Abstract
OBJECTIVES:
Variations in the prevalence of sex-hormone-related diseases have been observed between Asian ethnic groups living in the same country; however, available data concerning their sex hormone levels are limited. The present study aimed to determine the influence of ethnicity and age on the sex hormone levels of Malay and Chinese men in Malaysia.
METHODS:
A total of 547 males of Malay and Chinese ethnicity residing in the Klang Valley Malaysia underwent a detailed screening, and their blood was collected for sex hormones analyses.
RESULTS:
Testosterone levels were normally distributed in the men (total, free and non-sex hormone-binding globulin (SHBG) bound fractions), and significant ethnic differences were observed (p<0.05); however, the effect size was small. In general, testosterone levels in males began to decline significantly after age 50. Significant ethnic differences in total, free and non-SHBG bound fraction estradiol levels were observed in the 20-29 and 50-59 age groups (p<0.05). The estradiol levels of Malay men decreased as they aged, but they increased for Chinese men starting at age 40.
CONCLUSIONS:
Small but significant differences in testosterone levels existed between Malay and Chinese males. Significant age and race differences existed in estradiol levels. These differences might contribute to the ethnic group differences in diseases related to sex hormones, which other studies have found in Malaysia.
Keywords: Testosterone, Estradiol, Ethnicity, Male
INTRODUCTION
Sex hormones play a vital role in maintaining the health status of men. In addition to sexual function, androgens are important in maintaining bone health (1), muscle strength (2), body composition (2,3) and cognitive status (4) in men. Estrogens are also important determinants of bone health status in men (5,6). Fractures (7), osteoporosis (8), cancers (9) and diabetes (10) are associated with variations in sex hormones. Differences in the prevalence of hormone-related diseases have been observed among distinct ethnic populations living in the same community, including Malaysia (11-13). Many studies have explored the differences in sex hormones levels between African Americans and Caucasians, but their results have proven inconclusive (14-16).
Orwell et al. conducted a large epidemiological study and found significant differences in the sex hormone levels of elderly men from various ethnic backgrounds across a wide geographical range (17). However, data are limited concerning the differences in sex hormone levels among Asian ethnic groups who live in the same country and share similar lifestyles. The present study compared sex hormone levels between Chinese and Malay men in Malaysia because these groups represent the two major ethnicities in Malaysia (92% of the total population; http://www.statistics.gov.my). Furthermore, substantial differences in the prevalence of fracture (11) and prostate cancer (13) between these groups have been recorded. No study has evaluated between-group differences in their sex hormone levels. Bridging this knowledge gap would provide valuable information concerning the sex hormone variation between ethnic groups, which might help to explain the differences in disease prevalence between ethnic groups.
Normative values of sex hormones in men have seldom been evaluated because men lack an abrupt decline in sex hormones, such as menopause in women. However, a decline in the bioavailability of testosterone transpires because of an increase of sex hormone-binding globulin (SHBG) in aging males; this effect might cause certain pathological conditions, such as involutional osteoporosis (18). Testosterone replacement therapy effectively improves the bone density of hypogonadal males who are osteoporotic (19). Data on the normative value of sex hormones in men would help physicians to diagnose androgen deficiencies and decide whether to initiate sex hormone replacement therapy. Information concerning ethnic differences in sex hormone levels might also aid decisions regarding whether different androgen deficiency cutoff points are needed for separate ethnic groups in a multiracial setting.
This study examined the distributions and the ethnic and age-related differences in sex hormones between Malay and Chinese men living in Malaysia who ranged in age from young adults to the elderly. This study extends our previous work on the ethnic differences regarding the sex hormone levels between young Chinese and Malay men in Malaysia (20).
METHODS AND MATERIALS
Design
This cross-sectional study adopted a purposive sampling method. Participants who visited the health screening facility at Universiti Kebangsaan Malaysia were recruited between September 2009 and September 2011. Participants were informed of this screening program via advertisements in major newspapers, radio broadcasts, flyers and public announcements in community and religious centers. The Ethics Committee of Universiti Kebangsaan Malaysia Medical Centre (UKMMC) reviewed and approved the study protocol.
