SUMMARY
Loss of FMRP causes Fragile X syndrome (FXS), but the physiological functions of FMRP remain highly debatable. Here we show that FMRP regulates neurotransmitter release in CA3 pyramidal neurons by modulating action potential (AP) duration. Loss of FMRP leads to excessive AP broadening during repetitive activity, enhanced presynaptic calcium influx and elevated neurotransmitter release. The AP broadening defects caused by FMRP loss have a cell-autonomous presynaptic origin and can be acutely rescued in postnatal neurons. These presynaptic actions of FMRP are translation-independent and are mediated selectively by BK channels via interaction of FMRP with BK channel’s regulatory β4 subunits. Information-theoretical analysis demonstrates that loss of these FMRP functions causes marked dysregulation of synaptic information transmission. FMRP-dependent AP broadening is not limited to the hippocampus, but also occurs in cortical pyramidal neurons. Our results thus suggest major translation-independent presynaptic functions of FMRP that may have important implications for understanding FXS neuropathology.
INTRODUCTION
Transcriptional silencing of the Fmr1 gene encoding Fragile X mental retardation protein (FMRP) is the most common inheritable cause of mental disability, known as FXS (Pfeiffer and Huber, 2009). FXS is also the leading single-gene disorder linked to autism (Bassell and Warren, 2008). Extensive research efforts have focused on elucidating the functions of FMRP at synapses to uncover the molecular basis of FXS. However, the physiological functions of FMRP are incompletely understood and remain a subject of ongoing debate (Pfeiffer and Huber, 2009).
FMRP is an RNA-binding protein that is thought to act primarily as a regulator of local protein synthesis in dendrites (Bassell and Warren, 2008). Research to date has extensively focused on the postsynaptic effects of FMRP loss leading to altered long-term forms of synaptic plasticity. The currently dominant view, known as “mGluR theory” of FXS (Bear et al., 2004), suggests that FMRP’s principal role in synaptic function is controlling postsynaptic mGluR-dependent forms of long-term plasticity, which require local translation of mRNAs in dendrites (Huber et al., 2002).
Recent evidence points to additional important sites of FMRP function beyond the dendritic compartment. (i) Immunoelectron microscopy localized FMRP in axons and presynaptic terminals (Akins et al., 2012; Christie et al., 2009). (ii) Analysis of mRNA translational profiles in Fmr1 knock-out (KO) mice revealed a wide variety of presynaptic FMRP targets (Brown et al., 2001; Darnell et al., 2012; Miyashiro et al., 2003), and proteomic analyses showed that the levels of many presynaptic proteins are affected by FMRP loss (Klemmer et al., 2011; Liao et al., 2008). (iii) Our recent studies revealed changes in the sizes of synaptic vesicle pools and in vesicle recycling kinetics in mouse hippocampal terminals lacking FMRP (Deng et al., 2011). (iv) Recent studies using mosaic Fmr1 KO mice revealed extensive connectivity defects in the hippocampal circuit that have a cell-autonomous presynaptic origin (Hanson and Madison, 2007). Together, these findings suggest that several major neuronal and circuit abnormalities attributed to loss of FMRP arise from the requirement for FMRP in presynaptic functions.
Among the presynaptic mechanisms, the potential role of FMRP in modulating neurotransmitter release and synaptic strength during neuronal activity is of particular interest. Indeed, rapid activity-dependent modulation of synaptic strength, also known as short-term plasticity (STP), is widely believed to serve several essential neural functions such as information processing, working memory and decision making (Deng and Klyachko, 2011). Moreover, our recent information-theoretic analyses have shown that STP plays a critical role in synaptic information transmission by determining the optimal amount of information that synapses transmit in response to specific patterns of neuronal activity (Rotman et al., 2011). Major STP deficits have been implicated in many cognitive disorders, including Rett syndrome (Moretti et al., 2006; Nelson et al., 2011), TSC-related autism (von der Brelie et al., 2006) and schizophrenia (Earls et al., 2011). Recent studies in our and other labs have also uncovered major STP deficits associated with loss of FMRP (Deng et al., 2011; Klemmer et al., 2011; Olmos-Serrano et al., 2010). Yet the functions of FMRP in synaptic mechanisms regulating neurotransmitter release, synaptic strength and STP have received little attention and remain largely unexplored.
On rapid time scales relevant to information processing, the release of neurotransmitter is determined in large part by the shape, frequency and pattern of presynaptic action potentials (APs) (Bean, 2007). In particular, AP duration is an important determinant of release, controlling the amount of presynaptic calcium influx, which translates in ~4th power to the release magnitude. Modulation of AP duration thus represents a precise and powerful mechanism to control and regulate neurotransmitter release. The AP duration is controlled primarily by the activity of voltage-gated K+ channels (VGKCs) (Bean, 2007). In central neurons, the large conductance Ca2+-activated (BK) K+ channels are among the major determinants of AP duration during repetitive activity, owing to their activation being both voltage- and calcium-regulated (Salkoff et al., 2006).
Here we demonstrate that FMRP regulates neurotransmitter release and STP in CA3 hippocampal pyramidal neurons by modulating AP duration via BK channels. By combining electrophysiological, biochemical and genetic evidence with rescue and mimicking experiments, we show that this function of FMRP is translation-independent, cell-autonomous presynaptic and is mediated by FMRP interactions with the BK channels’ regulatory β4 subunits. We further demonstrate the axonal/presynaptic locus of these FMRP actions using recordings of compound APs in the proximity of CA3-CA1 terminals and by direct two-photon measurements of presynaptic calcium influx. Our information theory-based analysis suggests that changes in neurotransmitter release and STP due to loss of FMRP lead to marked defects in synaptic information transmission. Importantly, FMRP-dependent AP broadening appears to be a widespread phenomenon that occurs to even larger extent in cortical pyramidal neurons. Our study thus uncovers a major translation-independent role of FMRP in rapid modulation of neurotransmitter release and synaptic information transmission.
RESULTS
FMRP Regulates Action Potential Duration in CA3 Pyramidal Neurons
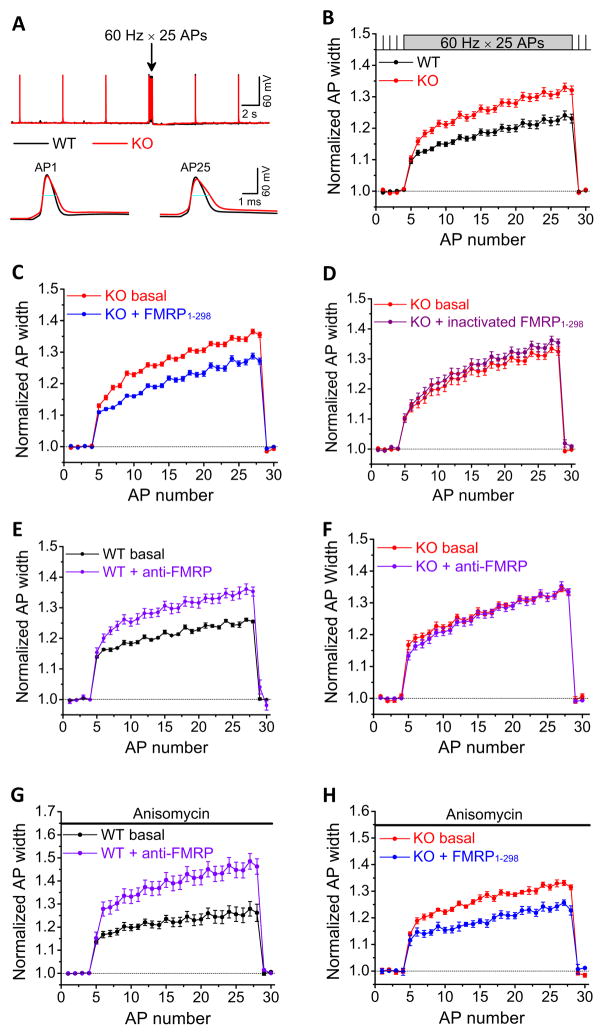
FMRP has been recently shown to play a crucial role in the maintenance of normal STP in excitatory CA3-CA1 synapses (Deng et al., 2011; Klemmer et al., 2011). Since the AP duration is one of the main determinants of release during repetitive activity, we examined the effects of FMRP loss on AP properties in CA3 pyramidal cells (PCs) during repetitive firing. APs were recorded from the current-clamped CA3 PCs in slices from 15- to 25-day-old Fmr1 KO mice and WT controls by repetitive injection of a 1-ms current to evoke a 25-AP train at 60 Hz. The resting membrane potential was set at −65 mV via automatic slow current injection to avoid spontaneous AP firing. We found that AP duration was significantly increased in Fmr1 KO mice both for baseline APs (evoked at 0.2 Hz) and particularly during AP bursts (n=19(WT),18(KO); p=0.013(baseline), p=0.011(burst); Figure 1A,B, Table S1). In contrast, the AP amplitude was not significantly affected by FMRP loss (p=0.56(baseline), p=0.23(burst), Table S1). These results indicate that loss of FMRP leads to excessive AP broadening in CA3 PCs, particularly during repetitive activity, suggesting a role for FMRP in modulating AP duration.
Figure 1. FMRP Regulates Action Potential Duration in CA3 Pyramidal Neurons in a Translation-Independent and Cell-Autonomous Presynaptic Manner.
(A) Sample traces of AP trains recorded in CA3 PCs of WT (in black, here and throughout) or Fmr1 KO mice (in red, here and throughout) in response to 25 stimuli at 60 Hz. 4 APs at 0.2 Hz were evoked prior to each train to determine baseline AP duration. Blue lines show the AP width measured at −10 mV level.
