Abstract
Many water treatment technologies for arsenic removal that are used today produce arsenic-bearing residuals which are disposed in non-hazardous landfills. Previous works have established that many of these residuals will release arsenic to a much greater extent than predicted by standard regulatory leaching tests (e.g. the toxicity characteristic leaching procedure, TCLP) and, consequently, require stabilization to ensure benign behavior after disposal. In this work, a four-step sequential extraction method was developed in an effort to determine the proportion of arsenic in various phases in untreated as well as stabilized iron-based solid matrices. The solids synthesized using various potential stabilization techniques included: amorphous arsenic-iron sludge (ASL), reduced ASL via reaction with zero valent iron (RASL), amorphous ferrous arsenate (PFA), a mixture of PFA and SL (M1), crystalline ferrous arsenate (HPFA), and a mixture of HPFA and SL (M2). The overall arsenic mobility of the tested samples increased in the following order: ASL > RASL > PFA > M1 > HPFA > M2.
Keywords: Arsenic-bearing residual, Sequential extraction, Arsenic leaching, Ferrous arsenate, Iron oxide, Crystallization
Introduction
The deleterious impact of arsenic in drinking water is now recognized even at relatively low concentrations (Bodwell et al., 2004, Ahsan et al., 2006, Smith et al., 2006). As a consequence, the maximum contaminant level (MCL) of arsenic in drinking water was reduced from 50 µg/L to 10 µg/L by the U.S. Environmental Protection Agency (EPA), with compliance beginning March 2006. It is estimated that about 4,000 tons of arsenic bearing solid residuals (ABSR) will be generated annually by arsenic removal processes applied to drinking water treatment (Ela, 2006). Although dissolution of naturally occurring arsenic-containing minerals is the primary source of contamination, mining activities, wood preservation processes and pesticide production are other major arsenic waste sources.
Regulations by the US EPA suggest assessment of potential toxic waste mobilization using the TCLP test. However, a number of studies have shown that the TCLP significantly underestimates arsenic leaching from ABSR disposed in landfills due to the poor simulation of landfill conditions with regards to pH and redox potential (Ghosh et al., 2004). Because of this, more aggressive and specific tests are needed for the particular assessment of arsenic mobilization. A method that evaluates the long-term leachability potential of arsenic is the use of continuous column tests within a simulated landfill environment in terms of pH, redox potential, and biological activity. Although column leach testing provides a realistic simulation tool to examine the interactions between ABSR and particular surroundings, the long testing time, months to years, limits its application for routine risk assessment of contaminated solid waste (Tan et al., 2006, Ghosh et al., 2006). Compared with column tests, a relatively simple and more widely used method is sequential extraction, which can provide information on proportion of a contaminant in different phases and consequently on its leachability and bioavailability (Tessier et al., 1979, Shiowatana et al., 1006). A sequential extraction test employs increasingly aggressive leaching fluids to target specific chemical or physical aspects of the solid waste. In this process, the extraction fluid is sequentially removed and replaced with progressively more aggressive extractants. The concentrations of the contaminants measured in the serial leachate samples provide phase-association information about the contaminant distribution within a solid.
Although sequential extraction procedures have been applied to a variety of metal contaminants including Hg, Se, Cu, and Pb, the study of the stability of ABSR has only received attention since the new regulation for arsenic in drinking water was issued in 2001 (Krysiak and Karczewska, 2007). Due to the similarity of arsenic and phosphorus in bonding properties, most sequential extraction methods for extracting arsenic from arsenic contaminated soil and sediment use phosphorous containing extractants (Shaw, 2004, Van Herreweghe et al., 2003, Mihaljevič et al., 2003). Ideally, a sequential extraction test would be such that one extraction fluid targets only one phase of the arsenic mineral matrix. The fraction of arsenic in soil and sediment has been classified as: (1) exchangeable, (2) bonded to carbonate, (3) bonded to iron oxide, (4) bonded to organic matter, and (5) residuals (Tessier et al., 1979). The overall strength of the extractants has been reported as: H2O < 1.0 M MgCl2 < 1.0 M NaH2PO4 < 0.20 M Ammonium oxalate < 10 M HF < 16 M HNO3 < 16 M HNO3 + 30% H2O2 (Keon et al., 2001). Although the sequential extraction method has been used for arsenic extraction from natural contaminated soil and sediment, to our knowledge there have been no reported applications to synthesized arsenic residuals, such as arsenic sludge generated from water treatment facilities and synthetic arsenic minerals. In this research, we apply a simplified sequential extraction method to a suite of arsenic solid wastes for identification of arsenic phases including adsorbed, amorphous, and crystalline mineral phases.
The main aim of this work is to understand the distribution of arsenic in various potential phases in the arsenic-bearing solids and, as a consequence, to better understand and predict their stability under particular conditions. For this purpose, we develop a sequential extraction test for application to arsenic solid wastes, which potentially might include the arsenic sludge generated by iron-based adsorption processes, crystallized arsenic solids produced from arsenic treatment sludges, and synthetic iron-arsenic minerals. The development of the sequential extraction test required the selection of proper extractants, determination of appropriate contact times, and correlation of the test results with other characterization techniques (e.g., XRD, SEM, standard leaching tests, isotherms). Once the test procedures were developed, experiments were performed to determine the relative contributions of the different arsenic phases associated with the iron-arsenic minerals that are of interest as potential stable repositories for arsenic in water treatment residuals. With this information, it is possible to evaluate and predict the feasibility of the proposed arsenic sludge treatment technology and, eventually, to establish appropriate strategies to stabilize arsenic waste subjected to long-term disposal.
Materials and methods
All the reagents were analytical grade and used as purchased without any further purification. All solutions were made with deoxygenated deionized (DI) water obtained by boiling DI water for about 1 h with continuous flushing by argon or nitrogen gas. All reactors, glassware and tools were flushed for 10 minutes using nitrogen gas to remove oxygen.
In what follows, we describe the preparation methods for the solids that were subjected to the sequential extraction procedure developed in this work. Amorphous ferrihydrite was prepared as a control solid, and arsenic-iron sludge was used as a basis for comparison to other, more stable solids, because it is a common waste generated in arsenic separation processes. Reduced sludges were prepared as potential candidates for arsenic immobilization.
Amorphous (PFA) and Crystalline (HPFA) Ferrous Arsenate Preparation
Ferrous arsenate materials were prepared by mixing 0.05 M FeSO4.7H2O and 0.05 M Na2HAsO4.7H2O solutions at a Fe:As molar ratio of 3:2 to a total volume of 300 mL in a 500 mL beaker under a nitrogen atmosphere. The mixture was continuously stirred using a magnetic stirrer until the pH of the solution varied less than 0.05 units within 30 minutes, and a light green precipitate was observed. The resultant slurries were transferred to a Teflon-lined Parr reactor (Parr 5500) and cured for (1) 2 days at 25°C, which generates PFA, and (2) 2 days at 160°C, which generates HPFA. Nitrogen was used to maintain a pressure of 8 bars within the reactor to ensure oxygen free conditions. At the end of the synthesis process, the precipitates were filtered using 0.45-µm membrane filters and washed subsequently three times using 0.5 L deoxygenated DI water. The washed precipitates were then dried in a nitrogen evaporator (Evaporator Associate Inc., N_EVAP 112) or a glove box (Terra Universal 100) at room temperature. Dried samples were stored in glass vials at room temperature for solid phase characterization and solubility tests.
