Abstract
Cytokinesis, the final stage of cell division, bisects the cytoplasm into two daughter cells. In mitotic cells, this process depends on the activity of non-muscle myosin II (NMII), a family of actin-binding motor-proteins that participate in the formation of the cleavage furrow. The relevance of NMII for meiotic cell division, however, is poorly understood. The NMII family consists of three members, NMIIA, NMIIB, and NMIIC, containing different myosin heavy chains (MYH9, MYH10, and MYH14, respectively). We find that a single non-muscle myosin II, NMIIB, is required for meiotic cytokinesis in male but not female mice. Specifically, NMIIB-deficient spermatocytes exhibit cytokinetic failure in meiosis I, resulting in bi-nucleated secondary spermatocytes. Additionally, cytokinetic failure at meiosis II gives rise to bi-nucleated or even tetra-nucleated spermatids. These multi-nucleated spermatids fail to undergo normal differentiation, leading to male infertility. In spite of the presence of multiple non-muscle myosin II isoforms, we demonstrate that a single member, NMIIB, plays an essential and non-redundant role in cytokinesis during meiotic cell divisions of the male germline.
Keywords: Spermatogenesis, Meiosis, Cytokinesis, NMIIB, MYH10, Mouse
Introduction
Cytokinesis, the final step of cell division in all animal cells, partitions the cytoplasm into two separate daughter cells. This process is regulated and executed by molecular machines that have been well characterized in eukaryotic cells (Glotzer, 2005). Attachment of a contractile ring, consisting of a network of actin and myosin filaments, to the cytoplasmic membrane induces the formation of a cleavage furrow that partitions the cytoplasm into two lobes. The assembly and constriction of the contractile ring is regulated by the RhoA GTPase, which stimulates actin polymerization and induces myosin activity. The final abscission requires cytoplasmic membrane fusion at the cleavage sites, involving the membrane vesicle trafficking system. The molecular mechanisms of cytokinesis have mainly been identified from studies on mitotic cell division. Genetic screens in Drosophila have begun to dissect the role of various proteins for cytokinesis during meiosis, a cell division mechanism unique to germ cells (Giansanti et al., 2001,2004). However, genetic studies of cytokinesis in mammalian meiosis are lacking, possibly hampered by the developmental lethality of mutants exhibiting cytokinetic failure in somatic tissues.
Unlike somatic cells that exhibit complete abscission, dividing germ cells of most organisms undergo incomplete cytokinesis and remain interconnected by cytoplasmic connections that serve various functions. In Drosophila, stable ring canals allow transfer of essential factors from associated nurse cells into the transcriptionally inactive oocyte (Mahowald, 1971). In the mammalian male germ line, all germ cells derived from a single spermatogonial stem cell remain connected by intercellular cytoplasmic bridges that allow passing of factors essential for germ cell synchronization (Dym and Fawcett, 1971; Greenbaum et al., 2011; Huckins, 1971). In addition to ubiquitous cytokinesis factors of the centralspindlin complex, these intercellular bridges also contain germ-cell specific factors. TEX14 was the first of these factors found to be essential for germ cell development (Greenbaum et al., 2006). TEX14 interacts with the centrosomal protein CEP55 to block cell abscission, the final step of cytokinesis, such that stable intercellular bridges remain between germ cells (Iwamori et al., 2010). In the absence of TEX 14, the formation of intercellular bridges is disrupted, and males become sterile due to meiotic arrest before the first meiotic cell division. Other components of intercellular bridges in germ cells include KIF23 and RBM44 (Greenbaum et al., 2007, 2009; Iwamori et al., 2011). Unlike TEX14, RBM44 is neither required for the formation of intercellular bridges nor for fertility (Iwamori et al., 2011). Citron kinase (CIT), a downstream effector molecule of Rho, localizes to the cleavage furrow and the midbody (Madaule et al., 1998). Although CIT is widely expressed, its ablation in mice causes cytokinetic failure in spermatogonia (mitotic germ cells) and proliferating neurons only, but does not affect the division of other cell types (Di Cunto et al., 2000, 2002).
In mammals, meiotic cytokinesis in germ cells is sexually dimorphic in respect to timing and symmetry. In the male germ line, cytokinesis begins with puberty and results in symmetric divisions. Cytokinesis of primary spermatocytes (during meiosis I) generates two secondary spermatocytes of equal size, which again divide symmetrically, such that each primary spermatocyte ultimately gives rise to four haploid round spermatids of equal size. In females, meiosis is initiated during embryogenesis, however, primary oocytes become arrested at the prophase of meiosis I around birth and only resume meiosis I upon ovulation, with highly asymmetrical cytokinesis that produces a large secondary oocyte and a small polar body almost devoid of cytoplasm. Only the secondary oocyte proceeds with the second meiotic division and becomes arrested at the metaphase stage of meiosis II. Only upon fertilization, the secondary oocyte resumes meiosis II and cytokinesis is again highly asymmetric, producing a large ovum and a small second polar body.
