SUMMARY
Approximately 10% of patients with chronic lymphocytic leukaemia (CLL) have a family history of the disease or a related lymphoproliferative disorder, yet the relationship of familial CLL to genomic abnormalities has not been characterized in detail. We therefore studied 75 CLL patients, half familial and half sporadic, using high-resolution array comparative genomic hybridization (CGH), in order to better define the relationship of genomic abnormalities to familial disease and other biological prognostic factors. Our results showed that the most common high-risk deletion in CLL, deletion 11q, was significantly associated with sporadic disease. Comparison of familial to sporadic disease additionally identified a copy number variant region near the centromere on 14q, proximal to IGH@, in which gains were associated both with familial CLL, and with mutated IGHV and homozygous deletion of 13q. Homozygous deletion of 13q was also found to be associated with mutated IGHV and low expression of ZAP-70, and a significantly longer time to first treatment compared to heterozygous deletion or lack of alteration. This study is the first high resolution effort to investigate and report somatic genetic differences between familial and sporadic CLL.
Keywords: CLL, familial, deletion 11q
INTRODUCTION
One of the strongest biological predictors of survival in chronic lymphocytic leukaemia (CLL) is the presence or absence of certain chromosomal abnormalities identifiable by fluorescence in situ hybridization (FISH), first described by Dohner et al (2000) who proposed a hierarchical classification . The highest risk abnormality was found to be loss of 17p13, followed by loss of 11q22, both of which were associated with shorter time to treatment and overall survival (Dohner, et al 2000, Fegan, et al 1995, Geisler, et al 1997, Neilson, et al 1997). Trisomy 12 has also been associated with advanced stage disease and shorter survival time (Matutes, et al 1996), but was found to have an overall survival similar to a normal FISH analysis (Dohner, et al 2000). The most common chromosomal abnormality, seen in approximately half of all CLL cases, is deletion of 13q14, which is associated with a favourable prognosis in the absence of other abnormalities (Juliusson, et al 1990).
Despite the identification of these chromosome abnormalities and their clear association with survival, the mechanism by which these abnormalities contribute to disease causation remains largely unknown. Although several likely candidate genes have been identified, including TP53 in 17p13 (Fenaux, et al 1992, Gaidano, et al 1991, Stankovic, et al 1999) and ATM in 11q22.3 (Stankovic, et al 1999), their role in the pathogenesis of CLL has not been elucidated. The potential function of the micro-RNA genes MIR15A and MIR16-1 (Calin, et al 2002, Grubor, et al 2009, Tyybakinoja, et al 2007), located at 13q14, is better understood, given their ability to target BCL-2 (Cimmino, et al 2005).
One of the major risk factors for developing CLL is a family history of CLL or another B cell malignancy (Cuttner 1992, Ishibe, et al 2001). In fact familial CLL occurs in 10-15% of all cases (Brown, et al 2008, Yuille, et al 2000). Despite this, most work in familial CLL to date has been epidemiological or has focused on small family studies investigating primarily germline traits. Relatively little work has focused on studying familial CLL patients and their disease biology in an effort to identify pathogenetic mechanisms that could be unique or shared with sporadic CLL. In particular, very little data exists on the association between familial CLL and the occurrence of particular chromosome abnormalities. The advent of highly sensitive array-based genome-wide technologies for identifying genomic abnormalities is allowing fine mapping of both previously known and newly identified genomic copy number changes associated with CLL (Gunn, et al 2008, Gunnarsson, et al 2008, Kujawski, et al 2008, Pfeifer, et al 2007). Two studies have further sought to delineate additional abnormalities associated with deletion of 17p specifically (Forconi, et al 2008, Rudenko, et al 2008). Our objective was to characterize the genomic changes associated with familial and sporadic CLL as a first step towards addressing their pathogenetic mechanisms. To accomplish this objective, we performed array Comparative genomic hybridization (CGH) using Agilent 244k oligonucleotide CGH arrays on two groups of patients, a cohort of unrelated CLL patients with no family history of lymphoproliferative disorders, and a cohort of patients with a familial history of CLL or lymphoma.
MATERIALS AND METHODS
CLL patients
A total of 75 CLL DNA specimens were used in this study. Consecutive familial and sporadic cases were used, in order to balance the numbers of each. Forty-seven (47) were obtained from Dana Farber Cancer Institute (DFCI) tissue banking protocols. One of these tissue banks (#99-224) enrolls any individual seen at DFCI who is 18 years or older and has CLL/small lymphocytic leukaemia (SLL). The other tissue bank (#04-165) enrolls any individual 18 years or older who has a lymphoproliferative disorder (LPD; defined as CLL/SLL, any non-Hodgkin lymphoma [NHL] or Hodgkin lymphoma [HL]) and at least one first degree relative also with a lymphoproliferative disorder (Brown, et al 2008). Both tissue banking protocols have been approved by the DFCI Institutional Review Board, and all subjects sign informed consent prior to participation. All subjects were screened for a family history of a LPD using a dedicated questionnaire (Brown, et al 2008). In previous work we have validated the accuracy of this questionnaire by obtaining pathology reports (Brown, et al 2008). Our previous study and others have described the co-occurrence of CLL and lymphomas within families, and we therefore included these families in our definition of familial disease (Brown, et al 2008, Mauro, et al 2006). Subjects with an ambiguous family history, for example a history of leukaemia not otherwise specified, were excluded from either group. The remaining 28 samples, deliberately chosen to be half familial (again, defined as at least one affected first degree relative with LPD) and half sporadic (no family history of LPD) and approximately evenly divided by IGHV mutational status and ZAP-70 status, were provided by the CLL Research Consortium Tissue Bank (La Jolla, CA, USA). Only one affected individual from a given family was included in the study.
