Abstract
OBJECTIVE:
Ophthalmologic examination for retinopathy of prematurity is a painful procedure. Pharmacological and non-pharmacological interventions have been proposed to reduce pain during eye examinations. This study aims to evaluate the analgesic effect of 25% glucose using a validated pain scale during the first eye examination for retinopathy of prematurity in preterm infants with birth weight ≤1,500 g and/or gestational age ≤32 weeks.
METHODS:
A masked, randomized clinical trial for one dose of 1 ml of oral 25% glucose solution 2 minutes before the first ophthalmologic examination for retinopathy of prematurity was conducted between March 2008 and April 2010. The results were compared to those of a control group that did not receive oral glucose solution. Pain was evaluated using a Neonatal Infant Pain Scale immediately before and immediately after the ophthalmologic examination in both groups. Clinicaltrials.gov: NCT00648687
RESULTS:
One hundred and twenty-four patients who were examined for the first time for retinopathy of prematurity were included. Seventy were included in the intervention group and 54 in the control group. The number of patients with pain immediately before the procedure was similar in both groups. The number of patients with pain after ophthalmologic examination was 15.7% in the intervention group and 68.5% in the control group (p<0.001).
CONCLUSIONS:
One ml of oral 25% glucose solution given 2 minutes before an ophthalmologic examination for retinopathy of prematurity was an effective measure for pain relief.
Keywords: Premature, Retinopathy of Prematurity, Pain, Pain Measurement, Stress, Neonatal Infant Pain Scale (NIPS)
INTRODUCTION
Retinopathy of prematurity (ROP) is a leading cause of childhood preventable blindness in middle-income countries (1). Ophthalmologic examination is the best way to detect ROP but is a painful procedure and causes stress and physical debilitation, especially because of the use of the eyelid speculum or scleral indentation (2-6). The use of local anesthetic eye drops before an eye examination is a partially effective therapeutic procedure (7).
Recent studies have proposed the use of pharmacological and non-pharmacological interventions, such as sweet solutions (oral sucrose or glucose solutions) with or without non-nutritive suck, swaddling and a pacifier to minimize pain during eye examinations for ROP. The influence of nesting without topical anesthesia to minimize pain during the screening for ROP was previously evaluated with controversial results. Fewer body movements were observed in the nested group, including movements of the distal limb, proximal limb and trunk, relative to the non-nested group prior to, during and after the ROP examination (8). The authors suggested that the benefits of some of the non-pharmacological pain measures were dependent on the age of the neonate at the time the examination was performed (8,9). Although the efficacy of these approaches is clearly evident, they cannot provide analgesia for moderate or severe pain in the neonate (8-)(12). Pharmacological interventions used to treat neonatal pain include opiates, benzodiazepines, barbiturates, ketamine, propofol, acetaminophen and local and topical anesthetics. However, the indications, advantages and disadvantages of commonly used analgesic drugs and non-pharmacolgical interventions in the neonatal period are controversial and require further study (8,11).
The effect of oral glucose for analgesia in newborns is known. Glucose solutions are routinely used for intravenous infusions because they are readily available in neonatal intensive care units (NICU) and they are the preferred sweet solution used in our institution (13,14).
The objective of this study was to analyze the analgesic effect of 25% glucose to reduce the frequency of pain during the first eye examination for ROP in preterm infants with birth weight (BW) ≤1,500 g and/or gestational age (GA) at birth ≤32 weeks. Pain was evaluated by using a validated Neonatal Infant Pain Scale (NIPS).