Amorphous Arsenic-iron Sludge Preparation (ASL)
The arsenic-iron sludge was prepared by mixing 1 L of 0.169 M NaH2AsO4.7H2O and 1 L of 0.96 M FeCl3.6H2O solutions in a 3 L beaker with a Fe:As molar ratio of 5.5:1. The mixture was rapidly stirred at 200 rpm using an overhead stirrer (Arrow 1750). This mixture was allowed to equilibrate for 1 h before the solution was adjusted to pH of 7 using 10 M NaOH. After pH adjustment, the sludge mixture was shaken on a reciprocating shaker table (Orbit, reciprocating speed of 30 rpm) for 30 minutes to ensure homogenization without causing breakup of the flocculated particles. The sludge mixture was allowed to settle and equilibrate for 14 days at room temperature. During this equilibration period, pH fluctuations of ± 0.5 units were observed. The fluctuations were mitigated by periodic adjustment with 0.1 M HCl or 0.1 M NaOH. At the end of the precipitation process, the precipitate was filtered using 0.45-µm membrane filters and a vacuum filtration system, to achieve a water content of 65% wt-wt. The semi-dried sludge was stored in glass-stoppered bottles at 4°C for further characterization and extraction tests.
Reduced Arsenic-iron Sludge Preparation (RASL)
The reduced arsenic-iron sludge was prepared by mixing 100 g of prepared ASL sample and 4 g of zero-valent iron (ZVI) powder (average particle size = 35 µm) in 300 mL DI water under a nitrogen environment. The mixture was then continuously stirred using a magnetic bar at the rotation speed of 500 rpm for 24 hours. During this period, the pH of the suspension was maintained at 7 by adding 0.1 M NaOH or 0.1 M HCl periodically. At the end of the process, the liquid and solid phases were separated using a centrifuge system (Beckman J2-21) at 5000 rpm for 10 minutes. The liquid phase was analyzed for chemical constituents. The solid phase was tested for water content, and was also digested for chemical analysis.
Amorphous Ferrihydrite Sludge Preparation (SL)
A ferrihydrite sludge without arsenic was synthesized to use as a baseline material against which to compare the rest of the synthesized solids and to prepare mixtures with other solids. This sludge was prepared by titrating 1 L of 0.96 M FeCl3.6H2O solution using 10 M NaOH to the pH of 7 within 1 hour. The suspension was then allowed to settle and equilibrate for 14 days at room temperature. During this period of time, pH fluctuated within ± 0.5 units. These fluctuations were mitigated by periodic adjustment using 0.1 M HCl or 0.1 M NaOH. The same filtration and storage protocols as for the preparation of arsenic-iron sludge were used in preparing the AFH (amorphous ferrihydrite) sludge samples.
Sequential Extraction Test
Step 1: A 1.0 M MgCl2 solution at pH 7 was used to extract exchangeable arsenic phases. MgCl2 is a typical extractant used for trace metals, and was adopted to extract the iron-bound arsenic through anion exchange of Cl for arsenate (Ruttenberg, 1992).
Step 2: 1 M NaH2PO4 solution at pH of 5 was used to target strongly adsorbed As phase on the surface of iron oxide (ferrihydrite, Fe(OH)3). Due to the similarity between P and As, a high concentration of phosphate solution has been considered as the best candidate to remove arsenic from most sorbent materials such as iron oxides and aluminum oxides (Alam et al., 2001).
Step 3: 1.0 M HCl solution was applied to solubilize the amorphous iron (Fe (III) and Fe(II)) arsenate species (Keon et al., 2001). Common amorphous minerals phases presented in our samples are scorodite (FeAsO4.2H2O) and ferrous arsenate (Fe3(AsO4)2).
Step 4: 1.0 M Na-citrate at pH 5 was used to extract the crystalline arsenic via coordination with iron.
The sequential extraction test was applied to each of the materials generated in this work.
Continuous Column test vs. Batch Test Set-up
Both column and batch tests were investigated to determine the best method for extraction of the prepared samples. Amount of sample used and conditions are specified in the Results and Discussion section. Extraction fluid was pumped up-flow through the column. A small filter disc prevented solids leaving the column and a nitrogen purge was maintained in the collection container to minimize ferrous iron oxidation. The batch sequential extraction experiment was conducted in a sealed reactor shaken on a Lab-line orbital shaker. After a predetermined contact time (see Results and Discussion), the sample was centrifuged and decanted. The continuous-flow method had the advantages of simplicity (no need for filtration for each step), less risk of contamination, and less variation in extraction conditions, yet it was discontinued due to the slow flow rate of the extraction fluid and ineffective mixing inside the system, which prevented the arsenic and iron concentrations from reaching an equilibrium within a reasonable time period. The batch experiment was then determined to be the best option and was utilized in all subsequent trials.
Characterization and Analytical Methods
To identify the crystalline phases present in the solid samples, X-ray diffraction patterns were collected at room temperature using a Bruker D8 ADVANCE powder diffractometer, using Cu radiation. The diffractometer was equipped with scintillation and energy dispersive detectors to handle fluorescent material. Diffraction patterns for selected samples were recorded by continuous scans from 5° to 90° at 2.0 s per 0.01° step with 40 kV and 40 mA. This results in a 283-minutes scan. The machine is equipped with a 0.6 mm incident beam divergence slit, a 0.6 mm diffracted beam anti-scatter slit, a diffracted beam Ni filter, and a 0.1 mm detector receiving slit. The powdered samples were mounted on a round sample holder 5 mm in diameter and 8 mm thickness. Crystalline phases were identified using the Bruker software and the International Centre for Diffraction Data (ICDD) database.
Scanning Electron Microscope (SEM) images and Energy Dispersive X-Ray Spectroscopy (EDS) spectra of the samples were recorded using a Hitachi S-2460N Scanning Electron Microscope. The SEM images allow for identification of the microstructures of the solid samples, and EDS identifies the elemental composition of the samples. Most elements are detected at concentrations on the order of 0.1%.
The total concentration of dissolved arsenic and iron in solution was determined by a Perkin-Elmer Optima 5300 DV inductively-coupled plasma optical emission spectrometer (ICP-OES). The gas flow to the nebulizer was 0.60 L/min and the sample flow rate was 1.5 mL/min. The OES detector has detection limits of 0.01 ppm and 0.005 ppm for arsenic and iron, respectively. Samples with arsenic concentration less than 20 ppb were rerun in a Perkin-Elmer graphite-furnace Atomic Absorption (AA) spectrometer. The instrument was operated using a Perkin-Elmer software interface and protocols adapted from EPA testing method SW846 6020A for ICP-MS (EPA, 2007). The samples were diluted to the measurable range of the instrument using 2% HCl solution. Dilution factors varied depending on the constituent concentration. Typically, a 10 mL solution was prepared for analysis. An analytical blank was prepared for every batch test. The primary chemicals used to prepare the calibration and quality control standards and reagents were of analytical grade.
