Abstract
Background
Increasing epithelial chloride (Cl−) secretion in the upper airways represents a putative method for promoting MCC through augmentation of airway surface liquid depth. Several naturally occurring flavonoid compounds, including quercetin, have demonstrated the capacity to increase transepithelial Cl− transport. Quercetin exhibits well-known antioxidant and anti-inflammatory activity and is now recognized as a potent activator of the cystic fibrosis transmembrane conductance regulator (CFTR) anion channel activity in a fashion largely independent of cAMP signaling. The present study investigates whether this compound activates Cl− secretion and ciliary beat frequency (CBF) in well characterized culture models of sinonasal epithelium.
Methods
CF and non-CF primary human sinonasal (HSNE) and murine nasal septal epithelial (MNSE) cultures were studied for transepithelial ion transport in Ussing chambers under voltage clamp conditions and CBF was performed using pharmacologic manipulation.
Results
Change in short circuit current (ΔISC -expressed as μA/cm2) in response to quercetin were significantly greater than controls in both MNSE (23.23+/−5.44 vs. 2.47 +/− 1.62, p<0.0001) and HSNE (−8.72+/−1.88 vs. −1.88+/−0.66, p<0.01) cultures. CBF was significantly increased in quercetin-treated cells (expressed as fold-change over baseline) in w.t. [1.65+/−0.13 vs. 1.23+/−0.05 (control), p<0.01), but not CFTR−/− (1.65+/−0.29 vs. 1.48+/−0.38, p = 0.23).
Conclusions
Quercetin significantly increased transepithelial Cl− transport and CBF in MNSE and HSNE cultures. Future studies investigating quercetin as a means to promote mucociliary transport in individuals with rhinosinusitis are warranted.
BACKGROUND
Sinonasal mucosal inflammation and decreased mucociliary clearance (MCC) are major contributing factors to the pathophysiology of chronic rhinosinusitis (CRS). MCC is primarily determined by the sinonasal respiratory epithelium, a highly regulated barrier. Respiratory epithelium modifies the volume of the periciliary fluid layer[1] and the viscoelastic properties of the mucus through vectorial ion transport.[2] Decreased epithelial chloride (Cl−) transport decreases MCC through dehydration of airway surface liquid (ASL) and is clearly demonstrated in the lethal, inherited disease, cystic fibrosis. Pharmacologic agents designed specifically to enhance ASL hydration and MCC represent a novel approach to treating CRS, but have not been characterized in models representative of the sinus and nasal airway. One promising therapeutic group of naturally occurring compounds that may have salutary effects on sinus infections are flavonoids, although the activity of these agents in the upper airways is not well understood.
Flavonoids are comprised of a wide array of ubiquitous plant compounds that have the basic structural feature of a 2-phenylbenzo(-y)pyrone nucleus. The class of agents exhibits a number of biological effects critical to human health, and has been shown previously to confer antibacterial, antiviral, anti-inflammatory and antiallergic effects.[3, 4] Owing to its well-established safety profile in humans and its anti-inflammatory effects, the flavonoid quercetin has been tested in a variety of clinical trials.[5–7] Recently, our group has characterized the Cl− secretory properties of quercetin in cultured cell lines and in vivo airways.[8] While this finding has important implications for promoting Cl− secretion and hydration of airway surface liquid, the properties of quercetin have yet to be fully investigated in primary cultured sinonasal epithelia, and the downstream effects on cilia beating have not been tested. Furthermore, little is known about the effects of flavonoid compounds on ciliary beating. The present study aimed to measure the effects of quercetin in augmenting transepithelial Cl− transport and CBF in primary sinonasal epithelial cell cultures.
METHODS
Institutional animal care and use committee and institutional review board approval were obtained prior to the initiation of the study.
Cell Culture
Primary murine nasal septal (MNSE) and human sinonasal epithelial (HSNE) cultures were utilized in the current study. MNSE filters were derived from genetically matched (congenic) C57 wild type (w.t.) mice. Transgenic CFTR−/− (knockout) MNSE cultures were also investigated to test a CFTR dependent mechanism. Culturing of epithelia at an air-liquid interface is well described in our prior studies.[9–20] Briefly, MNSE cells were harvested and grown on Costar 6.5-mm-diameter permeable filter supports (Corning Life Sciences, Lowell, MA) submerged in culture medium. The apical surfaces of the epithelial monolayers were exposed to air on day 4 after reaching confluence and cells fed via the basal chamber. Under these conditions, differentiation and ciliogenesis occurs within 10 to 14 days, generating cultures with >90% cilia in each monolayer.
Insert Summary
A total of 205 MNSE and 42 HSNE cell cultures were used in the completion of these studies. Cultures with transepithelial resistances (Rt) > 500 Ω*cm2 were used to obtain Ussing chamber measurements and monitor ciliary beating with an inverted microscope. A minimum of 6 wells were used per condition.
Solutions and Chemicals
The bath solution contained (mM): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose. The pH of this solution is 7.3–7.4 when gassed with a mixture of 95% O2-5% CO2 at 37°C. Chemicals were obtained from Sigma (St. Louis, MO). Each solution was made as 1,000x stock and used at 1x in the Ussing chamber or for CBF analysis. All Ussing chamber experiments were performed with low Cl− (6 mM) in the mucosal bath. Pharmacologic preparations were as follows: amiloride (100μM); forskolin (100 nM); H89 dihydrochloride (20μM); quercetin (10μM, 50μM, and 200μM); CFTRinh-172 (10μM), UTP (150 μM). Amiloride was used to block ENaC activity, ensuring that the change in short-circuit current (ΔISC) from subsequent manipulation is secondary to effects on Cl− transport. Forskolin activates CFTR via a cyclic adenosine monophosphate (cAMP)-mediated mechanism. H89 is a PKA inhibitor that prevents cAMP-dependent pathway phosphorylation (activation) of the regulatory domain (RD) in CFTR. CFTRinh-172 is a specific inhibitor of CFTR[12] and is designed to allow comparisons of the relative contribution of CFTR to the ISC in different systems.