METHODS
Study design
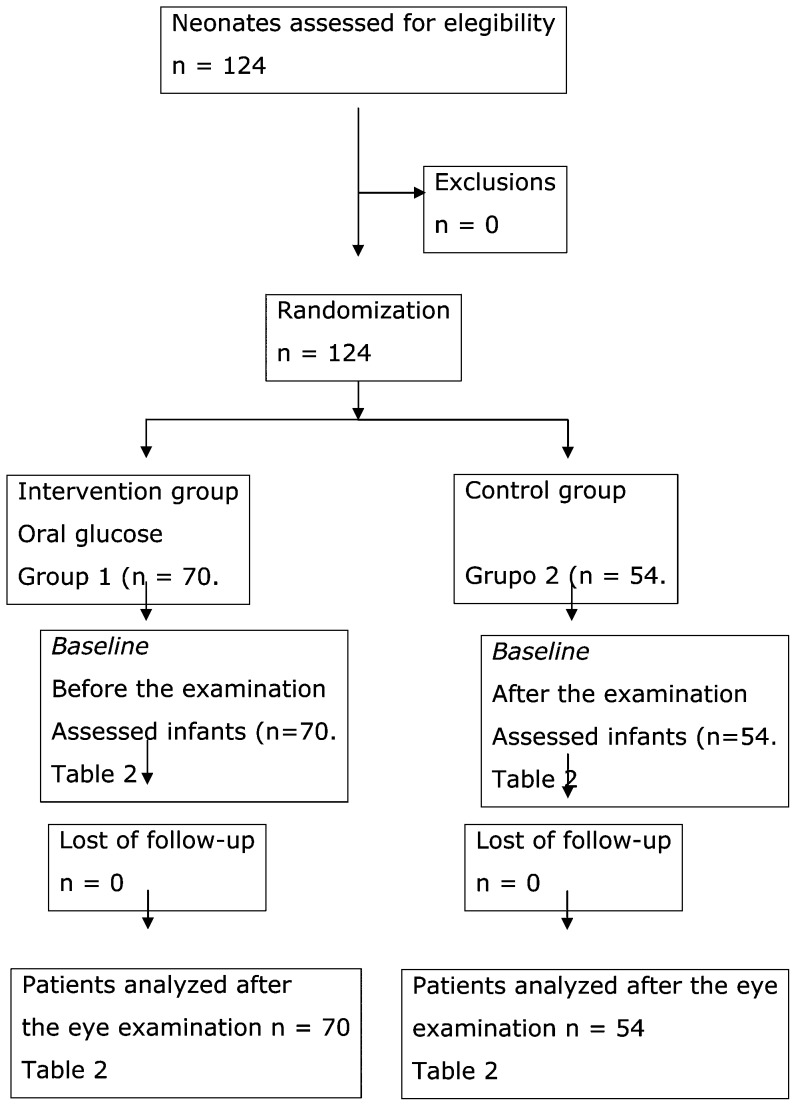
A masked randomized clinical trial with or without the oral administration of 1 ml of 25% glucose solution 2 minutes before an ophthalmologic examination for ROP in preterm infants with BW ≤1,500 g and/or GA ≤32 weeks at birth was conducted between March 2008 and April 2010. Patients were randomly assigned to one of the two groups. The randomization was computerized. We included patients that received oral glucose solution in the intervention group (Group 1) and patients that did not receive oral glucose in the control group (Group 2). A placebo was not used in the control group so that it would be as similar as possible to the ophthalmological examination routinely performed in our NICU. The study flow diagram followed the CONSORT statement (Figure 1).
Figure 1.
Flow diagram for the included patients in each step of the study.
Population
Screening to detect ROP is performed weekly in 93 to 95% of all admitted very-low-birth-weight (VLBW) preterm infants. We included all patients with BW ≤1,500 g and/or GA ≤32 weeks who were examined for the first time for ROP in this clinical trial. There were no exclusion criteria.