Results and Discussion
Characterization of Solid Samples
Table 1 summarizes the samples synthesized for subsequent use in the sequential extraction tests. The solids encompass the range of ferrous arsenate materials that were synthesized as potential materials for arsenic solid waste stabilization. Sample SL is poorly crystalline ferrihydrite (AFH, Fe(OH)3) with no arsenic present, which is formed when Fe(III) precipitates from neutral to alkaline solutions at ambient temperature (Cornell and Schwertmann, 2003). Both XRD and SEM results revealed that this type of material was completely amorphous with no distinguishable crystalline structure.
Table 1.
Summary of samples for sequential extraction tests
| Sample | Sample Description | Water content (wt %) |
Crystallinity | Possible arsenic phase |
|---|---|---|---|---|
| SL | Ferric hydroxide sludge | 61.2 | Amorphous | -- |
| ASL | Arsenic-iron sludge | 64.6 | Amorphous | Exchangeable, adsorbed |
| RASL | Reduced arsenic-iron sludge | 62.3 | Amorphous | Exchangeable, adsorbed, ferrous arsenate |
| PFA | Pure ferrous arsenate synthesized at T = 25°C | dry | Amorphous | Exchangeable, amorphous, ferrous arsenate |
| HPFA | Pure ferrous arsenate synthesized at T = 160°C | dry | Crystalline | Exchangeable, ferrous arsenate |
| M1 | Mixture of SL and PFA | -- | Amorphous | Exchangeable, adsorbed, ferrous arsenate |
| M2 | Mixture of SL and HPFA | -- | Partially crystalline | Exchangeable, Adsorbed, Ferrous arsenate |
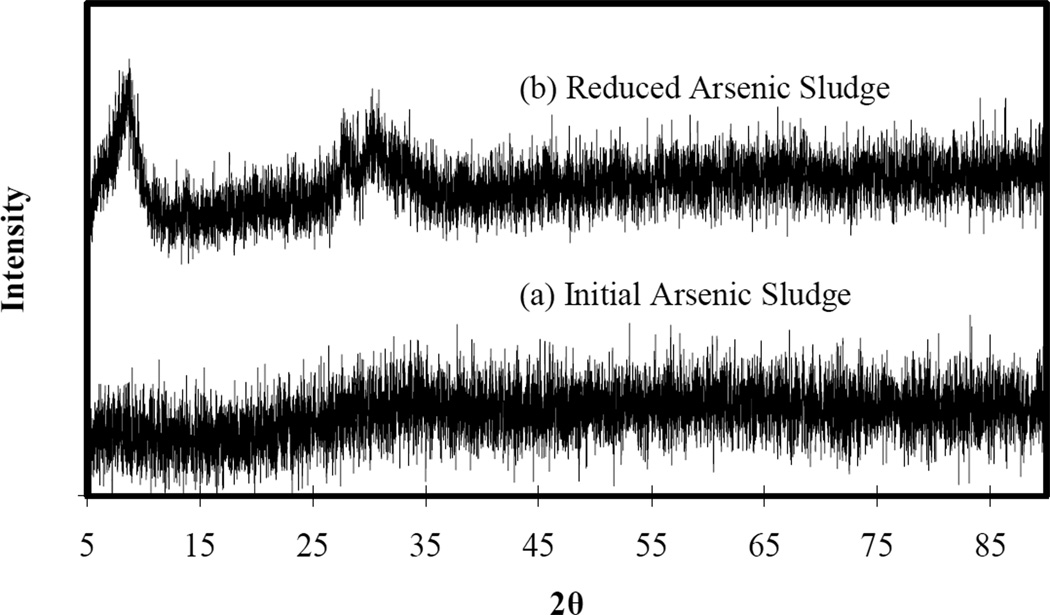
Sample ASL, arsenic-iron sludge, is a typical iron rich, arsenic-bearing solid waste form of AFH, but with arsenic present as arsenate. It is the solid residual, with varying arsenic content, of a number of arsenic removal processes for treating drinking water and brines (e.g., coagulation-assisted microfiltration, ion exchange regenerant purification, enhanced coagulation/flocculation) (Jekel and Amy, 2006). There was no crystalline phase identified in XRD analysis of ASL (results not shown). Addition of zero valent iron to ASL produced a reduced form of arsenic sludge. Our work has indicated (results not shown) that RASL contains various combinations of iron oxides (both ferric and ferrous forms) and ferrous arsenate (Fe3(AsO4)2), depending on the conditions during the contacting of ASL with zero valent iron. For this particular sample, XRD results show that poorly crystalline phase(s) appeared during the reduction process, although they are not identifiable from the ICDD database as to whether they are arsenic-containing materials or just a more crystalline iron oxide structure (Figure 1).
Figure 1.
XRD pattern of initial and reduced arsenic-iron sludge (RASL).
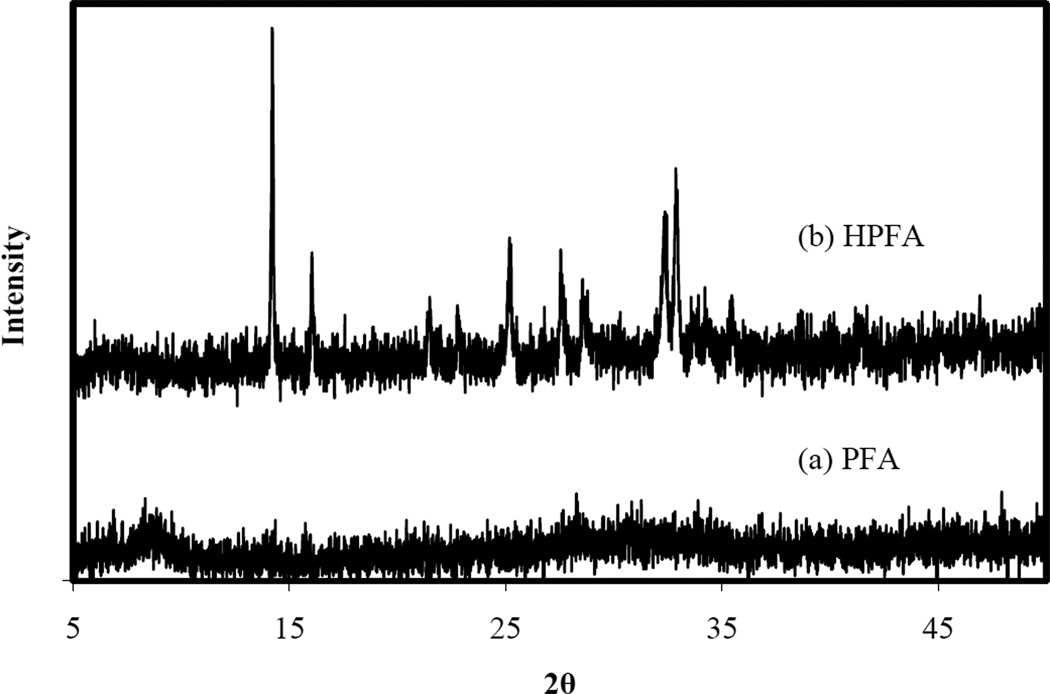
Sample PFA is a ferrous arsenate mineral synthesized at 25°C. It contains only Fe3(AsO4)2 as confirmed by SEM-EDS analysis, but with no distinguishable crystal structure evident in XRD scans (Figure 2). Sample HPFA has the same chemical composition as PFA but was synthesized at high temperature (160°C). Results of XRD show that the bulk of HPFA was a crystalline phase as evidenced by the sharp, high intensity XRD peaks (Figure 2). Although the dominant arsenic phase in both samples PFA and HPFA is ferrous arsenate, iron oxide and adsorbed arsenic might be present, yet not detected by XRD due to their low relative concentration or poorly crystalline nature. Samples M1 and M2 were mixtures of SL with PFA and HPFA, respectively. It is worth noting that all the arsenic-containing solids samples for sequential extraction tests in Table 2 contained the same total mass of arsenic, so as to facilitate comparison of the extractability of arsenic from the different minerals. For samples ASL, M1, and M2, not only was the mass of arsenic same but the molar ratio of iron to arsenic was also fixed at 5.5 (Table 2).