Cytokinesis involves a set of approximately 20 proteins that are evolutionarily conserved in diverse organisms including Drosophila, C. elegans, and vertebrates (Glotzer, 2005). Among these factors, non-muscle myosin II is a central component of the contractile ring that provides the motor activity for contraction (Mabuchi and Okuno, 1977; Straight et al., 2003). Non-muscle myosin II is essential for cytokinesis during mitosis. Injection of myosin II antibodies blocks cleavage of blastomeres in starfish (Mabuchi and Okuno, 1977). Blebbistatin, a specific inhibitor of non-muscle myosin II, inhibits the closure of the cleavage furrow in cultured cells by inhibiting non-muscle myosin II when it is in the detached state from actin (Kovacs et al., 2004; Straight et al., 2003). Found in all eukaryotic cells (Vicente-Manzanares et al., 2009), myosin II is a hexamer composed of a dimer of myosin heavy chains (MYH), a pair of essential light chains, and a pair of regulatory light chains. Non-muscle myosin II assembles to form bipolar myosin filaments and exert tension on actin filaments to initiate cytokinesis (Ma et al., 2012; Zhang and Robinson, 2005). In mammals, three different genes encode the non-muscle myosin heavy chain proteins MYH9, MYH10, and MYH14, which, in combination with common light chains, form the non-muscle myosin II isoforms NMIIA, NMIIB, and NMIIC, respectively (Vicente-Manzanares et al., 2009). While MYH9 and MYH10 share 80% amino acid identity, they both share 64% amino acid identity with MYH14 (Golomb et al., 2004). These three Myh genes have been studied by targeted gene inactivation. While mice lacking MYH14 are viable and display no obvious abnormalities (Ma et al., 2010), inactivation of MYH9 or MYH10 causes embryonic lethality (Conti et al., 2004; Tullio et al., 1997). MYH9-deficient embryos die by E7.5 (Conti et al., 2004). Inactivation of MYH10 causes embryonic lethality relatively late during gestation (between E14.5 and birth), and leads to cytokinetic failure in cardiac myocytes (Takeda et al., 2003; Tullio et al., 1997).
In mouse meiotic germ cells, MYH10 localizes to the contractile region of testicular spermatocytes (Manandhar et al., 2000). Although both MYH9 and MYH10 are expressed in oocytes, meiotic cell divisions are unaffected by microinjection of anti-MYH9 and/or anti-MYH10 antibodies into oocytes, leaving the functional requirement of non-muscle myosin II in meiosis unknown (Simerly et al., 1998). Here, we report the functional characterization of MYH10 in mouse germ cells and demonstrate that, in male mice, MYH10 is required for cytokinesis during meiosis I and meiosis II.
Materials and methods
Mice
Mice bearing the conditional Myh10fl allele, in which exon 2 is flanked with loxP sites, were generated previously (Ma et al., 2009) and are available from the Mutant Mouse Regional Resource Centers (Stock number: 016981-UNC; Allele symbol: Myh10tm7Rsad). Ddx4-Cre and Hspa2-Cre mice were obtained from the Jackson Laboratory (Stock numbers: Ddx4-Cre, 006954; Hspa2-Cre, 008870) (Gallardo et al., 2007; Inselman et al., 2010). Fig. S1 details the breeding strategy that relied on exclusively paternal transmission of the Ddx4-Cre allele. Ddx4-Cre is expressed in oocytes, such that its transmission through the female germline would cause recombination of all floxed alleles, including those from the sperm, at the zygote stage (Gallardo et al., 2007). Genotyping for Myh10 and Cre alleles was performed separately on genomic DNA isolated from tails. Mice were maintained and used for experimentation according to the guidelines of the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Western blotting analyses
Adult testes or ovaries from 2-month-old mice were homogenized in SDS-PAGE sample buffer using a glass homogenizer. 30 µg of protein lysates were used for gel electrophoresis. Western blotting was performed with the following antibodies: anti-MYH9 (1:500; Sigma-Aldrich), anti-MYH10 (1:1000, Sigma-Aldrich), anti-MYH11 (1:500; Abcam), and anti-β-actin (1:5000; Sigma-Aldrich).
Histology, electron microscopy (EM), and immunofluorescence
For histology, testes and epididymis were fixed in Bouin’s solution, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. EM of testes (fixed in 2.5% glutaraldehyde and 2% paraformaldehyde) was performed at the Biomedical Imaging Core facility at the University of Pennsylvania, as previously described (Yang et al., 2006). For immunofluorescence, testicular cells were fixed in 4% paraformaldehyde and stained with anti-β-tubulin (DSHB) and anti-ACRV1 antibodies (gift from P.P. Reddi, University of Virginia, Charlottesville, VA). Immunostaining with anti-ACRV1 or anti-TEX14 antibodies (gift from M.M. Matzuk, Baylor College of Medicine, Houston, TX) was also performed on 8-µm cryosections of testes that were fixed in 4% paraformaldehyde overnight, dehydrated in 30% sucrose solution, embedded with TBS tissue freezing medium, and frozen in ethanol/dry ice.
Measurement of DNA content
After dissection of cauda epididymides, cells were squeezed out of the tubules using forceps, fixed in 4% paraformaldehyde, adhered to glass slides, and stained with DAPI in antifade mounting medium (Vector Laboratories). Imaging was performed on an Axioskop 40 microscope (Carl Zeiss, Inc.) with a digital camera (Evolution QEi; MediaCybernetics). The DNA content in each cell was measured by quantifying the total DAPI signal using ImagePro software (Phase 3 Imaging Systems).