Sample Processing
Peripheral blood mononuclear cells were isolated by density centrifugation through Ficoll and viably frozen for each DFCI participant. Samples provided by the CLL Research Consortium were shipped overnight to San Diego from the participating sites, separated through Ficoll and viably frozen. DNA was then isolated at DFCI using the QIAamp DNA Blood Maxi Kit (Qiagen, Valencia, CA, USA) and preserved at -20°C until use.
Characterization of CLLs
CD38 expression was determined by routine flow cytometry and considered positive if expressed by > 30% of CLL cells. IGHV homology (unmutated defined as greater than or equal to 98% homology to the closest germline match) and ZAP-70 expression (positive defined as >20%) were determined by the CLL Research Consortium tissue core as previously described (Rassenti, et al 2004). Cytogenetics were evaluated by FISH for the most common abnormalities (del 13q, trisomy 12, del 11q, del 17p, 14 rearrangements) at DFCI (Dohner, et al 2000) or at the participating sites of the CLL Research Consortium for the samples obtained from the CLL Research Consortium.
Array-CGH
Array-CGH analysis was performed on 38 familial CLL samples and 37 sporadic CLL samples using Agilent's Human Genome CGH Microarray Kit 244A (Agilent Technologies, Santa Clara, CA, USA). The array for 244k consists of ~244,000 60-mer oligonucleotide probes that span both coding and non-coding sequences with an average spatial resolution of ~35Kb. Commercial male DNAs (Promega, Madison, WI) were used as a reference DNA with the same protocol. All DNAs were quantitated with a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA).
Briefly, 500ng of genomic reference or patient DNA was digested with AluI and RsaI (Invitrogen, Carlsbad, CA) or sonication. The reference and test DNAs were labeled with Cy3- and Cy5-dCTP (Perkin Elmer, Boston, MA, USA) by Bioprime Array CGH Genomic Labeling kit (Invitrogen). Following the labelling reaction, the individually labelled test and reference samples were combined and concentrated using Microcon YM-30 filters (Millipore, Billerica, MA, USA). The hybridization mixture contained the labeled DNAs, 2x hybridization buffer, 10x blocking agent (Agilent Technologies), and human Cot-1 DNA (Invitrogen). Both labeled DNAs were simultaneously hybridized onto the microarray slides for 40 h at 37°C. The array slides were washed and then scanned using an Agilent DNA microarray scanner (Agilent Technologies). For each patient sample, dye-swap experiments were performed with reversal of the dye. Microarray images were extracted using feature extraction software (Version 9.2, Agilent Technologies) and data were analysed using Nexus Copy Number software from BioDiscovery (El Segundo, CA, USA).
Data Analysis
Nexus 3.1 software was used to detect the most common regions in multiple samples. The probe coordinates on the Agilent 244K array were remapped to the National Center for Biotechnology Information human genomic hg18 build using a liftOVER PERL script. Analysis was carried out with this version of the dataset. The Rank Segmentation algorithm was used for detection of regions of copy number change. The significance threshold was set at 1.0-6. In order to define segments, we considered a minimum of 5 contiguous probes and the maximum contiguous probe spacing was set to 1000kb. Thresholds were set at log2 ratios of 0.2, 0.5, -0.3 and -1.0 to call gain, high copy gain, one copy loss and homozygous (two-copy) loss. The threshold for two-copy loss was chosen to be very stringent in order to minimize the effects of mosaicism. Any significant findings were manually reviewed.
All significant findings detected by the Nexus 3.1 software are reported, except for copy number variants (CNVs), which were filtered out [based on Database of Genomic Variants (projects.tcag.ca/variation)] unless they were located in a region of known cytogenetic interest in CLL. Table S1 shows those abnormalities that were both present in 2% or more of the samples and significant based on a STAC frequency p value less than 0.05. This p value was calculated according to Diskin et al (2006) using the Nexus Copy Number software.
Statistical Analysis
Descriptive statistical analysis was performed to compare the sporadic and familial subgroups. The two-sided Fisher Exact test was used for 2 × 2 table analysis and the two-sided Wilcoxon-Rank-Sum test was used for two-sample comparison of continuous variables. The relationship between chromosomal abnormalities identified by CGH and categorical prognostic factors was assessed using Fisher's exact test. Analyses of predictors of timing of treatment took into account the timing of sampling in relation to first treatment, dividing subjects into never treated, treated prior to sampling or treated after sampling. The method of Kaplan and Meier was used to estimate the duration of time from diagnosis to first treatment based on a given potential predictor, and log rank p values were calculated. Correction for multiple comparisons was not performed as this study was a small exploratory study designed to be hypothesis-generating.
RESULTS
Patient Characteristics
The baseline characteristics of the patient cohort are outlined in Table 1. 75 patients were analysed, of whom 38 had familial CLL and 37 had sporadic CLL. All patients characterized as familial had at least one affected first-degree relative, 31 with CLL (82%), 6 with NHL (16%) and one with HL (2%). Those patients with familial CLL had a younger age at diagnosis(Ishibe, et al 2001) and a longer time to sample collection, but were otherwise similar to the sporadic patients in terms of clinical and biological parameters.
TABLE 1.