Figure 2.
XRD patterns of ferrous arsenate samples synthesized at 25°C (PFA) and 160°C (HPFA).
Table 2.
Summary of initial chemical compositions of testing samples. Composition of each solid was determined after microwave digestion of a sample.
| Sample | SL | ASL | RASL | PFA | HPFA | M1 | M2 |
|---|---|---|---|---|---|---|---|
| Mass (g) | 29 | 40 | 43.44 | 2.2 | 2.20 | SL: 29 PFA: 2.2 |
SL: 29 HPFA: 2.2 |
| Total As (mg) | -- | 831 | 831 | 831 | 831 | 831 | 831 |
| Total Fe (mg) | 2845 | 3415 | 6815 | 931 | 931 | 3415 | 3415 |
| Fe/As (Molar) | -- | 5.5 | 11.0 | 1.5 | 1.5 | 5.5 | 5.5 |
Sequential extraction
The sequential extraction concept is based on the premise that, ideally, each extraction fluid targets only one phase of the arsenic mineral matrix. Most sequential extraction procedures have been developed for soil or sediment that contains mutable mineral phases. The arsenic-associated phases in these type of matrices normally are categorized as exchangeable, bound to carbonates, bound to Mn and Fe oxyhydroxides, bound to crystalline iron oxides, bound to sulfides, bound to organic mater, and bound to silicates (Tessier et al., 1979, Borovec, 1993). One extractant chemical with proper concentration and contact time is assumed to dissolve a single solid phase and allow for measurement of the arsenic associated with that phase by sampling the final extractant liquid. It is often necessary to determine the appropriate reaction time for each step in a preliminary rate study to demonstrate that the dissolution of the target phase is complete or nearly complete and little is dissolved from other phases. Compared with natural soil and sediment, the synthetic samples used in this study are less complex in that they contain fewer potential solid phases. The possible arsenic-associated phases in the samples include soluble arsenate ion, arsenic adsorbed on the surface of iron oxides, amorphous ferric arsenate (FeAsO4), amorphous and/or crystalline ferrous arsenate (Fe3(AsO4)2), and other residuals.
To evaluate the optimum length of time for extraction of the exchangeable arsenic phase, the prepared solids were contacted with 1 M MgCl2 solution at pH of 7 and aqueous samples were withdrawn as a function of time. The mixed slurry was continuously stirred by a magnetic stirrer. The results of the amount of As and Fe leached as a function of time for ASL (arsenic sludge) and RASL (reduced arsenic sludge) show that the extraction of As and Fe was a relatively slow process. For both samples, the equilibrium between solid phase and soluble phase was approximated after 16 hours of contact. Subsequent extractions used 16 hours as the minimum time required for extraction of the exchangeable arsenic phase.
To evaluate the required contact time for the adsorbed arsenic extraction by phosphate, the solids that had been previously extracted with MgCl2 solution were contacted with 1 M NaH2PO4 at pH of 5 and samples were withdrawn as a function of time. One significant observation was that much more arsenic than iron was leached out for both samples. This can be explained by the nature of the extractant, which contains phosphate that competes strongly with arsenate for adsorption sites (Ghosh et al., 2006, Dixit and Hering, 2003, Manning and Goldberg, 1996) on iron-based media, but has little effect on the solid iron phase. Although the extraction process did not reach complete equilibrium during the testing period of 24 hours, this extraction time was considered long enough to extract most of adsorbed arsenic phase.
Following similar procedures as for exchangeable and sorbed arsenic extraction, required leaching times were determined for 1.0 M HCl solution and 2.0 M Na-citrate solution at pH of 5. The results indicate that extraction of amorphous iron arsenate minerals can be completed within 4 hours and the crystalline arsenic minerals can be fully extracted in 10 hours.
Table 3 summarizes the sequential extraction test procedures developed for arsenic-bearing solid materials. For the sequential extraction tests, the amount of solid varied depending on its water content and chemical composition, however, the ratio of dried solid to liquid was kept constant at 1:50. The mixture of extraction solution and solid sample was continuously stirred using a magnetic stirrer at 400 rpm. After each step of extraction, the liquid phase was separated from the solids by centrifugation and a liquid sample was taken for analysis of As and Fe. The next extractant was then added and mixed thoroughly to repeat the process. Once all extractants had been used, the solid sample was dried in an oxygen free glove box (Terra Universal 100) at room temperature for 7 days. The dried samples were then stored and preserved in a 4 degree freezer for later solid phase characterization.