Results and discussion
MYH10 is required for fertility in males but not females
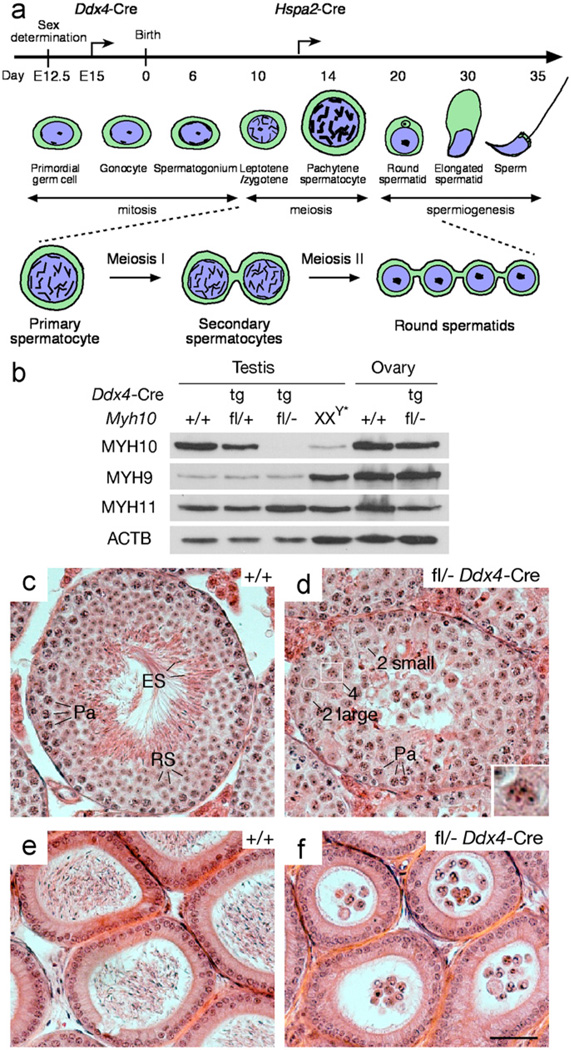
As germline ablation (ubiquitous deletion) of Myh10 in mice causes embryonic lethality between E14.5 and birth (Takeda et al., 2003), we evaluated the function of Myh10 in male and female germ cells by intercrossing mice bearing a conditional Myh10 allele (Myh10fl) with mice expressing Cre recombinase under the control of the germ cell-specific Ddx4 promoter through a two-generation breeding scheme (Fig. S1) (Gallardo et al., 2007; Ma et al., 2009). The conditional Myh10fl allele contains two loxP sites flanking exon 2, such that Cre-mediated recombination results in a frame shift upon deletion of this exon (Ma et al., 2009). Ddx4-Cre expression is restricted to the germline lineage and begins at embryonic day 15 (E15) in both testis and ovary (Fig. 1a) (Gallardo et al., 2007). MYH10 was abundantly detected in wild type testes but only at very low levels in XXY* testes, which are completely devoid of germ cells, showing that MYH10 is predominantly expressed in germ cells (Fig. 1b). Western blot analysis confirmed efficient ablation of MYH10 in the testes of Myh10fl/− Ddx4-Cre mice (Fig. 1b).
Fig. 1.
MYH10 is required for cytokinesis during male meiosis. (a) Developmental timeline of mouse spermatogenesis. Arrows mark the onset of expression of the Ddx4-Cre and Hspa2-Cre transgenes. Meiotic germ cells, including primary spermatocytes, secondary spermatocytes, and round spermatids are represented schematically, and intercellular bridges, resulting from incomplete cytokinesis, are shown. (b) Absence of MYH10 protein in Myh10fl/− Ddx4-Cre testes. Western blot analysis was performed on testicular or ovarian protein extracts from 2 month-old adult mice. The genotype of each sample, determined by analysis of tail DNA, is indicated above each lane. MYH11, the smooth muscle myosin II heavy chain, and ACTB served as loading controls (Deng et al., 1993). (c) Testicular seminiferous tubule from a 2-month-old wild type mouse contains a full spectrum of germ cells. (d) Tubules from 2-month-old Myh10fl/− Ddx4-Cre mice exhibit spermiogenic arrest. Germ cells with four nuclei (Inset, enlarged view of boxed area), two small nuclei, or two large nuclei are marked. (e) Epididymal tubules from 2-month-old wild type mice are filled with sperm. (f) Epididymal tubules from 2-month-old Myh10fl/− Ddx4-Cre mice lack sperm but contain round multi-nucleated cells. Sections (c–f) are stained with H&E. Abbreviations: Pa, pachytene spermatocytes; RS, round spermatids; ES, elongated spermatids. Scale bar, 50 µm.
Myh10fl/− Ddx4-Cre mice were viable without overt defects, but exhibited a sexually dimorphic fertility phenotype. Myh10fl/− Ddx4-Cre males were sterile, whereas Myh10fl/− Ddx4-Cre females were fertile. All 15 pups from Myh10fl/− Ddx4-Cre females sired by wild type males were of Myh10+/− genotype, suggesting that Cre-mediated ablation of Myh10 in oocytes is complete. However, as both MYH9 and MYH10 are expressed in oocytes (Simerly et al., 1998), MYH9 may compensate for MYH10 function in the oocytes of Myh10fl/− Ddx4-Cre females. In the testis, MYH9 is abundantly expressed in somatic cells, but mostly absent from germ cells, demonstrated by high levels of expression in germ-cell deficient XXY* testes, and very low levels in normal or Myh10 mutant testes (Fig. 1b). Therefore, absence of MYH10 is probably not compensated for by MYH9 in the male germ cells of Myh10fl/− Ddx4-Cre mice. In conclusion, these genetic studies demonstrate that MYH10 is required for male fertility.