Ciliary Beat Frequency Analysis
Images were visualized using a 20X objective on an inverted scope (Fisher Scientific, Pittsburgh, Pa.). Data was captured using a Model A602f-2 Basler area scan high-speed monochromatic digital video camera (Basler AG, Ahrensburg, Germany) at a sampling rate of 100 frames per second and a resolution of 640 × 480 pixels. Images were analyzed using the Sisson-Ammons Video Analysis (SAVA) system version 2.1.6. For each experiment, a large area of beating cilia on air-liquid interface cultures was identified with the inverted microscope. The digital image signal was then routed from the camera directly into an acquisition board (National Instruments) within a Dell Workstation running the Windows XP Professional operating system. Images were analyzed with virtual instrumentation software for CBF analysis. All recordings were made at 200× magnification.
Experiments were all performed at ambient temperature (23°C). A baseline recording of CBF was conducted for each cell monolayer prior to apical administration of test solution, since apical fluid addition, by itself, can increase CBF. Additional fluid depth overlying the respiratory epithelial cell surface stimulates CBF due to improved hydration and optimal fluid dynamics. Thus, all experiments compared the effects of agonists to that of a PBS control solution. In some studies, quercetin was mixed with the basal culture media. Whole field analysis was performed with each point measured representing one cilia. The reported frequencies describe arithmetic means of these values, followed by standard deviations. Each analysis was normalized to fold-change over baseline.
Statistics
For Isc and CBF (fold-change), descriptive statistics (mean, SD, and SEM) were compared using Student’s t-test or ANOVA, as appropriate. All statistical tests were two-sided and were performed at a 5% significance level (i.e., α = 0.05) using Microsoft Excel (Seattle, WA).
RESULTS
Transepithelial Cl− transport
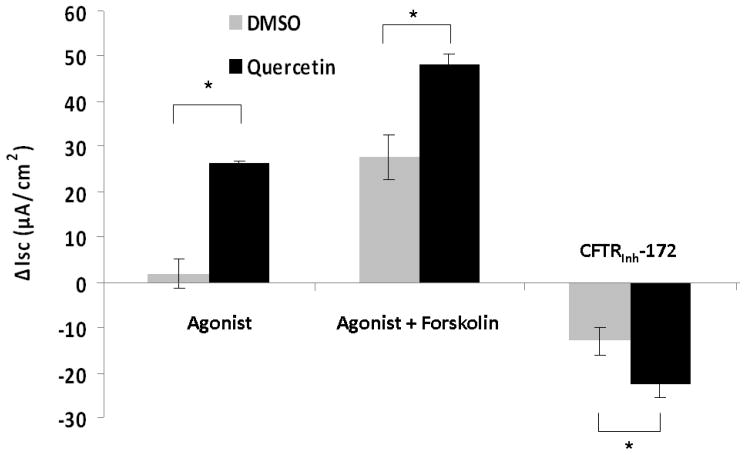
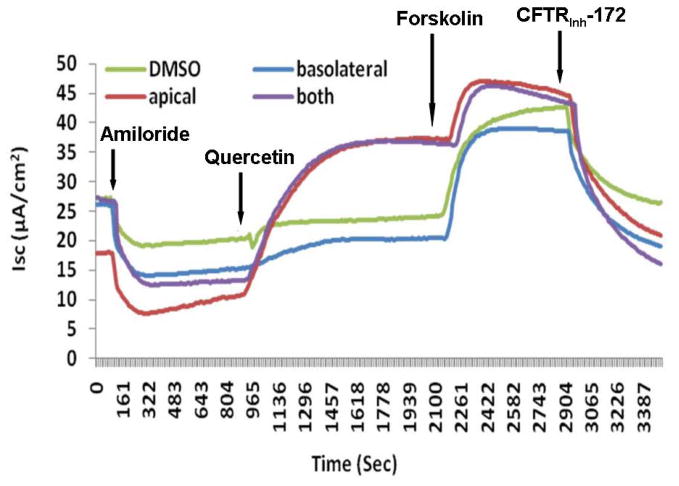
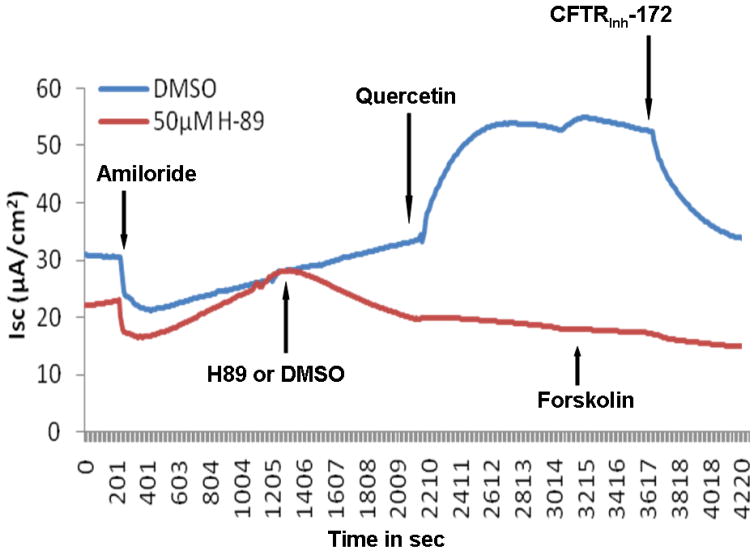
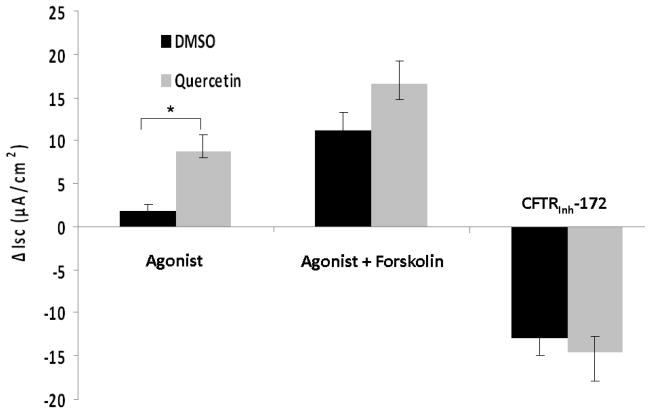
Changes in short circuit current (ΔISC, measured in μA/cm2) were significantly higher when MNSE cultures were exposed to 10μM quercetin (4.94+/−1.93) than when exposed to DMSO control (1.45+/−1.52, p<0.0001). Maximal effects were demonstrated with 50μM quercetin (26.26+/−8.03) when compared to the control vehicle (DMSO) (2.02+/−1.18, p<0.0001) (Figure 1A). In addition, 50 μM quercetin markedly increased ΔISC after the administration of 100nM forskolin (23.23+/− 5.44) compared to 100nM forskolin followed by control solution (2.47 +/− 1.62, p<0.0001) demonstrating