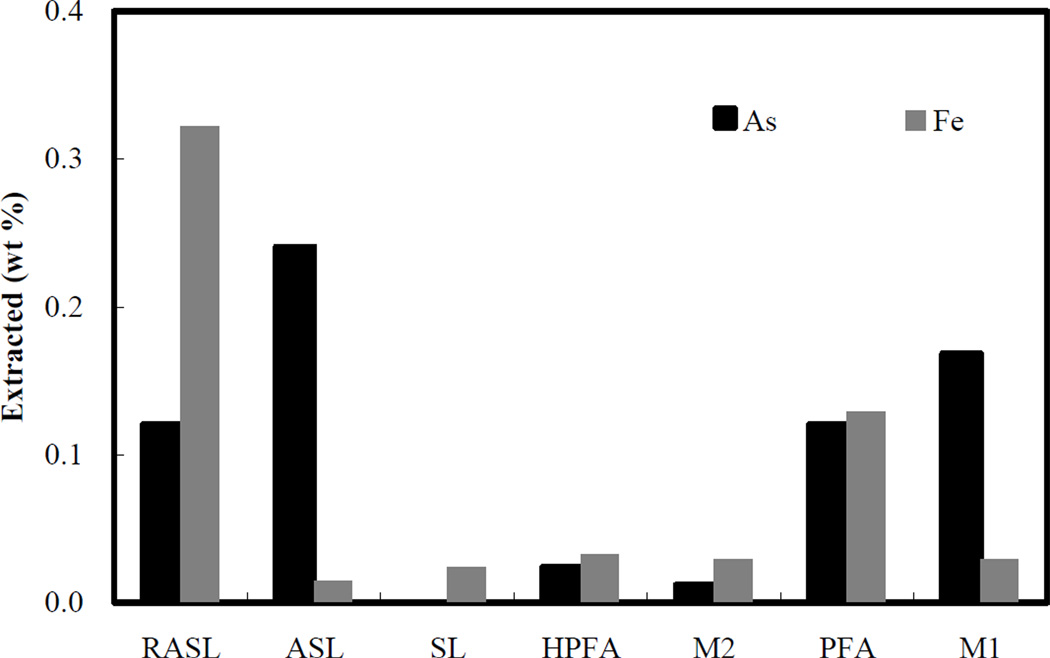
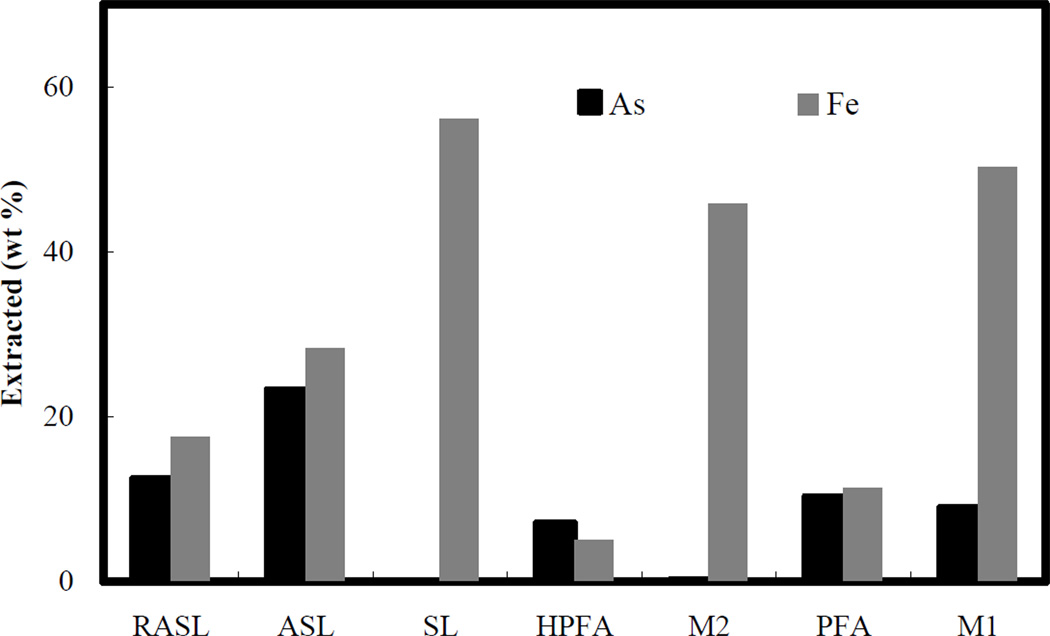
Step 1 (treatment with MgCl2). The mass of arsenic and iron extracted by MgCl2 is shown in Figure 3 as a percentage of the total arsenic and iron masses in the original samples. The extracted arsenic in this stage represents the exchangeable arsenic phase. Since all samples were rinsed with DI water after the synthesis process, there should not be any highly soluble arsenic salts associated with any of the samples. In addition, MgCl2 in solution is essentially an indifferent electrolyte with respect to ionic bound arsenic and iron-arsenic solids, as chloride is only a very weak anion competitor with arsenic (Ruttenberg, 1992). Consequently, the amount of arsenic extracted in this step can be considered to represent the equilibrium concentration dictated by the water/solid adsorption isotherm at pH 7. For all samples, the extracted arsenic fraction is less than 0.3%, indicating that nearly all of the arsenic in the solids was strongly sorbed or precipitated within the solid matrix. The arsenic solubility of different samples followed the sequence ASL > M1 > PFA > RASL > HPFA > M2, which suggests that as the proportion of reduced iron and solid crystallinity increases, the proportion of arsenic extracted in this step decreases. The larger faction of iron extracted by MgCl2 from RASL is explained by the greater solubility of Fe(II) with respect to Fe(III) (compare RASL vs. ASL). A decreased solubility of iron is also evident as the crystallinity of the ferrous solids increases (RASL>PFA>HPFA).
Step 2 (treatment with NaH2PO4). The fraction of arsenic extracted by phosphate varies from 0.1% (M2) to 32% (ASL), indicating a significant difference in the proportion of adsorbed arsenic in the different samples (Table 4). The order of samples based on arsenic extraction by phosphate is ASL>RASL>M1>PFA>HPFA>M2. Phosphate solubilizes more arsenic from ASL and RASL, the two arsenic-containing sludges, than from the other samples. This stands to reason as the arsenic is originally introduced as a sorbed/co-precipitated phase with ferryhydrite and the conditions after mixing this solid with ZVI, low temperature and an excess of ferric iron, are not conducive to incorporation of most of arsenic in a more crystalline phase. The extracted arsenic from ASL (32%) is also somewhat higher than from RASL (26%). This difference suggests that some of the adsorbed arsenic phase in ASL has been transformed to stronger bonded phases during the reduction process. The very low extractability of arsenic by phosphate from HPFA and M2 is consistent with the arsenic being present as part of a well-crystallized mineral matrix (as opposed to adsorbed) and, for the case of M2, the co-presence of a good sorbent (unloaded AFH) that would potentially re-sorb most of whatever arsenic might be leached from the crystalline ferrous arsenate. Although the major arsenic phase in PFA and M1 is also ferrous arsenate, these two samples yield higher amounts of phosphate extractable arsenic due to their smaller particle size, larger surface area and less crystalline nature (see Figure 2). Although phosphate shows a strong capacity for solubilizing arsenic from some of the samples, as expected, it extracts a negligible fraction of the iron (<3%).
