Abstract
We highlight our recent applications of functional peptide nanotubes, self-assembled from short peptides with recognition elements, as building blocks to develop sensors. Peptide nanotubes with high aspect ratios are excellent building blocks for directed assembly into device configurations, and their combining structures with the nanometric diameters and the micrometric lengths enables to bridge the nano-world and the micro-world.
Keywords: peptide nanotube, biosensor, self-assembly, pathogens, heavy metals, bionanotechnology, electrochemistry
1. Introduction
Peptides are emerging as versatile materials whose function can be programmed to perform specific tasks via genetic engineering and chemical functionalization. Molecular recognition and robust assembling nature of peptides are very powerful features for the application of device building blocks.[1] For instance, peptides can be designed to self-assemble and yield nanostructures with defined shapes such as tubes, rings, sheets, and particles.[2–7] By incorporating molecular recognition motifs, directed assemblies of these peptide building blocks are possible by patterning complementary binding units on substrates.[8, 9] When the molecular recognitions are orthogonal, the immobilization of multiplex building block assemblies at respective locations on substrates can be accomplished, useful for the long sought-after bottom-up fabrication technique of complex nanoarchitectures in device configurations.[10, 11] The molecular recognition can also be applied to bind specific ions for material synthesis and sensing. For example, by carefully selecting peptide sequences and conformations to have high affinity and catalytic activity for specific metals, their crystals can be grown by triggering the crystallization function of these peptides in defined sizes and crystalline structures.[12–17] In some cases, peptides could recognize particular crystalline faces to block/catalyze the crystal growth, resulting in tailoring crystal shapes with peptide recognition functions.[18–20] Alternatively, high affinity of peptides for particular inorganics and ions can be used for sensing applications by anchoring the target ions at concrete locations on transducer platforms.[21, 22] Complex functions of proteins, which result from millenniums of evolution, can serve as an endless source of inspiration for the design of functional peptides with shorter sequences and more predictable behaviors. Moreover, when these short and mono-functional peptide-based building blocks are assembled, their functions could be more than just a sum of single functions, and the resulting peptide assemblies might behave as “meta-functional” materials, where the integration generates new functions. As more functions are added to the hybrid peptide assemblies, higher degree of complexity will be obtained and the final structure and the function of the assemblies become more difficult to predict.
In this feature article we highlight our recent applications of functional peptide nanotubes, self-assembled from short peptides with recognition elements, as building blocks to develop sensors (Figure 1). Sensors are essential in myriads of applications including the detection of hazardous pathogens, environmental pollutants and disease markers, all of them scoring high for social impacts. These devices are aimed to be applied in-field so that particular hazardous analytes can be detected fast with high specificity and low detection limit before they reach alarming levels. Consequently, for this type of sensor applications, compact designs integrating simple readouts are desirable. It is also important to develop sensors whose transducers have high affinity and high sensitivity for a target molecule in order to avoid tedious purification steps. Traditionally, antibodies have been employed as molecular recognition elements in biosensors for the highly selective detection of analytes, and more recently, aptamers are also emerging as an alternative to be incorporated in biosensors.[23] The peptide nanotubes are superior building blocks for biosensors because robust and directed self-assembly nature enables to generate miniaturized sensor chip platforms in the bottom-up process without using sophisticated lithographic methodologies.[24] When peptide nanotubes are coupled with adequate transducers, the resulting hybrid bio-inorganic devices can improve signal-to-noise ratio and specificity of the sensor.
Figure 1.
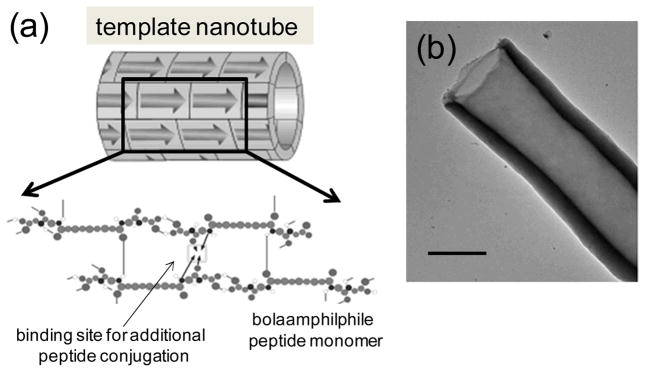
(a) Structural illustration of the template nanotube self-assembled from bolaamphiphile peptide monomers via 3D intermolecular hydrogen-bonding. (b) TEM image of the template nanotube in (a). Scale bar = 100 nm.
We expect that readers are wondering why peptides in the nanotube form are used instead of the ones in the spherical form. Tubular structures have received much attention because they combine nanometric diameters with micrometric lengths, and therefore they are able to bridge the nano- and micro-worlds. This feature is crucial to integrate the tubular nanostructures with microfabrication processes such as photolithography, which is a key factor for a large-scale production of materials that still retain nanoscale physical properties. The anisotropic tubular shape also makes the directed assembly of peptide nanotube easier at targeted positions on substrates via molecular recognition, chemical interactions, and dielectrophoresis. For example, these biological nanotubes can be assembled between interdigitated electrodes by applying an AC field of 5 V peak-to-peak and 10 Hz frequency (Figure 2a).[24] By this non-destructive positive dielectrophoresis process it is possible to fabricate complex sensor nanoarchitectures without using sophisticated nanolithography equipments such as e-beam lithography. Moreover, when the nanotubes incorporate molecular recognition units such as antibodies, the directed assembly is possible by patterning complementary proteins on substrates (Figure 2b).[8, 10, 11, 25] The combination of these assembly methods enables to place multiple recognition-transducer units at desired locations on the detection platform more precisely, generating multi-sensors on a chip.