Sample
A total of 840 men visited the osteoporosis screening facility during the study period, and 597 (71.07%) consented to blood collection and were recruited for this study. The participants consisted of Chinese and Malay men aged 20 years or older who resided in the Klang Valley located in central Peninsular Malaysia (Kuala Lumpur, Gombak, Petaling Jaya, Shah Alam and Klang). The participants were screened using a detailed demographic questionnaire that included their medical history. Qualified physicians collected patient histories and basic medical examination. The following exclusion criteria were applied: 1) unable to complete the questionnaire; 2) refused or did not appear for blood collection; 3) diagnosed with major chronic systemic diseases that indicated a change in sex hormone levels, such as osteoporosis, cancers, sexual dysfunction, etc.; or 4) consumed medication known to affect somatic sex hormone levels, such as corticosteroids and testosterone. All of the participants were provided with detailed information concerning the present study, and written consent was obtained from each patient.
Demographic Data
Data regarding age, ethnicity, social background, lifestyle (i.e., smoking status and alcohol intake) and existing medical conditions were collected using a self-administered questionnaire. The participants were advised to bring their previous medical records and their current medical prescriptions to the screening to be examined by qualified physicians.
Body Anthropometric Measurements
The standing height of participants without shoes was determined using a portable stadiometer and recorded to the nearest 0.1 cm. The weight of participants with light clothing was determined using a standardized balance beam scale and recorded to the nearest 0.1 kg. Each participant's body mass index (BMI) was then calculated using the following formula: BMI (kg/m2) = weight (kg)/height squared (m2).
Hormone Measurements
Blood collection was conducted between 8:30 and 10:30 a.m. on the screening day. The participants fasted for at least eight hours prior to the blood collection. During the fasting period, the participants were allowed to consume water but no other beverages or food. Serum was extracted immediately after the blood collection, and a portion of it was sent for total testosterone, total estradiol and albumin assays. The remaining serum was stored in polypropylene tubes at -70 °C for one to three months for sex-hormone binding globulin assays.
A competitive immunoassay with a direct chemiluminescent technique using ADVIA Centaur (Siemens Healthcare Diagnostics, Illinois, USA) was used to evaluate the total testosterone and total estradiol levels. Non-SHBG-bound and free fractions of sex hormones were calculated using the formula introduced by Södergård et al. (21). Albumin was measured using the bromocresol green method with ADVIA 2400 (Siemens Healthcare Diagnostics, Illinois, USA). Solid phase enzyme-linked immunosorbent assay (ELISA) kits (IBL International, Hamburg, Germany) based on the sandwich ELISA principal were used to determine the SHBG level. All of the test principles and procedures were performed according to the manufacturer's instructions. The inter-assay coefficient of variation (CV) for total testosterone, total estradiol, albumin and SHBG assays were 1.79-6.32%, 1.99-2.03%, 1.37-1.70% and 7.2-11.6%, respectively.
Data analyses
A visual inspection of the histogram (plotted as the distribution frequency of sex hormone values) and the Shapiro-Wilk test were used to determine the normality of the data. The SHBG level was square root transformed and reverted to normal. Total, free and non-SHBG-bound fraction estradiol levels were skewed, and no conventional transformation methods were suitable to normalize them. The relationship between the hormonal variables and body anthropometry were analyzed using Pearson's correlation (normal data) or Spearman's correlation (skewed data).
A factorial analysis of variance (ANOVA) was used to assess the effects of normally distributed data, including age (in decades) and ethnicity, on sex hormones. A Kruskal-Wallis H-test was used for skewed data, and post hoc analyses were conducted using multiple Mann-Whitney U-tests with Bonferroni adjustments to reduce Type I errors. The significance threshold of the post hoc analyses was set at p<0.005 for between-age comparisons and p<0.01 for between-ethnicity comparisons within each age group. Otherwise, the significance threshold was set at p<0.05. All of the statistical analyses were conducted using the Statistical Package for Social Sciences Version 16.0 (SPSS Inc., Chicago, USA).
RESULTS
A total of 597 males volunteered for the study and consented to provide their blood for analysis. Three participants were excluded because they did not complete the questionnaire, and the data from 44 participants were excluded because their data lied outside the upper and lower hinges of the outlier analysis. The data of 547 participants (91.63% of the original recruitment) were used for the analysis. In this sample, 313 (57.22%) men were Chinese, and 234 (42.78%) men were Malay. The participants' ages ranged from 20-79 years, with a mean of 45.15 years (SD = 14.91 years). The Mann-Whitney U-test revealed that significant differences did not exist between Chinese and Malay participants with regard to age (p>0.05); however, they differed significantly in height, weight, and BMI (p<0.05). Chinese men were significantly taller and lighter; thus, they had significantly lower BMIs than their Malay counterparts (p<0.05, Table 1).
Table 1.