(B) Analysis of AP duration in (A). AP duration was normalized to its own baseline for WT or Fmr1 KO mice.
(C) Effect of intracellular perfusion of FMRP1-298 in Fmr1 KO CA3 PCs on AP broadening.
(D) Heat-inactivated FMRP1-298 failed to affect AP broadening in Fmr1 KO mice.
(E) Effect of intracellular perfusion of anti-FMRP Ab in WT CA3 on AP broadening.
(F) Intracellular perfusion of Anti-FMRP Ab had no effect on AP duration in Fmr1 KO mice.
(G, H) Anisomycin failed to block the effects of anti-FMRP on AP duration in WT mice (G), or the effects of FMRP1-298 on AP duration in Fmr1 KO mice (H).
FMRP Regulates Action Potential Duration in Cortical Pyramidal Neurons
Since loss of FMRP impacts neural function in several brain areas (Pfeiffer and Huber, 2009), we examined whether FMRP regulation of AP duration also occurs in other brain regions. Given the importance of cortical processing in cognitive brain functions, we focused on cortical excitatory neurons. Analysis of AP duration in Layer 5 PCs in the entorhinal cortex revealed excessive AP broadening in Fmr1 KO relative to WT mice (Figure S1). In respect to the magnitude of the effect of FMRP loss on AP duration, PCs in this cortical area appeared to fall into two different populations. In one, excessive AP broadening due to FMRP loss was similar to that in the hippocampal PCs, while another population exhibited greatly exaggerated broadening up to ~300% of baseline at the end of the train (Figure S1, Table S1). These data suggest that FMRP regulation of AP duration is not limited to the hippocampus, but is also present in cortical neurons.
Because of our earlier findings of abnormal STP in the hippocampal CA3-CA1 synapses of Fmr1 KO mice (Deng et al., 2011) and because hippocampal circuit organization allows us to examine not only somatic, but also axonal/presynaptic APs (see below), we focused our studies on CA3 PCs as a model system.
FMRP Regulation of Action Potential Duration Has a Cell-Autonomous Presynaptic Origin
To determine the cellular origin of FMRP actions on AP waveform, we examined whether acute reintroduction of FMRP via intracellular perfusion into individual CA3 Fmr1 KO neurons (presynaptic from the perspective of CA3-CA1 synaptic connections) can rescue the abnormal AP broadening in these neurons. We used the recombinant FMRP fragment containing amino acids 1–298 (100 nM, FMRP1-298), which was included in the recording pipette. To establish “baseline” AP duration in the same neuron, electrode tips were filled with the same internal solution without FMRP1-298, and APs were recorded shortly after formation of whole-cell recordings. AP duration was then examined again after a ~30-min period to allow for intracellular diffusion of FMRP1-298. Only the data recorded from the same neurons before and after 30 min perfusion of FMRP1-298 were used in this analysis. We found that introduction of FMRP1-298 into CA3 Fmr1 KO neurons rapidly reduced excessive broadening of both baseline and burst APs (Figure 1C, Table S1; n=11; p=0.006 (baseline), p=0.0095 (burst)). The heat-inactivated FMRP1-298 had no effect on AP duration in the Fmr1 KO mice (Figure 1D, Table S1; n=6; p=0.79(baseline), p=0.33(burst)). This result indicates that FMRP modulation of AP duration in CA3 neurons has a cell-autonomous presynaptic origin.
If this is the case, we further reasoned that acute neutralization of FMRP in WT CA3 neurons should mimic the abnormal AP broadening observed in Fmr1 KO mice. We thus used an anti-FMRP polyclonal antibody (Ab) to acutely neutralize FMRP in the WT CA3 neurons. Anti-FMRP Ab (1:200) was included in the recording pipette and its effects on AP duration were examined as described above. In agreement with our prediction, anti-FMRP Ab significantly increased both the baseline and burst AP widths in WT mice (Figure 1E, Table S1; n=8; p=0.025(baseline), p=0.031(burst)). To verify the specificity of anti-FMRP Ab, we performed the same experiments in Fmr1 KO mice. Anti-FMRP Ab failed to alter the duration of both baseline and burst APs in Fmr1 KO mice (Figure 1F, Table S1; n=6; p=0.65(baseline); p=0.76(burst)), indicating that anti-FMRP Ab broadens AP duration in WT neurons specifically by neutralizing FMRP. Taken together these results indicate that FMRP rapidly regulates AP duration in CA3 PCs in a cell-autonomous manner.
FMRP Modulation of Action Potential Duration is Independent of Protein Synthesis
Since FMRP is thought to function primarily as regulator of protein synthesis, we next examined whether the rapid effects of FMRP on AP duration are protein synthesis dependent. Slices from WT mice were pre-incubated with the protein synthesis inhibitor anisomycin (20 μM) for >2 hrs and continuously perfused with anisomycin during recording. We found that anisomysin failed to block the effects of anti-FMRP Ab on increasing the AP duration in WT neurons (Figure 1G, Table S1; n=6, p=0.028(baseline), p=0.031(burst)). Similarly, in Fmr1 KO mice, pre-incubation and continuous perfusion of slices with anisomycin failed to block the effect of FMRP1-298 on shortening AP duration (Figure 1H, Table S1; n=6; p=0.018(baseline), p=0.015(burst)). We verified the effectiveness of the anisomycin treatment in blocking translation, by showing that anisomycin completely abolished a translation-dependent form of LTD induced by activation of Group 1 mGluRs in CA3-CA1 synapses (Huber et al., 2002) (Figure S2). These results strongly suggest that the rapid modulation of AP duration by FMRP is independent of protein synthesis.
FMRP Modulates TEA-Sensitive Voltage-Gated K+ Conductances in CA3 Pyramidal Neurons
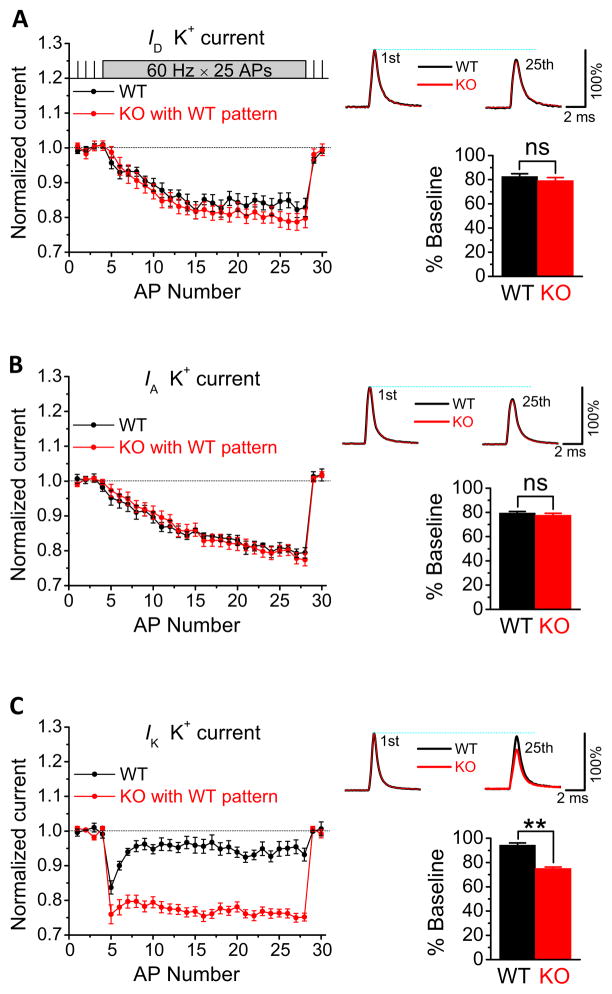
AP duration in central neurons is mostly determined by the activity of VGKCs (Bean, 2007). We therefore used an AP-clamp approach to test the hypothesis that FMRP modulation of AP duration is mediated by the regulation of VGKC activity. In this technique, the membrane voltage follows an actual AP trajectory, and thus the time course and amplitude of ionic currents closely approximate what occurs during the AP trains. Due to excessive AP broadening in the absence of FMRP, changes in AP-evoked currents in Fmr1 KO mice may result from either prolongation of AP waveform or, independently, from FMRP modulation of channel activity, or both. To disentangle these possibilities and reveal changes in K+ currents that are independent of changes in AP waveform, we used the same WT AP waveforms (25 APs at 60 Hz) as voltage commands for both WT and Fmr1 KO neurons.
Based on the pharmacological properties and kinetics of VGKCs, three major K+ current components can be isolated in CA3 neurons: ID, IA and IK (Vacher et al., 2008). We isolated each of these components in CA3 PCs by sequentially adding 30 μM 4-AP, 3 mM 4-AP and 25 mM TEA (Figures 2A–C and S3) in the presence of 1 μM TTX, but omitting Ca2+ channel blockers to ensure that Ca2+-activated K+ currents are included. We found that only IK was altered in Fmr1 KO relative to WT mice during the same WT AP trains and was significantly reduced by FMRP loss (Figure 2C; n=7(WT),9(KO); p=0.0029). Neither ID nor IA were significantly affected by FMRP loss (Figures 2A,B). These results suggest that among the VGKCs, the TEA-sensitive, K+ conductance IK is the main target of FMRP regulation in CA3 PCs.
Figure 2. FMRP modulates TEA-Sensitive Voltage-Gated K+ Conductances in CA3 Pyramidal Neurons.