Figure 2.
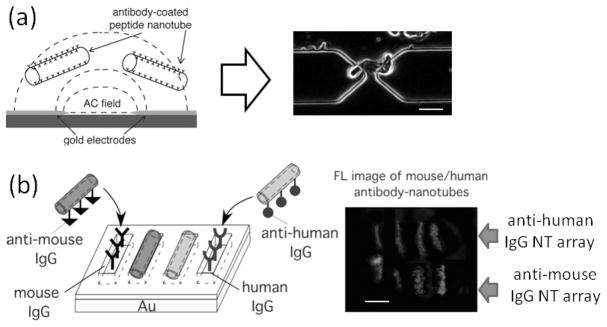
(a) Peptide nanotubes are injected onto the electrode-patterned platform while applying an AC field (left), and then peptide nanotubes are trapped at the gap between adjacent electrodes by positive dielectrophoresis (right in an optical micrograph). Scale bar = 2 μm. (b) (left) Scheme to assemble anti-mouse IgG-coated nanotubes and anti-human IgG-coated nanotubes onto their antigen-patterned substrates via biomolecular recognition; Location-specific immobilization of Alexa Fluor 546-labeled anti-mouse IgG nanotubes onto the mouse IgG trenches and FITC-labeled anti-human IgG nanotubes onto the human IgG trenches. (right) Fluorescence image of anti-mouse IgG nanotubes (in red) and anti-human IgG nanotubes (in green), attached onto four upper trenches filled with mouse IgG and four bottom trenches filled with human IgG, respectively, scale bar = 2 μm. (a, reproduced with permission from ref. 23, copyright Wiley-VCH Verlag GmbH & Co. KGaA. b, reprinted with permission from ref. 10, copyright 2005 American Chemical Society)
It is also advantageous that molecular recognition units such as binding peptides and antibodies can be easily conjugated with the peptide nanotubes. Recently, we found evidence that the shape of substrates plays a significant role to control peptide conformations and their binding strength toward target molecules as observed in nature. For example, when the mineralizing peptides for Au were immobilized on the cylindrical peptide nanotube surface, more Au ions were bound and the morphologies of the coatings became very sensitive with pH of the growth solution[13] while the same degrees of binding efficiency and morphology control were not observed on the monolayers of the same peptide on the flat Au substrates.[26] This comparison indicates that non-flat substrates have certain advantages in binding target analytes efficiently and controlling the morphology of coatings, however this is not very surprising since the natural mineralization template is rarely flat. These flexibilities in binding strength and morphology on the peptide-conjugated nanotubes could be due to the ease of the conformation change of peptide on the cylindrical surfaces.[14]
Another advantage of using the peptide nanotubes in sensor platforms is that they are not conductive, as opposed to inorganic/carbon nanotubes frequently used for the development of electrochemical biosensors. This attribute is responsible for a unique transduction mechanism of peptide nanotubes for label-free detection, as explained in Section 3. Finally, the facile synthesis of bolaamphiphile peptide monomers along with their room-temperature assembly makes the device fabrication inexpensive and environmentally friendly, which is an important aspect in their mass-scale production.
These characteristic features of functional peptides are key aspects to design new peptide nanotube-based biosensors and this article pays special attention to their integration with electrochemical chip sensors. After reviewing several peptide building blocks and their integration with electrochemical transducers, the basic transduction mechanism of peptide nanotube-based electric sensors will be highlighted in Section 2. Then, the practical detections of pathogens with the peptide nanotube sensor platforms will be reviewed in details, followed by the application of peptide nanotubes as antennas in optical pathogen sensing systems in Section 3.
2. Special Features of Peptide Nanowires for Sensing
Functionalized building blocks of peptide assemblies used in our group are peptide nanowires; this nanowire consists of a templating peptide nanotube as a core and recognition peptides/antibodies conjugated on the surfaces of the peptide template. For the templates of the functionalized building blocks, glycine-rich peptide monomers that have the characteristics of bolaamphiphiles (i.e., two hydrophilic head groups connected by one hydrophobic tail group) are self-assembled into the tubular structure with intermolecular hydrogen bonding. The advantages of using these peptide nanotubes are robustness of assembly with high yield and high affinity to biological molecules for the functionalization.
The assembly function of several peptides is well known to yield a plethora of structures, whose 3D arrangement depends on the chemical composition of the peptide sequence and the physicochemical properties of the environment such as temperature, pH and ionic strength. Therefore, even if the amino acid sequence is designed to carry out desired functions, the assembled structure may not be under control and it could be difficult to assemble these peptides into nanotubes. Our strategy is to use the template nanotubes that have non-specific affinity for biological molecules so that desired binding peptides and antibodies can be immobilized on these cylindrical templates independent of their chemical compositions.
Among various bolaamphiphile peptides, we focused on the tubular assembly of bis(N-α-amidoglycylglycine)-1,7-heptane dicarboxylate (Figure 1a).[27, 28] This monomer can undergo robust and stable assembly into tubular structures with hydrophobic interactions between hydrocarbon chains and the entire structure is reinforced by intricate networks of hydrogen bonds between carboxylic acid and amide groups in acidic conditions. Once this semi-crystalline nanotube is assembled in the diameters of 50 ~500 nm and length over 1 μm, in solution and then dried (Figure 1b), this structure is very stable and the rigidity is maintained even at 200°C and in strong acid/base solutions.