Interventions
The main intervention was the administration of 1 ml of oral 25% glucose solution in a single dose by syringe without sucking 2 minutes before the eye examination. The ophthalmologic examination was conducted according to the Brazilian guidelines for ROP screening (15) and was always performed by the same author (JBFF), who was blinded to the intervention and blinded to the NIPS scores. The eyes were evaluated by binocular indirect ophthalmoscopy using a 28 D Nikon lens (Nikon®, Melville, NY, USA) and a lid speculum for newborns (Alfonso Eye Speculum, Storz®, Bausch & Lomb Inc., San Dimas, CA, USA) after dilation of the pupils with eye drops containing tropicamide 0.5% and phenylephrine 2.5%. Scleral indentation was used to achieve a better examination of peripheral zone III. Local anesthetic eye drops (proxymetacaine 0.5%) were used in all patients prior to the introduction of the eyelid speculum. All infants were first examined between four and six weeks of life.
For the study, we considered only the first ophthalmologic examination to avoid the influence of "pain memory". The same type of speculum and indentor were used to maintain consistency throughout the study period. The mean examination time for each patient was approximately 2 minutes per eye.
Pain response evaluation and variables
We used the NIPS scale to evaluate the presence of pain (16). The NIPS scale considers facial expression, crying, breathing pattern, arms, legs and state of arousal as variables. Scores equal or greater than 4 were considered to be positive for pain. The NIPS score evaluation was performed 2 minutes before and 2 minutes after the ophthalmologic examination by two different evaluators. The NIPS evaluators were independent and unaware of each other's results. The NIPS evaluators were also blinded to the intervention. We considered the mean score of both examiners before and after examination. The examiners were NICU nurses who had extensive experience with the NIPS score in our NICU. We used the Kappa coefficient to analyze the concordance between both evaluators regarding the NIPS scores (NIPS <4 and NIPS >4) before and after the eye examination.
Data on BW, GA, postconceptional age (PCA), weight of the preterm and the NIPS score at baseline was evaluated in all patients included in this study (Group 1 Intervention and Group 2 Control). The main outcome was the variation of the NIPS scores before and after the ophthalmological examination to detect ROP.
Statistical methods
A sample size of 50 patients in each group was calculated after a pilot trial with 20 patients in each group to obtain a 90% study power and a 5% significance level. The initial sample size was calculated to detect a mean difference of 0.6 in the NIPS score from before and after the eye examination in both groups.
The NIPS score was evaluated as a continuous variable by repeated-measures ANOVA and as a categorical variable (NIPS≥4) by Chi-square analysis. Repeated-measures ANOVA was used to evaluate the difference between the two groups regarding time (before and after the eye examination). Analyses for the evolution of the groups before and after the eye examination (P interaction), time (P time), and comparison between groups (P group) are presented. Differences were considered significant if p<0.05.
The statistical analysis was performed using Statistical Package for Social Sciences software (SPSS 15.0 for Windows, SPSS Inc., Chicago, IL, USA).
Ethics
The parents or legal representatives signed a consent form for all newborns included in the study. The study protocol was approved by the Ethics Committee of the HCPA (number 07-437) and it was registered with the Clinical Trials of the US National Institutes of Health (number NCT00648687).
The authors certify that the protocol for the research project conformed to the provisions of the Declaration of Helsinki of 1995 (as revised in Edinburgh 2000) and declare no financial support or relationships that may pose a conflict of interest.
RESULTS
In total, 124 VLBW preterm infants were included in the study: 70 newborns were included in the intervention group and 54 in the control group. A CONSORT-based diagram showing patient flow through each step of the trial is shown in Figure 1.
The mean BW and GA of the 124 included newborn infants were 1,261.3±273.1 g and 30.2±2.0 weeks, respectively. Thirty patients (24.2%) had a BW ≤1,000 g and 48 patients (38.7%) were small for GA (<10th percentile). The PCA at the first ophthalmological examination in the entire cohort ranged from 31 to 37 weeks (mean 34.6±1.2 weeks). The data regarding BW, GA, PCA and weight of the preterm infant on the examination day in each group of the study are displayed in Table 1. On the scheduled day of the ophthalmological examination, all of the patients were evaluated by the neonatologist-in-charge and were considered to be sufficiently healthy to undergo screening for ROP. None of the patients were on mechanical ventilation and all were clinically stable. After the eye examination, none of the patients required any clinical or pharmacological intervention to minimize pain.