(A, B, C) Three major VGKC components, ID, IA and IK isolated in WT and Fmr1 KO CA3 PCs using AP-clamp. Currents during the AP burst were normalized to their own baseline. Representative traces show averaged currents evoked by the first and 25th APs of the burst, scaled for comparison to the normalized current evoked by the first AP of the burst in a corresponding neuron. Bar graphs show averaged currents evoked by the last two APs of the burst, expressed as percentage of baseline. ns, not significant; **p <0.01.
FMRP Effects on Action Potential Duration are Mediated by BK Channels
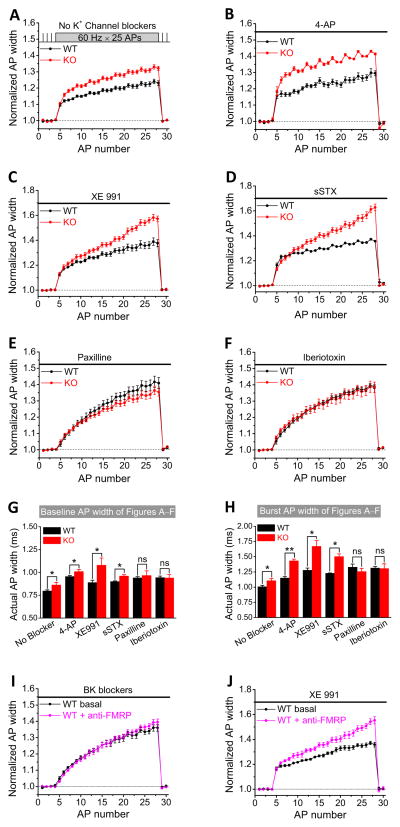
We next sought to identify the specific K+ channels that mediate FMRP actions on AP duration. Based on the above results, we focused on the VGKCs that are both sensitive to TEA and known to contribute to AP repolarization and synaptic transmission in the hippocampus: KCNQ/Kv7 (M channels), Kv2.1/Kv2.2 (delay rectifier K+ channels), and BK channels (Vacher et al., 2008). We examined the roles of these channels in FMRP actions on AP duration using channel-specific blockers. Both M-channel blocker XE991 (10 μM) and Kv2 channel blocker sStromatoxin-1 (sSTX, 100 nM) increased the baseline and burst AP duration, but neither of them abolished the differences in AP duration between WT and Fmr1 KO mice (Figure 3C,D and see summary data in Figure 3G,H). Conversely, the specific BK channel blockers paxilline (10 μM) or iberiotoxin (100 nM) not only increased AP duration in WT and Fmr1 KO mice, but more importantly, either of these blockers abolished the differences in AP duration between WT and Fmr1 KO mice both for baseline APs and APs during bursts (Figure 3E,F and 3G,H; Table S1; paxilline: n=6(WT),6(KO); p=0.94(baseline), p=0.25(burst); iberiotoxin: n=7(WT);6(KO), p=0.77 (baseline), p=0.94(burst)). This analysis suggests that BK channels are the major K+ conductance mediating FMRP effects on AP duration in CA3 PCs.
Figure 3. FMRP Regulation of Action Potential Duration is Mediated by BK Channels.
(A–F) Normalized AP width in WT and Fmr1 KO CA3 PCs in the absence of K+ channel blockers (A), or in the presence of: 3 μM 4-AP (B); M channel blocker XE991 (C); Kv2 channel blocker sStromatoxin-1 (sSTX) (D); BK channel blocker paxilline (E); BK channel blocker iberiotoxin (F).
(G,H) Summary of the data in (A–F) showing actual width of baseline AP (G) or of burst AP (averaged from the last 2 APs of burst) (H). ns, not significant; *p<0.05.
(I, J) BK channel blockers (I), but not M channel blocker XE 991 (J) masked the effect of anti-FMRP Ab on AP broadening in WT mice.
If FMRP indeed regulates AP duration via BK channels, then BK channel blockers should occlude the effects of anti-FMRP Ab on AP broadening observed in WT neurons (Figure 1E). Indeed, we found that when slices from WT mice were pre-incubated with paxilline (10 μM) or iberiotoxin (100 nM) for at least 10 min and continuously perfused during recordings, these BK channel blockers completely occluded the effect of anti-FMRP Ab on AP duration (Figure 3I, Table S1; pooled data for paxilline and iberiotoxin; n=6; p=0.59(baseline), p=0.70(burst)). As a negative control, we performed the same experiment with M-channel blocker XE 991 (10 μM). XE 991 failed to occlude the effect of anti-FMRP Ab on AP duration in WT neurons (Figure 3J, Table S1; n=6; p=0.0005(baseline), p=0.0062(burst)). These results provide further evidence that FMRP modulation of AP duration in CA3 PCs is mediated specifically by BK channels.
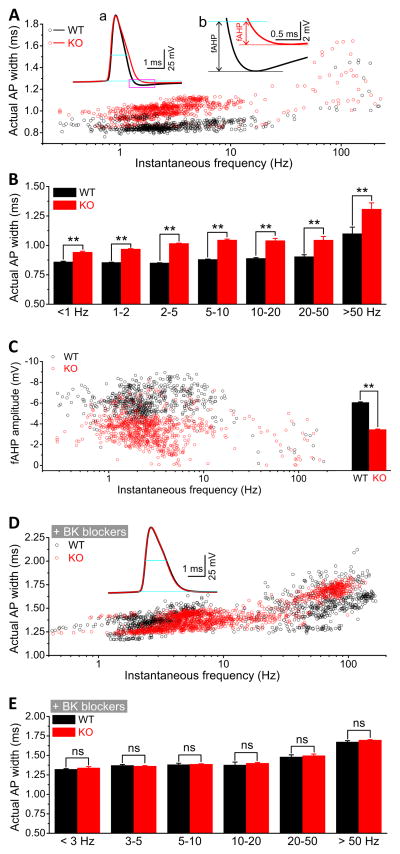
To extend the above results, we next examined the effects of BK channel blockers on AP duration during spontaneous firing. Spontaneous APs were recorded in CA3 PCs at a membrane potential of ~ −51 mV set via automatic slow current injection. Under this condition, the actual rest membrane potential (RMP) and AP amplitudes were not significantly different between WT and Fmr1 KO mice (Table S1; n=17(WT),29(KO); p=0.60(RMP), p=0.18(AP amplitude)). Similarly to evoked APs, duration of spontaneous APs was significant larger in the Fmr1 KO mice regardless of firing frequency (Figures 4A,B). In agreement with the above results, either paxilline or iberiotoxin abolished the differences in spontaneous AP duration between WT and Fmr1 KO mice (Figures 4D,E).
Figure 4. FMRP Modulates Spontaneous Action Potential Duration and fAHP in CA3 Pyramidal Neurons via BK Channels.
(A) Duration of spontaneous APs for WT and Fmr1 KO neurons. Insert (a): sample spontaneous APs; blue lines denote AP width and RMP. Insert (b): Enlargement of box in (a) indicating measurements of fAHP amplitude. Black and red lines indicate the lowest points of fAHP for WT and KO mice, respectively.
(B) Data from (A) averaged within frequency bands shown on X-axis. **p<0.01.
(C) Scatter plots of fAHP amplitude against firing frequencies for WT and Fmr1 KO neurons. The bar graph represents the mean fAHP amplitudes. **p<0.01.
(D) Scatter plots of actual AP widths against firing frequencies in the presence of BK channel blockers. Insert: sample APs.
(E) Data from (D) presented as frequency-banded mean AP width. ns, not significant.
To further confirm the role of BK channels in FMRP modulation of AP waveform, we examined the AP afterhyperpolarization (AHP). In pyramidal neurons, the fast AHP (fAHP) is mediated largely by BK channels (Storm, 1987). Based on the above results, we expect that loss of FMRP should lead to a reduction of fAHP amplitude. Indeed, the fAHP amplitudes were significantly reduced in Fmr1 KO relative to WT mice (Figure 4C, Table S1; n=17(WT), 29(KO); p<0.0001). A significantly reduced fAHP amplitude was also observed in Layer 5 cortical PCs of Fmr1 KO mice (Figure S1C, n=6(WT),9(KO, Group I), p<0.00001; n=5(KO, Group 2), p<0.00001). Consistent with the predominant role of BK channels in the fAHP, there was no evident fAHP in the presence of paxilline or iberiotoxin within 5 ms after the AP peaks in both WT and Fmr1 KO mice (Figure 4D insert).
Taken together, these experiments indicate that FMRP modulation of AP waveform is mediated by BK channels.