2.1 Functionalizations of peptide nanotubes with recognition groups
We generally applied two types of recognition units for the nanotube functionalizations, antibodies and metal-binding peptides, for different purposes. By the antibody-conjugation, we aim the directed assembly of peptide nanotubes on substrates via molecular recognition. Previously we demonstrated that the antibody nanotubes could be assembled on an array of complementary antigen-filled trenches on Au substrates, patterned by nanolithography (Figure 2-b).[8, 10, 11, 25] After the trenches were drawn by AFM nanolithography (i.e., removing protective alkyl SAMs on Au substrate by the tip of atomic force microscope (AFM)), mouse IgG was deposited on these trenches via the thiol-Au interaction. Then anti-mouse IgG-coated nanotubes were immobilized selectively onto the mouse IgG-patterned trenches via the antigen-antibody interaction. Antibody recognition on the nanotube could locate different nanotubes on the respective substrates where complimentary antigens were marked and the biomolecular recognition was very specific; As shown in Figure 2-b, anti-mouse IgG nanotubes and anti-human IgG nanotubes were attached only at their complimentary antigen-patterned areas.[10] It should be noted that the high yield of this location-specific attachment of antibody nanotubes was accomplished when the antibody concentration on the nanotubes was carefully optimized to maximize the antibody-antigen interaction.[25]
Metal-binding peptides are growing interest in materials sciences since a wide variety of metal/semiconductor materials could be grown in mild conditions, as inspired by crystal growth phenomena in biological systems.[29] In nature, biomineralizing organisms such as starfish and sea urchin achieve an exquisite control over inorganic structures by using peptides with high affinity for the precursors of the reaction, among other factors.[30, 31] These biomineralization processes have inspired scientists to search for peptide sequences with high affinity for a particular material to catalyze crystal growth and to produce hybrid organic-inorganic structures. Among various approaches for peptide designs, genetic engineering protocols are gaining much attention for their intrinsic capability to build a large peptide sequence libraries in the field of materials sciences.[32–34] In this approach, a large number of peptides are expressed in a host microorganism and peptides possessing high affinity to a target material are selected by stringent protocols. In the field of biosensing, peptides with high affinity to a target metal ion were identified as useful biorecognition elements in their integration with optical and electrochemical transducers. However, we found more values on metal-binding peptides as target-specific transducers; if the metal-binding peptides can also catalyze the nucleation, the resulting crystal growth triggers the electrical signal for sensing, which adds another reaction specificity to the binding specificity of peptides. And if the degree of metallization changes conductivity of the hybrid nanotube proportionally, sensing platforms containing the peptide nanotubes can be used to detect metal ions. For example, when the peptide having high affinity and mineralization function for Pb2+ is used as the recognition element in the nanotube-based chip sensor, this hybrid sensor is not only sensitive to detect Pb ions through the generation of Pb crystals but it can also simultaneously eliminate interference by other cations such as Ca2+ or Mg2+, whose reduction potentials are higher than that of the target ions.[22] If this affinity function is coupled with the mineralization function of peptides, the peptide nanotube transducers can have the significant electric signal amplification to achieve extremely high sensitivity for the detection of heavy metal ions.
2.3. The basic detection mechanism for peptide nanotube-based electric sensors
Peptide assemblies are essentially non-conductive and this electric property apparently limits their applications in electrochemical biosensors in traditional designs since these sensors measure conductivity changes with DC probes. However, this feature can be turned into an advantage when the non-conductive peptide nanotubes are coupled with AC probes on the sensor platforms. When semiconductor nanowires are used as transducers of the electric signal, the contact resistance between the nanowire and the DC probes dominates the measurement and it will have significant influence on the reproducibility of the measurements among different sensor platforms. The use of DC current in an aqueous environment also requires an exquisite control of excitation voltages in order to avoid unwanted electrochemical phenomena such as the oxidation of water. Moreover, although the transduction of the conductance signal at the nanowire/solution interface can be extremely sensitive with DC probes, it is also prone to suffer from interference from small molecules such as proteins and lipids, which are present in real samples and adsorb non-specifically onto the surface of transducers. Non-conductive peptide nanotube-based sensors coupled with AC probes do not have these problems since these impedimetric sensors measure the change in electric properties of the solution close to the nanotube transducers but not the one at the nanotube/solution interface. In the case of AC impedance probes, when a small AC excitation voltage is applied between two neighboring electrodes in the design of planar micro-fabricated transducer in electrolyte solutions, the distribution of the electric field lines and currents is dictated by the geometry of the electrodes and this distribution is confined within a few micrometers around the electrodes.[24] Because the transmission of the electric field is through the solution and not through a nanowire, the contact resistance is irrelevant for the peptide nanotube-based sensing circuits. Under this condition, the detection is not limited to the transducer/solution interface and hence the impact of non-specific adsorption phenomena is significantly reduced. Moreover, the small excitation voltages in impedimetric detection do not perturb the system to induce interfering electrochemical processes. The transmission of the electric field through the solution and not through a nanowire eliminates the contact resistance from the sensing circuit. As a drawback, parasitic components of the sensor circuit may have a greater impact on AC measurements, and the transducers need to be designed carefully to reduce their contribution so that the signal from analytes can be extracted from measured impedimetric values.