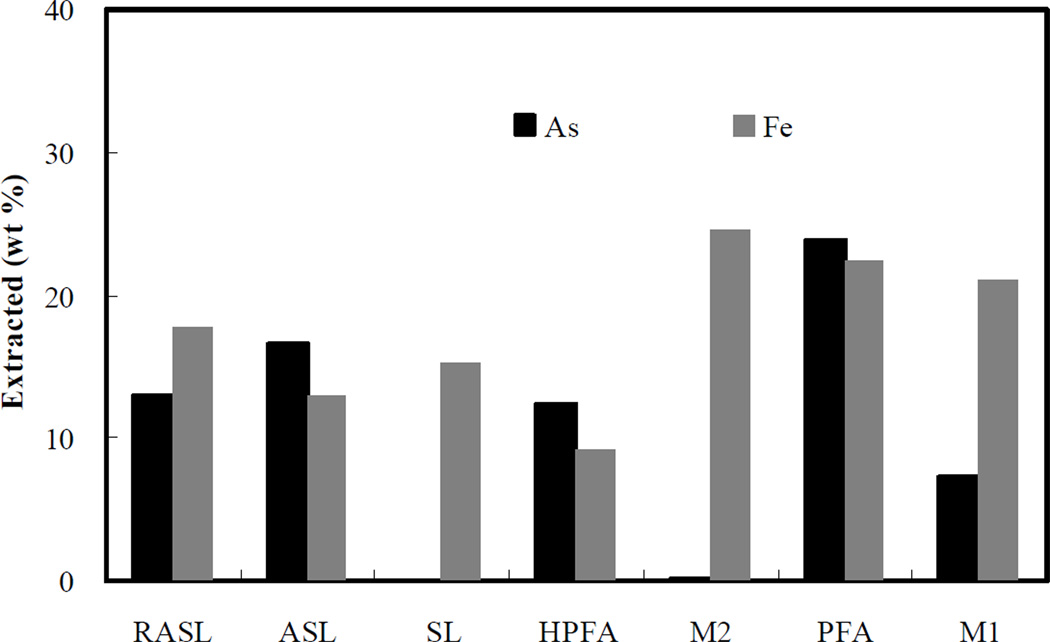
Step 3 (treatment with HCl). Figure 4 shows that the fraction of arsenic incorporated in amorphous iron-arsenic minerals in all samples is relatively large (7% to 23%), with the exception of M2, which is surprisingly low. Although the target of HCl extraction is the amorphous phase of iron-arsenic and iron oxyhydroxide minerals, the resulting arsenic fraction released is not representative of the amount of arsenic in the amorphous phase. The primary reason is that the HCl solution results in low pH solution, in which most arsenic and iron minerals have relatively high solubility. For example, based on XRD and SEM characterization, HPFA is a highly crystalline ferrous arsenate, yet about 12% of the arsenic is extracted in this step. However, the sample M2 contains HPFA but yields almost no arsenic extraction (0.12%). Similar behavior is seen for PFA (23.8%) compared to M1 (7.2%). The explanation is that much of the amorphous iron sludge (73% for M2 and 79% for M1) did not dissolve, based on the fractional iron extraction results. The remaining AFH is a good arsenic sorbent that increases its loading as the pH decreases (Jain et al., 1999). Thus for M1 and M2, the presence of undissolved AFH allows for readsorption of much of the arsenic released from the ferrous arsenate mineral. As expected, the greater arsenic extraction from PFA than HPFA indicates that amorphous ferrous arsenate (PFA) has about a factor of 2 higher solubility at low pH than its crystalline analog (HPFA). By comparing arsenic-iron sludge (ASL) with reduced arsenic-iron sludge (RASL), it is seen that the reduction treatment generated only slightly more recalcitrant iron-arsenic minerals, resulting in the fraction of extracted arsenic dropping from 16.6% to 13%.
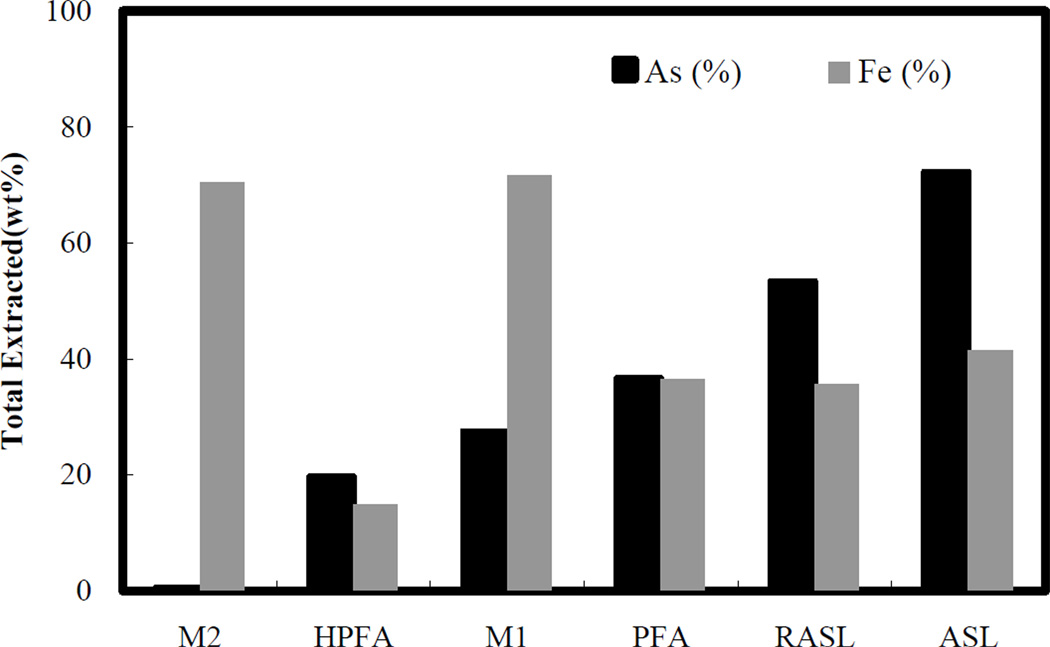
Step 4 (treatment with sodium citrate). The last step of the sequential extraction procedure targets the crystalline form of arsenic minerals by adding a strong iron complexing agent, citrate, to solubilize the iron crystalline structure and release any arsenic in the crystal matrix. Theoretically, all crystalline phase of arsenic minerals should be removed in this step. Although Figure 5 shows that sodium citrate iss effective at releasing arsenic from all samples. The results presented on Figure 6 indicate that this extraction step does not remove all arsenic with 27% to 99% arsenic left in residuals after citrate extraction. Despite the limitation of the citrate extraction step, the fractions distinguished in this step are still useful for estimating relative arsenic mobility as discussed in the following section.
Table 3.
Summary of sequential extraction procedure for synthetic arsenic solids
| Target arsenic phase | Extraction procedure | |||||
|---|---|---|---|---|---|---|
| Extractant | Concentration | pH | T (°C) |
Time (hr) |
Mixing | |
| Exchangeable (soluble arsenate) | MgCl2 | 1 M | 7 | 25 | 16 | All steps were continuously mixed by magnetic stirrer at 400 RPM. |
| Adsorbed phase on the surface of iron oxide | NaH2PO4 | 1 M | 5 | 25 | 24 | |
| Amorphous arsenic mineral phase, FeAsO4 and Fe3(AsO4)2 | HCl | 1 M | -- | 25 | 4 | |
| Crystalline arsenic-iron mineral, FeAsO4 and Fe3(AsO4)2 | Na-citrate | 2 M | 5 | 25 | 10 | |
| Residuals | Dry and conserved for characterization analysis | |||||
Figure 3.
Percent of original arsenic and iron extracted by 1.0 M MgCl2 solution.
Table 4.
Fraction of arsenic and iron released by 1.0 M NaH2PO4 solution
| Sample | RASL | ASL | SL | HPFA | M2 | PFA | M1 |
|---|---|---|---|---|---|---|---|
| As (wt %) | 27.54 | 32.16 | 0.00 | 0.13 | 0.11 | 2.43 | 11.10 |
| Fe (wt %) | 0.15 | 0.05 | 0.19 | 0.71 | 0.13 | 2.70 | 0.12 |
| Fe/As (molar) | 0.007 | 0.002 | -- | 7.222 | 1.695 | 1.483 | 0.0139 |
Figure 4.
Percent of original arsenic and iron extracted by 1.0 M HCl solution
Figure 5.
Percent of original arsenic and iron extracted by 2.0 M Na-Citrate solutions
Figure 6.