Sample characteristics.
| Malay | Chinese | Overall | ||
| Mean (SD) | Mean (SD) | Mean (SD) | p-values | |
| Age (years) | 44.09 (17.23) | 45.94 (12.87) | 45.15 (14.91) | n.s. |
| Height (cm) | 165.94 (6.09) | 168.40 (6.31) | 167.35 (6.33) | <0.05 |
| Weight (kg) | 71.82 (13.93) | 69.17 (12.60) | 70.30 (13.24) | <0.05 |
| BMI (kg/m2) | 26.06 (4.65) | 24.35 (3.90) | 25.08 (4.32) | <0.05 |
| T (nmol/l) | 18.19 (5.57) | 18.38 (5.87) | 18.30 (5.74) | n.s. |
| Free T (nmol/l) | 0.39 (0.12) | 0.38 (0.12) | 0.39 (0.12) | n.s. |
| Non-SHBG T (nmol/l) | 11.00 (3.39) | 10.76 (3.35) | 10.86 (3.36) | n.s. |
| Median (IQR) | Median (IQR) | Median (IQR) | p-value | |
| SHBG (nmol/l) | 36.56 (24.76) | 39.44 (25.39) | 38.31 (25.01) | n.s. |
| E (pmol/l) | 86.00 (82.50) | 82.00 (111.50) | 85.00 (99.00) | n.s. |
| Free E (pmol/l) | 2.07 (1.91) | 1.96 (2.54) | 2.03 (2.35) | n.s. |
| Non-SHBG E (pmol/l) | 56.03 (78.00) | 61.78 (53.74) | 58.84 (70.45) | n.s. |
Abbreviations: E = estradiol; SHBG = sex hormone binding globulin; T = testosterone; n.s. = not significant. P indicates the significance value for the differences in the variables tested between the Malay and Chinese participants. Normally distributed data are presented using means and standard deviations (SDs), whereas skewed data are presented using medians and interquartile ranges (IQRs).
Total, free and non-SHBG-bound testosterone levels were normally distributed; however, total, free and non-SHBG-bound estradiol levels assumed a skewed distribution. After excluding the participants with total estradiol levels below the detection limit of the ADVIA Centaur (<30 pmol/L), the distribution of estradiol levels remained skewed. No conventional transformation was able to convert the estradiol levels to a normal distribution. Hence, nonparametric tests were used to analyze the estradiol levels.
Correlations were used to assess the association between the sex hormones and body anthropometric parameters (Table 2). These tests revealed that testosterone and SHBG levels were significantly associated with participant height, weight and BMI, but the estradiol levels were not associated with any anthropometric parameters. Based on these results, the testosterone level analysis was adjusted for BMI, but the estradiol level analysis was not adjusted.
Table 2.
The correlations between sex hormones and body anthropometry.
| Variable | Height | Weight | BMI |
| Total T | 0.096 (0.025) | -0.358 (<0.001) | -0.424 (<0.001) |
| Free T | 0.162 (<0.001) | -0.174 (<0.001) | -0.257 (<0.001) |
| Non-SHBG T | 0.177 (<0.001) | -0.187 (<0.001) | -0.278 (<0.001) |
| Total E | 0.067 (0.118) | -0.054 (0.209) | -0.080 (0.062) |
| Free E | 0.079 (0.063) | 0.030 (0.483) | >0.001 (0.997) |
| Non-SHBG E | 0.087 (0.043) | 0.020 (0.633) | -0.013 (0.765) |
| SHBG | -0.129 (0.02) | -0.361 (<0.001) | -0.328 (<0.001) |
The correlation coefficient and significance value are expressed as r and (p), respectively. Normally distributed data (all T measurements) were analyzed using Pearson's correlation, and skewed data (all E measurements) were analyzed using Spearman's correlation. SHBG was square-root-transformed and analyzed as normally distributed data
After adjusting for BMI, a factorial ANOVA indicated that significant differences existed between the age groups with regard to testosterone (T) levels (p-value for total T = 0.017, free T = <0.001, non-SHBG T = <0.001, SHBG level p<0.001). Marginally significant differences also existed between Chinese and Malay participants with regard to all testosterone measurements (p-value for total T = 0.041, free T = 0.031 and non-SHBG T = 0.035). The post-hoc analyses revealed that the effect sizes for the ethnic-group comparisons were low (total T = 0.008, free T = 0.009 and non-SHBG T = 0.008), and the power was below the generally accepted value (<0.80). Hence, the inter-racial differences between Chinese and Malay participants with regard to testosterone levels might not be clinically significant (Table 3). Significant differences did not exist between the two ethnic groups with regard to SHBG level across all of the age groups (p = 0.788, Table 4).
Table 3.