Two transducer designs integrating non-conductive peptide nanotubes were applied. The first sensor platform includes a pair of microfabricated electrodes separated by a gap of 1 μm (Figure 3a).[24] This type of electrode design is suitable to concentrate nanomaterials efficiently via positive dielectrophoresis because electric field lines converge at the tip of the electrodes, enabling the positioning of peptide nanotubes between the electrodes as bridges. For sensing, the path of the electric field and currents is the shortest at the gap between the electrodes and this device geometry makes the impedimetric detection of analytes at this location most sensitive. Therefore, the incorporation of recognition units such as antibodies and aptamers onto the nanotube effectively concentrate the target analytes at the sensitive spot. When the analyte is an insulating object with low dielectric constant, the capacitance of the solution between the electrodes decreases and hence the impedance at high frequency increases. If the analyte is a conductive material, the impedance decreases and a resistor is observed in the equivalent electric circuit. In general pathogens and cells behave as the former case while reduced metal ions are detected as the later case.
Figure 3.
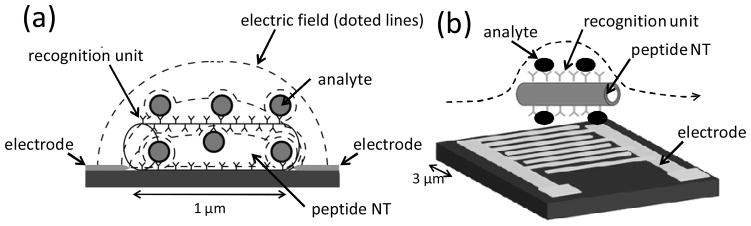
(a) The distribution of the electric field lines and currents is confined within a few micrometers around the electrodes in this device geometry. The presence of dielectric bioparticles in this region where the electric field strength is at maximum results in a decrease of the capacitance between the electrodes. (b) Free-flowing antibody-conjugated peptide nanotubes recognize and bind cells and then fast sedimentation of these complexes onto interdigitated electrodes generates an impedimetric signal for pathogen detection.
While the device design in Figure 3a is very versatile to assemble peptide nanotubes via positive dielectrophoresis and the impedimetric detection can be enhanced, another device design with interdigitated electrode arrays (Figure 3b) can be very effective for impedimetric detection when electric pre-concentration of nanotubes between electrodes is not necessary.[35] The interdigitated electrodes can create well-defined electric field line distribution patterns around the electrodes with a limited electric field penetration depth, and hence it is adequate to detect changes in the electrical properties of the solution locally. This feature can be extended to larger surface areas since the electric field line distribution is mainly determined by the digit width and interdigital distance of electrodes. Since in this device configuration the sensitive region of the device is not limited to a small area on the detection platform, pre-concentration steps of analytes are not very critical for their detection.
When insulating objects are placed onto the electrodes in the device geometry of Figure 3b, surface confined electric fields are obstructed and the cell constant of the sensor increases with the relationship:
| (1) |
where k is the cell constant, Rsol is the resistance of the solution, ρ is the resistivity, ε is the permittivity and Csol is the capacitance of the solution. Upon the adsorption of cells on the interdigitated electrodes, k increases and then Csol decreases. This response appears as an increase of the impedance at the high frequency region of the impedance spectrum. This transduction mechanism in Figure 3b presents the great advantage of being insensitive to contaminants in samples such as cell debris; the magnitude of signals depends on the volume of insulating object and the penetration depth of electric fields and smaller insulating objects are not detected even when they are located near the transducers. Of course, these contaminants are not detected when they are far from the transducers (> 1 μm). Recently, this volume-sensitive detection mechanism was also exploited for selective detection of cancer cells without any labels by monitoring their volume changes as osmotic pressure was applied to these cells.
Both sensor chips use electrochemical transducers with the straightforward transduction of electric signals. The standard microfabrication processes are applied for their fabrications, which facilitate the mass production and miniaturization of sensors as well as its integration with complex microprocessors for signal treatment. The reusing of the transducers will have a significant impact for their future applications and recently this type of reusable peptide nanotube-based electric sensor design was introduced targeting point-of-care applications.[35]
3. Practical applications of peptide nanotube-based electric sensors
3.1 Virus sensors with nanotube-based electric transducers
The detection of pathogens is a matter of great concern due to the high rate of growth of microorganisms, which can lead to alarming levels of hazardous agents rapidly. Similarly, detecting the identity of the pathogen in infected patients is crucial to administrate a proper cure and stop the disease. Consequently, robust detection devices that are suitable for point-of-care applications are required to detect pathogens quickly. In this context, we launched the peptide nanotube detection platform for virus detection (Figure 3a).[24] This peptide nanotube detection platform consists of a pair of electrodes separated by a 1 μm gap, which are bridged by a peptide nanotube modified with biomolecules with specific recognition function. As described in the previous section, peptide nanotubes are modified with antibodies for target viruses and the nanotube assembly at the gap between the electrodes is accomplished by positive dielectrophoresis. The major role of antibodies is to specifically concentrate viruses onto the most sensitive location of the impedimetric transducers via molecular recognition. Viral particles can be envisioned as a conductive electrolyte core surrounded by an insulating shell. When in presence of an electric field of the appropriate frequency, AC field lines do not penetrate the outer layer and viruses can be considered as purely insulating objects. Therefore, the binding of low permittivity viruses onto the nanotube decreases the capacitance of the solution measured between the electrodes and this change can be used as a signal for virus detection. Figure 4a shows impedance spectra of the peptide nanotube detection platform before and after binding herpes simplex virus type 2 (HSV-2) with the concentration of 104 cpu/ml between 1MHz and 10 kHz. At the higher frequency range, the capacitance of the solution dominates the spectrum and the impedance increases as the frequency decreases. At the lower frequency range, the value of the impedance modulus is almost constant, therefore revealing the presence of a resistor associated to the conductivity of the solution. After binding the viruses, the impedance of the system increases due to the attachment of low permittivity viruses at the gap between electrodes. By fitting the curves to the equivalent circuit show in the inset of Figure 4a, the capacitance of the solution (Csol) can be derived as the signal of target viruses. In Figure 4b, the decrease of Csol with respect to control sensors that contains no specific recognition function is used for the assay of viruses. As the virus concentration increases, −ΔCsol increases because the inclusion of more low permittivity objects drops the capacitance lower. By this sensor platform, the virus concentration as low as 102 pfu/ml could be detected within 1 hr, therefore demonstrating the suitability of the peptide nanotube detection platform for the highly sensitive detection of virus in a short period of time.