potentiation of cAMP dependent CFTR activity. However, at higher doses (200 μM) of quercetin, there was an initial peak, followed by inhibition of Cl− secretion. Regardless of the order in which forskolin and quercetin were administered, total CFTR stimulation was significantly higher in the quercetin (dose) treated group (48.51 +/− 9.32) than DMSO controls (26.79 +/− 7.65, p<0.0001) (Figure 1B). Quercetin’s effects were specific to apical application of the agent (Figure 1C). Blockade of anion transport by the CFTR chloride channel inhibitor CFTRinh-172 demonstrated a significant difference between groups (quercetin, −27.07 +/− 8.89 vs. DMSO control, 15.71 +/− 8.35, p<0.01), indicating the effects of quercetin are CFTR dependent. Blockade of PKA with H89 (20 μM) eliminated the effects of quercetin, indicating that at least some baseline PKA activity (activation/phosphorylation of the R domain in CFTR) is necessary for quercetin’s potentiating action (Figure 1D). Quercetin also significantly increased CFTR-mediated ISC in HSNE cultures (−8.72+/−1.88 vs. −1.88+/−0.66, p<0.01) indicating the effects of quercetin are applicable to both murine and human CFTR (Figure 2). The ion transport phenotype of HSNE cultures is of smaller magnitude when compared to MNSE.[12]
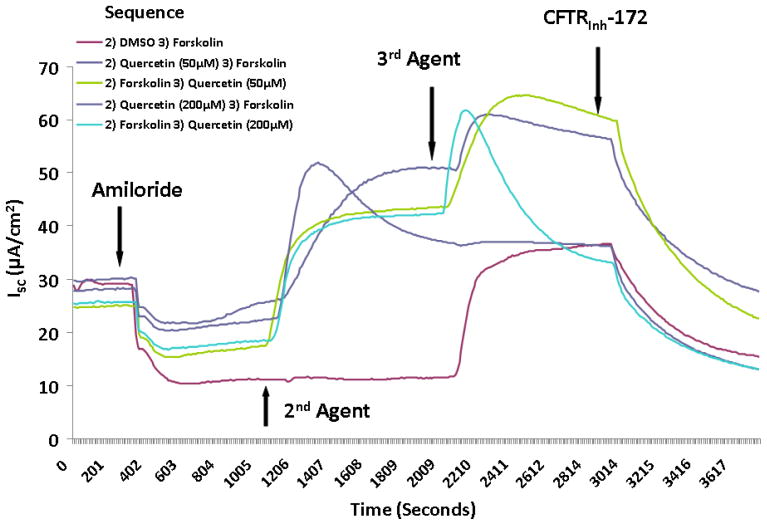
Figure 1. Quercetin stimulates transepithelial Cl− transport in murine nasal septal epithelium.
Representative Ussing chamber tracings demonstrating pharmacologic manipulation of ion transport. (A) A positive deflection in the tracing (ΔISC) represents anion (i.e. Cl−) movement from the serosal to mucosal direction. Quercetin (50μM) increases anion transport both with (green tracing) and without (blue tracing) forskolin (100 nM) pretreatment when compared to DMSO control (red tracing). Increasing the concentration of quercetin (200μM) inhibits overall Cl− secretion with both pre (light blue tracing) and post (purple tracing) treatment with forskolin. CFTRinh-172 (10 μM) is added to confirm CFTR dependence. (B) Summary data indicate both quercetin (50 μM) and quercetin + forskolin (100 nm) significantly increased Cl− transport over the corresponding controls (p<0.0001). Stimulated currents were sensitive to CFTRInh-172 indicating CFTR dependence (p<0.01). (C) Ussing chamber tracings measuring ΔISC in response to apical, basolateral, and apical + basolateral application of quercetin (50 μM). Apical administration is required to induce a significant Cl- secretory response. (D) The PKA inhibitor H89 (50μM) eliminates the Cl− secretory response by quercetin (red tracing) indicating that phosphorylation of the CFTR RD is necessary for CFTR activation by quercetin.