Total original arsenic and iron extracted in the 4-step sequential extraction test
Discussion
The total arsenic extracted by the 4-step sequential procedure ranged from 0.5% to 72.2% among the tested samples, which initially contained the same mass of arsenic (Figure 6). Comparison of the total extracted arsenic from ASL with that from RASL illustrates that the reductive treatment was successful in stabilizing arsenic for the range of extractants tested, yet over 50% of the arsenic was released from the RASL. Comparison of sample M2 with HPFA and M1 with PFA indicates that the overall stability of arsenic in the solid phase relies on not only its bonding phase, but also on the combination of matrix materials. For example, much lower arsenic removal from M2 was observed than from HPFA. The reason causing this remains unclear due to limited experiments and characterization tests performed. Possible mechanisms can be hypothesized as follows. (1) There might be a passive layer formed on the surface of ferrous arsenate which protects arsenic leaching. The possible chemical component of this layer could be magnetite (Fe4O3), because ferrous arsenate contains Fe(II) and iron sludge (FeOOH) is made of Fe(III). A mixture of this two phases has the possibility to form magnetite on the surface of ferrous arsenate. (2) Amorphous iron sludge is more favorable to be extracted out by extraction agents compare to the ferrous arsenate phase. As long as the iron sludge is not totally consumed, most extractants are used up by iron sludge rather than by the ferrous arsenate phase. If this is true, M1 and M2 will have similar performance as PFA and HPFA once no more amorphous iron sludge is left in the system. Unfortunately, for all experiments performed in this study, the amorphous sludge was never totally extracted. The fact that much higher iron extraction for M1 and M2 (71.5% and 70.5% respectively) occurred as compared to ASL (41.5%) supports both hypotheses. Further work is needed to reveal the mechanism by which much smaller amounts of arsenic were extracted from M1 and M2.
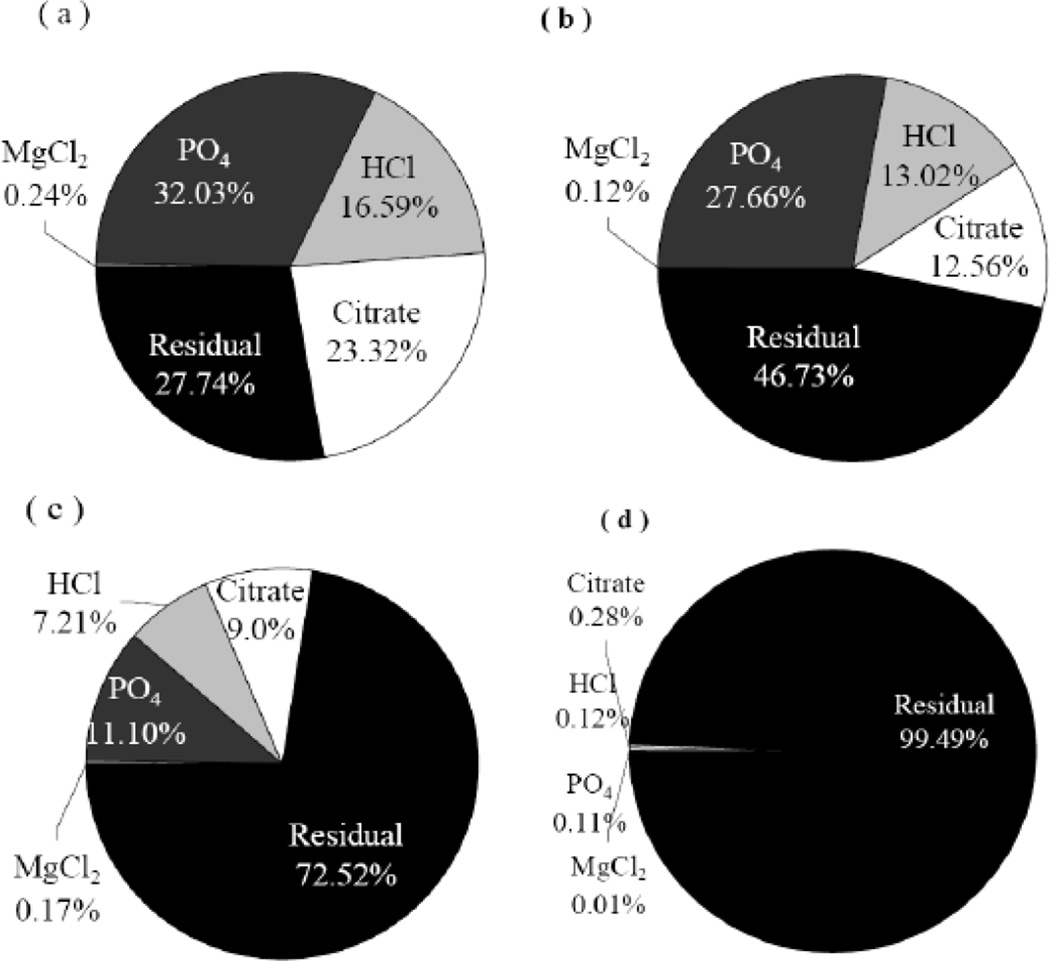
Although the sequential extraction procedure developed in this work provides useful information regarding the relative stability of arsenic in various synthesized iron-arsenic solids, limitations exist to adopt this technique as a standard procedure. First, the 1 M HCl solution was not as effective as expected in extracting arsenic from amorphous iron solids because amorphous iron oxy/hydroxides were not completely solubilized and acted as a potential sink (by sorption) for released arsenic. In addition, using sodium citrate to extract crystalline arsenic needs more study regarding the necessary concentration and contact time requirements. Despite these limitations, the operational fractionation of arsenic phases generates a good picture of the relative variation in arsenic phase distribution for the samples analyzed (Figure 7). It is worthwhile to point out that despite the fact that various arsenic phases are identified by the sequential extraction test, the results do not allow for a quantitative assessment of arsenic phases present in the tested samples, even though each step of extraction targets a specific arsenic phase.
Figure 7.
Distribution of arsenic between different fractions based on the sequential extraction test delineations: (a) ASL, (b) RASL, (c) M1, and (d) M2.
The low levels of arsenic extraction from the samples M2 and HPFA implies that stabilization of arsenic in the amorphous iron sludges generated by water treatment processes may be achieved by transforming the adsorbed arsenic phase into a crystalline ferrous-arsenic form with excess ferric iron. Reduction treatment alone of the arsenic sludge improves the arsenic stability in the solids, lowering the fraction of extracted As from 72% (ASL) to 53% (RASL). Due to the high stability of the pure crystalline ferrous arsenate, it seems desirable to optimize the treatment of the reduced sludge to produce the maximum yield of the crystalline phase. Results of arsenic fractionation also suggest that addition of matrix materials such as amorphous iron oxide may reduce the mobility of arsenic significantly, yet the limitations of the current sequential extraction approach do not allow unequivocal demonstration of this point.