Figure 4.
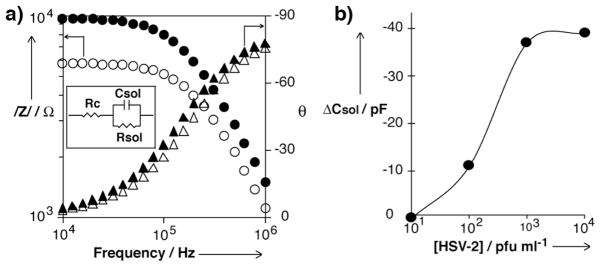
(a) Impedance spectra of peptide nanotube-assembled sensing platform. Hollow circles are impedance modulus values before incubating HSV-2, and filled circles are impedance modulus values after incubating HSV-2. Hollow triangles are phase angles before incubating HSV-2, and filled triangles are phase angles after incubating HSV-2. Impedance data was fitted to the equivalent electric circuit in the inset. Rc is the resistance of the contacts, Rsol is the resistance of the solution, and Csol is the capacitance of the solution, which is proportional to the permittivity of the solution. (b) Correlation between capacitance and the concentration of HSV-2 in the sample solution. ΔCsol is the corrected capacitance value by subtracting the blank measurement. (reproduced with permission from ref. 23, copyright Wiley-VCH Verlag GmbH & Co. KGaA)
3.2 Heavy metal ion detection with nanotube-based transducers
The interest in developing sensors for heavy metal ions at low detection limits raises from the highly toxic nature of these ions even at ultra-low concentrations. To detect these hazardous environmental pollutants with extreme sensitivity, we improved the peptide nanotube detection platform with the same strategy as the pathogen sensing system; the metal-binding peptide with molecular recognition function is incorporated into the nanotube at the gap between electrodes to concentrate the target analytes at the most sensitive location on the transducer (Figure 5a).[22] In this case, to achieve high affinity and selectivity for Pb2+, the nanotubes were conjugated with the Pb-binding peptide TAR-2-Asp (DHHTQQHD). After the peptides concentrate Pb2+on the nanotube, a reducing agent is added to accelerate the nucleation of Pb crystals on the peptide nanotubes (Figure 5b), and the presence of the metallic junction of the Pb-coated nanotube between the electrode is detected as a resistor in the equivalent circuit (inset of Figure 5b). This peptide nanotube-based sensor does not detect other heavy metal ions such as Co2+, Cu2+, Hg2+ or Zn2+ in the same detection condition because molecular recognition by the peptide does not assist developing their crystalline depositions even after the reduction step. When the electric signal was amplified by the supportive Ag deposition that can improve the metallic contact, this sensor could detect Pb2+ with an unprecedented sensitivity in the range from 0.01 nM to 10 nM even under the interference with other ions (Figure 5c). This concept could be adapted to detect various heavy metal ions provided that peptides that can bind them with high affinity and specificity are available for the fabrication of the sensor.
Figure 5.
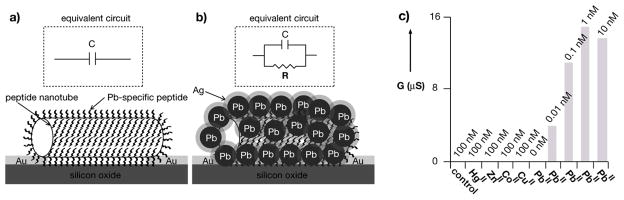
Strategy for the detection of ultra-low levels of PbII with the peptide nanotube detection platform; (a) in the absence of PbII nanowires are not templated by the nanotubes and no conductance is measured between the electrodes; (b) in the presence of PbII the peptide nucleate the crystallization of Pb; after signal enhancement by Ag reduction, the metallic Pb/Ag coating on the nanotube that bridges the electrodes appears as a resistor in the circuit and the conductance between the electrodes is detected as the signal for PbII. (c) Conductance (G) of the peptide nanotube between the electrodes after the incubation with different heavy metal ions with the concentration indicated on the bars. “Control” is the experiment in the presence of 100 nM Pb (II) without the TAR-2-Asp peptide conjugated with the peptide nanotube. (reproduced with permission from ref. 34, copyright Wiley-VCH Verlag GmbH & Co. KGaA)
3.3. Point-of-care peptide nanotube biochips: multiple detection with reusable sensors
While the peptide nanotube detection platform demonstrated a breakthrough in pathogen biosensing, cost-effectiveness of this approach for its application in real samples could be improved by making sensor chips reusable. The problem is that the nanotubes are firmly immobilized onto the transducers and the antibody-pathogen binding is difficult to reverse so that the substrates cannot be cleaned well for the removal of pathogens. To overcome this issue, multiple pathogen cells are detected by arrays of interdigitated electrodes as antibody-conjugated nanotubes are circulated to catch target pathogens and sediment quickly via the pathogen binding around the nanotube (Figure 3b).[35] In this sensor configuration, the presence of insulating bacteria-nanotube complexes on the interdigitated transducers deviates the path of electric field lines and currents between electrodes and the impedance increases as these complexes fall onto the transducers (Figure 6a). Conversely, when control tubes that do not recognize specifically the cells are present in solution, sedimentation occurs at lower rates and the impedance change is too slow to be detected (Figure 6b). Since more nanotubes sediment onto the transducers as the concentration of pathogens increases, the impedimetric signal can be used to assay these pathogens. This sensor’s detection mechanism is similar to previous peptide nanotube detection platforms and the electric signal is generated as the analytes are concentrated onto the transducers, although in this case the concentration of analytes is induced by the mass increase. Since the recognition step takes place in solution and not on the transducers and the peptide nanotubes are not immobilized on the sensor platform, the sensor can be reused many times by simple rinsing the surface with an aqueous solution after the removal of both measured pathogens and the nanotube catchers.