Patient Characteristics
| Familial (N = 38) | Sporadic (N = 37) | P-value | |
|---|---|---|---|
| Age at diagnosis (years) | 51 (41-76) | 56 (36-71) | 0.03 |
| Sex | |||
| Female | 17 (45%) | 12 (32%) | 0.35 |
| Male | 21 | 25 | |
| Rai Stage at Sampling | |||
| Low (0) | 6 | 8 | 0.77 |
| Intermediate (1-2) | 19 | 15 | |
| High (3-4) | 11 | 9 | |
| Time from diagnosis to Sample (months) | 61 (25-99) | 39 (15-64) | 0.004 |
| Time from diagnosis to Therapy (months) | 51 (30-94) | 50 (17-74) | 0.13 |
| Previously Treated at Sampling | 8 (21%) | 5 (14%) | 0.26 |
| Untreated at Sampling | 30 (79%) | 32 (86%) | |
| Treated Since Sampling | 9 | 15 | |
| IGHV Mutational Status | |||
| Unmutated | 14 (37%) | 21 (57%) | 0.24 |
| Mutated, <98% | 20 (53%) | 16 (43%) | |
| ZAP-70 Expression | |||
| Positive (>20%) | 14 (37%) | 16 (43%) | 1.0 |
| Negative (20% or less) | 18 | 20 | |
| CD38 Expression | |||
| Positive (30% or more) | 11 (29%) | 11 (30%) | 1.0 |
| Negative (less than 30%) | 24 | 25 | |
| Interphase FISH (n= 56) | |||
| Del 17p | 6 | 8 | |
| Del 11q | 2 | 10 | |
| Trisomy 12 | 5 | 4 | |
| Normal | 7 | 5 | |
| Del 13q | 10 | 15 | |
| Homozygous del 13q | 8 | 2 | |
| Multiple | 9 | 12 |
FISH, fluorescent in situ hybridization
Correspondence of FISH and CGH
Table 1 shows the chromosome abnormalities identified by FISH in the familial and sporadic patients. Table 2 shows the abnormalities identified by CGH. Of the 56 samples with complete FISH results, 44 had completely concordant FISH and CGH results, for an overall concordance of 79% (90% CI 68-87%). Ten of the twelve discordant samples were discordant because of mosaic lesions present in 5-27% of cells by FISH, a level previously found to be poorly detected by CGH (Gunn, et al 2008, Kujawski, et al 2008). Eight of these twelve discrepant cases had higher frequency abnormalities identified by FISH that were also identified by CGH. Thus most of the discrepant cases resulted from abnormalities found in a subset of cells by FISH that were probably below the threshold of array CGH detection.
TABLE 2.
CGH Summary
| N | % of Total | Familial | Sporadic | P values | |
|---|---|---|---|---|---|
| 2p Gain | 3 | 4% | 0 | 3 | |
| 2p Loss | 39 | 52% | 16 | 23 | 0.11 |
| 4p Loss | 3 | 4% | 0 | 3 | |
| 4q Loss | 2 | 3% | 1 | 1 | |
| 6q Loss | 5 | 7% | 1 | 4 | 0.2 |
| 8p Loss | 2 | 3% | 1 | 1 | |
| 10q Loss | 3 | 4% | 3 | 0 | |
| 11q Loss | 10 | 13% | 1 | 9 | 0.007 |
| 12 Gain | 14 | 19% | 8 | 6 | 0.77 |
| 13q Loss | 40 | 53% | 20 | 20 | 0.15 |
| Heterozygous | 26 | 35% | 10 | 16 | |
| Homozygous | 14 | 19% | 10 | 4 | |
| 14q11.2 Gain | 11 | 15% | 7 | 4 | 0.52 |
| 14q Region 1 | 0.02 | ||||
| Gain | 6 | 8% | 6 | 0 | |
| Loss | 13 | 17% | 4 | 9 | |
| 14q Region 2 Loss | 2 | 3% | 1 | 1 | |
| 14q Region 3 Loss | 67 | 89% | 36 | 31 | 0.26 |
| Heterozygous | 56 | 75% | 29 | 27 | |
| Homozygous | 11 | 15% | 7 | 4 | |
| 15q Loss | 3 | 4% | 2 | 1 | |
| 17p Loss | 4 | 5% | 2 | 2 | |
| 17q Gain | 2 | 3% | 1 | 1 | |
| 18p Loss | 2 | 3% | 0 | 2 | |
| 19q Gain | 2 | 3% | 2 | 0 | |
| 21p Gain | 6 | 8% | 1 | 5 | 0.09 |
Overview of Chromosome Abnormalities Identified
The frequency of the common chromosomal abnormalities normally identified by FISH was as expected in our patient population using CGH, 53% del 13q, 19% trisomy 12, 13% del 11q and 5% del 17p (Table 2 and Figure S1). In addition, five patients (7%) showed loss of 6q, which has been previously described in CLL (Grubor, et al 2009). Three of these five patients had large deletions. Three patients also showed gain of 2p16, including the REL and BCL11 genes, which has also been described in CLL using CGH (Pfeifer, et al 2007). Three patients showed loss of 4p, and loss of 2p11.2 was seen in 73% of patients (55 of 75), probably corresponding to the kappa immunoglobulin light chain locus. Three regions of abnormality were noted in 14q, with region 1 (at 14q11.1 – q11.2) showing gains in six patients (8%) and losses in 13 (17%) patients; region 2 (at 14q24.1 - 32.33) showing losses in two (3%) patients; and region 3 (at 14q32.33) showing loss in 67 (89%) patients. Region 1 has not been reported previously in association with CLL. The losses in region 3 are probably related to rearrangements of IGHV, as has been suggested previously (Gunn, et al 2008). Rare losses were seen in 4q, 8p, 9q, 18p, 20p and 21p, with rare gains in 8q, 9q and 21p (Figure S1, Table 2, Table S2). The genes mapping to the genomic alterations and their minimal regions are shown in Table S3. The frequency distribution of the number of abnormalities found in each sample is depicted in Figure S2.