Testosterone levels between Malay and Chinese participants.
| Ethnicity | Malay | Chinese | Overall | ||||
| Variable | Mean | SD | Mean | SD | Mean | SD | |
| Total T (nmol/l) | 20-29 years | 20.07 | 5.31 | 21.11 | 5.43 | 20.43 | 5.35 |
| 30-39 years | 19.23 | 5.49 | 17.33 a | 6.14 | 17.95 a | 5.97 | |
| 40-49 years | 17.58 | 4.84 | 18.91 | 5.41 | 18.55 | 5.28 | |
| 50-59 years | 17.09 a | 5.59 | 17.87 a | 5.91 | 17.59 a | 5.79 | |
| ≥60 years | 16.68 a | 5.78 | 17.01 a | 6.13 | 16.82 a | 5.91 | |
| Overall | 18.19 | 5.57 | 18.38 | 5.87 | 18.30 | 5.74 | |
| Free T (nmol/l) | 20-29 years | 0.43 | 0.11 | 0.46 | 0.11 | 0.44 | 0.11 |
| 30-39 years | 0.41 | 0.11 | 0.38 a | 0.11 | 0.39 a | 0.11 | |
| 40-49 years | 0.41 | 0.12 | 0.40 | 0.11 | 0.41 | 0.11 | |
| 50-59 years | 0.38 | 0.12 | 0.36 a, c | 0.11 | 0.36 a, c | 0.11 | |
| ≥60 years | 0.33 a, b, c | 0.11 | 0.32 a, b, c | 0.10 | 0.32 a, b, c | 0.11 | |
| Overall | 0.39 | 0.12 | 0.38 | 0.12 | 0.39 | 0.12 | |
| Non-SHBG T (nmol/l) | 20-29 years | 12.65 | 3.15 | 13.41 | 3.34 | 12.92 | 3.23 |
| 30-39 years | 11.67 | 2.89 | 10.85 a | 3.14 | 11.12 a | 3.06 | |
| 40-49 years | 11.39 | 3.26 | 11.40 a | 3.10 | 11.40 a | 3.13 | |
| 50-59 years | 10.27 a | 3.13 | 9.90 a, c | 3.07 | 10.03 a, c | 3.08 | |
| ≥60 years | 8.97 a, b, c | 3.03 | 8.58 a, b, c | 2.71 | 8.80 a, b, c, d | 2.89 | |
| Overall | 11.00 | 3.39 | 10.76 | 3.35 | 10.86 | 3.36 | |
Significant differences in sex hormones were tested within each age group and indicated using the following designations: “a” indicates significant differences (p<0.05) between the relevant age group and the 20-29 year group; “b” compares the relevant age group with the 30-39 year group; “c” compares the relevant age group with the 40-49 year group, and “d” compares the relevant age group with the 50-59 year group.
Table 4.
SHBG and estradiol levels between the ethnic groups.
| Ethnicity | Malay | Chinese | Overall | ||||
| Variable | Median | IQR | Median | IQR | Median | IQR | |
| SHBG (nmol/l) | 20-29 years | 35.49 | 21.76 | 32.09 | 22.29 | 32.93 | 21.54 |
| 30-39 years | 37.03 | 21.83 | 31.96 | 22.76 | 35.37 | 21.80 | |
| 40-49 years | 27.23 | 22.59 | 37.46 | 21.79 | 36.29 | 21.80 | |
| 50-59 years | 36.21 | 19.76 | 44.19 a, b, c | 28.11 | 40.93 a, b, c | 25.34 | |
| ≥60 years | 45.98 a, c | 32.92 | 52.07 a, b, c | 22.07 | 49.07 a, b, c, d | 27.47 | |
| Overall | 36.56 | 24.76 | 39.44 | 25.39 | 38.31 | 25.01 | |