Figure 6.
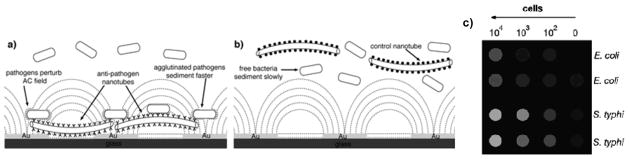
Label-free detection of pathogens with antibody-modified peptide nanotubes; a) bacteria agglutinated by the interaction with antibodies on peptide nanotubes sediment fast, and the increased number of the insulating cells on the transducer via the sedimentation increases the impedance at 316 kHz; b) control nanotubes modified with rabbit IgG that do not interact specifically with E. coli do not sediment these cells fast enough to generate the impedance signal; c) Multiplexed detection of pathogens with the peptide nanotube biochip; variations of the real part of the impedance (Z′) of samples containing E. coli and S. typhi in various concentrations was converted into a color chart. (reproduced with permission from ref. 22, copyright Wiley-VCH Verlag GmbH & Co. KGaA)
To demonstrate the proof-of-concept of the multiplex pathogen detection, the array of electrodes was incubated with solutions containing E. Coli cells and S. Typhi cells at different concentrations. When both anti E Coli- and anti S. Typhi-conjugated nanotubes were circulated in these samples, E. Coli cells and S. Typhi cells could be assayed simultaneously. In Figure 7c, impedimetric signals for E. Coli cells and S. Typhi cells were converted to an easy-to-read color code for its simple interpretation by non-specialists (Figure 6c). In this color chart, both E. Coli and S. Typhi cells can be differentiated from the blank signal in the range of concentrations from 102 to 104 cells, which is well below infectious doses of these pathogens, therefore demonstrating the suitability of this approach for the early detection of multiple pathogens.
Figure 7.
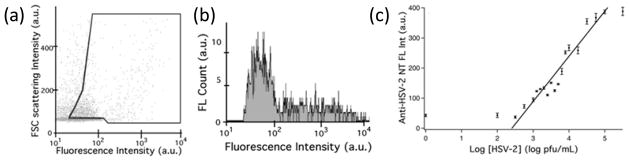
Distribution of forward light scattering (FSC) intensity and fluorescence intensity of (a) anti-HSV nanotubes with 106 pfu/mL HSV-2 in solution. (b) Integrated fluorescence intensities inside the gates in (a). (c) Linear correlations and fittings between the integrated fluorescence intensities of the antibody nanotube-virus aggregates and the concentrations on a log scale (R2 = 0.9010). (Reproduced by permission of The Royal Society of Chemistry).
3.4. Peptide nanotube optical probes
While so far the binding events between analytes and the functional nanotubes were probed by electrochemical transducers, they can also be detected optically as the nanotubes are tagged by fluorescent dyes. For example, when the nanotubes are conjugated by antibodies for viruses at the ends and fluorescent dyes on the sidewalls, the recognition element at the tips of the nanotubes amplifies the aggregation size of nanotubes in dendrimer-like structures with increased viral concentrations. Since fluorescence intensity is observed to increase proportionally with the viral concentration, target viruses can be assayed by flow cytometry.[36] Because the distribution of forward light scattering (FSC) intensity and fluorescence intensity reflects the size of the virus-nanotube aggregates, we can discriminate unbound nanotubes from virus-nanotube complexes by gating the characteristic distribution of neat nanotubes (i.e., dots inside the gate in Figure 7a). Viruses can be assayed by integrating fluorescence intensities from virus-bound nanotubes (Figure 7b). This method could assay HSV-2, influenza B, vaccinia, and adenovirus within 30 minutes after their incubation with the antibody-conjugated nanotubes (Figure 7c). The detection limit could be as low as 103 pfu/ml and non-specific cross-reactivity was not observed even in presence of an equivalent amount of other types of viruses, therefore demonstrating the usefulness of this approach for the specific detection of virus.
