Abstract
BACKGROUND & AIMS
Little is known about factors that regulate intestinal epithelial differentiation; microbial recognition receptors such as Toll-like receptor (TLR)4 might be involved. We investigated whether intestinal TLR4 regulates epithelial differentiation and is involved in development of necrotizing enterocolitis (NEC) of the immature intestine.
METHODS
Mice with conditional disruption of TLR4 in the intestinal epithelium and TLR4 knockout (TLR4−/−) mice were generated by breeding TLR4loxp/loxp mice with villin-cre and Ella-cre, respectively. Enterocytes that did not express or overexpressed TLR4 were created by lentiviral or adenoviral transduction. Intestinal organoids were cultured on tissue matrices. Bile acids were measured by colorimetric assays, and microbial composition was determined by 16S pyrosequencing. NEC was induced in 7- to 10-day-old mice by induction of hypoxia twice daily for 4 days.
RESULTS
TLR4−/− mice and mice with enterocyte-specific deletion of TLR4 were protected from NEC; epithelial differentiation into goblet cells was increased via suppressed Notch signaling in the small intestinal epithelium. TLR4 also regulates differentiation of goblet cells in intestinal organoid and enterocyte cell cultures; differentiation was increased on deletion of TLR4 and restored when TLR4 was expressed ectopically. TLR4 signaling via Notch was increased in intestinal tissue samples from patients with NEC, and numbers of goblet cells were reduced. 16S pyrosequencing revealed that wild-type and TLR4-deficient mice had similar microbial profiles; increased numbers of goblet cells were observed in mice given antibiotics. TLR4 deficiency reduced levels of luminal bile acids in vivo, and addition of bile acids to TLR4-deficient cell cultures prevented differentiation of goblet cells.
CONCLUSIONS
TLR4 signaling and Notch are increased in intestinal tissues of patients with NEC and required for induction of NEC in mice. TLR4 prevents goblet cell differentiation, independently of the microbiota. Bile acids might initiate goblet cell development.
Keywords: Development, Mouse Model, Microbiota, Pediatric Gastrointestinal Disorder
The external cues that regulate the development of the gastrointestinal tract remain incompletely understood. 1 The fact that the intestine develops in homeostatic balance with the various microbes that colonize it raises the exciting possibility that microbial recognition receptors themselves may somehow participate in the normal development of the gastrointestinal tract. Chief among such bacterial receptors are the Toll-like receptors (TLRs) of the innate immune system, including the lipopolysaccharide (LPS) receptor TLR4, which we2 and others3 have shown to be present on the intestinal epithelium. Given that the intestinal epithelial lining will ultimately interact with enteric bacteria, we hypothesized that intestinal epithelial TLR4 could regulate normal intestinal epithelial differentiation. To test this hypothesis, we generated mice that selectively lack TLR4 within the intestinal epithelium (TLR4ΔIEC) and observed a surprising effect on intestinal differentiation. Moreover, the physiological relevance of these findings was indicated by the striking resistance and altered intestinal Notch signaling of this epithelial-specific TLR4-deficient mouse to the development of experimental necrotizing enterocolitis (NEC), a severe and often fatal disease that affects premature infants,4 and in which intestinal epithelial TLR4 signaling has been shown to be markedly elevated in mice and humans.5, 6
Materials and Methods
Cell Culture and Reagents
IEC-6 enterocytes were obtained from American Type Culture Collection (Manassas, VA). Antibodies were chromogranin A, F4/80, green fluorescent protein (GFP), and Notch 1 (Abcam); lysozyme, Math1 (mouse atonal homolog 1), MucII, proliferating cell nuclear antigen (PCNA), and sucrose-isomaltase were from Santa Cruz Biotechnology (Santa Cruz, CA), TLR4 was from Imgenex, Alcian blue and 4′,6-diamidino-2- phenylindole (DAPI) were from Fisher Scientific, dibenzapine (DBZ) was from Calbiochem, and Myd88 and TRIF-deficient mice were from Jackson Research Laboratory.