| Total E (pmol/l) | 20-29 years | 135.00 | 82.25 | 92.50 | 106.50 | 121.00 | 82.25 |
| 30-39 years | 85.00 a | 60.00 | 59.00 | 116.00 | 66.00 a | 94.75 | |
| 40-49 years | 63.50 a | 75.00 | 75.00 | 103.00 | 69.50 a | 104.25 | |
| 50-59 years | 63.50 a | 53.75 | 116.00 b | 113.00 | 82.00 | 108.00 | |
| ≥60 years | 68.00 a | 61.00 | 91.00 | 120.00 | 75.00 | 80.75 | |
| Overall | 86.00 | 82.50 | 82.00 | 111.50 | 85.00 | 99.00 | |
| Free E (pmol/l) | 20-29 years | 2.95 | 1.95 | 2.52 | 2.43 | 2.72 | 2.13 |
| 30-39 years | 2.15 | 1.80 | 1.48 | 2.88 | 1.75 a | 2.17 | |
| 40-49 years | 1.58 a | 1.99 | 1.77 | 2.39 | 1.67 a | 2.42 | |
| 50-59 years | 1.74 a | 1.59 | 2.40 | 2.73 | 1.90 | 2.44 | |
| ≥60 years | 1.71 a | 1.56 | 2.21 | 2.87 | 1.80 | 1.88 | |
| Overall | 2.07 | 1.91 | 1.96 | 2.54 | 2.03 | 2.35 | |
| Non-SHBG E (pmol/l) | 20-29 years | 88.80 | 59.93 | 72.00 | 80.16 | 84.91 | 65.89 |
| 30-39 years | 63.59 a | 48.44 | 44.48 | 82.62 | 51.67 a | 63.98 | |
| 40-49 years | 45.52 a | 54.07 | 52.26 | 71.12 | 49.11 a | 71.78 | |
| 50-59 years | 45.31 a | 45.28 | 69.55 | 76.35 | 55.17 a | 69.79 | |
| ≥60 years | 46.68 a | 43.06 | 58.80 | 84.66 | 49.87 a | 50.69 | |
| Overall | 56.03 | 78.00 | 61.78 | 53.74 | 58.84 | 70.45 | |
Significant differences in sex hormones were tested within each age group and indicated using the following designations: “a” indicates significant differences (p<0.05) between the relevant age group and the 20-29 year group; “b” compares the relevant age group with the 30-39 year group; “c” compares the relevant age group with the 40-49 year group, and “d” compares the relevant age group with the 50-59 year group.
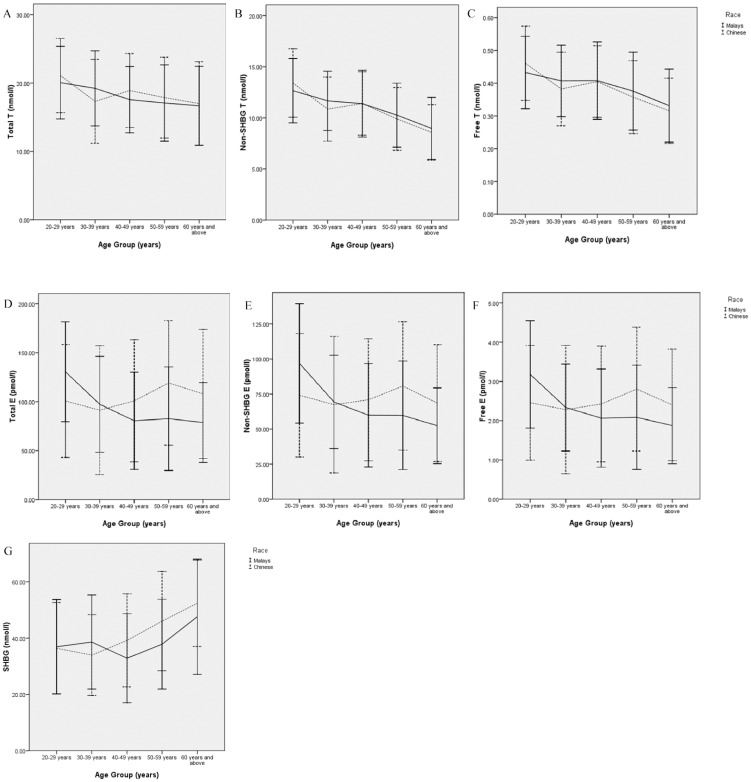
In general, the participants' testosterone levels decreased with age. This decline was most apparent with regard to non-SHBG-bound testosterone levels (Table 3, Figures 1A-1C). Likewise, the participants' SHBG levels increased with age, and this growth was most apparent in the group aged over 50 years (Table 4, Figure 1G).
Figure 1.
Age-trend of sex hormone and sex hormone binding globulin levels in Malay and Chinese men in Malaysia. All values are plotted as mean (standard deviation). Significances between ethnicity and among age groups are not indicated on the line graphs, but in (Tables 3 & 4). Abbr: SHBG = sex hormone binding globulin; T = testosterone; Free T = free testosterone; Non-SHBG T = non-SHBG-bound testosterone; E = estradiol; Free E = free estradiol; Non-SHBG E = non-SHBG-bound estradiol.