Conclusion
A modified 4-step sequential extraction scheme was developed to evaluate distribution of arsenic in seven, well-characterized, synthetic iron-arsenic minerals. The results show that the procedure is capable of highlighting certain relative differences between the solids, but is not sufficiently aggressive (particularly in solubilizing amorphous and crystalline iron-arsenate phases) to correlate with other solid characterization techniques such as XRD and SEM. Due to these limitations, it is difficult to interpret the data quantitatively step by step. For example, the HCl solution does not extract arsenic effectively if there is ferrihydrite present in the solids.
The overall mobilization of arsenic from the tested samples is listed in decreasing order as follows: amorphous arsenic sludge (ASL) > reduced ASL (via zero valent iron reaction) (RASL) > amorphous ferrous arsenate (PFA) > mixture of PFA and SL (M1) > crystalline ferrous arsenate (HPFA) > mixture of HPFA and SL (M2). The limited extraction of arsenic from the crystalline ferrous arsenate suggests that this material could be a good candidate for arsenic immobilization. The reduced arsenic sludge (RASL) is more stable against extraction than the initial arsenic sludge (ASL). However, the mineral structure that forms in the sludge contains a complex mixture of arsenic phases with adsorbed, amorphous, and crystalline ferrous arsenate phases present. Due to the greater stability of the pure crystalline ferrous arsenate, it is desirable to optimize the synthesis of the initial arsenic-bearing sludge to produce the maximum yield of the reduced iron crystalline phase.
The sequential extraction procedure developed allows for a comparative assessment of the leaching potential of the solid phases that contain arsenic in a given sample, which makes it useful for the evaluation of stabilization methods aimed at reducing arsenic mobilization from solid waste. In addition, the procedure has suggested crystalline ferrous arsenate as a potential candidate for arsenic stabilization. This will be subject of further research.
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) grant #P42 ES04940. This paper’s contents are solely the responsibility of the authors and do not necessarily represent the official views of NIEHS. The authors want to thank the Arizona Department of Health for providing arsenic analysis on their ICP-OES instrument and Dr. Robert Downs and his research group in the University of Arizona, Geosciences Department for their help in arsenic mineral identification.
References
- Ahsan H, Chen Y, Parvez F, Zablotska L, Argos M, Hussain I, Momotaj H, Levy D, Cheng Z, Slavkovich V, van Geen A, Howe GR, Graziano JH. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the health effects of arsenic longitudinal study. Am. J. Epidemiol. 2006;163:1138–1148. doi: 10.1093/aje/kwj154. [DOI] [PubMed] [Google Scholar]
- Alam MGM, Tokunaga S, Maekawa T. Extraction of arsenic in a synthetic arsenic-contaminated soil using phosphate. Chemosphere. 2001;43:1035–1041. doi: 10.1016/s0045-6535(00)00205-8. [DOI] [PubMed] [Google Scholar]
- Bodwell JE, Kingsley LA, Hamilton JW. Arsenic at very low concentrations alters glucocorticoid receptor (GR)-mediated gene activation but not GR-mediated gene repression: complex dose-response effects are closely correlated with levels of activated GR and require a functional GR DNA binding domain. Chem. Res. Toxicol. 2004;17:1064–1076. doi: 10.1021/tx0499113. [DOI] [PubMed] [Google Scholar]
- Borovec Z. The uptake of trace amounts of uranium and radium on some river suspension components. Proc. 11th Conf. Clay Mineral Petrol. 1993;251:258. [Google Scholar]
- Cornell RM, Schwertmann U. The iron oxides: structure, properties, reactions, occurrences and uses. 2nd ed. New York: Wiley; 2003. [Google Scholar]
- Dixit S, Hering JG. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ. Sci. Technol. 2003;37:4182–4189. doi: 10.1021/es030309t. [DOI] [PubMed] [Google Scholar]
- Ela W. Disposal of arsenic-bearing water treatment residuals: assessing the potential for environmental contamination. Washington DC: NIEHS; 2006. [Google Scholar]
- EPA. Method 6020A. 2007 http://www.epa.gov/epaoswer/hazwaste/test/pdfs/6020a.pdf.
- Ghosh A, Mukiibi M, Ela WP. TCLP underestimates leaching of arsenic from solid residuals under landfill conditions. Environ. Sci. Technol. 2004;38:4677–4682. doi: 10.1021/es030707w. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Mukiibi M, Sáez AE, Ela WP. Leaching of arsenic from granular ferric hydroxide residuals under mature landfill conditions. Environ. Sci. Technol. 2006;40:6070–6075. doi: 10.1021/es060561b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Sáez AE, Ela WP. Effect of pH, competitive anions and NOM on the leaching of arsenic from solid residuals. Sci. Total Environ. 2006;363:46–59. doi: 10.1016/j.scitotenv.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Jain A, Raven KP, Loeppert RH. Arsenite and arsenate adsorption on ferrihydrite: surface charge reduction and net OH- release stoichiometry. Environ. Sci. Technol. 1999;33:1179–1184. [Google Scholar]
- Jekel M, Amy GL. Arsenic removal during drinking water treatment. Interface Sci. Technol. 2006;10:193–206. [Google Scholar]
- Keon NE, Swartz CH, Brabander DJ, Harvey C, Hemond HF. Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environ. Sci. Technol. 2001;35:2778–2784. doi: 10.1021/es001511o. [DOI] [PubMed] [Google Scholar]
- Krysiak A, Karczewska A. Arsenic extractability in soils in the areas of former arsenic mining and smelting, SW Poland. Sci. Total Environ. 2007;379:190–200. doi: 10.1016/j.scitotenv.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Mihaljevič M, Poňavič M, Ettler V, Šebek O. A comparison of sequential extraction techniques for determining arsenic fractionation in synthetic mineral mixtures. Anal. Bioanal. Chem. 2003;377:723–729. doi: 10.1007/s00216-003-2115-7. [DOI] [PubMed] [Google Scholar]
- Manning BA, Goldberg S. Modeling arsenate competitive adsorption on kaolinite, montmorillonite and illite. Clays Clay Miner. 1996;44:609–623. [Google Scholar]
- Ruttenberg KC. Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol. Oceanography. 1992;37:1460–1482. [Google Scholar]
- Shaw D. Mobility of arsenic in saturated, laboratory test sediments under varying pH conditions. Proc. 4th Geoenviron. Eng. Conf.; Stratford-upon-Avon; United Kingdom. 2004. [Google Scholar]
- Shiowatana J, Tantidanai N, Nookabkaew S, Nacapricha D. A novel continuous-flow sequential extraction procedure for metal speciation in solids. J. Environ. Qual. 2001;30:1195–1205. doi: 10.2134/jeq2001.3041195x. [DOI] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, Steinmaus C, Bates MN, Selvin S. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ. Health Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K, Yong RN, Thomas HR. Leaching column test on arsenic-soil interactions. Geotech. Spec. Publ. 2006;148:306–314. [Google Scholar]
- Tessier A, Campbell PGC, Bisson M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979;51:844–851. [Google Scholar]
- Van Herreweghe S, Swennen R, Vandecateele C, Cappuyns V. Solid phase speciation of arsenic by sequential extraction in standard reference materials and industrially contaminated soil samples. Environ. Pollution. 2003;122:323–342. doi: 10.1016/s0269-7491(02)00332-9. [DOI] [PubMed] [Google Scholar]