Abstract
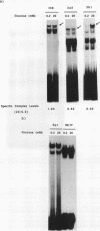
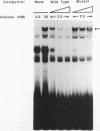
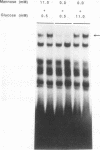
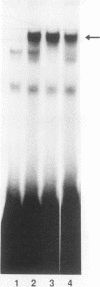
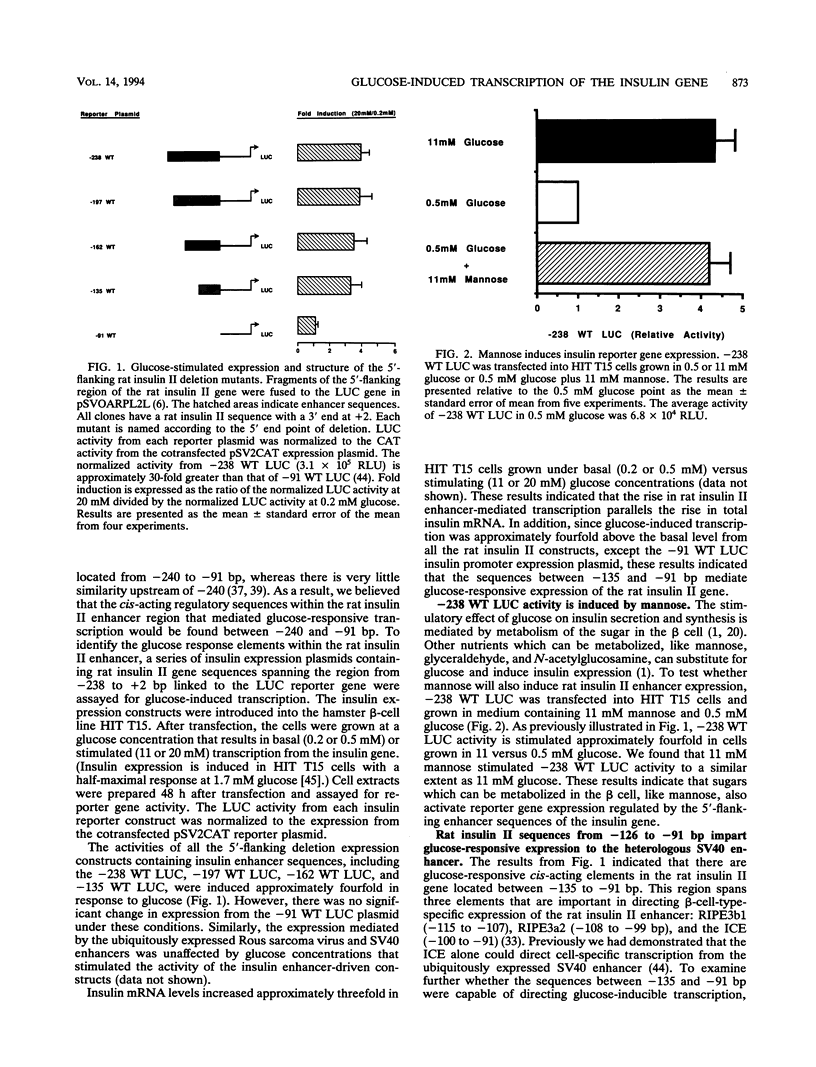
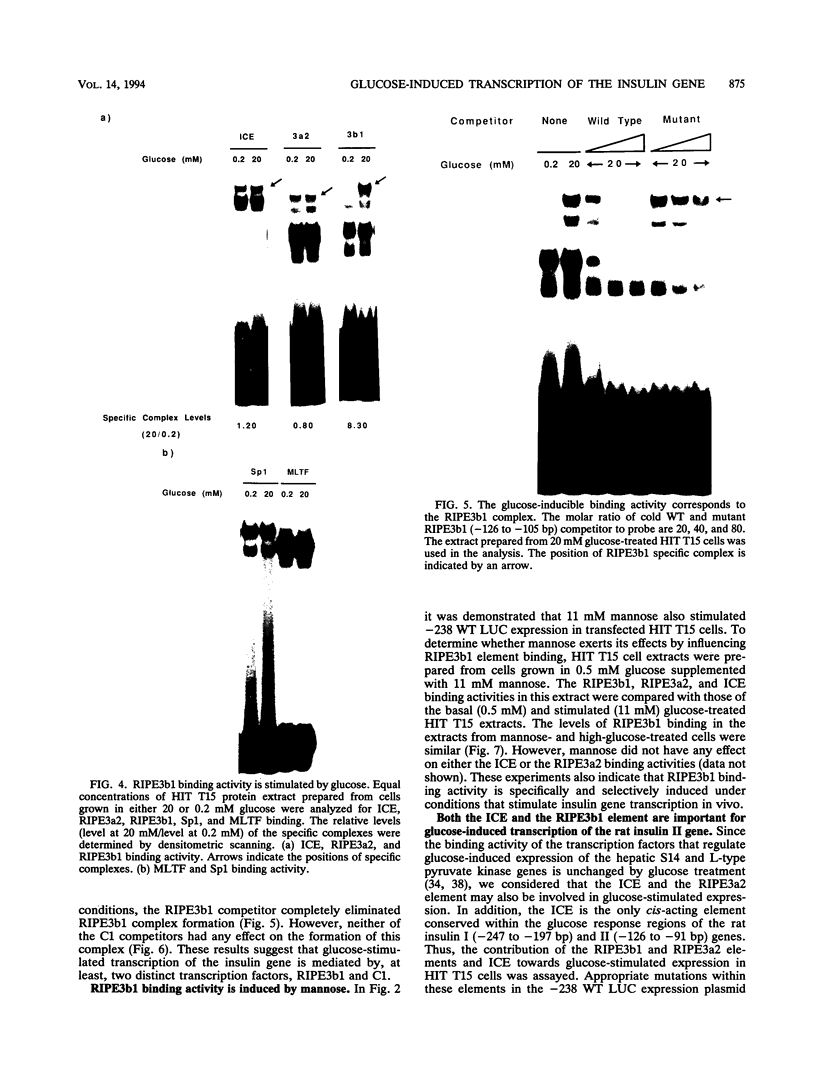
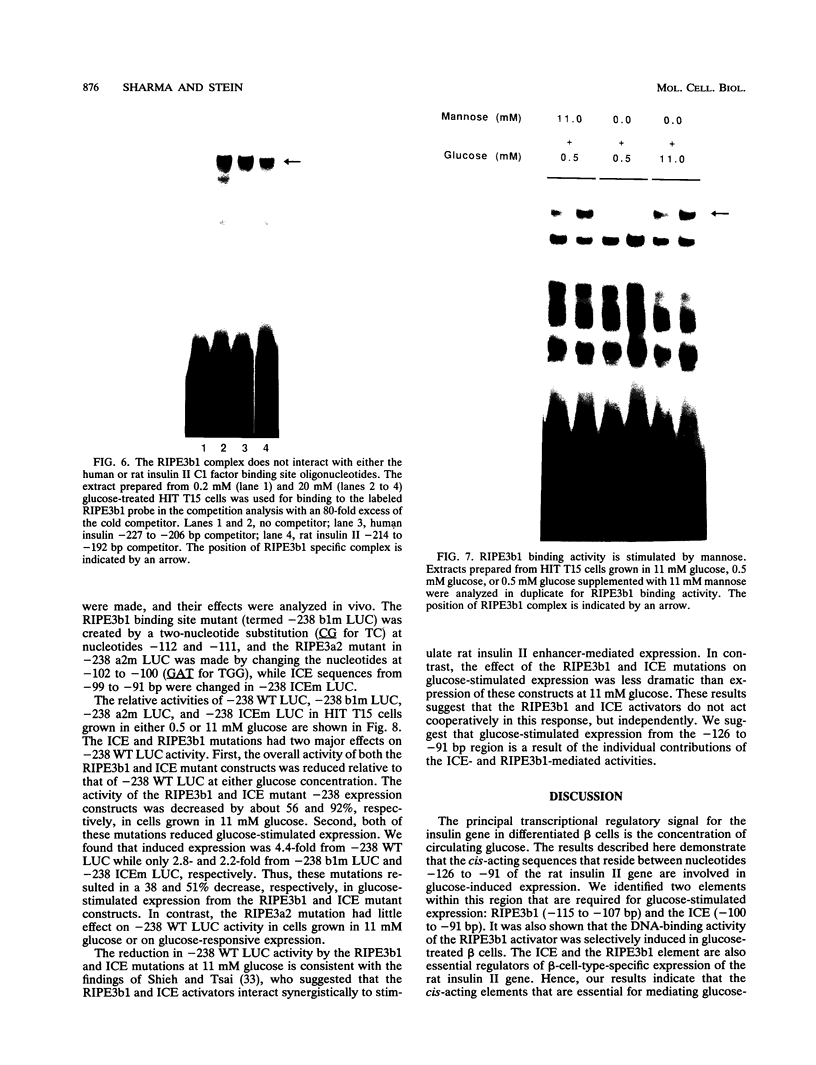
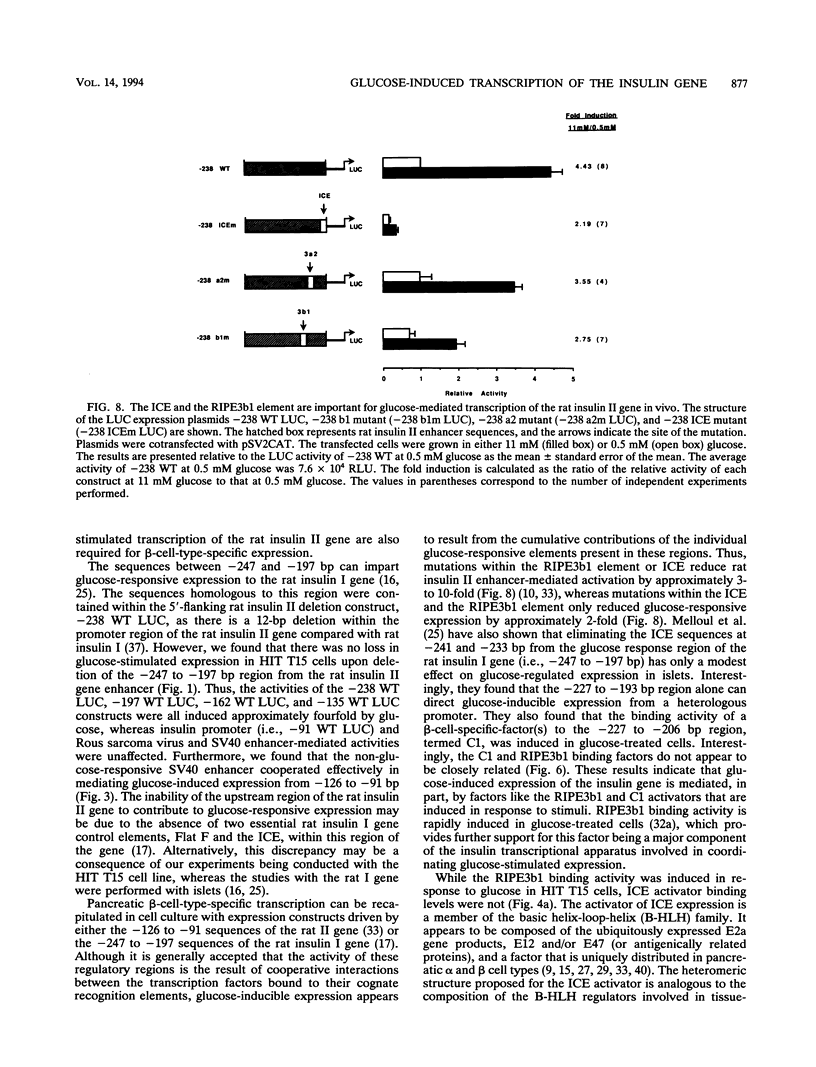
The insulin gene is expressed exclusively in pancreatic islet beta cells. The principal regulator of insulin gene transcription in the islet is the concentration of circulating glucose. Previous studies have demonstrated that transcription is regulated by the binding of trans-acting factors to specific cis-acting sequences within the 5'-flanking region of the insulin gene. To identify the cis-acting control elements within the rat insulin II gene that are responsible for regulating glucose-stimulated expression in the beta cell, we analyzed the effect of glucose on the in vivo expression of a series of transfected 5'-flanking deletion mutant constructs. We demonstrate that glucose-induced transcription of the rat insulin II gene is mediated by sequences located between -126 and -91 bp relative to the transcription start site. This region contains two cis-acting elements that are essential for directing pancreatic beta-cell-type-specific expression of the rat insulin II gene, the insulin control element (ICE; -100 to -91 bp) and RIPE3b1 (-115 to -107 bp). The gel mobility shift assay was used to determine whether the formation of the ICE- and RIPE3b1-specific factor-DNA element complexes were affected in glucose-treated beta-cell extracts. We found that RIPE3b1 binding activity was selectively induced by about eightfold. In contrast, binding to other insulin cis-acting element sequences like the ICE and RIPE3a2 (-108 to -99 bp) were unaffected by these conditions. The RIPE3b1 binding complex was shown to be distinct from the glucose-inducible factor that binds to an element located between -227 to -206 bp of the human and rat insulin I genes (D. Melloul, Y. Ben-Neriah, and E. Cerasi, Proc. Natl. Acad. Sci. USA 90:3865-3869, 1993). We have also shown that mannose, a sugar that can be metabolized by the beta cell, mimics the effects of