MYH10 is essential for cytokinesis at meiosis I and meiosis II
Germ-cell specific inactivation of Myh10 resulted in sharply reduced testis size. Testes from 2 to 3-mo-old Myh10fl/− Ddx4-Cre mice weighed significantly less than those from age-matched Myh10+/− mice (122.2 ± 46.2 mg/pair versus 200.0 ± 15.7 mg/pair; n = 7, P < 0.0012). Seminiferous tubules of testes from adult mice typically contain a full spectrum of spermatogenic cells including spermatogonia, spermatocytes, and spermatids (Fig. 1c). In contrast, seminiferous tubules from adult Myh10fl/− Ddx4-Cre mice contained early germ cells, including spermatogonia and spermatocytes, but lacked mature spermatozoa, suggesting spermatogenic arrest (Fig. 1d and Fig. S2). In wild type seminiferous tubules, round spermatids contain a single nucleus per cell (Fig. 1c and Fig. S2a). Remarkably, MYH10-deficient seminiferous tubules contained many multi-nucleated cells with either two or four nuclei. The size of the nuclei of binucleated cells was either comparable to that of nuclei of round spermatids, or to that of secondary spermatocytes (Fig. 1d; Fig. S2b and Fig. S3). Consistent with the testicular histology, epididymal tubules from adult Myh10fl/− Ddx4-Cre mice lacked sperm but were filled with multi-nucleated round cells, including “tetrad” round cells with four nuclei (Fig. 1f).
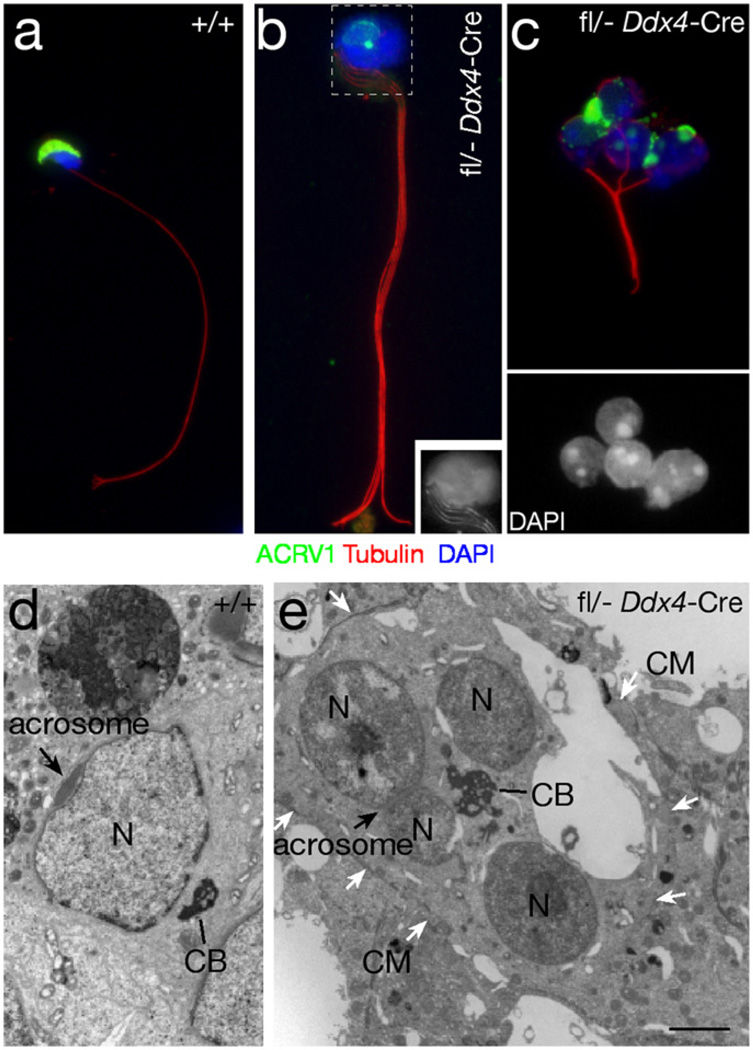
To further characterize these cells, we ascertained whether “tetrad” round cells, like haploid round spermatids, would undergo spermiogenesis, including flagellum assembly, nuclear elongation, and acrosome formation (Fig. 2a). Multi-nucleated cells contained abnormal acrosomal structures (Fig. 2b and c), and each cell assembled multiple axonemes (flagella), which were arranged in a “pony tail” (Fig. 2b) or a “bouquet” fashion (Fig. 2c). Most multi-nucleated cells did not undergo nuclear elongation (Fig. 2c). We next performed ultrastructural analysis of testis sections to visualize the acrosome and the chromatoid body, a prominent electron-dense multi-lobular nuage located in the perinuclear space of wild type round spermatids (Fig. 2d) (Fawcett et al., 1970). Multi-nucleated germ cells from adult Myh10fl/− Ddx4-Cre testes contained a large chromatoid body per cell and abnormal acrosomal structures (Fig. 2e). A single cell membrane encompassed the nuclei of multi-nucleated cells, illustrating that these cells differ from normal spermatids with surrounding membranes that are only connected by electrondense cytoplasmic bridges (Fig. 2e). These findings suggest that the multi-nucleated round cells adopt features of spermatids, and hence result from cytokinetic failure during meiosis I of male germ cell formation.
Fig. 2.
Structural analysis of multi-nucleated germ cells from the testes of 2-month-old Myh10fl/− Ddx4-Cre mice. (a–c) Immunofluorescence analysis of testicular germ cells, stained with an antibody against ACRV1 (green), a component of the acrosome (Reddi et al., 1995), and with an anti-tubulin antibody to visualize the axoneme (red). Nuclei are stained with DAPI (blue). (a) Wild type sperm. (b) MYH10-deficient germ cell with a bundle of four flagella. Inset: grayscale image showing the head (boxed area) with DAPI stained nuclei and four flagella (four bundles of microtubules). (c) MYH10-deficient germ cell with four nuclei (DAPI staining shown in bottom panel), bundles of microtubules, and defective acrosomes. (d and e) Ultrastructural analysis of a wild type spermatid (d), and a MYH10-deficient germ cell with four nuclei (e). N, nucleus; CB, chromatoid body; CM, cell membrane. White arrows mark the cell membrane. Black arrow marks the acrosome. Scale bar (d and e), 2 µm.