Replication-deficient recombinant adenoviruses that express GFP-tagged mouse TLR4–complementary DNA and control adenovirus that expresses only GFP were prepared using the Adeno-X Expression System 2 Kit (Clontech, Mountain View, CA).7 TLR4-deficient IEC-6 enterocytes were generated by transduction of lentiviral particles (Invitrogen, Grand Island, NY) containing TLR4–small hairpin RNA (shRNA) (Open Biosystems, Lafayette, CO), using the 4-plasmid lentiviral packaging system in HEK293. Stable integration of lentivirus was obtained by selection using puromycin-containing media (5 µg/mL), and complete knockdown of TLR4 was verified by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Primary intestinal cultures (organoids) were isolated and maintained in culture according to Sato et al.8 Cells were treated with scrambled lentivirus as described.9 Bile acids were measured using the total bile acids assay kit (Diazyme, Poway, CA),10 and cells were exposed to deoxycholic acid (50–250 µmol/L; Sigma, St Louis, MO).
Generation of Mice Lacking TLR4
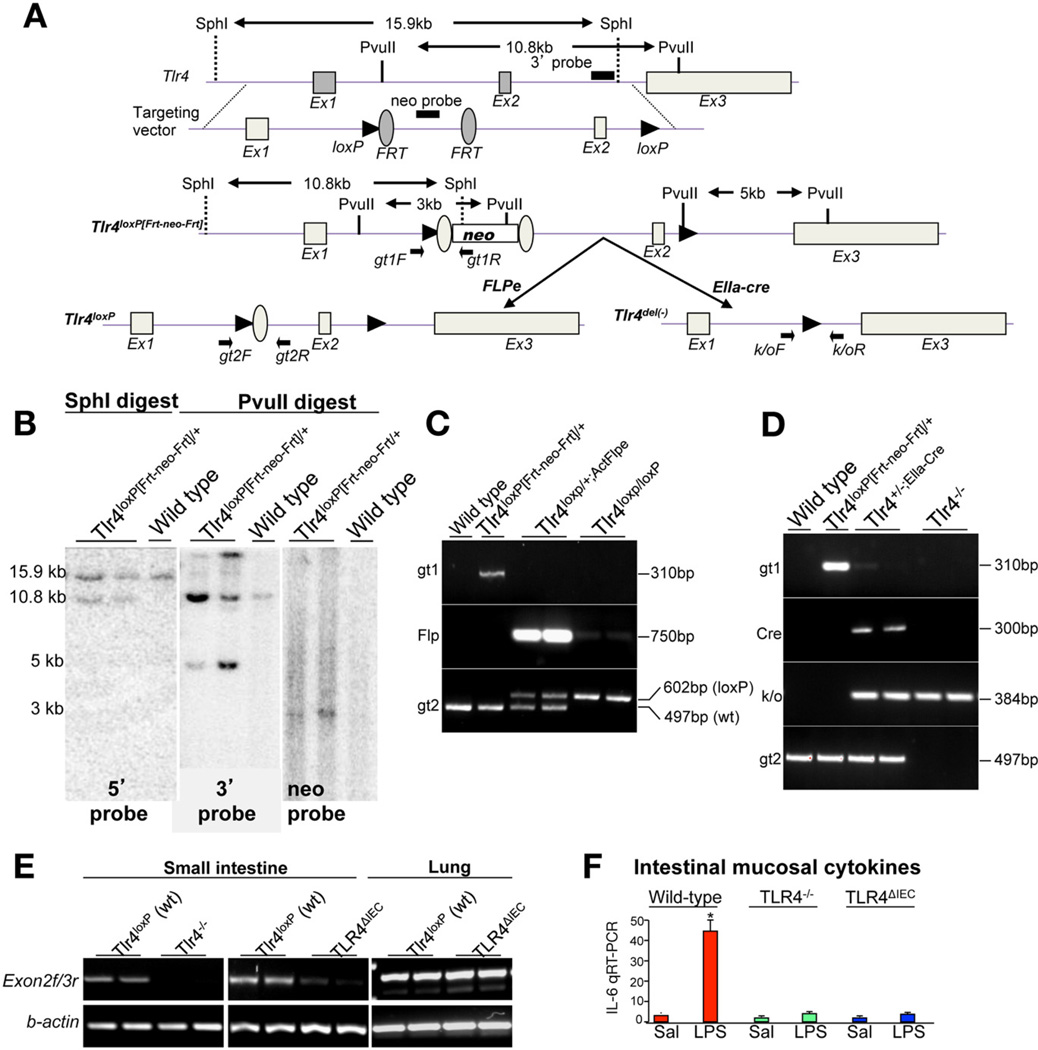
Mice harboring a floxed allele of TLR4 (Tlr4loxP[FRT-neo-FRT]/+) were generated in collaboration with Ozgene (Bentley, Bentley DC, WA). The Tlr4loxP[FRT-neo-FRT]/+ mice carrying the positive selection neomycin phosphotransferase (neo) gene were mated to transgenic B6;SJL-Tg(ACTFLPe) 9205Dym/J mice expressing Flp recombinase under the control of the human β-actin gene promoter to remove the neo gene. The Flp recombinase (flp deleter), which effectively recombines the Frt sites flanking the neo gene, leaves behind the loxP sites flanking the exon2 of Tlr4 and thus created a TLR4loxP/+ allele. Correct excision of the neo cassette was confirmed in offspring by polymerase chain reaction (PCR)-based genotyping using multiple primer pairs as shown in the schematic analyses of Figure 1. TLR4loxP/+ mice were interbred to generate Tlr4loxP/loxP mice, which were found to be healthy and fertile and to reach maturity. Tlr4loxP/loxP mice were then bred with Villin-cre transgenic mice to generate intestinal epithelialspecific TLR4 conditional knockout mice (TLR4ΔIEC mice). In parallel, a mouse line harboring the floxed allele of TLR4 (Tlr4loxP[Frt-neo-Frt]/+) was also mated with an EIIa-Cre transgenic mouse (B6.FVBTg [EIIa-cre]C5379Lmgd/J) to generate a null allele of TLR4 (TLR4del/+ or TLR4+/−). The transgenic EIIa-Cre mouse expresses Cre recombinase in nearly all tissues, including those of preimplantation embryos, and has been used previously to mediate recombination between loxP sites in germ cells.11 TLR4+/− progeny were identified by PCR genotyping using multiple primer pairs as shown in the schematic analyses of Figure 1. TLR4+/− mice were finally interbred to generate TLR4−/− mice, which were unresponsive to LPS (Figure 1). TLR4-modified mice showed no differences in overall health or viability as compared with wild-type littermates.