glucose in the in vivo transfection assays and the in vitro RIPE3b1 binding assays. These results suggested that the RIPE3b1 transcription factor is a primary regulator of glucose-mediated transcription of the insulin gene. However, we found that mutations in either the ICE or the RIPE3b1 element reduced glucose-responsive expression from transfected 5'-flanking rat insulin II gene constructs. We therefore conclude that glucose-regulated transcription of the insulin gene is mediated by cis-acting elements required for beta-cell-type-specific expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J. Glucoreceptor mechanisms and the control of insulin release and biosynthesis. Diabetologia. 1980 Jan;18(1):5–15. doi: 10.1007/BF01228295. [DOI] [PubMed] [Google Scholar]
- Bengal E., Ransone L., Scharfmann R., Dwarki V. J., Tapscott S. J., Weintraub H., Verma I. M. Functional antagonism between c-Jun and MyoD proteins: a direct physical association. Cell. 1992 Feb 7;68(3):507–519. doi: 10.1016/0092-8674(92)90187-h. [DOI] [PubMed] [Google Scholar]
- Boam D. S., Docherty K. A tissue-specific nuclear factor binds to multiple sites in the human insulin-gene enhancer. Biochem J. 1989 Nov 15;264(1):233–239. doi: 10.1042/bj2640233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstedt J., Chan S. J. Direct effect of glucose on the preproinsulin mRNA level in isolated pancreatic islets. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1383–1389. doi: 10.1016/0006-291x(82)91267-0. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J. A., Knust E. Genetics of early neurogenesis in Drosophila melanogaster. Annu Rev Genet. 1990;24:387–407. doi: 10.1146/annurev.ge.24.120190.002131. [DOI] [PubMed] [Google Scholar]
- Chrapkiewicz N. B., Davis C. M., Chu D. T., Caldwell C. M., Granner D. K. Rat gene 33: analysis of its structure, messenger RNA and basal promoter activity. Nucleic Acids Res. 1989 Aug 25;17(16):6651–6667. doi: 10.1093/nar/17.16.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. L., Steiner D. F. Insulin biosynthesis in the rat: demonstration of two proinsulins. Proc Natl Acad Sci U S A. 1969 Jan;62(1):278–285. doi: 10.1073/pnas.62.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Diamond D., Smith S., Pünter J., Schöne H. H., Goodman H. M. Disproportionate expression of the two nonallelic rat insulin genes in a pancreatic tumor is due to translational control. Cell. 1982 Dec;31(3 Pt 2):531–542. doi: 10.1016/0092-8674(82)90309-9. [DOI] [PubMed] [Google Scholar]