Table 1.
Demographic data of eligible VLBW infants.
| Group 1 Intervention n = 70 patients | Group 2 Control n = 54 patients | p-value | |
| BW g* | 1,287.80±255.1 | 1,227.00±293.6 | 0.229 |
| GA weeks * | 30.5±1.9 | 29.9±2.0 | 0.073 |
| Weight at examination day * | 1,904.40±330.2 | 1,801.00±363.8 | 0.101 |
| PCA at examination day * | 34.9±1.1 | 34.3±1.2 | 0.754 |
VLBW: very low birth weight; *: Data in mean ± standard deviation; BW: birth weight; GA: gestational age, PCA: postconceptional age.
According to the data displayed in Table 1, both groups were similar at baseline in terms of BW, GA, PCA and weight on the day of the eye examination.
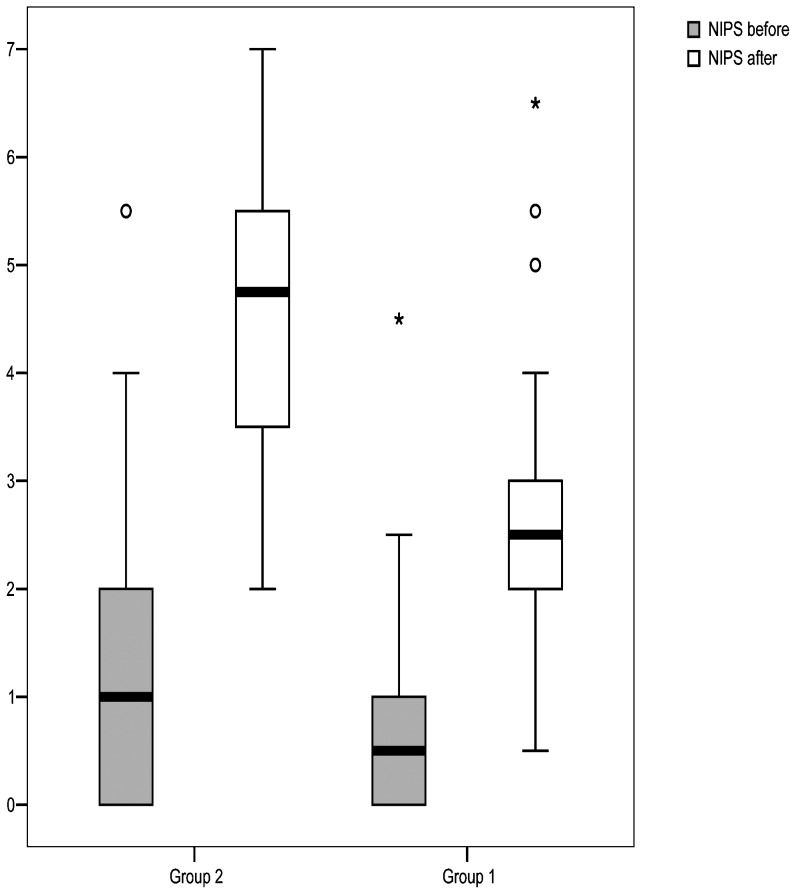
Prior to the examination, the mean NIPS score was 0.8±0.8 and 1.2±1.2 (p = 0.100) in the intervention and control groups, respectively. After the examination, the mean NIPS score increased to 2.6±1.1 in the intervention group, whereas the score increased to 4.5±1.3 in the control group (p<0.001). The inter-examiner reliability of the NIPS scale (kappa) in our study was 0.70. With the inclusion of 70 patients in the intervention group and 54 patients in the control group, our study achieved a power of 100% to detect a difference of 1.9 in the mean NIPS score between the groups (4.5-2.6) after examination for ROP with a standard deviation of 1.1 (intervention group) and 1.3 (control group).