4. Conclusions
Here we summarize our recent applications of functional peptide nanotubes, self-assembled from short peptides with recognition elements, as building blocks to develop sensors. Peptide nanotubes with high aspect ratios are excellent building blocks for the directed assembly into device configurations, and their combining structures with nanometric diameters and micrometric lengths enables to bridge the nano-world and the micro-world. This feature is crucial to integrate the tubular nanostructures with microfabrication processes and the resulting building blocks can be assembled into micron-scale materials but still remain the nanoscale physical properties. The anisotropic tubular shape also makes the directed assembly of peptide nanotubes easier at targeted positions and angles on substrates with molecular recognition, chemical interactions, and dielectrophoresis. Cylindrical surfaces of the nanotubes displaying recognition units enhance the binding efficiency toward target pathogens and heavy metal ions for their sensing.
These characteristic features of functional peptides are key aspects to design new peptide nanotube-based biosensors. When these biosensors that incorporate molecular recognition units apply AC probes to detect impedance signals, the peptide nanotubes behave as excellent building blocks of the transducer for the detection of target analytes such as pathogens, cells, and heavy metal ions with high specificity. In some sensor configurations, the electric signal could be amplified by coupling them with ion-specific mineralization via molecular recognition of peptides. In general the detection limit of peptide nanotube chip sensors is very low and the dynamic range of detection can be widened by improved device designs. The advance in sampling techniques such as the integration with microfluidics will also help improve the dynamic ranges of the nanotube-based chip sensors.
While the large aspect ratio of peptide nanotubes is advantageous for device fabrications and detections, control of the aspect ratio is difficult to achieve. In fact, the majority of self-assembling systems face this problem when they are applied to practical devices; to mass-produce certain designs, all of incorporated nanotubes need to have a uniform diameter and length to minimize the fluctuation in the device-to-device performance. When the strict dimensional control is necessary, the monodisperse 1D peptide structure can be generated genetically. For example, the collagen-based triple helix peptide is a biomolecular nanowire whose diameter and length are monodisperse and tunable; the length of the triple helix nanowire is determined by the number of amino acid residues in the triple helix, controllable with the well-established recombinant technology.[37, 38] The genetic engineering approach is also more advantageous than the chemical approach when the functionalization/derivatization of peptide nanowires is necessary at the specific position of the peptide; the recombinant technology allows this type of specific functionalization whereas chemical synthesis require blocking undesired positions due to the lack of specificity of chemical reactions. The efficiency and the yield of the genetic production can be very high once expression and amplification processes with bacteria are optimized.
Acknowledgments
This research was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award No. DE-FG-02-01ER45935 (device fabrication, electric measurements) and the National Science Foundation under Award No. ECCS-082390 (biological materials). R.R. acknowledges a postdoctoral fellowship from the Spanish Ministerio de Innovación y Ciencia and Fundación Española para la Ciencia y la Tecnología. Hunter College infrastructure is supported by the National Institutes of Health, the RCMI program (G12-RR003037-245476).
Biographies
Hiroshi Matsui
Hiroshi Matsui received his MS in Materials Science and Engineering from Stanford University and PhD in Chemistry from Purdue University in 1996 after completion of his BS in Chemistry at Sophia University. He worked at Columbia University as a postdoctoral associate in Chemistry Department for two years, and currently he is a Professor in Chemistry Department at City University of New York – Hunter College. His research focuses on self-assemblies of biological molecules and their applications in biosensors, photovoltaics, medical imaging, tissue engineering, genetic engineering and cancer research.

Roberto de la Rica
Roberto de la Rica joined Prof. Matsui’s group after receiving his MS in Chemistry and in Biochemistry and Molecular Biology from the Autonomous University of Barcelona, and completing his PhD at the National Microelectronics Center in Barcelona, Spain. His current research interest is in the integration of biological processes with well-known technologies for the design of complex functional devices such as sensors, circuits and functional materials.

Christophe Pejoux
After receiving his MS in Materials Engineering from the University of Evry (France) in 2001, Christophe Pejoux went to work for several years as a visting student on organic-modified Schottky diodes and nanocrystals-based Grätzel cells at the Weizmann Institute of Science (Israel). He has been in the laboratory of Professor Matsui at Hunter College (CUNY) since 2005 to complete a PhD in Nanotechnology. His work includes the synthesis of semicondcutor nanoparticles via biomineralization and the development of biosensors.

Contributor Information
Dr. Roberto de la Rica, Email: roberto.delarica@gmail.com, Department of Chemistry and Biochemistry, City University of New York, Hunter College-CUNY, 695 Park Avenue, New York, NY 10065 (USA), Fax: (+1) 212-650-3918
Christophe Pejoux, Department of Chemistry and Biochemistry, City University of New York, Hunter College-CUNY, 695 Park Avenue, New York, NY 10065 (USA), Fax: (+1) 212-650-3918.