Although adjustments for confounds were not performed for the estradiol value comparisons, Bonferroni adjustments for significant values were applied to avoid Type I error inflation caused by multiple between-group comparisons. Total, free and non-SHBG-bound estradiol (E) levels did not significantly differ between Chinese and Malay participants aged 30-39 years (p-value for total E = 0.173, free E = 0.276 and non-SHBG E = 0.230), 40-49 years (p-value for total E = 0.135, free E = 0.228 and non-SHBG E = 0.222) and ≥60 years (p-value for total E = 0.066, free E = 0.123 and non-SHBG E = 0.105); however, significant differences existed in participants aged 20-29 years (p-value for total E = 0.007, free E = 0.006 and non-SHBG E = 0.006) and 50-59 years (p-value for total E = 0.001, free E = 0.011 and non-SHBG E = 0.008). Calculations indicated that these differences were associated with large effect sizes (0.49-4.91) and sufficiently high power (0.69-1.00). The estradiol levels of Malay men were greater than their Chinese counterparts for participants aged 20-39 years. However, the estradiol levels of Chinese men were greater than their Malay counterparts for the participants ≥40 years (Table 4, Figures 1D-1F).
A Kruskal Wallis H-test revealed that significant overall differences existed among the age groups with regard to estradiol levels (p-values for all estradiol measurements <0.001). A specific age pattern was not observed with regard to the estradiol levels; nevertheless, participants aged 20-29 years had significantly greater estradiol levels compared with older participants (p<0.05). After segregating the data by ethnicity, a distinct trend was observed between the Malay and Chinese participants. The estradiol levels of Malay men gradually decreased as they aged; however, these levels began to increase in Chinese men in their forties and peaked between 50-59 years of age before marginally declining after age 60 (Table 4, Figures 1D-1F). An omnibus effect size and power analysis was not conducted for the Kruskal Wallis H-test because the statistical software used was limited.
DISCUSSION
Variations in sex hormone levels are associated with an increased risk for disease. Grossman et al. found that testosterone deficiency was common among diabetic men regardless of the type of diabetes (10). Tsai et al. reported that significant inverse associations existed between testosterone and fasting insulin levels (22). Kuchuk et al. observed that reduced bioavailable estradiol was independently associated with increased fracture risk in men, and reduced bioavailable testosterone was an age-dependent risk factor (1). Meier et al. reported similar findings; specifically, reduced serum testosterone was associated with increased fracture risk in men (23). Gann et al. indicated that increased testosterone and reduced SHBG levels were associated with an increased risk of prostate cancer (9).
In Malaysia, ethnic differences have been observed in the prevalence of hormone-related diseases. Chinese men have an increased incidence of hip fracture compared with Malay men after age 50. The National Cancer Registry (2007) reported that Chinese men have a greater prevalence of prostate cancer than Malay men in Malaysia (13). However, data concerning the between-ethnic-group differences in sex hormones are lacking; hence, inferences cannot be drawn regarding the relationship between variations in sex hormones and disease prevalence differences between ethnic groups.
Differences in sex hormone levels among males of disparate ethnicities have been explored in Western countries, particularly between Caucasians and African Americans. In a large cross-sectional study, Rohrmann et al. found that the estradiol levels of non-Hispanic blacks were significantly greater than that of non-Hispanic Whites; however, no differences were observed with regard to testosterone levels (15). These findings differed from the observation of Winters et al. who found that African American men had significantly greater testosterone levels compared with Caucasians in a small-scale cross-sectional study; however, the differences in estradiol levels were not significant (16).
In the present study, the testosterone levels between Chinese and Malay men were marginally (but significantly) different. However, this result might not be clinically important because the effect size was small. A separate normative testosterone value for Chinese and Malay men living in Malaysia might not be necessary, particularly when evaluating androgen deficiency. In general, testosterone (particularly the non-SHBG-bound and free fraction levels in both ethnic groups) decreased with age. The decline in non-SHBG-bound and free fraction levels was more obvious than that of total testosterone because the SHBG levels increased in men as they aged. Other researchers have demonstrated this finding (24). Increased SHBG levels in normal young men are usually followed by an increase in testosterone level. However, this feedback mechanism is not as efficient in elderly males because the testosterone production of the testes is compromised. Consequently, the bioavailability of testosterone is reduced in elderly men (18,25). The age-dependent increases in SHBG have been linked to decreases in growth hormone and insulin-like growth factor 1, but these relationships have not been validated (25-27).