Figure 2. Quercetin (50μM) stimulates Cl− transport in human sinonasal epithelium in vitro.
−ΔISC was measured following amiloride blockade of ENaC channels in a Cl− secretory gradient using primary human sinus epithelial cultures studied in an Ussing chamber under voltage clamp conditions. Following addition of quercetin (50 μM),ΔISC significantly increased in human cultures (p<0.01).
Ciliary Beat Frequency
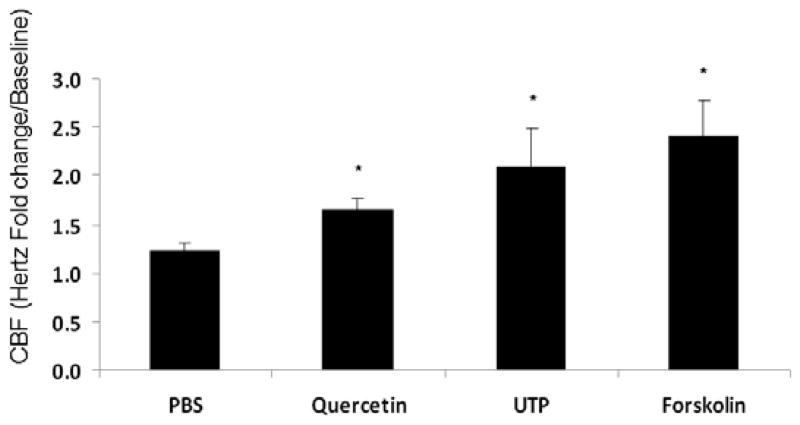
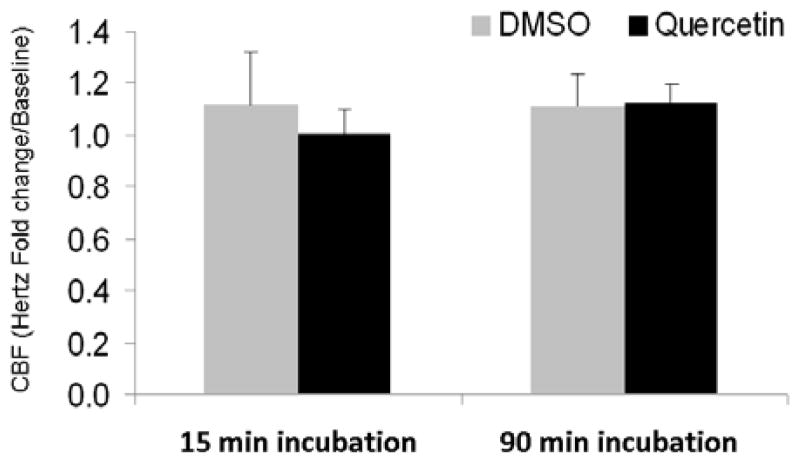
Robust stimulation of CBF was observed with concentrations of quercetin up to 10μM, but no further elevation was encountered despite increasing doses up to 200μM. Thus, 10μM was used as the working concentration for CBF analysis. CBF (expressed as fold-change over baseline) significantly increased following quercetin addition to the apical surface in comparison to control (PBS) solution (1.65+/−0.13 vs. 1.23+/−0.05, respectively; p<0.01), although efficacy was less than that observed with known CBF stimulants UTP (150 μM) and forskolin (100 nM); (2.1 +/−0.38 and 2.41+/−0.37, respectively). (Figure 3A) The effects of quercetin on CBF were specific to apical addition. Quercetin delivered to the basal media did not significantly increase CBF following either immediate administration (15 minutes) [n = 6, 1.01+/−0.16 vs. 1.12+/−0.35 (control); p=0.64] or more sustained treatment (90 minutes) [n = 8, 1.12+/−0.14 vs. 1.11+/−0.25 (control); p=0.93]. (Figure 3B)
Figure 3. Quercetin (10 μM) activates ciliary beat frequency following apical, but not basolateral exposure.
(A) CBF was significantly increased following quercetin (10 μM) addition to the apical surface when compared to controls (PBS) (p<0.01). Alternate agents that directly activate CBF through P2Y2 receptors (UTP, 150 μM) or cAMP (forskolin, 100 nM) are shown for comparison. All measurements were recorded following 15 minutes of exposure. (B) Quercetin administered to the basal media demonstrated no significant increase in CBF following acute administration (15 minutes) (p=0.64) or longer duration (90 minutes) (p=0.93).