Overview of Abnormalities Associated with Familial and Sporadic CLL
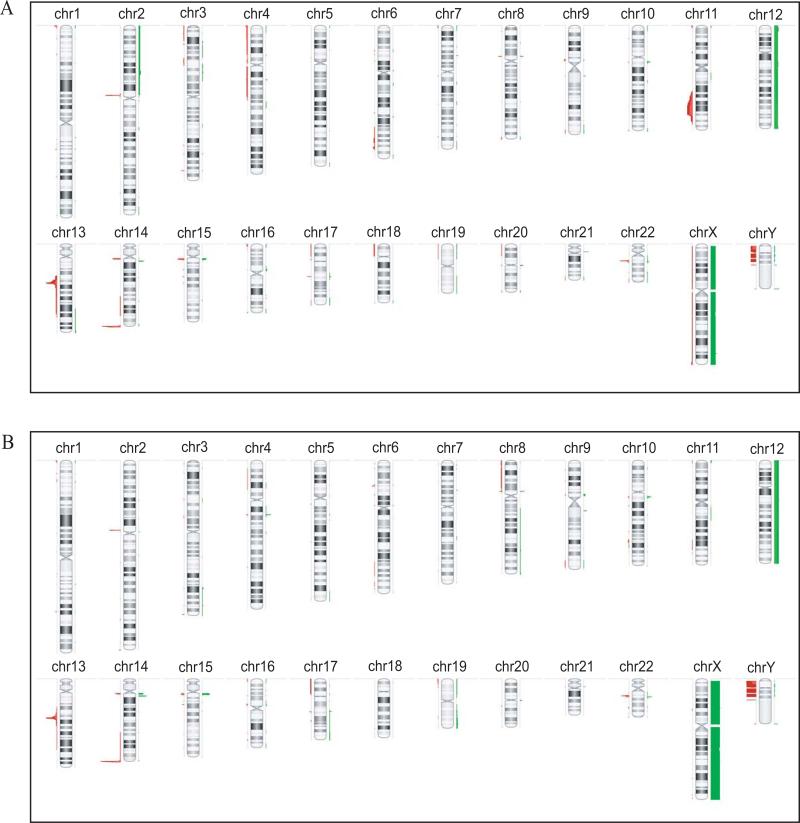
As noted above, chromosomal abnormalities varied between sporadic (Figure 1A) and familial CLL (Figure 1B). Sporadic cases showed a significant association with 11q loss (with nine sporadic and one familial case showing this loss, Figure 1, p=0.01). Deletion of 6q was seen in 4 sporadic cases and only 1 familial case. Also seen only in sporadic cases, albeit in small numbers, were 2p gain (n=3), 4p loss (n=3), and 4q loss (n=2) (Figure 1A, Table S2).
Figure 1. Chromosomal Abnormalities in Sporadic and Familial CLL.
Sporadic cases (panel A) showed gains in 2p and losses in 4q, 6q, 11q. Familial cases (panel B) showed gains in 14q region1 and homozygous loss of 13q.
In contrast, gains of 14q region 1 were seen only in familial cases (n=6), while losses were more common in sporadic cases (p=0.02) (Figure 1B). This region showed both gains and losses and is probably a region of CNV. However alterations of 14q region 1 were also associated with mutated IGHV (p=0.005; Table S4) and gains were associated with homozygous deletions of 13q (p=0.04) (Table S5).
Features of Specific Chromosomal Regions
Deletion of 13q14.12 - q14.3
We found that 40 (53%) cases in our cohort showed deletion of 13q, of which 26 (65%) showed a heterozygous (one-copy) loss, while 14 (35%) showed some level of homozygous (two-copy) loss (Figure S3). All cases with a homozygous deletion of 13q by FISH were also found to have homozygous deletions by array CGH. Using a very stringent log2 ratio threshold of -1.0 to call the homozygous deletions, array CGH identified an additional five cases with similar homozygous deletions, three that lacked FISH data and two that showed heterozygous (one-copy) loss of 13q by FISH. Using a threshold of -0.7, which allowed detection of mosaics (containing a mixture of cells with homozygous and heterozygous deletions), the percentage of samples that showed some component of homozygous loss increased to 43% of the total number of cases and 80% of cases with 13q loss. Of the cases that showed varying degrees of homozygous loss, 50% showed homozygous loss with apparently consistent breakpoints, while the remaining samples had a homozygous deletion within varying lengths of an apparent one-copy deletion. The minimal deleted area (chr13:49,501,120-49,877,565) includes miRNAs MIR15A and MIR16-1 as previously reported (Calin, et al 2002, Cimmino, et al 2005, Tyybakinoja, et al 2007) (Figure S3).
No difference was observed between familial and sporadic cases in the frequency of heterozygous or homozygous deletions of 13q. However, those patients with homozygous deletions were more frequently IGHV mutated (p=0.03), and negative for ZAP-70 (p=0.01) (Table 3). Homozygous deletions of 13q were mutually exclusive with loss of 17p, 6q, 4p and 11q (p=0.04), as well as with trisomy 12 (p=0.004) (Figure S4, Table S5). Of the samples heterozygous for deletion of 13q, only two samples showed trisomy 12. The less frequent event of 2p gain was also mutually exclusive with deletion 13q.
TABLE 3.