- Cordle S. R., Henderson E., Masuoka H., Weil P. A., Stein R. Pancreatic beta-cell-type-specific transcription of the insulin gene is mediated by basic helix-loop-helix DNA-binding proteins. Mol Cell Biol. 1991 Mar;11(3):1734–1738. doi: 10.1128/mcb.11.3.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe D. T., Tsai M. J. Mutagenesis of the rat insulin II 5'-flanking region defines sequences important for expression in HIT cells. Mol Cell Biol. 1989 Apr;9(4):1784–1789. doi: 10.1128/mcb.9.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Efrat S., Surana M., Fleischer N. Glucose induces insulin gene transcription in a murine pancreatic beta-cell line. J Biol Chem. 1991 Jun 15;266(17):11141–11143. [PubMed] [Google Scholar]
- Falvey E., Schibler U. How are the regulators regulated? FASEB J. 1991 Mar 1;5(3):309–314. doi: 10.1096/fasebj.5.3.2001790. [DOI] [PubMed] [Google Scholar]
- German M. S., Blanar M. A., Nelson C., Moss L. G., Rutter W. J. Two related helix-loop-helix proteins participate in separate cell-specific complexes that bind the insulin enhancer. Mol Endocrinol. 1991 Feb;5(2):292–299. doi: 10.1210/mend-5-2-292. [DOI] [PubMed] [Google Scholar]
- German M. S., Moss L. G., Rutter W. J. Regulation of insulin gene expression by glucose and calcium in transfected primary islet cultures. J Biol Chem. 1990 Dec 25;265(36):22063–22066. [PubMed] [Google Scholar]
- German M. S., Moss L. G., Wang J., Rutter W. J. The insulin and islet amyloid polypeptide genes contain similar cell-specific promoter elements that bind identical beta-cell nuclear complexes. Mol Cell Biol. 1992 Apr;12(4):1777–1788. doi: 10.1128/mcb.12.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings S. J., Chirgwin J., Permutt M. A. Effects of glucose on proinsulin messenger RNA in rats in vivo. Diabetes. 1982 Jul;31(7):624–629. doi: 10.2337/diab.31.7.624. [DOI] [PubMed] [Google Scholar]