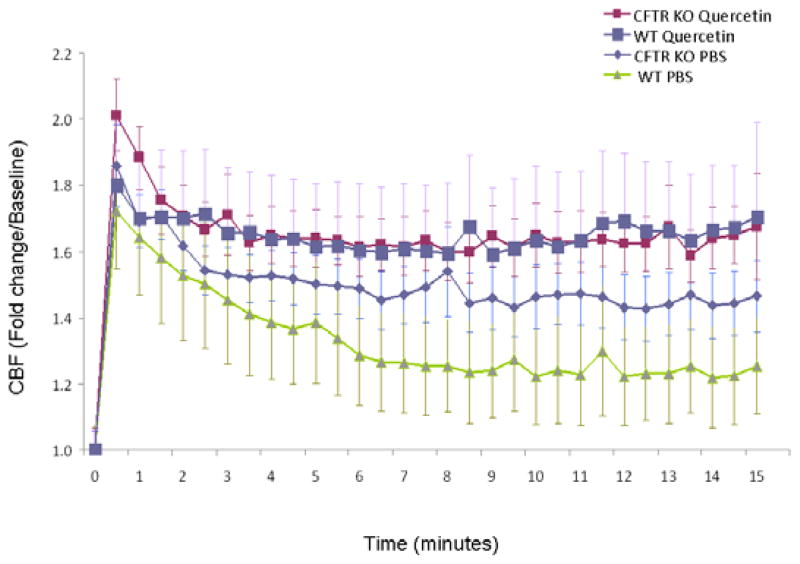
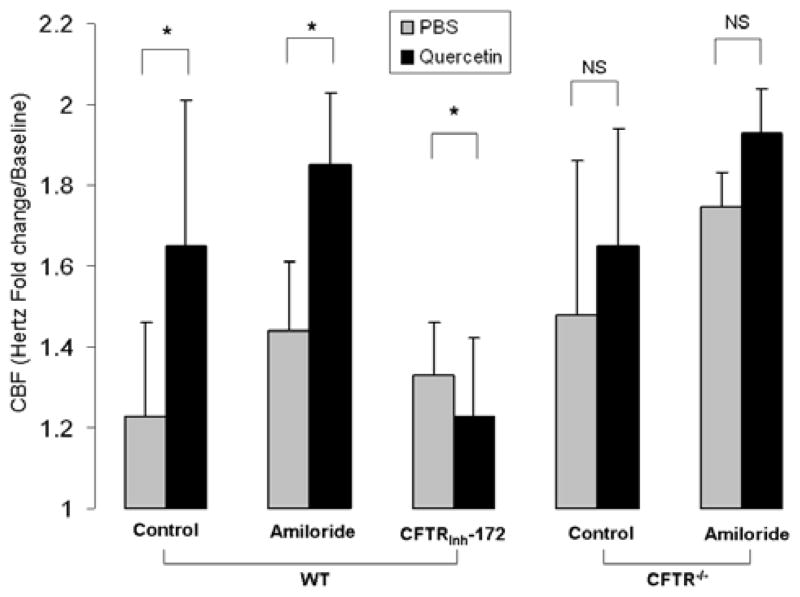
To isolate the effects of quercetin on anion transport, we next tested CBF in the presence of amiloride to inhibit ENaC. In the presence of amiloride (100 μM), quercetin stimulation of CBF was observed [1.85+/−0.18 vs. 1.44+/−0.17 (control); p<0.01], and was similar to the effect on cultures tested without sodium-channel blockade. (Figure 4) Pharmacologic blockade of CFTR with CFTRinh-172 completely abrogated stimulation [1.23+/−0.19 vs. 1.33+/−0.13 (control)] indicating effects of quercetin on CBF are dependent upon CFTR. To further examine the specificity of quercetin on CFTR expressing cultures, we tested the effects of quercetin in cultures derived from CFTR (−/−) mice. CBF did not significantly increase compared to PBS [1.65+/−0.29 vs. 0.1.48+/−0.38 (control); p=0.23). Because increased sodium hyperabsorption relative to Cl− is thought to dehydrate the ASL in CF airway disease, [21] we initially attributed the relative increase in CBF with PBS application in CFTR−/− cells (in comparison to w.t.) to restoration of the apical fluid volume. Subsequent tests supporting this hypothesis included amiloride in CFTR−/− cells to block excess Na transport in CFTR−/− cultures. Results of these experiments demonstrated no significant increase in CBF with the administration of quercetin (1.93+/−0.11) over PBS control (1.75+/−0.11). indicating the effects of quercetin on CBF are primarily due to augmenting Cl− transport (as opposed to altering Na+ conductance).
Figure 4. The effects of quercetin on ciliary beat frequency in wild type and CFTR knockout cultures.
(A) Time dependent effects of stimulated CBF following a single application of apical quercetin (10 μM) in wild type and CFTR knockout cultures. (B) Quercetin (10 μM) significantly increased CBF in wild type nasal airway epithelial cultures both with and without the sodium-channel blocker, amiloride (100 μM). Relative stimulation of CBF was slightly enhanced in the presence of amiloride (1.85+/−0.18 vs. 1.44+/−0.17, p<0.01), although not significantly different when compared to cultures without sodium-channel blockade. Cells blocked with the CFTR inhibitor CFTRinh-172 completely suppressed quercetin-stimulated CBF. In addition, quercetin did not activate CBF in cultures derived from CFTR knockout mice over that observed with PBS alone (p=0.23). Incubation with amiloride increased CBF when exposed to both PBS (1.75+/−0.08) and quercetin (1.93+/−0.11). All CBF rates were obtained at 15 minutes.