Association of Key CGH Abnormalities with Biological Features
| 11q loss | 12 gain | 13q loss, heterozygous | 13q loss, homozygous | 17p loss | |
|---|---|---|---|---|---|
| IGHV Status (N=71) | N=10 | N=13 | N=25 | N=13 | N=4 |
| IGHV Mutated n = 36 | 2 (20%) | 5 (38%) | 10 (40%) | 11 (85%) | 1 (25%) |
| IGHV Unmutated n=35 | 8 (80%) | 8 (62%) | 15 (60%) | 2 (15%) | 3 (75%) |
| ZAP70 Status (N=68) | N=9 | N=11 | N=25 | N=13 | N=4 |
| ZAP70+ n= 30 | 8 (89%) | 6 (55%) | 14 (56%) * | 1 (8%) | 2 (50%) |
| ZAP70- n=38 | 1 (11%) | 5 (45%) | 11 (44%) | 12 (92%) | 2 (50%) |
| CD38 Status (N=71) | N=10 | N=13 | N=24 | N=13 | N=4 |
| CD38+ n=22 | 3 (30%) | 7 (54%) | 7 (29%) * | 1 (8%) | 2 (50%) |
| CD38- n=49 | 7 (70%) | 6 (46%) | 17 (71%) | 12 (92%) | 2 (50%) |
| Family History (N=75) | N=10 | N=14 | N=26 | N=14 | N=4 |
| Familial n=38 | 1 (10%) | 8 (57%) | 10 (39%) | 10 (71%) | 2 (50%) |
| Sporadic n=37 | 9 (90%) | 6 (43%) | 16 (61%) | 4 (29%) | 2 (50%) |
All the long deletions are in Zap70-, CD38-
Deletion of 11q22.1-23.2
Deletion of 11q22-23 was observed in 10 (13%) patients and significantly associated with unmutated IGHV status (p < 0.05) and overexpression of ZAP-70 (p=0.01) (Table 3, Table S6). 11q loss was mutually exclusive with trisomy 12 although this finding was not statistically significant (Figure S5). However, a significant association was observed with 4p loss, which was seen in 3 patients all of whom had sporadic disease and deletion 11q (p=0.002). 4p loss was also associated with deletion 6q (p=0.01). Gain of 21p was also associated with 11q loss (p=0.004) and 4p loss (p=0.03). 2p gain was not found in samples with loss of 11q, 4p or 6q.
Correlation of CGH Results with Treatment Status and Time to First Treatment
We assessed predictors of whether patients remained untreated at the time of analysis, or had been treated prior to or after study sampling (Table S7). Treatment of these patients was based on National Cancer Institute-96 criteria, which include symptomatic disease or significant cytopenias (Cheson, et al 1996). As expected, IGHV unmutated status, as well as positivity for ZAP70 or CD38, were all associated with whether patients had been treated. No significant difference was observed between the familial and sporadic subgroups with respect to treatment status, with a median time to first treatment of 50-51 months for both groups (p=0.13).
As expected, deletion of 17p was associated with earlier treatment (p=0.006), as were deletion 11q (p=0.06) and deletion 6q (p=0.05). In addition, several of the less well characterized abnormalities, including gain of 2p (p<0.03), loss of 4p (p=0.03) or 18p (p=0.03), and abnormalities of 9q (p=0.01) and 21p (p=0.008), were associated with treatment status in our cohort (Table S7). These findings are not surprising given that several of these abnormalities were associated with other high-risk abnormalities, namely 4p loss and 21p abnormalities with deletion 11q, and 9q abnormalities with deletion 6q. Deletion of 13q, considered as homozygous, heterozygous or unaltered, was not associated with treatment status, nor was trisomy 12.
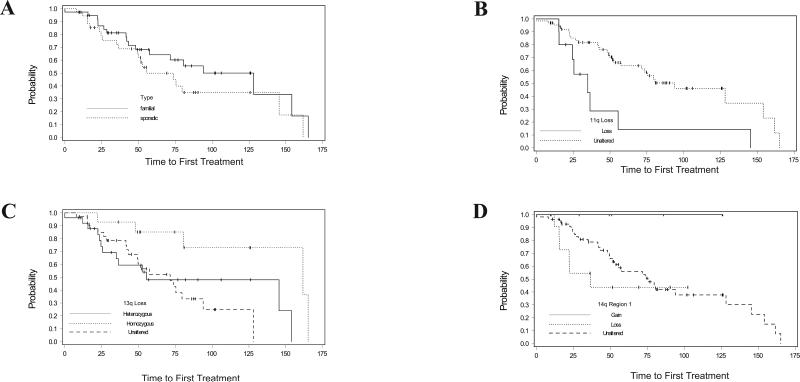
Analysis of time to first treatment using the method of Kaplan and Meier again found that unmutated IGHV, positive ZAP70, and positive CD38 were all associated with shorter time to first therapy, as expected (data not shown). No difference between familial and sporadic CLL was observed in time to first therapy (Figure 2A). However, chromosomal losses in 11q (median 35 months vs 94 months; p=0.005; Figure 2B) and 6q (median 41 months vs 81 months; p=0.04; data not shown) were predictive of shorter time to first treatment. Abnormalities of chromosome 9, though seen in only 3 patients with one gain and two losses, were significantly associated with shorter time to first treatment (p<0.0001, data not shown). Abnormalities of 21p were also associated with a trend to a shorter time to first treatment (p=0.06, data not shown).
Figure 2. Time to First Treatment (TTFT).
Panel (A) There was no significant difference (p value = 0.18) between TTFT in sporadic (dashed line, 55.53 months) and familial cases (solid line, 128.16 months). Deletion of 11q (panel B) resulted in a significantly shorter TTFT (p=0.0002, solid line, 34.93 months) compared to unaltered cases (dashed line, 94 months). Panel (C) shows the TTFT for the 13q samples that are unaltered (dashed line, 71.51 months), have homozygous deletion (dotted line, 161.65 months) or have heterozygous deletion (solid line, 55.53 months). The samples with homozygous deletion had a significantly longer TTFT (p value = 0.03). Panel (D) shows that gains in 14q region 1 are associated with a trend to a longer TTFT, while losses were associated with a shorter TTFT (p value = 0.07). (Dashed line- unaltered, 73.68 months; dotted line- loss, 29.42 months, solid line- gain).
A significantly longer time to first treatment was observed with homozygous deletion of 13q (162 months), compared to heterozygous deletion of 13q (55.5 months) or unaltered 13q (71.5 months) (p 0.03; Figure 2C). Gains in 14q region 1, which were associated with familial CLL and IGHV mutated disease in our cohort, were also associated with a trend to longer time to first treatment, while losses were associated with shorter time to treatment (p=0.07; Figure 2D).