The estradiol levels of the current study population present a more complex scenario. Estradiol levels declined as Malay participants aged; however, they increased beginning at age 40 and continued for Chinese participants as they aged. The estradiol levels of the Malay participants were greater than the Chinese men aged 20-39 years; however, the estradiol levels of the Chinese men were greater after age 40. The exact reason for this discrepancy is unknown. Bjørnerem et al. discovered a positive association between age and total and free fraction estradiol levels in men. A 33% increase in total estradiol and a 12% increase in free estradiol were observed in men aged >70 years compared with men <40 years (28). Ferrini and Barrett-Connor observed a decline in total estradiol as men aged (29). Orwell et al. confirmed this result by finding an inverse correlation between age and the total and free fraction estradiol levels in men ≥65 years (30).
In the present study, the increased levels of estradiol in young Malay men may help to explain their lower incidence of hip fracture compared with Chinese men. Because estradiol has beneficial effects on bone, Malay men might be helped by achieving greater peak bone mass during their 20s. The greater total testosterone and SHBG levels after the age of 40 among Chinese participants might help to explain why they have an increased prevalence of prostate cancer compared with Malays in Malaysia. This observation agrees with Gann et al., as described above: high levels of total testosterone and SHBG were associated with a greater risk of prostate cancer (9). However, these opinions remain speculative. To establish the relationship between sex hormones and the risk of disease, a prospective cohort study must be conducted.
This study has several limitations that must be considered when interpreting the presented results. This study adopted a purposive, non-randomized sampling method, and substantial selection bias might have been introduced during recruitment. The sample consisted of Chinese and Malay males; therefore, the results might not reflect the sex hormones of other minorities in Malaysia. The detection limit of total estradiol levels was 30 pmol/l; thus, the non-SHBG-bound and free fraction levels of participants with total estradiol levels below this cutoff were not accurately depicted. Varicocele is a significant risk factor for hypogonadism. Tanrikut et al. indicated that men with varicocele had significantly lower testosterone levels compared with a control group (31); however, this condition was not considered during the screening of participants in the present study. Thus, the prevalence of varicocele and its effects on the sex hormone levels of the sample are unknown.
In conclusion, small but significant differences exist in the testosterone levels between Malay and Chinese men in Malaysia. Significant differences in estradiol levels were found in specific age groups. Testosterone levels gradually declined as men aged, but different age trends in estradiol levels were observed between Malay and Chinese men. Differences in the sex hormone variations between Malay and Chinese men might help to explain the ethnic differences in the prevalence of hormone-related diseases.
ACKNOWLEDGMENTS
We thank Universiti Kebangsaan Malaysia for providing a research fund and the Pharmacoepidemiology and Drug Safety Unit for providing statistical consultation. This project received financial support from the Arus Perdana Grant (UKM-AP-TKP-09-2009) and a postgraduate research grant (FF-376-2010) from Universiti Kebangsaan Malaysia Medical Centre.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Kuchuk NO, Van Schoor NM, Pluijm SMF, Smit JH, De Ronde W, Lips P. The association of sex hormone levels with quantitative ultrasound, bone mineral density, bone turnover and osteoporotic fractures in older men and women. Clin Endocrinol. 2007;67(2):295–303. doi: 10.1111/j.1365-2265.2007.02882.x. [DOI] [PubMed] [Google Scholar]
- 2.van den Beld AW, de Jong FH, Grobbee DE, Pols HAP, Lamberts SWJ. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85(9):3276–82. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- 3.Svartberg J, von Muhlen D, Sundsfjord J, Jorde R. Waist circumference and testosterone levels in community dwelling men. The tromso study. Eur J Epidemiol. 2004;19(7):657–63. doi: 10.1023/b:ejep.0000036809.30558.8f. [DOI] [PubMed] [Google Scholar]
- 4.Beauchet O. Testosterone and cognitive function: Current clinical evidence of a relationship. Eur J Endocrinol. 2006;155(6):773–81. doi: 10.1530/eje.1.02306. [DOI] [PubMed] [Google Scholar]