FMRP Modulates BK Channel Calcium Sensitivity
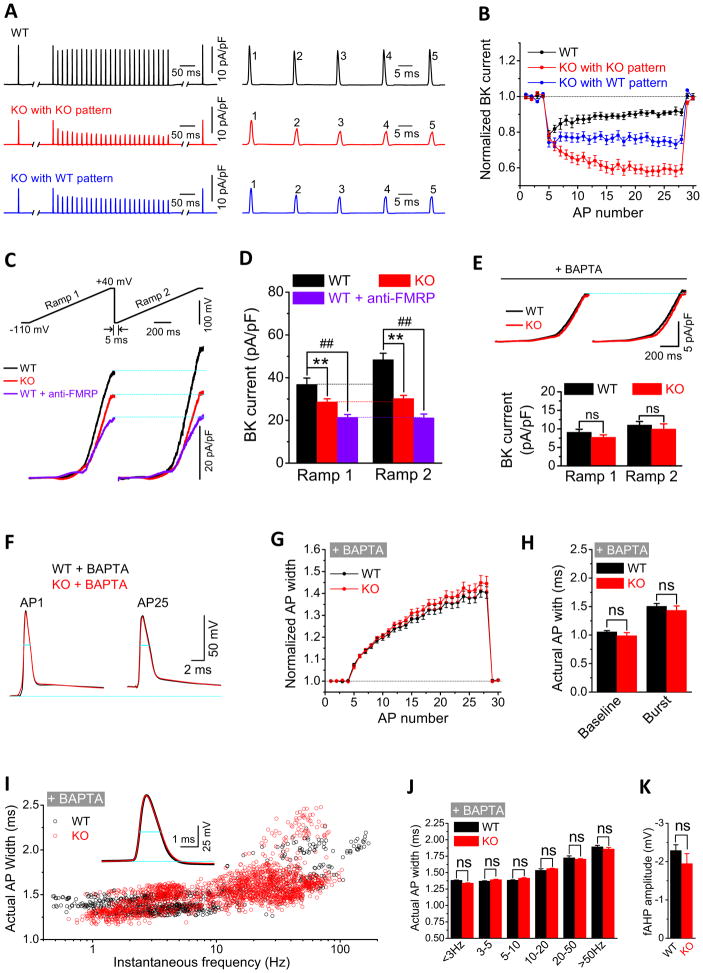
To examine the specific mechanisms by which FMRP modulates BK channel function, we isolated BK currents (as a paxilline or iberiotoxin sensitive component) using AP clamp. Both the baseline BK currents and BK currents during bursts were significantly smaller in Fmr1 KO than in WT mice (Figure 5A,B). This was the case for the native AP trains (n=8(WT), 10(KO); p=0.00065 (baseline), p=0.00038 (burst); Table S1) and when the same WT AP-trains were used in both WT and Fmr1 KO mice (p=0.00065(baseline), p=0.0068(burst); Table S1). Activity of BK channels is thus decreased in the absence of FMRP, and this effect is independent of changes in AP waveform.
Figure 5. FMRP Modulates Calcium Sensitivity of BK Channels.
(A,B) Sample traces (A) and summary data (B) for BK currents isolated using AP clamp in WT and Fmr1 KO neurons. Right panels in (A) are BK currents evoked by the first 5 APs of the train.
(C) Sample BK currents evoked using the paired-ramp protocol shown. Two identical ramps were from −110mV to +40mV with a rate of 0.1mV/ms and 50ms hold at +40mV. Time between ramps (5ms) is expanded for presentation. Blue lines indicate maximal BK currents of the 1st ramp.
(D) Summary of the data from (C) **, p < 0.01; ##, p < 0.01.
(E) Same as (C, D) in the presence of BAPTA. ns, not significant.
(F–H) Sample AP traces (F), normalized AP widths (G) and actual AP widths (H) during 25 AP trains at 60Hz in Fmr1 KO and WT mice in the presence of BAPTA.
(I) Scatter plots of actual AP width against firing frequencies for spontaneous APs in the presence of BAPTA. Insert shows sample APs.
(J) Frequency-banded mean spontaneous AP duration (data from (I)). ns, not significant.
(K) Mean fAHP amplitude in the presence of BAPTA. Data are from APs at instantaneous firing frequencies <20 Hz, since no measurable fAHP was evident above ~20 Hz in the presence of BAPTA. ns, not significant.
Because BK channels are both voltage and Ca2+ dependent, we further examined which of these BK channel properties are regulated by FMRP, using a ramp protocol. In agreement with the above results, BK current measured using the ramp was significantly smaller in the Fmr1 KO than in WT mice (Figures 5C,D, Table S1; n = 6(WT), 6(KO); p=0.0039 at the end of ramp). Similarly, neutralization of FMRP by anti-FMRP Ab perfusion in WT neurons also reduced BK currents during the ramp (Figure 5C,D, Table S1; n=6; p=0.0026). We note that stronger reduction of BK currents by anti-FMRP Ab in WT mice than that in Fmr1 KO mice might be due to compensatory upregulation of BK channel activity by other regulatory mechanisms under the long-term absence of FMRP. We further reasoned that if FMRP regulates BK channel Ca2+-sensitivity, then minimizing the intracellular calcium elevation during stimulation should reduce or eliminate differences in BK current between WT and Fmr1 KO neurons. Indeed, we found that a fast calcium chelator BAPTA (10 mM in recording pipette) completely abolished the differences of BK currents between WT and Fmr1 KO mice during the ramp (Figure 5E, Table S1; n=6(WT),6(KO); p=0.22). Since the remaining ramp-evoked BK current was the same in WT and Fmr1 KO mice in the presence of BAPTA, these data suggest that reduction in BK channel activity in the absence of FMRP is due primarily to the altered calcium sensitivity of the channel, with its voltage-dependence remaining largely unaffected.
We extended these results by using a paired-ramp protocol with two identical ramps (as above) separated by 5 ms. This protocol is equivalent in principle to the paired-pulse protocol commonly used in synaptic studies in that the only difference between the first and the second ramp is the elevated intracellular Ca2+ ([Ca2+]i) during the 2nd ramp due to the residual [Ca2+]i remaining from the first ramp. We found that BK current was indeed increased during the second ramp as would be expected from elevated calcium levels, but this increase was much larger in WT than in Fmr1 KO mice (Figure 5C,D, Table S1; n=6(WT),6(KO); p=0.0017), and this increase was also strongly reduced by anti-FMRP Ab perfusion in WT neurons (Figure 5C,D; n=6, p=0.00089). This increase in BK current during the second ramp was eliminated by BAPTA (Figure 5E) indicating that it is indeed due to elevated calcium levels. These results thus support the above findings that FMRP acts on BK channel activity by affecting the channel’s Ca2+sensitivity.
Finally if FMRP acts primarily by modifying BK channel calcium sensitivity, our results predict that BAPTA should abolish the differences in AP duration between WT and Fmr1 KO mice. We tested this prediction by examining spontaneous and evoked APs recorded from CA3 PCs in the present of BAPTA (10 mM) in the pipette. As we expected, BAPTA indeed abolished the differences of AP durations between WT and KO mice for both evoked APs (Figures 5F–H, Table S1; n=9(WT),11(KO); p=0.17(baseline); p=0.27(burst)) and spontaneous APs (Figures 5I,J). Furthermore, the fAHP amplitudes of both WT and KO mice were also significantly reduced and became indistinguishable (Figure 5K, Table S1; n=13(WT),19(KO); p = 0.18).
Taken together, these results suggest that FMRP regulates calcium sensitivity of the BK channels and that the AP broadening defects in the absence of FMRP are primarily due to reduced BK channel calcium sensitivity.
FMRP Modulates BK Channel Activity Via Interactions With the β4 Subunits
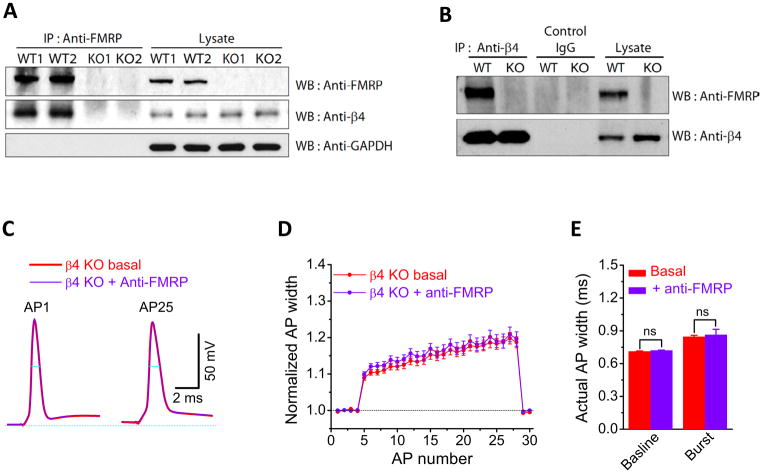
How does FMRP regulate the BK channel calcium sensitivity? A previous study had shown that FMRP does not interact with the pore-forming α subunit of BK channels (Brown et al., 2010). We therefore hypothesized that FMRP may modulate BK channels via a regulatory β subunit. Since β4 is the predominant β subunit expressed in CA3 PCs (Torres et al., 2007), we used a biochemical approach to test whether FMRP interacts with the BK channel’s β4 subunit. When FMRP was immunoprecipitated from hippocampi lysate from WT mice, or Fmr1 KO mice as a negative control, and analyzed by western blot for β4, we observed co-immunoprecipitation of β4 with FMRP in WT but not Fmr1 KO (Figure 6A). In the reverse experiment, when β4 was immunoprecipitated from WT or Fmr1 KO mice, FMRP co-immunoprecipitated with β4 but not with an IgG control (Figure 6B), and only in WT but not Fmr1 KO mice (Figure 6B). These results suggest that FMRP interacts with the β4 subunits of BK channels.
Figure 6. FMRP Interacts with the β4 Subunit of BK Channels to Regulate Action Potential Duration in CA3 Pyramidal Neurons.
(A). FMRP was immunoprecipitated from protein extract prepared from WT or Fmr1 KO mice hippocampi and analyzed by western blot with the indicated antibodies. GAPDH is used as a loading control for the lysate and as a negative control for the IP. A duplicate experiment is shown.
(B). β4 was immunoprecipitated from protein extract prepared from WT or Fmr1 KO mice hippocampi and analyzed by western blot with the indicated antibodies.
(C–E) Sample AP traces (C), normalized AP widths (D) and actual AP widths (E) during 25 AP trains at 60Hz in sloβ4 KO mice before and after intracellular perfusion of anti-FMRP Ab.