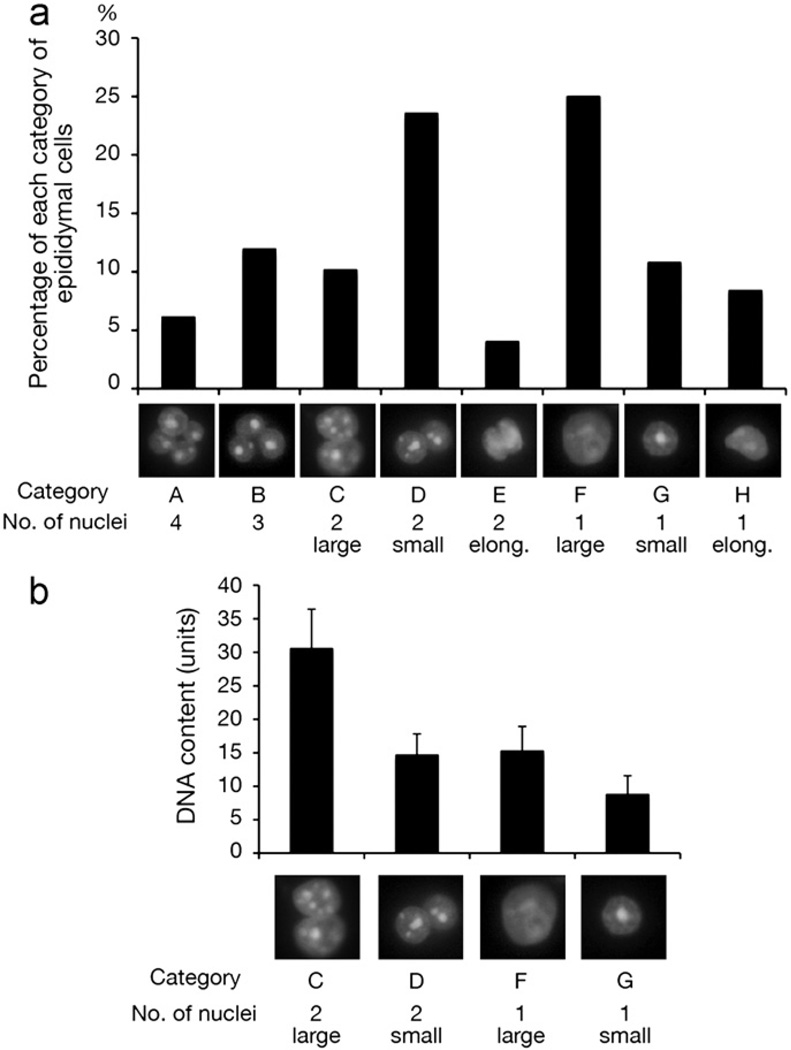
To determine whether MYH10 plays a role also during cytokinesis of male meiosis II, we characterized germ cells of Myh10fl/− Ddx4-Cre mice that had been sloughed off the testicular epithelium. Based on nuclear morphology, number of nuclei, and DNA content, the epididymal cells of MYH10-deficient testes could be grouped into eight categories (Fig. 3a). The majority of epididymal cells were germ cells, with the exception of cells with one large nucleus (Category F, Fig. 3a), which could also represent epididymal somatic cells. Consistent with our histological data (Fig. 1d and Fig. S3), a subset of cells contained four nuclei (Category A, Fig. 3a; 6% of all cells). We also detected cells with two large nuclei (category C) and cells with two small nuclei (category D). Cells with two large nuclei (category C) contained twice as much DNA as cells with two small nuclei (Fig. 3b), suggesting that category C cells contain two secondary spermatocyte nuclei as a result of cytokinetic failure in meiosis I (Fig. 1a). The DNA content of cells with two small nuclei (category D, Fig. 3b) was twice that of round spermatids (category G), indicating failure of cytokinesis during meiosis II in the absence of MYH10 (Fig. 1a). Intriguingly, a significant fraction (12%) of cells contained three nuclei (category B, Fig. 3a), suggesting that cytokinetic failure during meiosis I may often be followed by loss of one haploid nucleus during meiosis II. Such tri-nucleated spermatids have also been observed in Drosophila male meiotic mutants (Giansanti et al., 2004). Taken together, our results demonstrate that ablation of MYH 10 in male germ cells severely impairs cytokinesis during both meiosis I and meiosis II.
Fig. 3.
Nuclear morphology and DNA contents of MYH10-deficient epididymal germ cells. (a) Epididymal cells from 2-month-old Myh10fl/− Ddx4-Cre mice form eight distinct categories based on the number and size of nuclei. One representative nuclear image (DAPI) is presented for each category, and percentages refer to the total number of epididymal cells analyzed (n = 620). (b) Quantification of DNA content. The DNA content (shown in arbitrary units) was determined by quantification of the DAPI signal of 43 cells per category. Values shown represent the mean ± SD.
MYH10 is not required for cytokinesis of mitotic male germ cells (spermatogonia)
Spermatogenesis in Myh10fl/− Ddx4-Cre mice advanced to a late stage of meiosis, and seminiferous tubules did not exhibit detectable depletion of early germ cells, suggesting that cytokinesis during the preceding mitotic stages of germ cell development may not be impaired. To examine whether cytokinesis in mitotically dividing male germ cells occurred normally in the absence of MYH10, we visualized cytoplasmic bridges that connect dividing spermatogonia by immunostaining with anti-TEX14 antibodies (Fig. S4). TEX14 localizes to the intercellular cytoplasmic bridges in wild type germ cells (Fig. S4a) and is required for their formation (Greenbaum et al., 2006). In sections of postnatal day 10 testes and adult testes from Myh10fl/− Ddx4-Cre mice, cytoplasmic bridges were present between type A spermatogonia (Fig. S4b), between type B spermatogonia (Fig. S4c), and between preleptotene spermatocytes (Fig. S4d). Normal formation of cytoplasmic bridges between early spermatocytes and the absence of multi-nucleated spermatogonia in testes from Myh10fl/− Ddx4-Cre mice indicate that MYH10 is not essential for cytokinesis during mitotic divisions of spermatogonia.