DISCUSSION
To assess the relationship of genomic abnormalities to familial CLL, we applied high-resolution array CGH to well characterized CLL patients, half with familial disease and half with sporadic disease. We have found that deletion 11q was associated primarily with sporadic disease. In addition, in our cohort, homozygous deletion at 13q was more favorable than heterozygous deletion, and was associated with alterations in 14q that are associated with familial disease and a favorable prognosis.
We and many other groups note that array CGH does not reliably identify abnormalities present in less than 25-30% of cells (Gunn, et al 2008, Kujawski, et al 2008, Patel, et al 2008, Rudenko, et al 2008), with Rudenko et al (2008) reporting an even higher 50% threshold. This finding significantly reduces the potential clinical usefulness of array CGH, because clinically meaningful abnormalities can commonly occur in less than 30-50% of cells. Array CGH is therefore not likely to replace FISH for the identification of routine abnormalities until this limitation can be overcome.
In comparing familial to sporadic CLL, we found that high risk abnormalities were concentrated in the sporadic patient group; in particular, deletions of 11q, the most common high risk abnormality at diagnosis, was significantly associated with sporadic disease and rarely seen in familial disease. In addition, gain of 2p, as well as losses of 6q, both associated previously with aggressive disease (Chapiro, et al 2009, Dohner, et al 2000, Pfeifer, et al 2007), were seen primarily in sporadic disease. Prior studies looking at chromosomal abnormalities in familial compared to sporadic CLL have been limited, but the one prior study used conventional chromosomal CGH in 24 familial CLL cases, some from the same families, and reported a similar frequency of 11q deletion in familial and sporadic cases (Summersgill, et al 2002). The source of the different results between this study and ours may relate to our use of array CGH and our larger sample size that included all unrelated individuals.
Our other major finding in familial disease was alteration at 14q region 1. Gains in this region were seen only in familial disease, and four of thirteen losses were in familial disease. The presence of both gains and losses suggests that this region may be a CNV and in fact this CNV region has been previously described (Redon, et al 2006). If its association with familial disease is confirmed, this region may potentially be associated with germline susceptibility to CLL. Alteration in this region was also associated with favorable prognostic indicators, specifically mutated IGHV. Further work will be needed to confirm these findings, but our data suggest an association of familial disease with favorable prognosis cytogenetic abnormalities, while high-risk abnormalities, such as deletion 11q and 6q, are more prevalent in sporadic disease. Despite this suggestion in the data, however, no difference in time to first therapy was seen between familial and sporadic disease in this study. Larger patient numbers and larger studies that allow for multivariate analysis of prognostic factors will be required to definitively address this question in the future.
Although no association of heterozygous or homozygous deletions of 13q with familial CLL was observed in this study, homozygous deletions of 13q were associated with alterations in 14q region 1 discussed above. These homozygous deletions were found in approximately 30-40% of 13q deletions in our cohort, similar to prior reports (Corcoran, et al 1998). We chose to analyse all patients with 13q deletions as a group, regardless of additional abnormalities, rather than only those with sole 13q deletions. In doing so we observed that in our cohort homozygous 13q loss was mutually exclusive with other common CLL abnormalities including deletions of 11q, 17p, 6q, 4p, and trisomy 12 and gain of 2p, all of which were associated in this study with shorter time to treatment. Homozygous deletions of 13q were also associated with mutated IGHV, negative ZAP-70 and longer time to treatment compared to heterozygous 13q; similar findings were recently reported in another high resolution genomic study (Gunnarsson, et al 2010). These observations may be due in part to the common co-occurrence of heterozygous 13q deletion with higher risk cytogenetic abnormalities, as also observed in this study. This observation raises the possibility that a “second hit” at 13q may result in favorable phenotype disease, as compared to a “second hit” at a higher risk distant locus.
The primary limitation of our study is the relatively small sample size, and the multiple comparisons performed. These analyses were performed in order to characterize the dataset in detail and in so doing, to generate hypotheses for future work. As a result we were able to identify that deletion 11q is associated with sporadic CLL, and that alterations in a CNV region on 14q are associated with familial CLL and favorable prognostic markers. Alteration in this 14q region is further associated with homozygous deletion of 13q, which had a favorable prognosis in our study. Given the size of our study, these findings will all need to be confirmed in an independent dataset preferably with multivariate analysis. Such studies are in progress in our group and we expect that these larger studies will lead to new insights into the genetic pathogenesis of CLL.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to the patients who participated in this study and the clinic staff who support our research sample collection.