Since β4 works as a negative regulator of BK channel activity by modulating its calcium sensitivity (Brenner et al, 2000; Torres et al., 2007), our data in Figures 5 and 6A,B suggest that FMRP interactions with the β4 may be the main mechanism by which FMRP regulates BK channel activity and AP duration. If this is the case, then genetic ablation of sloβ4 should prevent the effects of FMRP loss on AP duration. We tested this idea using sloβ4 KO mice (Brenner et al, 2000). The AP duration was shorter in CA3 PCs from β4 KO than WT mice (n=19(WT),6(β4 KO); p=0.006(baseline), p=0.0001(burst), Table S1). Most importantly, perfusion of anti-FMRP Ab in CA3 neurons from β4 KO mice failed to induce the excessive AP broadening that we observed in WT neurons, for both baseline and burst APs (Figure 6C–E, Table S1; n=6; p=0.66(baseline), p=0.76(burst)).
These findings demonstrate that FMRP regulates AP duration by modulating BK channel calcium sensitivity via interactions with the channel’s regulatory β4 subunit.
FMRP Modulates Calcium Influx in Presynaptic Terminals of CA3 Pyramidal Neurons
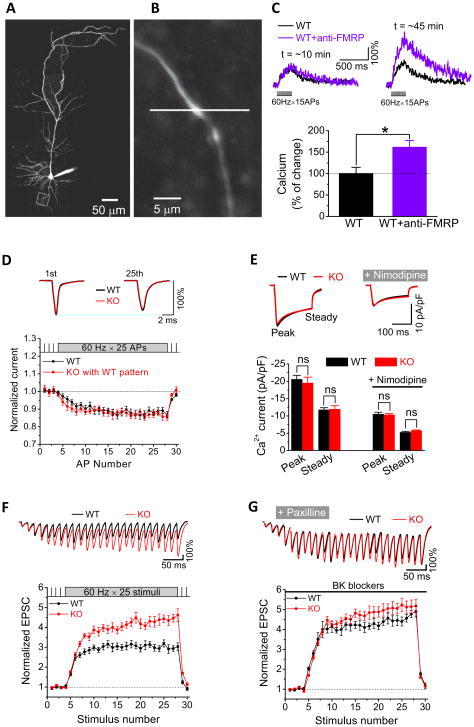
AP duration is one of the major determinants of presynaptic calcium influx (Bean, 2007). We thus tested whether FMRP regulation of AP waveform has an impact on calcium influx in presynaptic terminals of CA3 PCs using two-photon calcium imaging. CA3 neurons in slices from WT mice were loaded via a patch pipette with the fluorescent calcium indicator Fluo-5F and with the fluorescent dye Alexa Fluor 594 for detailed visualization of neuronal processes. Axons were identified as thin processes emanating from the cell body and having no spines, and presynaptic terminals were identified as boutons along the axons (Earls et al., 2011) (Figure 7A,B). Calcium changes were evoked by 15-AP trains at 60 Hz, measured using the line-scan mode and normalized to the average Alexa 594 fluorescence as it was described previously (Earls et al., 2011). To examine the role of FMRP in modulating presynaptic calcium dynamics, we used an intracellular perfusion of Anti-FMRP Ab to interfere with FMRP function, following the same approach as described above (Figure 1E,G). Filling the pipette tip with the solution not containing the Ab allowed us to establish the “baseline” calcium influx in the same terminal. In agreement with our earlier results, perfusion of Anti-FMRP Ab caused AP broadening (data not shown) and, most importantly, a significant increase of calcium influx in the presynaptic terminals during AP trains (Figure 7C) (n=3(WT),3(KO); p=0.04). We also used an Anti-FMRP Ab conjugated with Alexa-488 to verify that the Anti-FMRP Ab can reach remote presynaptic terminals (up to ~160μm from the soma) within the 30 min period after a break-in through intra-axonal diffusion (Figure S4). The delay in anti-FMRP Ab appearance in presynaptic terminals (Figure S4B) is consistent with the ~30min delay that we observed for the effects of the Ab on presynaptic calcium influx (Figure 7C), suggesting that FMRP action in the vicinity of presynaptic terminals mediates its effect on presynaptic calcium influx. This result thus provides direct evidence for the role of FMRP in the regulation of presynaptic calcium influx.
Figure 7. FMRP Regulates Presynaptic Calcium Influx and Synaptic Transmission in CA3 Pyramidal Neurons.
(A, B) Representative image of a CA3 PC filled with fluorescent dyes Fluo 5F and Alexa 594 (A). A high magnification image from the same neuron shows a presynaptic bouton on a CA3 axon (B). Square indicates the location of the presynaptic bouton in (A). Line indicates a direction of the line scan.
(C) Representative calcium transients measured in presynaptic terminals of CA3 PCs using two-photon microscopy in response to a 15-AP train (60-Hz) 10 min or 45 min following break-in in the absence (black) or presence (purple) of anti-FMRP Ab. Bar graph shows mean relative amplitudes of calcium transients measured 45 minutes after break-in for neurons without (black) or with (purple) anti-FMRP Ab. Data are normalized to respective values measured 10 min after break-in.
(D) VGCC currents isolated via AP clamp during 25-AP 60Hz WT trains. Sample averaged VGCC current traces are shown for the first and 25th AP in the train, scaled to normalized currents on the first AP of the train in a corresponding neuron.
(E) VGCC currents isolated via a step protocol (from −65 to 0 mV for 200 ms). Non-L-type Ca2+ currents were isolated in the presence of nimodipine (10 μM). “Steady” denotes the currents averaged from the last 25 ms of the step.
(F,G) Normalized EPSCs recorded in CA1 PC in response to SC stimulation with a train of 25-stimuli at 60 Hz in WT and Fmr1 KO mice under basal conditions (F) or in the presence of BK channel blockers. Upper panel shows EPSC traces during 60-Hz burst with the 1st EPSC of the burst scaled to the same value (100%) in WT and Fmr1 KO for visual comparison. Stimulus artifacts in traces were removed for clarity. Lower panel shows average synaptic responses normalized to their own low-frequency (0.2 Hz) baseline.
FMRP modulation of calcium influx could be mediated by prolongation of AP duration and, in addition, by changes in VGCC activity. We examined the latter possibility using the AP-clamp in CA3 PCs (Figure S3) and found that Ca2+ currents driven by the same WT AP trains were not significantly different in Fmr1 KO and WT mice (Figure 7D, Table S1; n=7(WT),9(KO); p=0.68). We also obtained the same results when Ca2+ currents (both total and non-L-type) were measured by a step protocol (from −65mV to 0mV for 200ms, Figure 7E). Since the bulk of synaptic transmission in CA3 neurons is mediated by non-L-type VGCCs, if FMRP acts similarly on somatic and presynaptic VGCCs, these results suggest that the FMRP modulation of presynaptic calcium influx is not mediated by changes in VGCC function per se.
FMRP Regulates Neurotransmitter Release and Short-Term Plasticity Via BK Channels at CA3-CA1 Synapses
Given a ~4th power relationship between calcium influx and neurotransmitter release, the above results suggest that BK channel-mediated modulation of AP duration by FMRP may play an important role in regulating neurotransmitter release. If this is the case, then BK channel blockers should reduce or abolish the differences in synaptic transmission and STP between WT and Fmr1 KO mice. To test this idea we recorded excitatory postsynaptic currents (EPSCs) from CA1 PCs evoked by stimulating axons of CA3 PCs (i.e., Schaffer collaterals (SC)). As we reported previously (Deng et al, 2011), loss of FMRP led to excessive enhancement of release during repetitive activity, and abnormally elevated STP (Figure 7F, Table S1; n=7(WT),9(KO); p<0.001). We found that BK channel blockers paxilline (10 μM) or iberiotoxin (100 nM) did not significantly alter the baseline EPSCs in either WT (n=7, p=0.16) or Fmr1 KO mice (n=9, p=0.34), indicating that BK channels may not play an important role under basal conditions, as previously suggested (Storm, 1987). However, BK channel blockers abolished the differences between WT and Fmr1 KO mice in synaptic transmission and STP during high-frequency trains (Figure 7G, Table S1; n=7(WT), 9(KO); p=0.60). We note that the action of BK blockers on EPSCs is unlikely to be postsynaptic, because postsynaptic BK channel inhibition failed to occlude the effect of iberiotoxin on paired-pulse ratio of EPSCs recorded from CA1 PCs (Hu et al., 2001). These results, corroborated further below, indicate that FMRP modulates neurotransmitter release and STP via BK channels.
Given that many of FMRP actions are mediated by Group I mGluRs, and particularly by mGluR5 (Bear et al., 2004), we tested whether mGluR5 signaling could contribute to the effects of FMRP loss on synaptic transmission and STP. Application of an mGluR5 antagonist MPEP (10 μM) failed to alter STP in Fmr1 KO mice (Figure S5B; n=11; p=0.75(baseline), p=0.69(burst)). Similarly, blocking translation with anisomycin (2 hour exposure, see positive control in Figure S2) had no significant effects on the differences in synaptic transmission and STP between Fmr1 KO and WT neurons (Figure S6; n=8(WT),6(KO); p=0.002), confirming that these rapid presynaptic FMRP actions are both mGluR5− and translation-independent.