Meiosis-specific ablation of MYH10 causes cytokinetic failures in spermatocytes
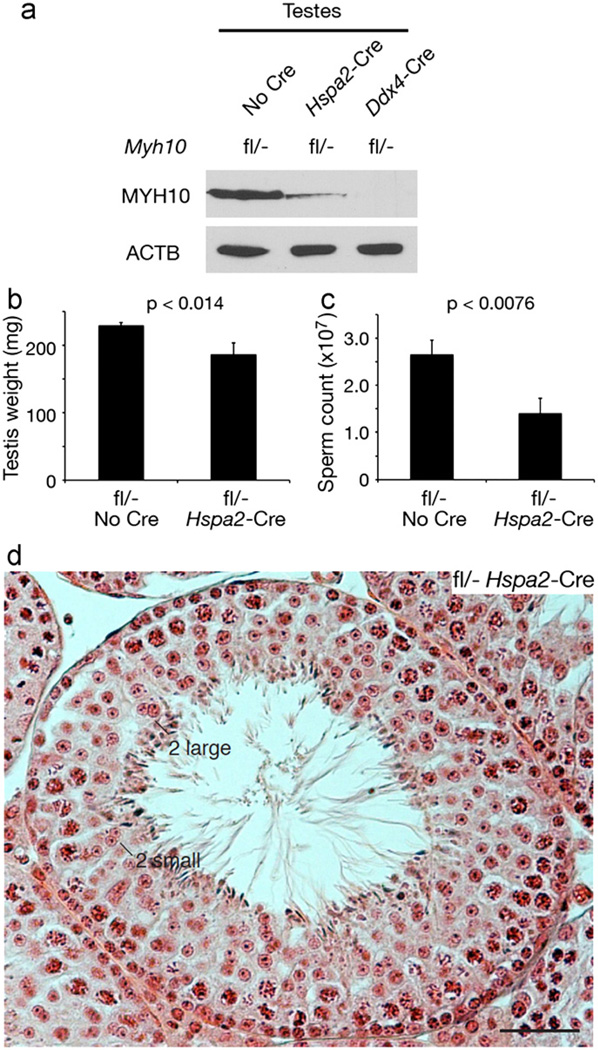
In Myh10fl/− Ddx4-Cre male mice, disruption of Myh10 begins in embryonic germ cells, prior to meiosis (Fig. 1a). We next examined the consequence of inactivation of Myh10 specifically during meiosis, by intercrossing Myh10fl/fl mice with Myh10fl/+ Hspa2-Cre mice, in which the onset of Cre expression occurs in leptotene/zygotene spermatocytes (Fig. 1a and Fig. S1) (Inselman et al., 2010). In testes from adult Myh10fl/− Hspa2-Cre mice, MYH10 protein was severely depleted but remained detectable at a low level (Fig. 4a). Testes from adult Myh10fl/− Hspa2-Cre mice weighed 19% less than those from controls (Fig. 4b), and sperm count in the cauda epididymides of mutant mice was reduced by nearly 50% (Fig. 4c). Seminiferous tubules of Myh10fl/− Hspa2-Cre mice (Fig. 4d) contained many bi-nucleated germ cells, including germ cells with two large nuclei, which may be equivalent to secondary spermatocytes, and germ cells with two small nuclei, which may be equivalent to round spermatids. In wild type seminiferous tubules, bi-nucleated spermatocytes or round spermatids were rarely observed. Myh10fl/− Hspa2-Cre testes also contained a substantial proportion of mono-nucleated secondary spermatocytes and round spermatids with normal appearance (Fig. 4d). Presumably, NMIIB from early germ cells is relatively stable, such that residual amounts of NMIIB protein (Fig. 4a) may be sufficient for cytokinesis in a subset of spermatocytes. Despite apparent defects in meiotic cytokinesis, Myh10fl/− Hspa2-Cre males were fertile. This was not caused by insufficient Cre activity, because all 34 offspring from wild type females sired by Myh10fl/− Hspa2-Cre males were Myh10+/− (tail DNA genotype) and none harbored the un-recombined floxed allele, demonstrating efficient Hspa2-Cre-mediated recombination. In conclusion, eliminating NMIIB specifically during meiosis, but not at earlier stages of germ cell development, confirms a specific role of NMIIB in male germline development restricted to the regulation of cytokinesis during meiosis.
Fig. 4.
Impaired meiotic cytokinesis in male germ cells of adult Myh10fl/− Hspa2-Cre mice. (a) Western blot analysis of MYH10 in testes from 2-month-old Myh10 mutant mice. ACTB served as a loading control. (b and c) Significant reduction in testis weight and sperm count in Myh10fl/−Hspa2-Cre mice at 2–3 months of age. Values are statistically significant by Student’s t-test. (d) Histological analysis (H&E) of testes from 2-month-old Myh10 mutant mice. Bi-nucleated germ cells with large and small nuclei are marked. Scale bar, 50 µm.