This work was supported in part by NIH grant K23 CA115682 to JRB, a leukemia and lymphoma translational grant (6161-05) and NIH grant CA111560 to CL, and by the Okonow-Lipton Fund. The work of LZR, TJK, DN and JRB in sample collection, analysis and determination of IGHV and ZAP70 status was supported by NIH grant PO1-CA081534 for the CLL Research Consortium (CRC). ASF is supported in part by NIH grants 2P01CA092625 and CA-103244.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
REFERENCES
- Brown JR, Neuberg D, Phillips K, Reynolds H, Silverstein J, Clark JC, Ash M, Thompson C, Fisher DC, Jacobsen E, LaCasce AS, Freedman AS. Prevalence of familial malignancy in a prospectively screened cohort of patients with lymphoproliferative disorders. Br J Haematol. 2008;143:361–368. doi: 10.1111/j.1365-2141.2008.07355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapiro E, Leporrier N, Radford-Weiss I, Bastard C, Mossafa H, Leroux D, Tigaud I, De Braekeleer M, Terre C, Brizard F, Callet-Bauchu E, Struski S, Veronese L, Fert-Ferrer S, Taviaux S, Lesty C, Davi F, Merle-Beral H, Bernard OA, Sutton L, Raynaud SD, Nguyen-Khac F. Gain of the short arm of chromosome 2 (2p) is a frequent recurring chromosome aberration in untreated chronic lymphocytic leukemia (CLL) at advanced stages. Leukemia Research. 2009;34:63–68. doi: 10.1016/j.leukres.2009.03.042. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, Rai KR. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran MM, Rasool O, Liu Y, Iyengar A, Grander D, Ibbotson RE, Merup M, Wu X, Brodyansky V, Gardiner AC, Juliusson G, Chapman RM, Ivanova G, Tiller M, Gahrton G, Yankovsky N, Zabarovsky E, Oscier DG, Einhorn S. Detailed molecular delineation of 13q14.3 loss in B-cell chronic lymphocytic leukemia. Blood. 1998;91:1382–1390. [PubMed] [Google Scholar]
- Cuttner J. Increased incidence of hematologic malignancies in first-degree relatives of patients with chronic lymphocytic leukemia. Cancer Invest. 1992;10:103–109. doi: 10.3109/07357909209032771. [DOI] [PubMed] [Google Scholar]
- Diskin SJ, Eck T, Greshock J, Mosse YP, Naylor T, Stoeckert CJ, Jr., Weber BL, Maris JM, Grant GR. STAC: A method for testing the significance of DNA copy number aberrations across multiple array-CGH experiments. Genome Res. 2006;16:1149–1158. doi: 10.1101/gr.5076506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, Dohner K, Bentz M, Lichter P. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- Fegan C, Robinson H, Thompson P, Whittaker JA, White D. Karyotypic evolution in CLL: identification of a new sub-group of patients with deletions of 11q and advanced or progressive disease. Leukemia. 1995;9:2003–2008. [PubMed] [Google Scholar]
- Fenaux P, Preudhomme C, Lai JL, Quiquandon I, Jonveaux P, Vanrumbeke M, Sartiaux C, Morel P, Loucheux-Lefebvre MH, Bauters F, Berger R, Kerckaert JP. Mutations of the p53 gene in B-cell chronic lymphocytic leukemia: a report on 39 cases with cytogenetic analysis. Leukemia. 1992;6:246–250. [PubMed] [Google Scholar]
- Forconi F, Rinaldi A, Kwee I, Sozzi E, Raspadori D, Rancoita PM, Scandurra M, Rossi D, Deambrogi C, Capello D, Zucca E, Marconi D, Bomben R, Gattei V, Lauria F, Gaidano G, Bertoni F. Genome-wide DNA analysis identifies recurrent imbalances predicting outcome in chronic lymphocytic leukaemia with 17p deletion. Br J Haematol. 2008;143:532–536. doi: 10.1111/j.1365-2141.2008.07373.x. [DOI] [PubMed] [Google Scholar]
- Gaidano G, Ballerini P, Gong JZ, Inghirami G, Neri A, Newcomb EW, Magrath IT, Knowles DM, Dalla-Favera R. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1991;88:5413–5417. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler CH, Philip P, Christensen BE, Hou-Jensen K, Pedersen NT, Jensen OM, Thorling K, Andersen E, Birgens HS, Drivsholm A, Ellegaard J, Larsen JK, Plesner T, Brown P, Andersen PK, Hansen MM. In B-cell chronic lymphocytic leukaemia chromosome 17 abnormalities and not trisomy 12 are the single most important cytogenetic abnormalities for the prognosis: a cytogenetic and immunophenotypic study of 480 unselected newly diagnosed patients. Leuk Res. 1997;21:1011–1023. doi: 10.1016/s0145-2126(97)00095-7. [DOI] [PubMed] [Google Scholar]
- Grubor V, Krasnitz A, Troge JE, Meth JL, Lakshmi B, Kendall JT, Yamrom B, Alex G, Pai D, Navin N, Hufnagel LA, Lee YH, Cook K, Allen SL, Rai KR, Damle RN, Calissano C, Chiorazzi N, Wigler M, Esposito D. Novel genomic alterations and clonal evolution in chronic lymphocytic leukemia revealed by representational oligonucleotide microarray analysis (ROMA). Blood. 2009;113:1294–1303. doi: 10.1182/blood-2008-05-158865. [DOI] [PubMed] [Google Scholar]
- Gunn SR, Mohammed MS, Gorre ME, Cotter PD, Kim J, Bahler DW, Preobrazhensky SN, Higgins RA, Bolla AR, Ismail SH, de Jong D, Eldering E, van Oers MH, Mellink CH, Keating MJ, Schlette EJ, Abruzzo LV, Robetorye RS. Whole-genome scanning by array comparative genomic hybridization as a clinical tool for risk assessment in chronic lymphocytic leukemia. J Mol Diagn. 2008;10:442–451. doi: 10.2353/jmoldx.2008.080033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson R, Staaf J, Jansson M, Ottesen AM, Goransson H, Liljedahl U, Ralfkiaer U, Mansouri M, Buhl AM, Smedby KE, Hjalgrim H, Syvanen AC, Borg A, Isaksson A, Jurlander J, Juliusson G, Rosenquist R. Screening for copy-number alterations and loss of heterozygosity in chronic lymphocytic leukemia--a comparative study of four differently designed, high resolution microarray platforms. Genes Chromosomes Cancer. 2008;47:697–711. doi: 10.1002/gcc.20575. [DOI] [PubMed] [Google Scholar]