Prof. Hiroshi Matsui, Email: hmatsui@hunter.cuny.edu, Department of Chemistry and Biochemistry, City University of New York, Hunter College-CUNY, 695 Park Avenue, New York, NY 10065 (USA), Fax: (+1) 212-650-3918
References
- 1.de la Rica R, Matsui H. Chem Soc Rev. 2010;38 doi: 10.1039/B917574C. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gazit E. Plenty of Room for Biology at the Bottom: An Introduction to Bionanotechnology. Imperial College Press; London: 2007. [Google Scholar]
- 3.Hartgerink JD, Beniash E, Stupp SI. Proc Natl Acad Sci USA. 2002;99:5133. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X, Djalali R, Haboosheh A, Samson J, Nuraje N, Matsui H. Adv Mater. 2005;17:1753. [Google Scholar]
- 5.Ulijn RV, Smith AM. Chem Soc Rev. 2008;37:664. doi: 10.1039/b609047h. [DOI] [PubMed] [Google Scholar]
- 6.Woolfson DN, Ryadnov MG. Curr Opin Chem Biol. 2006;10:559. doi: 10.1016/j.cbpa.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Ryu J, Park CB. Angew Chem Intl Ed. 2009;48:4820. doi: 10.1002/anie.200900668. [DOI] [PubMed] [Google Scholar]
- 8.Nuraje N, Banerjee IA, MacCuspie RI, Yu L, Matsui H. J Am Chem Soc. 2004;126:8088. doi: 10.1021/ja048617u. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee IA, Yu LT, Matsui H. J Am Chem Soc. 2003;125:9542. doi: 10.1021/ja0344011. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Z, Banerjee IA, Matsui H. J Am Chem Soc. 2005;127:8930. doi: 10.1021/ja051053p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Nuraje N, Bai H, Matsui H. J Pep Sci. 2008;14:203. doi: 10.1002/psc.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djalali R, Chen Y-F, Matsui H. J Am Chem Soc. 2002;124:13660. doi: 10.1021/ja028261r. [DOI] [PubMed] [Google Scholar]
- 13.Djalali R, Chen Y-F, Matsui H. J Am Chem Soc. 2003;125:5873. doi: 10.1021/ja0299598. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee IA, Yu LT, Matsui H. Proc Natl Acad Sci USA. 2003;100:14678. doi: 10.1073/pnas.2433456100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee IA, Yu LT, Matsui H. J Am Chem Soc. 2005;127:16002. doi: 10.1021/ja054907e. [DOI] [PubMed] [Google Scholar]
- 16.Yu LT, Banerjee IA, Matsui H. J Mater Chem. 2004;14:739. [Google Scholar]
- 17.Yu LT, Banerjee IA, Shima M, Rajan K, Matsui H. Adv Mater. 2004;16:709. [Google Scholar]
- 18.Naik RR, Stringer SJ, Agarwal G, Jones SE, Stone MO. Nature Mater. 2002;1:169. doi: 10.1038/nmat758. [DOI] [PubMed] [Google Scholar]
- 19.Dickerson MB, Sandhage KH, Naik RR. Chem Rev. 2008;108:4935. doi: 10.1021/cr8002328. [DOI] [PubMed] [Google Scholar]
- 20.Yu LT, Banerjee IA, Matsui H. J Am Chem Soc. 2003;125:14837. doi: 10.1021/ja037117i. [DOI] [PubMed] [Google Scholar]
- 21.Slocik JM, Zabinski JS, Phillips DM, Naik RR. Small. 2008;4:548. doi: 10.1002/smll.200700920. [DOI] [PubMed] [Google Scholar]
- 22.de la Rica R, Mendoza E, Matsui H. Small. 2010;6 doi: 10.1002/smll.201000489. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teller C, Shimron S, Willner I. Anal Chem. 2009;81:9114. doi: 10.1021/ac901773b. [DOI] [PubMed] [Google Scholar]
- 24.de la Rica R, Mendoza E, Lechuga M, Matsui H. Angew Chem Intl Ed. 2008;47:9752. doi: 10.1002/anie.200804299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Z, Matsui H. Small. 2007;3:1390. doi: 10.1002/smll.200700006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuraje N, Mohammed S, Yang LL, Matsui H. Angew Chem Intl Ed. 2009;48:2546. doi: 10.1002/anie.200805145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kogiso M, Ohnishi S, Yase K, Masuda M, Shimizu T. Langmuir. 1998;14:4978. [Google Scholar]
- 28.Matsui H, Gologan B. J Phys Chem B. 2000;104:3383. [Google Scholar]
- 29.Gao X, Matsui H. Adv Mater. 2005;17:2037. doi: 10.1002/adma.200401849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aizenberg J, Weaver JC, Thanawala MS, Sundar VC, Morse DE, Fratzl P. Science. 2005;309:275. doi: 10.1126/science.1112255. [DOI] [PubMed] [Google Scholar]
- 31.Meldrum FC, Colfen H. Chem Rev. 2008;108:4332. doi: 10.1021/cr8002856. [DOI] [PubMed] [Google Scholar]
- 32.Whaley SR, English DS, Hu EL, Barbara PF, Belcher AM. Nature. 2000;405:665. doi: 10.1038/35015043. [DOI] [PubMed] [Google Scholar]
- 33.Sarikaya M, Tamerler C, Jen AKY, Schulten K, Baneyx F. Nature Mater. 2003;2:577. doi: 10.1038/nmat964. [DOI] [PubMed] [Google Scholar]
- 34.Mao CB, Solis DJ, Reiss BD, Kottmann ST, Sweeney RY, Hayhurst A, Georgiou G, Iverson B, Belcher AM. Science. 2004;303:213. doi: 10.1126/science.1092740. [DOI] [PubMed] [Google Scholar]
- 35.de la Rica R, Pejoux C, Fernandez-Sanchez C, Baldi A, Matsui H. Small. 2010;7:1092. doi: 10.1002/smll.201000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacCuspie RI, Banerjee IA, Gummalla S, Mostowski HS, Pejoux C, Krause PR, Matsui H. Soft Matter. 2008;4:833. doi: 10.1039/b714470a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai H, Xu K, Xu Y, Matsui H. Angew Chem Intl Ed. 2007;46:3319. doi: 10.1002/anie.200605213. [DOI] [PubMed] [Google Scholar]
- 38.Bai H, Xu F, Anjia L, Matsui H. Soft Matter. 2009;5:966. [Google Scholar]