Notably, Myh10fl/− Ddx4-Cre mice exhibited a nearly complete failure of meiotic cytokinesis (Fig. 1d), and thus a much more severe phenotype than Myh10fl/− Hspa2-Cre mice (Fig. 4d). This difference in phenotype is likely associated with the continuous expression of Ddx4-Cre during germ cell development, from the embryonic germ cell through to the spermatocyte stage (Fig. 1a) (Gallardo et al., 2007). This should result in a complete depletion of MYH10 protein at the time when spermatogonia enter meiosis, rendering all spermatocytes in testes from Myh10fl/− Ddx4-Cre mice entirely MYH10-deficient. Consistent with this hypothesis, MYH10 protein was not detectable in testes from Myh10fl/− Ddx4-Cre mice by Western blot analysis (Fig. 1b and Fig. 4a).
Non-muscle myosin II is essential for mitotic cytokinesis in mammalian cells (Straight et al., 2003). Here, we demonstrate that of the three non-muscle myosin II isoforms in mammals (NMIIA, NMIIB, and NMIIC), a single myosin II, NMIIB, is essential for cytokinesis during mouse male meiosis. Consistent with such a role, MYH10 was previously shown to localize to the midbody in mouse spermatocytes (Manandhar et al., 2000). Conditional inactivation of Myh10 in cardiac myocytes causes cytokinetic defects in a subset of cells, resulting in multi-nucleation (Ma et al., 2009). Therefore, specific myosin II isoforms appear to play non-redundant roles in cytokinesis in certain cell lineages and during specific divisions. Citron kinase is a previously identified regulator of cytokinesis that is essential for mitotic division of male germ cells (Di Cunto et al., 2002). Here, we find that MYH10 is dispensable for mitotic cytokinesis in male germ cells, but required for meiotic divisions. The differences in MYH10 requirement between mitotic and meiotic cytokinesis could derive from compensation of MYH10 function by MYH9 during mitosis but not during meiosis. Alternatively, cytokinesis during male meiosis may have different MYH10-dependent requirements than during the preceding mitotic stages.
Our finding that MYH10 is not required for cytokinesis in female meiosis adds Myh10 to a large number of mouse meiotic mutants that cause male sterility but have little or no effect on female fertility (Handel and Schimenti, 2010; Hunt and Hassold, 2002). In most cases, the underlying mechanisms of such sexual dimorphism are not clear. Interestingly, neither TEX14 nor citron kinase are required for female fertility (Di Cunto et al., 2002; Greenbaum et al., 2009). Together with the studies on TEX14 and citron kinase, our study on MYH10 has uncovered a new aspect of sexual dimorphism in meiosis – regulation of cytokinesis. The underlying cause may lie in the different expression patterns of non-muscle myosin II isoforms: Myh10 is expressed in spermatocytes, whereas both Myh9 and Myh10 are expressed in oocytes and could thus be functionally redundant in female meiosis (Manandhar et al., 2000; Simerly et al., 1998). Alternatively, cytokinesis during meiosis differs in timing and symmetry between males and females, and may therefore be subject to sex-specific regulatory mechanisms and requirements that remain to be determined.
Supplementary Material
Acknowledgments
We thank Martin Matzuk for the anti-TEX14 antibody, Prabu Reddi for anti-ACRV1 antibodies, Xuefei Ma for technical assistance, Mary Anne Conti for comments on the manuscript, and Sigrid Eckardt for editing the manuscript. This work was supported by National Institutes of Health/National Institute of General Medical Sciences (Grant no. GM089893). The E7 anti-β-tubulin monoclonal antibody was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa.
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ydbio.2012.07.011.
References
- Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J. Biol. Chem. 2004;279:41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- Deng Z, Liu P, Marlton P, Claxton DF, Lane S, Callen DF, Collins FS, Siciliano MJ. Smooth muscle myosin heavy chain locus (MYH11) maps to 16p13.13-p13.12 and establishes a new region of conserved synteny between human 16p and mouse 16. Genomics. 1993;18:156–159. doi: 10.1006/geno.1993.1443. [DOI] [PubMed] [Google Scholar]
- Di Cunto F, Imarisio S, Camera P, Boitani C, Altruda F, Silengo L. Essential role of citron kinase in cytokinesis of spermatogenic precursors. J. Cell Sci. 2002;115:4819–4826. doi: 10.1242/jcs.00163. [DOI] [PubMed] [Google Scholar]
- Di Cunto F, Imarisio S, Hirsch E, Broccoli V, Bulfone A, Migheli A, Atzori C, Turco E, Triolo R, Dotto GP, Silengo L, Altruda F. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron. 2000;28:115–127. doi: 10.1016/s0896-6273(00)00090-8. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett DW. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol. Reprod. 1971;4:195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Eddy EM, Phillips DM. Observations on the fine structure and relationships of the chromatoid body in mammalian spermatogenesis. Biol. Reprod. 1970;2:129–153. doi: 10.1095/biolreprod2.1.129. [DOI] [PubMed] [Google Scholar]
- Gallardo T, Shirley L, John GB, Castrillon DH. Generation of a germ cell-specific mouse transgenic cre line vasa-cre. Genesis. 2007;45:413–417. doi: 10.1002/dvg.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti MG, Bonaccorsi S, Bucciarelli E, Gatti M. Drosophila male meiosis as a model system for the study of cytokinesis in animal cells. Cell Struct. Funct. 2001;26:609–617. doi: 10.1247/csf.26.609. [DOI] [PubMed] [Google Scholar]