- Gunnarsson R, Isaksson A, Mansouri M, Goransson H, Jansson M, Cahill N, Rasmussen M, Staaf J, Lundin J, Norin S, Buhl AM, Smedby KE, Hjalgrim H, Karlsson K, Jurlander J, Juliusson G, Rosenquist R. Large but not small copy-number alterations correlate to high-risk genomic aberrations and survival in chronic lymphocytic leukemia: a high-resolution genomic screening of newly diagnosed patients. Leukemia. 2010;24:211–215. doi: 10.1038/leu.2009.187. [DOI] [PubMed] [Google Scholar]
- Ishibe N, Sgambati MT, Fontaine L, Goldin LR, Jain N, Weissman N, Marti GE, Caporaso NE. Clinical characteristics of familial B-CLL in the National Cancer Institute Familial Registry. Leuk Lymphoma. 2001;42:99–108. doi: 10.3109/10428190109097681. [DOI] [PubMed] [Google Scholar]
- Juliusson G, Oscier DG, Fitchett M, Ross FM, Stockdill G, Mackie MJ, Parker AC, Castoldi GL, Guneo A, Knuutila S, Elonen E, Gahrton G. Prognostic subgroups in B-cell chronic lymphocytic leukemia defined by specific chromosomal abnormalities. N Engl J Med. 1990;323:720–724. doi: 10.1056/NEJM199009133231105. [DOI] [PubMed] [Google Scholar]
- Kujawski L, Ouillette P, Erba H, Saddler C, Jakubowiak A, Kaminski M, Shedden K, Malek SN. Genomic complexity identifies patients with aggressive chronic lymphocytic leukemia. Blood. 2008;112:1993–2003. doi: 10.1182/blood-2007-07-099432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matutes E, Oscier D, Garcia-Marco J, Ellis J, Copplestone A, Gillingham R, Hamblin T, Lens D, Swansbury GJ, Catovsky D. Trisomy 12 defines a group of CLL with atypical morphology: correlation between cytogenetic, clinical and laboratory features in 544 patients. Br J Haematol. 1996;92:382–388. doi: 10.1046/j.1365-2141.1996.d01-1478.x. [DOI] [PubMed] [Google Scholar]
- Mauro FR, Giammartini E, Gentile M, Sperduti I, Valle V, Pizzuti A, Guarini A, Giannarelli D, Foa R. Clinical features and outcome of familial chronic lymphocytic leukemia. Haematologica. 2006;91:1117–1120. [PubMed] [Google Scholar]
- Neilson JR, Auer R, White D, Bienz N, Waters JJ, Whittaker JA, Milligan DW, Fegan CD. Deletions at 11q identify a subset of patients with typical CLL who show consistent disease progression and reduced survival. Leukemia. 1997;11:1929–1932. doi: 10.1038/sj.leu.2400819. [DOI] [PubMed] [Google Scholar]
- Patel A, Kang SH, Lennon PA, Li YF, Rao PN, Abruzzo L, Shaw C, Chinault AC, Cheung SW. Validation of a targeted DNA microarray for the clinical evaluation of recurrent abnormalities in chronic lymphocytic leukemia. Am J Hematol. 2008;83:540–546. doi: 10.1002/ajh.21145. [DOI] [PubMed] [Google Scholar]
- Pfeifer D, Pantic M, Skatulla I, Rawluk J, Kreutz C, Martens UM, Fisch P, Timmer J, Veelken H. Genome-wide analysis of DNA copy number changes and LOH in CLL using high-density SNP arrays. Blood. 2007;109:1202–1210. doi: 10.1182/blood-2006-07-034256. [DOI] [PubMed] [Google Scholar]
- Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, Neuberg DS, Flinn IW, Rai KR, Byrd JC, Kay NE, Greaves A, Weiss A, Kipps TJ. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, Fiegler H, Shapero MH, Carson AR, Chen W, Cho EK, Dallaire S, Freeman JL, Gonzalez JR, Gratacos M, Huang J, Kalaitzopoulos D, Komura D, MacDonald JR, Marshall CR, Mei R, Montgomery L, Nishimura K, Okamura K, Shen F, Somerville MJ, Tchinda J, Valsesia A, Woodwark C, Yang F, Zhang J, Zerjal T, Zhang J, Armengol L, Conrad DF, Estivill X, Tyler-Smith C, Carter NP, Aburatani H, Lee C, Jones KW, Scherer SW, Hurles ME. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko HC, Else M, Dearden C, Brito-Babapulle V, Jones C, Dexter T, Fenwick K, Mackay A, Ashworth A, Matutes E, Gonzalez D, Catovsky D, Morgan GJ. Characterising the TP53-deleted subgroup of chronic lymphocytic leukemia: an analysis of additional cytogenetic abnormalities detected by interphase fluorescence in situ hybridisation and array-based comparative genomic hybridisation. Leuk Lymphoma. 2008;49:1879–1886. doi: 10.1080/10428190802345902. [DOI] [PubMed] [Google Scholar]
- Stankovic T, Weber P, Stewart G, Bedenham T, Murray J, Byrd PJ, Moss PA, Taylor AM. Inactivation of ataxia telangiectasia mutated gene in B-cell chronic lymphocytic leukaemia. Lancet. 1999;353:26–29. doi: 10.1016/S0140-6736(98)10117-4. [DOI] [PubMed] [Google Scholar]
- Summersgill B, Thornton P, Atkinson S, Matutes E, Shipley J, Catovsky D, Houlston RS, Yuille MR. Chromosomal imbalances in familial chronic lymphocytic leukaemia: a comparative genomic hybridisation analysis. Leukemia. 2002;16:1229–1232. doi: 10.1038/sj.leu.2402321. [DOI] [PubMed] [Google Scholar]
- Tyybakinoja A, Vilpo J, Knuutila S. High-resolution oligonucleotide array-CGH pinpoints genes involved in cryptic losses in chronic lymphocytic leukemia. Cytogenet Genome Res. 2007;118:8–12. doi: 10.1159/000106435. [DOI] [PubMed] [Google Scholar]
- Yuille MR, Matutes E, Marossy A, Hilditch B, Catovsky D, Houlston RS. Familial chronic lymphocytic leukaemia: a survey and review of published studies. Br J Haematol. 2000;109:794–799. doi: 10.1046/j.1365-2141.2000.02111.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.