- 5.Araujo AB, Travison TG, Leder BZ, McKinlay JB. Correlations between serum testosterone, estradiol, and sex hormone-binding globulin and bone mineral density in a diverse sample of men. J Clin Endocrinol Metab. 2008;93(6):2135–41. doi: 10.1210/jc.2007-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khosla S, Melton LJ, 3rd, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: A key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83(7):2266–74. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 7.LeBlanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett-Connor E, Ensrud KE, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab. 2009;94(9):3337–46. doi: 10.1210/jc.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paller CJ, Shiels MS, Rohrmann S, Basaria S, Rifai N, Nelson W, et al. Relationship of sex steroid hormones with bone mineral density (bmd) in a nationally representative sample of men. Clin Endocrinol. 2009;70(1):26–34. doi: 10.1111/j.1365-2265.2008.03300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88(16):1118–26. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 10.Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, MacIsaac RJ, Clarke S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93(5):1834–40. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 11.Lee JK, Khir ASM. The incidence of hip fracture in malaysians above 50 years of age: Variation in different ethnic groups. APLAR Journal of Rheumatology. 2007;10(4):300–5. [Google Scholar]
- 12.Letchuman GR, Wan Nazaimoon WM, Wan Mohamad WB, Chandran LR, Tee GH, Jamaiyah H, et al. Prevalence of diabetes in the malaysian national health morbidity survey iii 2006. Med J Malaysia. 2010;65(3):180–6. [PubMed] [Google Scholar]
- 13.Zainal AriffinO, Nor SalehaIT. National cancer registry report: Malaysia cancer statistics - data and figure 2007. Putrajaya: Ministry of Health Malaysia; 2011. [Google Scholar]
- 14.Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB. Serum androgen levels in black, hispanic, and white men. J Clin Endocrinol Metab. 2006;91(11):4326–34. doi: 10.1210/jc.2006-0037. [DOI] [PubMed] [Google Scholar]
- 15.Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92(7):2519–25. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 16.Winters SJ, Brufsky A, Weissfeld J, Trump DL, Dyky MA, Hadeed V. Testosterone, sex hormone-binding globulin, and body composition in young adult african american and caucasian men. Metabolism. 2001;50(10):1242–7. doi: 10.1053/meta.2001.26714. [DOI] [PubMed] [Google Scholar]
- 17.Orwoll ES, Nielson CM, Labrie F, Barrett-Connor E, Cauley JA, Cummings SR, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab. 2010;95(10):E151–60. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 19.Behre HM, Kliesch S, Leifke E, Link TM, Nieschlag E. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997;82(8):2386–90. doi: 10.1210/jcem.82.8.4163. [DOI] [PubMed] [Google Scholar]
- 20.Chin K, Soleiman I, Mohamed I, Johari H, Wan Ngah W. Ethnicity, smoking and body composition influence testosterone and estradiol levels in healthy young adult men in malaysia: A pilot study. Int J Endocrinol Metab. 2012;10(1):410–6. [Google Scholar]
- 21.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 22.Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance. Diabetes Care. 2004;27(4):861–8. doi: 10.2337/diacare.27.4.861. [DOI] [PubMed] [Google Scholar]
- 23.Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM, Rockwood AL, et al. Endogenous sex hormones and incident fracture risk in older men: The dubbo osteoporosis epidemiology study. Arch Intern Med. 2008;168(1):47–54. doi: 10.1001/archinternmed.2007.2. [DOI] [PubMed] [Google Scholar]
- 24.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26(6):833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 26.Erfurth EM, Hagm ar L, Sääf M, Hall K. Serum levels of insulin-like growth factor i and insulin-like growth factor-binding protein 1 correlate with serum free testosterone and sex hormone binding globulin levels in healthy young and middle-aged men. Clin Endocrinol. 1996;44(6):659–64. doi: 10.1046/j.1365-2265.1996.731552.x. [DOI] [PubMed] [Google Scholar]
- 27.Pfeilschifter J, Scheidt-Nave C, Leidig-Bruckner G, Woitge HW, Blum WF, Wüster C, et al. Relationship between circulating insulin-like growth factor components and sex hormones in a population-based sample of 50- to 80-year-old men and women. J Clin Endocrinol Metab. 1996;81(7):2534–40. doi: 10.1210/jcem.81.7.8675573. [DOI] [PubMed] [Google Scholar]
- 28.Bjørnerem Å, Straume B, Midtby M, Fønnebø V, Sundsfjord J, Svartberg J, et al. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: The tromsø study. J Clin Endocrinol Metab. 2004;89(12):6039–47. doi: 10.1210/jc.2004-0735. [DOI] [PubMed] [Google Scholar]
- 29.Ferrini RL, Barrett-Connor E. Sex hormones and age: A cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147(8):750–4. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 30.Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab. 2006;91(4):1336–44. doi: 10.1210/jc.2005-1830. [DOI] [PubMed] [Google Scholar]
- 31.Tanrikut C, Goldstein M, Rosoff JS, Lee RK, Nelson CJ, Mulhall JP. Varicocele as a risk factor for androgen deficiency and effect of repair. BJU Int. 2011;108(9):1480–4. doi: 10.1111/j.1464-410X.2010.10030.x. [DOI] [PubMed] [Google Scholar]