- Giansanti MG, Farkas RM, Bonaccorsi S, Lindsley DL, Wakimoto BT, Fuller MT, Gatti M. Genetic dissection of meiotic cytokinesis in drosophila males. Mol. Biol. Cell. 2004;15:2509–2522. doi: 10.1091/mbc.E03-08-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, Goldin E, Conti RS, Sellers JR, Adelstein RS. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J. Biol. Chem. 2004;279:2800–2808. doi: 10.1074/jbc.M309981200. [DOI] [PubMed] [Google Scholar]
- Greenbaum MP, Iwamori N, Agno JE, Matzuk MM. Mouse TEX14 is required for embryonic germ cell intercellular bridges but not female fertility. Biol. Reprod. 2009;80:449–457. doi: 10.1095/biolreprod.108.070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum MP, Iwamori T, Buchold GM, Matzuk MM. Germ cell intercellular bridges. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a005850. a005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum MP, Ma L, Matzuk MM. Conversion of midbodies into germ cell intercellular bridges. Dev. Biol. 2007;305:389–396. doi: 10.1016/j.ydbio.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum MP, Yan W, Wu MH, Lin YN, Agno JE, Sharma M, Braun RE, Rajkovic A, Matzuk MM. TEX14 is essential for intercellular bridges and fertility in male mice. Proc. Natl. Acad. Sci. USA. 2006;103:4982–4987. doi: 10.1073/pnas.0505123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat. Rev. Genet. 2010;11:124–136. doi: 10.1038/nrg2723. [DOI] [PubMed] [Google Scholar]
- Huckins C. The spermatogonial stem cell population in adult rats. I their morphology, proliferation and maturation. Anat. Rec. 1971;169:533–557. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Hassold TJ. Sex matters in meiosis. Science. 2002;296:2181–2183. doi: 10.1126/science.1071907. [DOI] [PubMed] [Google Scholar]
- Inselman AL, Nakamura N, Brown PR, Willis WD, Goulding EH, Eddy EM. Heat shock protein 2 promoter drives cre expression in spermatocytes of transgenic mice. Genesis. 2010;48:114–120. doi: 10.1002/dvg.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamori T, Iwamori N, Ma L, Edson MA, Greenbaum MP, Matzuk MM. TEX14 interacts with CEP55 to block cell abscission. Mol. Cell. Biol. 2010;30:2280–2292. doi: 10.1128/MCB.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamori T, Lin YN, Ma L, Iwamori N, Matzuk MM. Identification and characterization of RBM44 as a novel intercellular bridge protein. PLoS One. 2011;6:e17066. doi: 10.1371/journal.pone.0017066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- Ma X, Jana SS, Conti MA, Kawamoto S, Claycomb WC, Adelstein RS. Ablation of nonmuscle myosin II-B and II-C reveals a role for nonmuscle myosin II in cardiac myocyte karyokinesis. Mol. Biol. Cell. 2010;21:3952–3962. doi: 10.1091/mbc.E10-04-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Kovacs M, Conti MA, Wang A, Zhang Y, Sellers JR, Adelstein RS. Nonmuscle myosin II exerts tension but does not translocate actin in vertebrate cytokinesis. Proc. Natl. Acad. Sci. USA. 2012;109:4509–4514. doi: 10.1073/pnas.1116268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Takeda K, Singh A, Yu ZX, Zerfas P, Blount A, Liu C, Towbin JA, Schneider MD, Adelstein RS, Wei Q. Conditional ablation of non-muscle myosin II-B delineates heart defects in adult mice. Circ. Res. 2009;105:1102–1109. doi: 10.1161/CIRCRESAHA.109.200303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi I, Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J. Cell Biol. 1977;74:251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, Ishizaki T, Narumiya S. Role of citron kinase as a target of the small GTPase rho in cytokinesis. Nature. 1998;394:491–494. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- Mahowald AP. The formation of ring canals by cell furrows in drosophila. Z. Zellforsch. Mikrosk. Anat. 1971;118:162–167. doi: 10.1007/BF00341561. [DOI] [PubMed] [Google Scholar]
- Manandhar G, Moreno RD, Simerly C, Toshimori K, Schatten G. Contractile apparatus of the normal and abortive cytokinetic cells during mouse male meiosis. J. Cell. Sci. 2000;113(Pt 23):4275–4286. doi: 10.1242/jcs.113.23.4275. [DOI] [PubMed] [Google Scholar]
- Reddi PP, Naaby-Hansen S, Aguolnik I, Tsai JY, Silver LM, Flickinger CJ, Herr JC. Complementary deoxyribonucleic acid cloning and characterization of mSP-10: the mouse homologue of human acrosomal protein SP-10. Biol. Reprod. 1995;53:873–881. doi: 10.1095/biolreprod53.4.873. [DOI] [PubMed] [Google Scholar]
- Simerly C, Nowak G, de Lanerolle P, Schatten G. Differential expression and functions of cortical myosin IIA and IIB isotypes during meiotic maturation, fertilization, and mitosis in mouse oocytes and embryos. Mol. Biol. Cell. 1998;9:2509–2525. doi: 10.1091/mbc.9.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kishi H, Ma X, Yu ZX, Adelstein RS. Ablation and mutation of nonmuscle myosin heavy chain II-B results in a defect in cardiac myocyte cytokinesis. Circ. Res. 2003;93:330–337. doi: 10.1161/01.RES.0000089256.00309.CB. [DOI] [PubMed] [Google Scholar]
- Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc. Natl. Acad. Sci. USA. 1997;94:12407–12412. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, De La Fuente R, Leu NA, Baumann C, McLaughlin KJ, Wang PJ. Mouse SYCP2 is required for synaptonemal complex assembly and chromosomal synapsis during male meiosis. J. Cell Biol. 2006;173:497–507. doi: 10.1083/jcb.200603063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Robinson DN. Balance of actively generated contractile and resistive forces controls cytokinesis dynamics. Proc. Natl. Acad. Sci. USA. 2005;102:7186–7191. doi: 10.1073/pnas.0502545102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.