When the NIPS score was categorized for pain (NIPS≥4), only one patient (1.4%) in the intervention group and two patients (3.7%) in the control group had pain prior to the eye examination (p = 0.580). After the eye examination, we observed 11 patients with pain (NIPS≥4) in the intervention group (15.7%) and 37 patients with pain (68.5%) in the control group (p<0.001). The NIPS evaluations before and after the ophthalmologic examination in both groups are shown in Table 2 and Figure 2.
Table 2.
NIPS scores before and after the eye examination for ROP.
| BEFORE | p-value | AFTER | p-value | |||
| Group 1 Intervention n = 70 patients | Group 2 Control n = 54 patients | Group 1 Intervention n = 70 patients | Group 2 Control n = 54 patients | |||
| NIPS≥4 n (%) | 1 (1.4%) | 2 (3.7%) | 0.580 | 11 (15.7%) | 37 (68.5%) | <0.001 |
| NIPS mean±SD | 0.8±0.8 | 1.2±1.2 | 0.100 | 2.6±1.1 | 4.5±1.3 | <0.001 |
NIPS: Neonatal Infant Pain Scale; SD: standard deviation.Repeated-measures ANOVA: P time<0.001; P interaction<0.001; P group<0.001.
Figure 2.
NIPS score variation before and after the eye examination.
Repeated-measures ANOVA revealed different variations in the intervention group relative to the control group (P interaction <0.001, Table 2).
DISCUSSION
Our data showed that 1 ml of oral 25% glucose solution given 2 minutes before an ophthalmologic exam for ROP was efficient for pain relief. After the exam, patients in the control group had a mean NIPS score that was significantly higher than the score for patients in the intervention group. Furthermore, the control group had significantly more patients with pain (NIPS≥4) than the intervention group.
The ophthalmologic examination for ROP has been demonstrated to be a painful procedure that causes stress as well as physical and psychological distress in preterm infants (2,17,18). Anesthetic eye drops partially minimize the pain caused by eyelid speculum insertion, scleral indentation and the bright light of the binocular indirect ophthalmoscope during the eye exam for ROP (7,19,20). However, even with the use of anesthetic eye drops, pain is still present during the eye exam. In the present study, both groups were treated with anesthetic eye drops before the ophthalmologic examination and significantly more patients in the control group had a high NIPS score immediately after the examination.
Non-pharmacological measures have been proposed for neonatal pain relief, such as being held by nurse, use of maternal milk, nesting, non-nutritive sucking and the administration of oral glucose or sucrose solutions (8,11).
Samra et al. (9) and Sum et al. (11) reported studies on the administration of oral sucrose for pain relief during an ROP screening examination. In most other studies, the efficacy of 24% sucrose solution was evaluated; in one study, 33% sucrose solution was tested. The volume administered, time of administration and number of doses differed among the studies. Mitchell et al. (8) administered three doses of 0.1 ml of 24% sucrose solution before an eye examination in 15 patients. Gal et al. (21) used 2 ml of 24% sucrose solution in a cross-over placebo-controlled study with 22 patients. Grabska et al. (22) conducted a placebo-controlled study with 16 patients in the intervention group who received 24% sucrose solution and adjusted the sucrose dose from 0.12 to 0.48 ml according to the patient's weight. Boyle et al. (10) used 1 ml of 33% sucrose solution in 10 patients. These studies show that sucrose administration before ophthalmologic examination reduces pain, but the results were often confounded by the non-nutritive sucking effect. However, some studies did not show a significant effect of pain relief for sucrose or oral glucose (23,24).