- Goodison S., Kenna S., Ashcroft S. J. Control of insulin gene expression by glucose. Biochem J. 1992 Jul 15;285(Pt 2):563–568. doi: 10.1042/bj2850563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Henderson E., Stein R. c-jun inhibits transcriptional activation by the insulin enhancer, and the insulin control element is the target of control. Mol Cell Biol. 1994 Jan;14(1):655–662. doi: 10.1128/mcb.14.1.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Maekawa T., Sudo T., Ishii S., Seino Y., Imura H. c-Jun represses the human insulin promoter activity that depends on multiple cAMP response elements. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1045–1049. doi: 10.1073/pnas.89.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Chambard J. C., Karin M., Olson E. N. Fos and Jun repress transcriptional activation by myogenin and MyoD: the amino terminus of Jun can mediate repression. Genes Dev. 1992 Apr;6(4):676–689. doi: 10.1101/gad.6.4.676. [DOI] [PubMed] [Google Scholar]
- Melloul D., Ben-Neriah Y., Cerasi E. Glucose modulates the binding of an islet-specific factor to a conserved sequence within the rat I and the human insulin promoters. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3865–3869. doi: 10.1073/pnas.90.9.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen S. K., Green P. P., 3rd, Fowlkes D. M. A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA. 1987 Apr;6(2):173–178. doi: 10.1089/dna.1987.6.173. [DOI] [PubMed] [Google Scholar]
- Ohlsson H., Thor S., Edlund T. Novel insulin promoter- and enhancer-binding proteins that discriminate between pancreatic alpha- and beta-cells. Mol Endocrinol. 1991 Jul;5(7):897–904. doi: 10.1210/mend-5-7-897. [DOI] [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sartorelli V., Webster K. A., Kedes L. Muscle-specific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 1990 Oct;4(10):1811–1822. doi: 10.1101/gad.4.10.1811. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh S. Y., Tsai M. J. Cell-specific and ubiquitous factors are responsible for the enhancer activity of the rat insulin II gene. J Biol Chem. 1991 Sep 5;266(25):16708–16714. [PubMed] [Google Scholar]