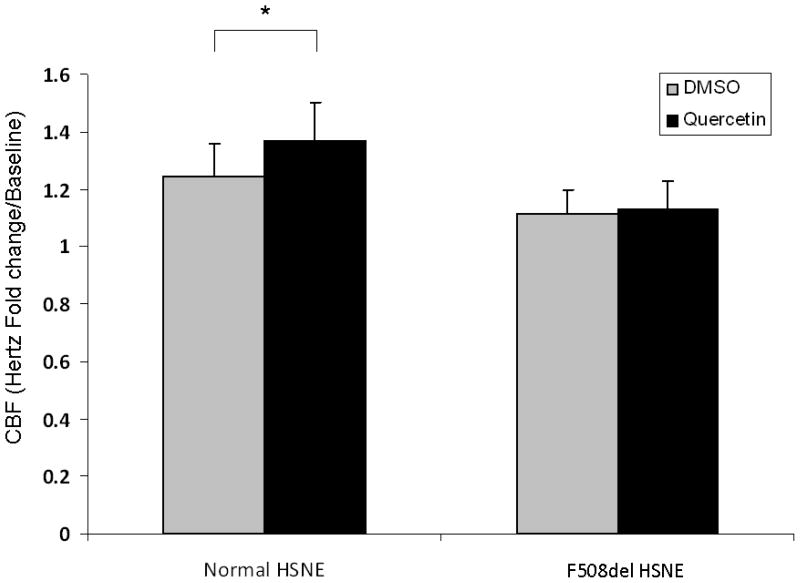
Primary HSNE cultures derived from individuals with functional CFTR had a modest, but significant increase in CBF following application of quercetin (10 μM) when compared to DMSO control (1.37 +/− 0.13 vs. 1.24 +/− 0.11, respectively; p<0.05). Analogous to explants in CFTR−/− mice, quercetin did not augment CBF in homozygous F508del HSNE (1.13 +/− 0.1 vs. 1.11 +/−0.08, control) (Figure 5).
Figure 5. Quercetin increases ciliary beat frequency in normal but not cystic fibrosis human sinonasal epithelial cultures.
Significant (although modest) CBF stimulation was demonstrated in primary cultures derived from individuals with functional CFTR ((1.24 +/−0.11, control vs. 1.37+/−0.13)), but not in cultures from patients homozygous for the F508del mutation.
DISCUSSION
CFTR is a Cl− channel in the apical membrane of respiratory epithelia and is crucial for modulating ASL via salt and water secretion and absorption. CFTR dysfunction results in severe respiratory disease in cystic fibrosis, but may also be dysfunctional in other conditions, such as cigarette smoke exposure[13] Drug discovery efforts have centered on small molecules that target CFTR[22, 23] to treat respiratory diseases. Flavonoids are one such group of molecules demonstrated to affect CFTR activity and CFTR-mediated currents.[22, 24, 25]
In the current study, we evaluated the propensity for quercetin to activate CFTR dependent Cl− transport in primary cell culture models of sinonasal epithelium. The findings of this study indicate quercetin robustly activates CFTR in upper airway cell monolayers. Similar to other studies, quercetin causes a dose-dependent increase in Cl− secretion with the maximal effect at 50 uM in MNSE cells. Interestingly, significant inhibition was seen at higher doses as also reported previously in lower airway cultures.[8, 24, 26] This biphasic response has been observed in other flavonoids, including quercetin. For example, in colon epithelial cell lines, quercetin activated Cl− currents at concentrations of 5 μmol/L, but had significant inhibition at higher doses.[27]
Our studies indicate that quercetin’s effects are CFTR dependent, based on inhibition of the response to CFTRinh-172 and dependence on CFTR expressing epithelium. In addition, the effects of quercetin were similar whether the agent was added before or after forskolin, demonstrating that pre-activation with an adenylate cyclase activator is not necessary for the observed effects. However, the PKA inhibitor H89 eliminated quercetin’s Cl− secretory properties indicating some baseline activation of CFTR (via phosphorylation of the R-domain) is required to augment Cl− transport. This is consistent with prior studies by our group indicating that the mechanistic effects of quercetin may differ from classic PKA dependent stimuli, such as forskolin (i.e. phosphorylation of the R domain)[8]. Further experiments will be required to determine the precise mechanism of action underlying robust stimulation of Cl− secretion conferred by this agent.
Quercetin also robustly activated CBF when applied to the respiratory epithelial cell surface – a finding not previously reported with this agent. Our studies were performed by submerging the entire epithelial cell surface with either quercetin or control solutions which should minimize the influence of augmented ASL depth alone as a mechanism underlying activation of CBF. However, it is plausible that the effects observed were compartmentalized changes in the ASL periciliary layer (sol), as increased CBF was observed following ENaC blockade[28]. Similarly, quercetin addition could confer small compartmentalized changes in the periciliary fluid layer not altered by the addition of exogenous apical surface fluid. Results indicating effects on CBF are dependent on CFTR with both pharmacologic blockade (CFTRinh-172) and transgenic CFTR−/− cultures provide additional support for this theory.