Oral glucose to relieve pain and minimize distress among newborns is commonly used in neonatology and it is the preferred solution used at our institution. Orally administered glucose has a clearly demonstrated pain-reducing effect in newborns, but the mechanisms underlying this effect remain unknown (25) and controversial. Slater et al. (26) stated that oral sucrose does not significantly affect activity in the neonatal brain or spinal cord nociceptive circuits. Therefore, it may not be an effective analgesic drug. The ability of sucrose to reduce clinical observational pain scores after noxious events in newborn infants should not be interpreted as pain relief. Wilkinson et al. (27) thus suggested that sucrose or sugar may be better understood not as an analgesic that is removing or relieving pain but rather as a compensating pleasure for the infant.
Olsson and Eriksson studied the pain relief effect of 1 ml of 30% glucose solution given 1 minute before an eye examination in 14 infants with gestational age less than 32 weeks and/or birth weight less than 1,500 grams relative to the effect in 15 control infants that received sterile water. They included patients who were previously examined and showed that glucose solution did not alleviate pain associated with the eye examination (23). We included 70 preterm infants that received 25% glucose solution 2 minutes before their first eye examination and compared them to 54 control infants. The extra minute before the eye examination and the exclusion of possible pain memory from a previous ophthalmologic examination differentiate our study from the previous study, and these differences may explain the difference in findings.
We chose to use the NIPS scale, which is appropriate for term and preterm infants. At the first ophthalmologic examination, the mean corrected gestational age was higher than 35 weeks and all of the patients were six weeks old. These characteristics of the included patients allowed us to use the NIPS scale for pain evaluation. The NIPS scale is currently used in most NICUs in Brazil (28-30).
The inter-examiner reliability of the NIPS scale (kappa coefficient) in our study was 0.70. An inter-examiner reliability of 0.80 or higher would provide better final results in our study. Nevertheless, the NIPS evaluators in our NICU were well-trained nurses with extensive experience in the use of the NIPS score, which is routinely used in our hospital.
Some identified limitations of our study were: a) we could not control for possible previous pain memory other than that caused by the ophthalmologic examination of each infant; b) because the NIPS score evaluation was performed 2 minutes after the ophthalmologic examination, it is possible that the infants were still recovering from pain, which could have resulted in under- or overestimation of the effects of glucose; and c) scales for pain evaluation are always subjective. These limitations did not affect the determination that 1 ml of oral 25% glucose alleviates pain responses to ophthalmologic examination in VLBW preterm infants.
Small volumes of glucose solution can be administered with drops and without the need for suction, which is useful for VLBW premature newborns that are not able to suck and for infants who are intubated at the time of their first ophthalmologic examination.
Currently, there are clear guidelines for ROP examination in many countries, but there are no established protocols for pharmacological or non-pharmacological pain management during ROP examinations (9). The findings of our study are relevant because they show that 1 ml of oral 25% glucose solution in a single dose administered 2 minutes prior to eyelid speculum insertion is effective for pain relief produced by the ROP examination.