Axonal/Presynaptic Actions of FMRP Modulate Action Potential Waveform and Neurotransmitter Release in CA3 Pyramidal Neurons
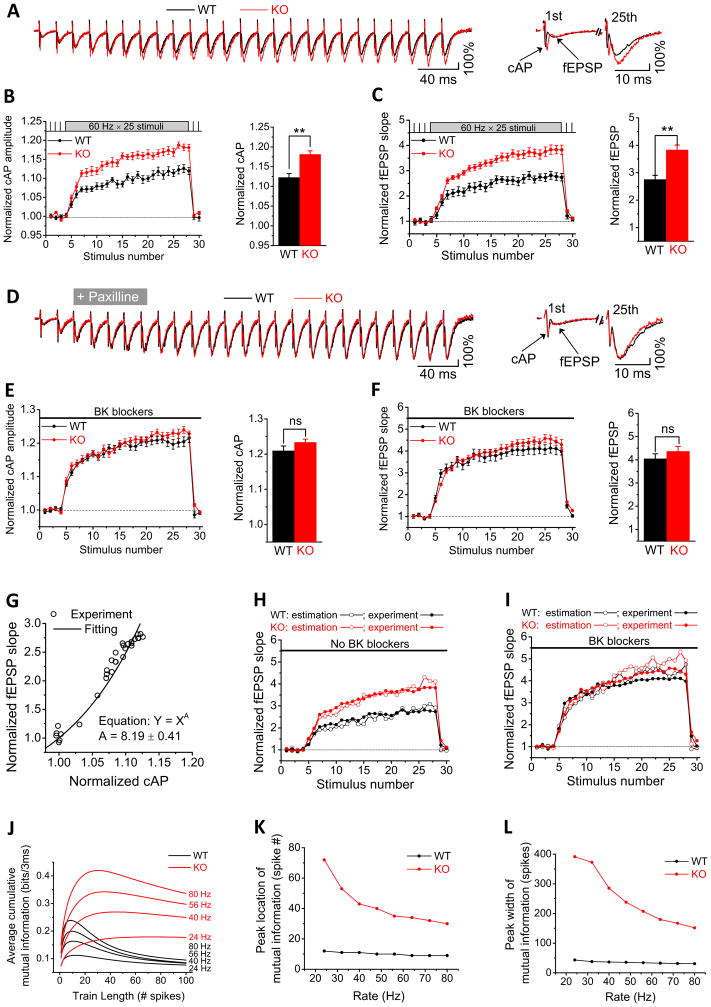
The above findings (Figure 7A–C) suggests that FMRP regulation of AP broadening is not limited to the soma, but also occurs in axons in the vicinity of presynaptic terminals. We tested this idea using extracellular recordings (Figure S7) of compound APs (cAPs, also known as fiber volleys) and resulting field EPSPs (fEPSPs) in the CA1 stratum radiatum, the location of CA3 PC axons and synaptic terminals. The cAP is essentially proportional to the time derivative of membrane voltage during AP firing and the cAP amplitude can be used as a surrogate for AP duration (Bean, 2007; Poage and Zengel, 2002). We found that cAP amplitude was excessively increased during high-frequency trains in the Fmr1 KO relative to WT mice (Figures 8A,B, Table S1; n=10(WT),11(KO); p=0.0015), in close agreement with our somatic AP recordings (Figure 1B). The simultaneously measured changes in fEPSPs, which reflect rapid synaptic dynamics, were also significantly larger in Fmr1 KO relative to WT mice during trains (Figures 8A,C, Table S1; n=10(WT), 11(KO); p=0.0010), in close agreement with our whole-cell recordings of synaptic transmission and STP in CA1 PCs (Figure 7F). If these effects of FMRP loss are indeed mediated by altered BK channel activity, then BK channel blockers should mask the differences in cAPs and fEPSPs between WT and Fmr1 KO mice during high-frequency trains. Indeed, we found that while BK channel blockers did not alter the baseline cAPs or fEPSPs (Table S1), the BK blockers abolished the differences in both cAPs and fEPSPs between WT and Fmr1 KO mice during trains (Figure 8D,E,F, Table S1; n=10(WT), 11(KO); p=0.96(cAP), p=0.77(fEPSP)). These results support our conclusion that FMRP modulation of AP duration, neurotransmitter release and STP is mediated by BK channels. Importantly, these recordings indicate that FMRP-dependent AP broadening occurs in the CA1 stratum radiatum, i.e in close proximity to presynaptic terminals.
Figure 8. Axonal/Presynaptic Actions of FMRP on BK Channel Activity Modulate Action Potential Waveform and Synaptic Information Transmission in CA3-CA1 Synapses.
(A) Sample traces of extracellular recordings of cAPs and fEPSPs in CA1 stratum radiatum in response to SC stimulation with a train of 25 stimuli at 60Hz in WT or Fmr1 KO mice. Traces are plotted with the first fEPSP scaled to the same value (100%) in WT and Fmr1 KO mice for visual comparison. Right panel shows the 1st and last (25th) responses on an extended time scale. Stimulus artifacts in traces were removed for clarity.
(B, C) Normalized cAP amplitude (B) and normalized fEPSP initial slope (C) during 25 stimuli at 60Hz in Fmr1 KO and WT mice. Bar graph compares the average of the last two measurements of the train. **p<0.01.
(D,E,F) Same as (A,B,C), respectively, in the presence of BK channel blockers.
(G) Plot of normalized fEPSP slope against normalized cAPs amplitude in WT mice without BK blockers (data from (B,C). The plot was fitted by Equation Y = XA, where Y is fEPSP and X is cAP.
(H, I) Prediction of fEPSP changes from corresponding cAP amplitude in the absence (H) or presence (I) of BK channel blockers.
(J–L). Information-theoretic analysis of synaptic information transmission in WT and Fmr1 KO mice. Mutual information is plotted as a function of input spike number for a range of input spike frequencies (J). Parameters of optimal information transmission are plotted for the peak location (K) and peak width (L). Input spike frequencies tested are shown in (J) above each trace.
An important question is whether changes in AP duration can fully account for the observed changes in release and STP in the absence of FMRP. To address this question we estimated the changes in release (evaluated by fEPSP) caused by excessive AP broadening (evaluated by cAP) when FMRP is absent. First, we established the precise relationship between cAP and fEPSP in WT mice by plotting them versus each other (data from Figures 8B,C) and fitting the plot by a power relationship Y = k·XA. Since X and Y are normalized, k=1, and we obtained the value of A=8.19±0.41 (Figure 8G). Since fEPSP slope reflects the amount of neurotransmitter release, it is expected to be proportional to the ~4th power of presynaptic [Ca2+]i. Our calculation then yields a 2nd power relationship between [Ca2+]i and cAP amplitude. This 2nd power relationship is expected, because we used a “1-dimensional parameter” cAP amplitude to predict the “2-dimensional parameter” the time-cumulative Ca2+ influx.
We then used this 8th power relationship between cAP amplitude and fEPSP slope to predict changes in synaptic transmission that would be expected from changes in corresponding cAPs. As shown in Figure 8H, changes in cAPs due to loss of FMRP predicted closely the corresponding changes in fEPSPs that were observed experimentally. We further used the same approach to predict the effects of BK channel blockers on fEPSPs in both WT and Fmr1 KO mice and also found a close agreement with the changes observed experimentally (Figure 8I). This analysis thus further confirms that abnormally elevated neurotransmitter release and STP caused by the absence of FMRP are mediated by BK channel dependent, excessive AP broadening that occurs in the axons in the vicinity of presynaptic terminals.
Implications of Abnormal Release and STP in the Absence of FMRP to Synaptic Information Transmission
Given the critical roles of STP in information processing (Deng and Klyachko, 2011), we examined what impact the abnormalities in synaptic transmission and STP due to FMRP loss have on the ability of synapses to transmit information. Synaptic information transmission can be estimated within the information-theoretic framework that we and others have developed based on computational modeling of synaptic dynamics (Rotman et al., 2011; Zador, 1998). In particular, our approach is based on a physiologically-relevant model of STP in the CA3-CA1 synapses (Kandaswamy et al., 2010). We first determined a set of model parameters to account for the changes in synaptic transmission and STP due to FMRP loss (see Methods for details). The model was then able to successfully reproduce changes in release and STP due to loss of FMRP during trains in the entire range of 20–80 Hz in which changes in STP were observed experimentally (data not shown). The model was then used to simulate STP during Poisson spike trains to estimate the amount of transmitted information, as we described previously (Rotman et al., 2011).
Using this approach, we have previously shown that STP maximizes information transmission for short high-frequency spike bursts (peak at ~10 spikes) at WT CA3-CA1 synapses (Figure 8J). Such spike bursts are indeed common in firing patterns of hippocampal PCs and are believed to carry information about an animal’s position in the environment (O’Keefe and Dostrovsky, 1971). In this study, our calculations showed that the increased neurotransmitter release and abnormally elevated STP due to FMRP loss had a marked effect on synaptic information transmission (Figure 8J–L). In the absence of FMRP, information transmission was elevated nearly indiscriminately at all frequencies in the range of 20–80 Hz and burst durations up to 400 spikes (Figure 8J). In addition, loss of FMRP caused a nearly complete loss of optimization of information transmission for spike bursts. Specifically, the peak of optimal information transmission was flattened and nearly eliminated, being broaden 4–9 fold to reach several hundred spikes in the same frequency range (Figure 8L). The peak was also shifted 3–5 fold to a larger spike number (Figure 8K). Since hippocampal neurons do not commonly fire such prolonged high-frequency bursts, this result suggests that processing properties of the synapses are greatly diminished due to loss of FMRP, since information transmission is no longer discriminative in regards to the input spike pattern within the physiologically relevant range. Moreover, these results suggest that the large amounts of “noise” that are normally “filtered out” during synaptic processing, are being transmitted indiscriminately in the absence of FMRP.