- Shih H. M., Towle H. C. Definition of the carbohydrate response element of the rat S14 gene. Evidence for a common factor required for carbohydrate regulation of hepatic genes. J Biol Chem. 1992 Jul 5;267(19):13222–13228. [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D. A., Birrer M., Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991 Dec 12;354(6353):494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Chan S. J., Welsh J. M., Kwok S. C. Structure and evolution of the insulin gene. Annu Rev Genet. 1985;19:463–484. doi: 10.1146/annurev.ge.19.120185.002335. [DOI] [PubMed] [Google Scholar]
- Thompson K. S., Towle H. C. Localization of the carbohydrate response element of the rat L-type pyruvate kinase gene. J Biol Chem. 1991 May 15;266(14):8679–8682. [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Park C. W., Rosen A., Aronheim A. A cDNA from a mouse pancreatic beta cell encoding a putative transcription factor of the insulin gene. Nucleic Acids Res. 1990 Mar 11;18(5):1159–1166. doi: 10.1093/nar/18.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Welsh M., Nielsen D. A., MacKrell A. J., Steiner D. F. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J Biol Chem. 1985 Nov 5;260(25):13590–13594. [PubMed] [Google Scholar]
- Whelan J., Cordle S. R., Henderson E., Weil P. A., Stein R. Identification of a pancreatic beta-cell insulin gene transcription factor that binds to and appears to activate cell-type-specific expression: its possible relationship to other cellular factors that bind to a common insulin gene sequence. Mol Cell Biol. 1990 Apr;10(4):1564–1572. doi: 10.1128/mcb.10.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J., Poon D., Weil P. A., Stein R. Pancreatic beta-cell-type-specific expression of the rat insulin II gene is controlled by positive and negative cellular transcriptional elements. Mol Cell Biol. 1989 Aug;9(8):3253–3259. doi: 10.1128/mcb.9.8.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. J., Walseth T. F., Robertson R. P. Insulin secretion and cAMP metabolism in HIT cells. Reciprocal and serial passage-dependent relationships. Diabetes. 1989 Jan;38(1):44–48. doi: 10.2337/diab.38.1.44. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]