckingham M. Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. A quantitative determination of the two actin isoforms. J Biol Chem. 1986 Feb 5;261(4):1838–1843. [PubMed] [Google Scholar]
- Volz A., Piper H. M., Siegmund B., Schwartz P. Longevity of adult ventricular rat heart muscle cells in serum-free primary culture. J Mol Cell Cardiol. 1991 Feb;23(2):161–173. doi: 10.1016/0022-2828(91)90103-s. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Wu J., LaPointe M. C., West B. L., Gardner D. G. Tissue-specific determinants of human atrial natriuretic factor gene expression in cardiac tissue. J Biol Chem. 1989 Apr 15;264(11):6472–6479. [PubMed] [Google Scholar]
- Yu Y. T., Breitbart R. E., Smoot L. B., Lee Y., Mahdavi V., Nadal-Ginard B. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992 Sep;6(9):1783–1798. doi: 10.1101/gad.6.9.1783. [DOI] [PubMed] [Google Scholar]
- Zeller R., Bloch K. D., Williams B. S., Arceci R. J., Seidman C. E. Localized expression of the atrial natriuretic factor gene during cardiac embryogenesis. Genes Dev. 1987 Sep;1(7):693–698. doi: 10.1101/gad.1.7.693. [DOI] [PubMed] [Google Scholar]
- Zhu H., Garcia A. V., Ross R. S., Evans S. M., Chien K. R. A conserved 28-base-pair element (HF-1) in the rat cardiac myosin light-chain-2 gene confers cardiac-specific and alpha-adrenergic-inducible expression in cultured neonatal rat myocardial cells. Mol Cell Biol. 1991 Apr;11(4):2273–2281. doi: 10.1128/mcb.11.4.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]