ACKNOWLEDGMENTS
The authors certify that the content has not been published or submitted for publication elsewhere. The authors also certify that the protocol for the research project has been approved by a suitably constituted ethics committee of the institution within which the work was undertaken under the number 03-248. The study conforms to the provisions of the Declaration of Helsinki of 1995 (as revised in Edinburgh in 2000). The authors declare no financial support or relationships that may pose a conflict of interest.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Fielder AR, Reynolds JD. Retinopathy of prematurity: clinical aspects. Semin Neonatol. 2001;6(6):461–75. doi: 10.1053/siny.2001.0091. [DOI] [PubMed] [Google Scholar]
- 2.Belda S, Pallas CR, De la Cruz J, Tejada P. Screening for retinopathy of prematurity: is it painful. Biol Neonate. 2004;86(3):195–200. doi: 10.1159/000079542. [DOI] [PubMed] [Google Scholar]
- 3.Rush R, Rush S, Ighani F, Anderson B, Irwin M, Naqvi M. The effects of comfort care on the pain response in preterm infants undergoing screening for retinopathy of prematurity. Retina. 2005;25(1):59–62. doi: 10.1097/00006982-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Rush R, Rush S, Nicolau J, Chapman K, Naqvi M. Systemic manifestations in response to mydriasis and physical examination during screening for retinopathy of prematurity. Retina. 2004;24(2):242–5. doi: 10.1097/00006982-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Laws DE, Morton C, Weindling M, Clark D. Systemic effects of screening for retinopathy of prematurity. Br J Ophthalmol. 1996;80(5):425–8. doi: 10.1136/bjo.80.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allegaert K, Casteels I, Tibboel D. Pain management during eye examinations for retinopathy of prematurity: what about procedural adaptations to blunt the pain response. Acta Paediatr. 2010;99(4):488–9. doi: 10.1111/j.1651-2227.2009.01672.x. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey E, McCreery K. Local anaesthetic eye drops for prevention of pain in preterm infants undergoing screening for retinopathy of prematurity. Cochrane Database Syst Rev. 2011;9:CD007645. doi: 10.1002/14651858.CD007645.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell A, Stevens B, Mungan N, Johnson W, Lobert S, Boss B. Analgesic effects of oral sucrose and pacifier during eye examinations for retinopathy of prematurity. Pain Manag Nurs. 2004;5:160–8. doi: 10.1016/j.pmn.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Samra HA, McGrath JM. Pain management during retinopathy of prematurity eye examinations: a systematic review. Adv Neonatal Care. 2009;9(3):99–110. doi: 10.1097/ANC.0b013e3181a68b48. [DOI] [PubMed] [Google Scholar]
- 10.Boyle EM, Freer Y, Khan-Orakzai Z, Watkinson M, Wright E, Ainsworth JR, et al. Sucrose and non-nutritive sucking for the relief of pain in screening for retinopathy of prematurity: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2006;91(3):F166–8. doi: 10.1136/adc.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Lemyre B, Barrowman N, O'Connor M. Pain management during eye examinations for retinopathy of prematurity in preterm infants: a systematic review. Acta Paediatr. 2010;99(3):329–34. doi: 10.1111/j.1651-2227.2009.01612.x. [DOI] [PubMed] [Google Scholar]
- 12.O'Sullivan A, O'Connor M, Brosnahan D, McCreery K, Dempsey EM. Sweeten, soother and swaddle for retinopathy of prematurity screening: a randomised placebo controlled trial. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F419–22. doi: 10.1136/adc.2009.180943. [DOI] [PubMed] [Google Scholar]
- 13.Hall RW. Anesthesia and analgesia in the NICU. Clin Perinatol. 2012;39(1):239–54. doi: 10.1016/j.clp.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skogsdal Y, Eriksson M, Schollin J. Analgesia in newborns given oral glucose. Acta Paediatr. 1997;86(2):217–20. doi: 10.1111/j.1651-2227.1997.tb08872.x. [DOI] [PubMed] [Google Scholar]