DISCUSSION
Our results demonstrate that FMRP regulates neurotransmitter release in CA3 pyramidal neurons via modulation of AP duration. Acute rescue experiments indicate that this function of FMRP is translation-independent and has a cell-autonomous presynaptic origin. The axonal/presynaptic locus of FMRP actions on AP waveform is supported by recordings of compound APs in the vicinity of presynaptic terminals and by direct measurements of presynaptic calcium influx. In WT neurons FMRP acts to limit AP broadening during repetitive activity by modulating the activity of BK channels, specifically their calcium sensitivity. This function of FMRP is mediated by an interaction between FMRP and the BK channel’s regulatory β4 subunit. Loss of FMRP causes reduced BK channel activity and excessive AP broadening, leading in turn to elevated presynaptic calcium influx, increased synaptic transmission and STP during repetitive activity. Information analysis suggests that the defects in neurotransmitter release and STP associated with FMRP loss cause marked abnormalities in synaptic information transmission. Taken together, these results reveal a major translation-independent presynaptic role of FMRP in regulating neurotransmitter release and synaptic information transmission. Our observation that FMRP modulates AP duration both in the hippocampal and cortical pyramidal neurons suggests that FMRP regulation of presynaptic function may be a widespread phenomenon that could play a role in the pathophysiology of FXS.
Translation-Independent Functions of FMRP
FMRP functions in synapses have been a focus of extensive research over the last two decades to uncover the molecular basis of FXS. Extensive research supports FMRP’s major function in controlling mRNA trafficking and translation, and evidence accumulated so far has led to the current dominant view that FMRP acts at synapses primarily by regulating local protein synthesis in dendrites (Pfeiffer and Huber, 2009).
In addition to its mRNA binding, FMRP possesses a N-terminal NDF domain, which is believed to serve as a platform for protein-protein interactions (Ramos et al., 2006). Several nuclear and ribosomal protein binding partners of FMRP have been identified, and these interactions are thought to support FMRP control of mRNA trafficking and translation (Bassell and Warren, 2008). Whether FMRP also interacts with proteins not involved in dendritic translation to regulate other neural functions remains largely unknown. Our results and recent evidence from others (Brown et al., 2010) suggest that FMRP indeed has additional and important functions in neurons. This notion is supported by several lines of evidence we provided in this study: interfering with FMRP function in WT CA3 neurons by intracellular perfusion of anti-FMRP Ab alters AP waveform within minutes, and this effect is not affected by the inhibition of protein synthesis. Furthermore, acute re-introduction of FMRP1-298 fragment containing the NDF domain into CA3 Fmr1 KO neurons via a patch pipette rapidly rescues excessive AP broadening and, most importantly, this action of FMRP1-298 is independent of protein synthesis. Finally, we find that FMRP co-immunoprecipitates with β4 subunit of the BK channels and that this FMRP-β4 interaction mediates FMRP regulation of AP duration. FMRP thus acts rapidly to modulate the AP waveform in a translation-independent manner. Together with the recent findings that FMRP interacts with and modulates activity of another ion channel, the Slack sodium-activated K+ channel (Brown et al., 2010), our results suggest a major translation-independent role of FMRP in synaptic function.
FMRP Regulation of Neurotransmitter Release via BK Channels
VGKCs are the main determinants of AP duration in central neurons. Among those, BK channels, which are both voltage- and calcium- regulated, are uniquely suited to control AP repolarization and play a major negative feedback role in limiting AP broadening during repetitive activity (Bean, 2007). Our experiments suggest that FMRP regulation of AP duration is mediated by BK channels, and this regulatory mechanism serves as a brake limiting AP broadening during bursts. Indeed, APs are abnormally broadened in the absence of FMRP, and BK channel blockers masked this effect of FMRP loss. Moreover, selective BK channel blockers, or genetic ablation of BK channel’s β4 subunit, occluded the AP broadening effects caused by acute neutralization of FMRP with anti-FMRP Ab perfusion. In addition, our results demonstrate that by regulating presynaptic AP duration via BK channels, FMRP also modulates neurotransmitter release and STP. BK channel blockers abolished the differences between WT and Fmr1 KO mice in presynaptic AP duration and simultaneously recorded synaptic transmission/STP measured in the vicinity of the CA3-CA1 presynaptic terminals. Moreover, our analysis indicate that changes in presynaptic cAPs associated with loss of FMRP fully accounted for the changes in synaptic transmission in Fmr1 KO mice, and also fully accounted for the effects of BK channel blockers on eliminating the differences in synaptic transmission and STP between WT and Fmr1 KO mice. The latter results indicate that BK-channel-mediated action of FMRP on AP duration is the predominant mechanism by which FMRP regulates synaptic transmission and STP. These results are in agreement with extensive evidence for the role of presynaptic BK channels in regulating neurotransmitter release in central neurons (Hu et al., 2001; Raffaelli et al., 2004). Our results support the emerging view that FMRP plays an important role in controlling neural excitability by regulating K+ channels via translation-dependent (Gross et al., 2011; Lee et al., 2011; Strumbos et al., 2010) as well as translation-independent mechanisms (Brown et al., 2010).
Mechanisms of FMRP Modulation of BK channel activity
How does FMRP regulate BK channel activity? Our results suggest that FMRP act on BK channels by modifying their calcium sensitivity. In support of this notion, the effect of FMRP loss on BK currents was completely abolished by a Ca2+ chelator BAPTA. BAPTA also abolished the differences in AP duration and fAHP amplitude between WT and Fmr1 KO mice. Paired-ramp experiments provided additional evidence that the calcium sensitivity of BK channels is reduced in Fmr1 KO mice or by acute neutralization of FMRP with anti-FMRP Ab perfusion in WT mice. Our analysis further indicates that FMRP modulation of the BK channels is indirect and is mediated by an interaction with the channel’s regulatory β4 subunit. Indeed, we found that FMRP co-immunoprecipitates with sloβ4 (but not slo1α (Brown et al., 2010)), which is known to regulate BK channel calcium sensitivity (Brenner et al, 2000; Torres et al., 2007). Furthermore, we found that the BK channel’s β4 subunit is required for FMRP modulation of AP duration. This opens a possibility that FMRP may modulate neural excitability not only by directly gating a subset of ion channels (Brown et al., 2010), but also via interactions with a variety of ion channel regulatory subunits thereby modulating channels’ properties indirectly.
We note that in addition to these rapid translation-independent effects, FMRP may in principle also regulate BK channel expression. Our observations that both the BK current and AP waveform are indistinguishable in the presence of BAPTA in WT and Fmr1 KO neurons argue against large changes in BK channel expression in the absence of FMRP. Recent proteomics analysis in hippocampal neurons also did not find BK channels among proteins which expression is altered by FMRP loss (Klemmer et al.,2011). In contrast, proteomics of cultured cortical neurons in Fmr1 KO mice suggest reduction in BK α subunit expression (Liao et al., 2008), yet the mRNA levels of BK channel α subunit has been reported to be increased in Fmr1 KO mice (Brown et al., 2001). Future studies will be needed to determine whether and to what extent FMRP regulates BK channel expression. Whatever the outcome, the rapid and translation-independent rescue or mimicking of FMRP effects on AP broadening during trains strongly argues that this effect of FMRP is independent of changes in BK channel expression that might occur in the absence of FMRP.
Cellular Locus of FMRP Actions at Synapses
One of the important unresolved questions in understanding FMRP functions at synapses is the cellular locus of the synaptic defects caused by FMRP loss. Extensive research supports the view that defects associated with FMRP loss have a principally postsynaptic origin (Bassell and Warren, 2008). Here we found that FMRP regulates synaptic transmission and STP at CA3-CA1 synapses via modulation of AP broadening in presynaptic neurons. While the synaptic mechanisms involved in controlling the AP waveform, neurotransmitter release and STP are widely believed to be predominately presynaptic in nature (Bean, 2007; Deng and Klyachko, 2011), modulation of these processes by FMRP might in principle also arise from homeostatic changes during development or due to trans-synaptic signaling mechanisms. Our ability to rapidly rescue or mimic the effects of FMRP loss on AP duration in individual CA3 neurons, however, strongly supports the cell-autonomous presynaptic origin of FMRP effects we observed. The mimicking experiments using anti-FMRP Ab in WT neurons also argue against the homeostatic/trans-synaptic nature of FMRP actions on AP broadening, since in these experiments all neurons, except for the one being perfused, are intact WT cells. Our findings, supported by evidence from other groups (Gatto and Broadie, 2008; Hanson and Madison, 2007) thus support the presence of major cell-autonomous presynaptic FMRP functions, in addition to its well-established postsynaptic ones. We note that while our results argue for the presynaptic origin of FMRP actions we described, our findings do not require nor indicate that FMRP acts directly at the presynaptic terminals, since FMRP can regulate release indirectly by acting on the AP duration in the axons. Indeed, there is evidence that even AP broadening that occurs selectively at the axon initial segment can spread far along an axon and cause a significant enhancement of release at presynaptic terminals (Kole et al., 2007).