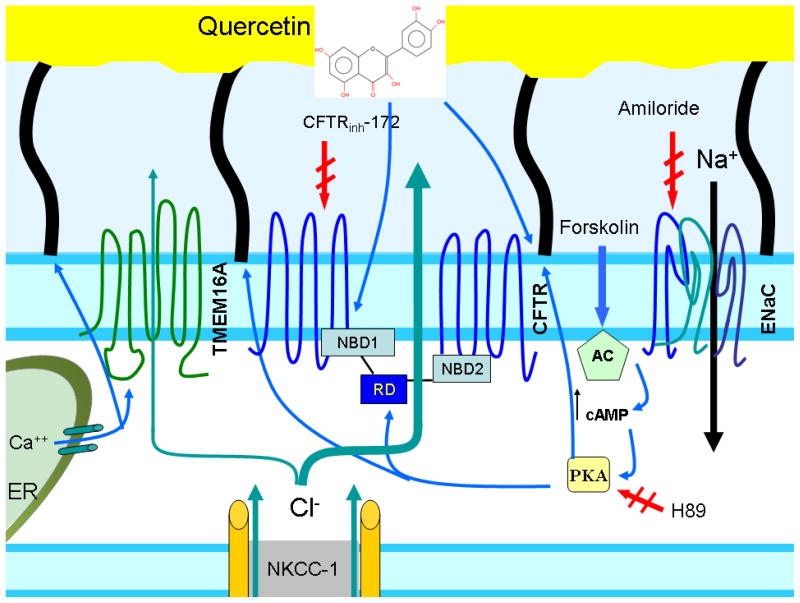
Flavonoids are ubiquitous compounds present in human foods, have excellent bioavailability, and have an excellent safety profile when administered through oral or intravenous means.[5, 6, 29] The flavonoid quercetin has undergone extensive preclinical and clinical testing for various human conditions. In a study examining quercetin as a tyrosine kinase inhibitor, intravenous doses exceeding 1700 mg/m2 induced transient elevations in serum creatinine, but doses below this threshold were well tolerated for 6–7 months.[5] In a hypertension trial, oral quercetin (730 mg daily) for 28 days produced no significant adverse events.[30] Prior studies indicate quercetin also has the ability to enhance Cl− transport through CFTR and, thus, has been investigated as an agent for differentiating between wild type and mutant CFTR in cystic fibrosis. Quercetin has previously been shown to efficiently activate CFTR in single cells, polarized cell lines, and stimulate CFTR when administered topically to the nasal mucosa as part of the in vivo nasal potential difference testing and was well tolerated with nasal perfusion.[24] Our model of quercetin stimulation of CFTR and CBF (Figure 6) is consistent with prior studies from our center demonstrating CFTR activation through interaction with the nucleotide binding domains (NBD) rather than activation (phosphorylation) of the Regulatory Domain (RD).[8] Based on studies presented here, stimulation of CBF appears to be dependent upon CFTR mediated anion transport and likely stimulates CBF through localized changes in the composition of the periciliary fluid, although other mechanisms such as altered viscosity of the mucus gel or direct activation of cilia beating may also contribute.
Figure 6. Pharmacologic manipulation of ion transport and ciliary activation pathways with quercetin’s suspected mechanism of action.
CBF and CFTR are activated through the PKA-dependent pathway. Cl− is transported via the basal membrane through basolateral potassium channels (NKCC-1) and secreted through CFTR at the apical surface. TMEM16A is a recently identified calcium-activated Cl− channel that also contributes to apical anion transport.[31] Activators (forskolin, quercetin) are indicated by the blue arrows; inhibitors by the red arrows (amiloride, CFTRinh-172, H89). The epithelial sodium channel (ENaC) is the primary apical Na+ channel. Quercetin is thought to act as a CFTR channel activator through its interactions with the nucleotide binding domains (NBD) rather than activation (phosphorylation) of the Regulatory Domain (RD).[8] Quercetin also stimulates CBF through either localized changes in the composition of the periciliary fluid or an unknown mechanism of direct activation.
While quercetin did not stimulate CBF or CFTR when cultures were exposed via the basal membrane, the effects with apical addition remained robust. These findings have relevance to the optimal route of quercetin administration (topical versus systemic) for use in nasal airways as a means to activate CFTR or augment cilia beating to improve MCC in diseased sinus airways.
CONCLUSION
In summary, the present study indicates quercetin is a robust CFTR dependent Cl− secretagogue in murine and human nasal airway cells in vitro and provides new evidence that the compound is an activator of CBF. Our results (and the well-established safety profile in other human studies) offer a rationale for further studies utilizing the agent in human protocols targeting topical administration to the sinuses, including individuals with relative deficiency in CFTR activity independent of congenital mutations in the CFTR gene.
Acknowledgments
This research was funded by the American Rhinologic Society New Investigator Award (2009), Flight Attendant’s Medical Research Institute Young Clinical Scientist Award (072218), NIH/NHLBI (1K08HL107142-01) to B.A.W.; NIH/NIDDK (5P30DK072482-03) to E.J.S.; U.S. National Institute of Health grants 1K23DK075788-01 R03 and Cystic Fibrosis Foundation grants ROWE08XX to S.M.R.
Footnotes
Findings in the current manuscript were presented at the American Academy of Otolaryngology – Head and Neck Surgery annual meeting and the American Rhinologic Society meeting, San Diego, Ca, October, 2009 and the North American Cystic Fibrosis Conference, Orlando, Fla., October 2009.
Competing Interests: Drs. Sorscher, Rowe, and Woodworth are inventors on a patent submitted regarding the possible activity of chloride secretagogues for therapy of sinus disease (Provisional Patent Application Under 35 U.S.C. § 111(b) and 37 C.F.R. § 1.53(c) in the United States Patent and Trademark Office).