- 15.Zin A, Florencio T, Fortes Filho JB, Nakanami CR, Gianini N, Graziano RM, et al. [Brazilian guidelines proposal for screening and treatment of retinopathy of prematurity (ROP)] Arq Bras Oftalmol. 2007;70(5):875–83. doi: 10.1590/s0004-27492007000500028. [DOI] [PubMed] [Google Scholar]
- 16.Gallo AM. The fifth vital sign: implementation of the Neonatal Infant Pain Scale. J Obstet Gynecol Neonatal Nurs. 2003;32(2):199–206. doi: 10.1177/0884217503251745. [DOI] [PubMed] [Google Scholar]
- 17.Figueras-Aloy J, Rodríguez-Miguélez JM, Salvia-Roiges MD, Botet-Mussons F. Concerning the article by s. Belda et al.: screening for retinopathy of prematurity: is it painful. Biol Neonate. 2006;89(3):197. doi: 10.1159/000089754. [DOI] [PubMed] [Google Scholar]
- 18.Rush R, Rush S, Ighani F, Anderson B, Irwin M, Naqvi M. The effects of comfort care on the pain response in preterm infants undergoing screening for retinopathy of prematurity. Retina. 2005;25(1):59–62. doi: 10.1097/00006982-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Marsh VA, Young WO, Dunaway KK, Kissling GE, Carlos RQ, Jones SM, Shocley DH, Weaver NL, Ransom JL, Gal P. Efficacy of topical anesthetics to reduce pain in premature infants during eye examinations for retinopathy of prematurity. Ann Pharmacother. 2005;39(5):829–33. doi: 10.1345/aph.1E476. [DOI] [PubMed] [Google Scholar]
- 20.Mehta M, Mansfield T, Vanderveen DK. Effect of topical anesthesia and age on pain scores during retinopathy of prematurity screening. J Perinatol. 2010;30(11):731–5. doi: 10.1038/jp.2010.36. [DOI] [PubMed] [Google Scholar]
- 21.Gal P, Kissling GE, Young WO, Dunaway KK, Marsh VA, Jones SM, et al. Efficacy of sucrose to reduce pain in premature infants during eye examinations for retinopathy of prematurity. Ann Pharmacother. 2005;39(6):1029–33. doi: 10.1345/aph.1E477. [DOI] [PubMed] [Google Scholar]
- 22.Grabska J, Walden P, Lerer T, Kelly C, Hussain N, Donovan T, et al. Can oral sucrose reduce the pain and distress associated with screening for retinopathy of prematurity. J Perinatol. 2005;25(1):33–5. doi: 10.1038/sj.jp.7211199. [DOI] [PubMed] [Google Scholar]
- 23.Olsson E, Eriksson M. Oral glucose for pain relief during eye examinations for retinopathy of prematurity. J Clin Nurs. 2011;20(7-8):1054–9. doi: 10.1111/j.1365-2702.2010.03529.x. [DOI] [PubMed] [Google Scholar]
- 24.Kandasamy Y, Smith R, Wright IM, Hartley L. Pain relief for premature infants during ophthalmology assessment. J AAPOS. 2011;15(3):276–80. doi: 10.1016/j.jaapos.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Gradin M, Schollin J. The role of endogenous opiods in mediating pain reduction by orally administered glucose among newborns. Pediatrics. 2005;115(4):1004–7. doi: 10.1542/peds.2004-1189. [DOI] [PubMed] [Google Scholar]
- 26.Slater R, Cornelissen L, Fabrizi L, Patten D, Yaxen J, Worley A, Boyd S, Meekt J, Fitzgerald M. Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomized controlled trial. Lancet. 2010;376(9748):1225–32. doi: 10.1016/S0140-6736(10)61303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson DJ, Savulescu J, Slater R. Sugaring the pill. Ethics and uncertainties in the use of sucrose for newborn infants. Arch Pediatr Adolesc Med. 2012;166(7):629–33. doi: 10.1001/archpediatrics.2012.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serpa ABM, Guinsburg R, Balda RCX, Santos AMN, Areco KCN, Peres CA. Multidimensional pain assessment of preterm newborns at the 1st, 3rd and 7th days of life. Sao Paulo Med J. 2007;125(1):29–33. doi: 10.1590/S1516-31802007000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira ALST, Guinsburg R, Almeida MFB, Monteiro AC, Santos AMN, Kopelman BI. Validity of behavioral and physiologic parameters for acute pain assessment of term newborn infants. Sao Paulo Med J. 1999;117(2):72–80. doi: 10.1590/s1516-31801999000200005. [DOI] [PubMed] [Google Scholar]
- 30.Nicolau CM, Pigo JDC, Bueno M, Falcão MC. Avaliação da dor em recém-nascidos prematuros durante a fisioterapia respiratória. Rev Bras Saude Mater Infant. 2008;8(3):285–90. [Google Scholar]