Implications of FMRP Loss for Synaptic Information Transmission and the Neuropathology of FXS
What are the implications of altered neurotransmitter release and STP in the absence of FMRP to synaptic information transmission? Using our recently developed information-theoretic approach (Rotman et al., 2011) we were able to estimate the impact of FMRP loss on information transmission at CA3-CA1 synapses. We found that processing properties of the synapses are greatly diminished due to loss of FMRP, since information transmission is no longer discriminative in regards to the input spike pattern within the physiologically relevant range. In particular, we found that while normal synapses are tuned precisely to maximize information transmission selectively for short high-frequency spike bursts, this optimization is nearly lost in the absence of FMRP. Instead of a sharp peak of optimization, loss of FMRP leads to marked shift and broadening of the optimization peak to several hundred spikes, which is largely outside of the physiological range of spiking intensity of hippocampal pyramidal cells. We also found a strong increase in information transmission across all frequencies tested in neurons lacking FMRP. This result further suggests that one abnormality in neural function associated with elevated STP in the absence of FMRP is indiscriminate transmission of large amounts of information, that otherwise would be “filtered” by healthy synapses. If presynaptic information processing defects in the hippocampus or other brain areas are found to contribute to the cognitive deficits in FXS, our results suggest that upregulation of BK channel activity could represent a promising strategy to alleviate some of these deficits in FXS.
EXPERIMENTAL PROCEDURES
Animals and Slice Preparation
Fmr1 KO and control strain mice were obtained from the Jackson Laboratory. sloβ4 KO mice were generously provided by Dr. Robert Brenner (University of Texas, San Antonio). All animal procedures conformed to the guidelines approved by the Washington University Animal Studies Committee. Horizontal brain slices (350 μm) were prepared as previously described (Deng et al., 2011).
Electrophysiology
APs were recorded using an Axopatch 700B or 200B amplifier (Molecular Devices) in whole-cell mode from CA3 PCs visually identified with infrared video microscopy and DIC. Recordings were conducted at 33–34°C in the presence of APV (5 0 μM), DNQX (10 μM) and gabazine (5 μM). AP duration was determined at the −10 mV level. In AP-clamp experiments, ionic currents were normalized by corresponding cell capacitance and expressed as pA/pF. Cell capacitance was compensated. Series resistance compensation was enabled with ~90% correction and 10–20 μs lag. Leak subtraction was done by P/5 protocol. EPSCs were recorded from CA1 PCs held at −65 mV by stimulating Schaffer Collaterals (SC) with a bipolar electrode in the presence of gabazine (5 μM) and APV (50 μM). Extracellular cAPs and fEPSPs were recorded from CA1 stratum radiatum by stimulating SC; stimulating and recording electrodes were set at least 350–400 μm apart. Recordings were filtered at 2 kHz, digitized at 20 kHz, acquired using custom software written in LabView, and analyzed using programs written in Matlab or MiniAnalysis.
Two-Photon Presynaptic Calcium Imaging
TPLSM was performed using an Ultima imaging system (Prairie Technologies), a Ti:sapphire Chameleon Ultra femtosecond-pulsed laser (Coherent), and 60× (0.9 NA) water-immersion IR objectives (Olympus). Alexa Fluor 594 and Fluo 5F were included in the internal solution and were excited at 820 nm. Alexa Fluor 594 fluorescence was used to image and reconstruct axonal morphology of CA3 neurons. Changes in Fluo 5F fluorescence were used to visualize changes in Ca2+ concentrations in presynaptic terminals. AP-evoked changes in the fluorescence of Fluo 5F were measured in current-clamp using line-scan mode (500 Hz) in CA3 presynaptic terminals.
Biochemistry
Anti-FMRP (Cell Signaling), anti-β4 (Abcam) or mouse IgG (Invitrogen) were incubated with Dynabeads (Invitrogen) in IP buffer containing 5% bovine serum albumin at 22°C for 30 min. Mouse hippocampi were homogenized in IP buffer containing protease inhibitor (Roche) and phosphatase inhibitor cocktail (Sigma). Protein extract was clarified by centrifugation and pre-incubated with protein G sepharose 4 beads (GE Healthcare) for 1 hour at 4°C. The supernatant was incubated with antibody-Dynabeads complex at 4°’C overnight. The immunoprecipited material was washed with IP buffer 5 times and analyzed by western blot.
Information-Theoretic Analysis of Synaptic Information Transmission
Information transmission analysis was performed using the approach we described previously (Rotman et al., 2011).
Data Analysis and Statistics
Data are presented as mean ± SEM. Student’s paired or unpaired t test or ANOVA were used for statistical analysis as appropriate; significance was set as p < 0.05. Statistical tests for burst recordings were determined for the averaged last two stimuli of the burst. The n number of measurements represents the number of cells examined, the number of animals examined is reported in Table S1.
Additional details are available in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
This work was supported in part by grants to VAK from the NINDS R01 NS081972, FRAXA Foundation, Edward Mallinckrodt Jr. Foundation, and McDonnell Center for Systems Neuroscience, and by NIMH R01 MH079079 and R01 MH095810 to SSZ, NINDS RO1 NS060709 to VC, NINDS R01 NS060706 to JC. We thank Dr. Brenner (University of Texas-San Antonio) for providing sloβ4 KO mice and Drs. Blumer, O’Malley, and Owyoung for their constructive comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akins MR, Leblanc HF, Stackpole EE, Chyung E, Fallon JR. Systematic mapping of Fragile X granules in the developing mouse brain reveals a potential role for presynaptic FMRP in sensorimotor functions. J Comp Neurol. 2012;520:3687–706. doi: 10.1002/cne.23123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula VR, Chen Y, et al. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci. 2010;13:819–821. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Christie SB, Akins MR, Schwob JE, Fallon JR. The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J Neurosci. 2009;29:1514–1524. doi: 10.1523/JNEUROSCI.3937-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2012;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Klyachko VA. The diverse functions of short-term plasticity components in synaptic computations. Commun Integr Biol. 2011;4:543–548. doi: 10.4161/cib.4.5.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Sojka D, Klyachko VA. Abnormal presynaptic short-term plasticity and information processing in a mouse model of fragile X syndrome. J Neurosci. 2011;31:10971–10982. doi: 10.1523/JNEUROSCI.2021-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls LR, Bayazitov IT, Fricke RG, Berry RB, et al. Dysregulation of presynaptic calcium and synaptic plasticity in a mouse model of 22q11 deletion syndrome. J Neurosci. 2011;30:15843–15855. doi: 10.1523/JNEUROSCI.1425-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto CL, Broadie K. Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure. Development. 2008;135:2637–2648. doi: 10.1242/dev.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Yao X, Pong DL, Jeromin A, Bassell GJ. Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci. 2011;31:5693–5698. doi: 10.1523/JNEUROSCI.6661-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE, Madison DV. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J Neurosci. 2007;27:4014–4018. doi: 10.1523/JNEUROSCI.4717-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shao LR, Chavoshy S, Gu N, et al. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandaswamy U, Deng PY, Stevens CF, Klyachko VA. The role of presynaptic dynamics in processing of natural spike trains in hippocampal synapses. J Neurosci. 2010;30:15904–15914. doi: 10.1523/JNEUROSCI.4050-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemmer P, Meredith RM, Holmgren CD, Klychnikov OI, et al. Proteomics, ultrastructure, and physiology of hippocampal synapses in a fragile X syndrome mouse model reveal presynaptic phenotype. J Biol Chem. 2011;286:25495–25504. doi: 10.1074/jbc.M110.210260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55:633–647. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Lee HY, Ge WP, Huang W, He Y, et al. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron. 2011;72:630–642. doi: 10.1016/j.neuron.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Park SK, Xu T, Vanderklish P, Yates JR., 3rd Quantitative proteomic analysis of primary neurons reveals diverse changes in synaptic protein content in fmr1 knockout mice. Proc Natl Acad Sci U S A. 2008;105:15281–15286. doi: 10.1073/pnas.0804678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, et al. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ED, Bal M, Kavalali ET, Monteggia LM. Selective impact of MeCP2 and associated histone deacetylases on the dynamics of evoked excitatory neurotransmission. J Neurophysiol. 2011;106:193–201. doi: 10.1152/jn.00751.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, et al. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15:549–567. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poage RE, Zengel JE. Repolarization of the presynaptic action potential and short-term synaptic plasticity in the chick ciliary ganglion. Synapse. 2002;46:189–198. doi: 10.1002/syn.10135. [DOI] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini E. BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J Physiol. 2004;557:147–157. doi: 10.1113/jphysiol.2004.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Hollingworth D, Adinolfi S, Castets M, et al. The structure of the N-terminal domain of the fragile X mental retardation protein: a platform for protein-protein interaction. Structure. 2006;14:21–31. doi: 10.1016/j.str.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Rotman Z, Deng PY, Klyachko VA. Short-term plasticity optimizes synaptic information transmission. J Neurosci. 2011;31:14800–14809. doi: 10.1523/JNEUROSCI.3231-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumbos JG, Brown MR, Kronengold J, Polley DB, Kaczmarek LK. Fragile X mental retardation protein is required for rapid experience-dependent regulation of the potassium channel Kv3.1b. J Neurosci. 2010;30:10263–10271. doi: 10.1523/JNEUROSCI.1125-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres YP, Morera FJ, Carvacho I, Latorre R. A marriage of convenience: beta-subunits and voltage-dependent K+ channels. J Biol Chem. 2007;282:24485–24489. doi: 10.1074/jbc.R700022200. [DOI] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Brelie C, Waltereit R, Zhang L, Beck H, Kirschstein T. Impaired synaptic plasticity in a rat model of tuberous sclerosis. Eur J Neurosci. 2006;23:686–692. doi: 10.1111/j.1460-9568.2006.04594.x. [DOI] [PubMed] [Google Scholar]
- Zador A. Impact of synaptic unreliability on the information transmitted by spiking neurons. J Neurophysiol. 1998;79:1219–1229. doi: 10.1152/jn.1998.79.3.1219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.