References
- 1.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95(7):1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 2.Knowles MR, Durie PR. What is cystic fibrosis? N Engl J Med. 2002;347(6):439–442. doi: 10.1056/NEJMe020070. [DOI] [PubMed] [Google Scholar]
- 3.Cook NC, Samman S. Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. Nutritional Biochemistry. 1996;7:66–76. [Google Scholar]
- 4.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, Baker J, Kerr DJ. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res. 1996;2(4):659–668. [PubMed] [Google Scholar]
- 6.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45(11):2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Shoskes DA, Zeitlin SI, Shahed A, Rajfer J. Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology. 1999;54(6):960–963. doi: 10.1016/s0090-4295(99)00358-1. [DOI] [PubMed] [Google Scholar]
- 8.Pyle LC, Fulton JC, Sloane PA, Backer K, Mazur M, Prasain J, Barnes S, Clancy JP, Rowe SM. Activation of CFTR by the Flavonoid Quercetin. Potential Use as a Biomarker of {Delta}F508 CFTR Rescue. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virgin FW, Azbell C, Schuster D, Sunde J, Zhang S, Sorscher EJ, Woodworth BA. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. Laryngoscope. 2010;120(7):1465–1469. doi: 10.1002/lary.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virgin F, Zhang S, Schuster D, Azbell C, Fortenberry J, Sorscher EJ, Woodworth BA. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. Laryngoscope. 2010;120(5):1051–1056. doi: 10.1002/lary.20871. [DOI] [PubMed] [Google Scholar]
- 11.Azbell C, Zhang S, Skinner D, Fortenberry J, Sorscher EJ, Woodworth BA. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol Head Neck Surg. 2010;143(3):397–404. doi: 10.1016/j.otohns.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Fortenberry JA, Cohen NA, Sorscher EJ, Woodworth BA. Comparison of vectorial ion transport in primary murine airway and human sinonasal air-liquid interface cultures, models for studies of cystic fibrosis, and other airway diseases. Am J Rhinol Allergy. 2009;23(2):149–152. doi: 10.2500/ajra.2009.23.3285. [DOI] [PubMed] [Google Scholar]
- 13.Cohen NA, Zhang S, Sharp DB, Tamashiro E, Chen B, Sorscher EJ, Woodworth BA. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009 doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 14.Woodworth BA, Tamashiro E, Bhargave G, Cohen NA, Palmer JN. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers. Am J Rhinol. 2008;22(3):234–238. doi: 10.2500/ajr.2008.22.3178. [DOI] [PubMed] [Google Scholar]
- 15.Khalid AN, Woodworth BA, Prince A, Quraishi SA, Antunes MB, Long FH, Bolger WE, Chiu AG, Palmer JN, Cohen NA. Physiologic alterations in the murine model after nasal fungal antigenic exposure. Otolaryngol Head Neck Surg. 2008;139(5):695–701. doi: 10.1016/j.otohns.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, Cohen NA. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. Am J Rhinol. 2008;22(1):7–12. doi: 10.2500/ajr.2008.22.3125. [DOI] [PubMed] [Google Scholar]
- 17.Woodworth BA, Antunes MB, Bhargave G, Xiong G, Aguilar JL, Ratner AJ, Kreindler JL, Rubenstein RC, Cohen NA. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques. 2007;43(2):195–204. doi: 10.2144/000112531. [DOI] [PubMed] [Google Scholar]
- 18.Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: A comparative study. Am J Rhinol. 2007;21(5):533–537. doi: 10.2500/ajr.2007.21.3068. [DOI] [PubMed] [Google Scholar]
- 19.Woodworth BA, Zhang S, Tamashiro E, Bhargave G, Palmer JN, Cohen NA. Zinc increases ciliary beat frequency in a calcium-dependent manner. Am J Rhinol Allergy. 2010;24(1):6–10. doi: 10.2500/ajra.2010.24.3379. [DOI] [PubMed] [Google Scholar]
- 20.Rowe SM, Pyle LC, Jurkevante A, Varga K, Collawn J, Sloane PA, Woodworth B, Mazur M, Fulton J, Fan L, et al. DeltaF508 CFTR processing correction and activity in polarized airway and non-airway cell monolayers. Pulm Pharmacol Ther. 2010;23(4):268–278. doi: 10.1016/j.pupt.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 22.Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol. 1995;268(4 Pt 1):C886–893. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]
- 23.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124(2):125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Illek B, Fischer H. Flavonoids stimulate Cl conductance of human airway epithelium in vitro and in vivo. Am J Physiol. 1998;275(5 Pt 1):L902–910. doi: 10.1152/ajplung.1998.275.5.L902. [DOI] [PubMed] [Google Scholar]
- 25.Illek B, Fischer H, Machen TE. Alternate stimulation of apical CFTR by genistein in epithelia. Am J Physiol. 1996;270(1 Pt 1):C265–275. doi: 10.1152/ajpcell.1996.270.1.C265. [DOI] [PubMed] [Google Scholar]
- 26.Illek B, Lizarzaburu ME, Lee V, Nantz MH, Kurth MJ, Fischer H. Structural determinants for activation and block of CFTR-mediated chloride currents by apigenin. Am J Physiol Cell Physiol. 2000;279(6):C1838–1846. doi: 10.1152/ajpcell.2000.279.6.C1838. [DOI] [PubMed] [Google Scholar]
- 27.Schuier M, Sies H, Illek B, Fischer H. Cocoa-related flavonoids inhibit CFTR-mediated chloride transport across T84 human colon epithelia. J Nutr. 2005;135(10):2320–2325. doi: 10.1093/jn/135.10.2320. [DOI] [PubMed] [Google Scholar]
- 28.Hirsh AJ, Sabater JR, Zamurs A, Smith RT, Paradiso AM, Hopkins S, Abraham WM, Boucher RC. Evaluation of second generation amiloride analogs as therapy for cystic fibrosis lung disease. J Pharmacol Exp Ther. 2004;311(3):929–938. doi: 10.1124/jpet.104.071886. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo M, Sasaki N, Saga K, Kaneko T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol Pharm Bull. 2005;28(2):253–259. doi: 10.1248/bpb.28.253. [DOI] [PubMed] [Google Scholar]
- 30.Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007;137(11):2405–2411. doi: 10.1093/jn/137.11.2405. [DOI] [PubMed] [Google Scholar]
- 31.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen TD, Canada AT, Heintz GG, Gettys TW, Cohn JA. Stimulation of secretion by the T84 colonic epithelial cell line with dietary flavonols. Biochem Pharmacol. 1991;41(12):1879–1886. doi: 10.1016/0006-2952(91)90127-q. [DOI] [PubMed] [Google Scholar]