Figure 1.
Generation of TLR4loxp/loxp and TLR4-deficient mice. (A) Schematic diagram showing the generation of TLR4loxp/loxp mice. The positions of exons, neo-cassette, loxP, and Frt sites are shown along with restriction sites and positions of primers. (B) Southern blot showing the correct ligation of the targeting vector under conditions of the digest indicated. (C and D) Genomic PCR of tail-tip DNA confirming the genotype under the indicated conditions. (E) RT-PCR showing the correct excision of exon 2 messenger RNA in intestinal and lung mucosa under the indicated genetic conditions. (F) RT-PCR showing expression of IL-6 in the intestinal mucosa of wild-type (red bars), TLR4−/− mice (green bars), and TLR4ΔIEC mice (blue bars) 6 hours after injection of saline (Sal) or LPS (1 mg/kg intraperitoneally); *P < .05 versus saline, wild-type mice.
Induction of NEC
All experiments were approved by the Animal Care and Use Committee of the University of Pittsburgh. NEC was induced in 7- to 10-day-old mice as described7, 12, 13 using formula gavage (Similac Advance infant formula [Ross Pediatrics, Columbus, Ohio]/Esbilac canine milk replacer 2:1) 5 times/day and hypoxia (5% O2, 95% N2) for 10 minutes in a hypoxic chamber (Billups-Rothenberg, Del Mar, CA) twice daily for 4 days. Mice were enterally treated with dibenzapine (4 µmol/L per kg; Calbiochem, San Diego, CA) once daily for 4 days. The expression of mucosal cytokines was assessed by reverse-transcription (RT)-PCR. The severity of disease was determined on histologic sections of the terminal ileum by a pediatric pathologist who was blinded to the study condition according to our previously published scoring system from 0 (normal) to 3 (severe).6 Intestinal samples were obtained from human neonates undergoing resection for NEC, for unrelated indications (control), or at the time of stoma closure and processed as we have described.14 All human tissue was obtained and processed as discarded tissue via waiver of consent with approval from the University of Pittsburgh Institutional Review Board and in accordance with the University of Pittsburgh anatomical tissue procurement guidelines. Mice were treated with ampicillin (1 g/mL), vancomycin (0.5 g/mL), neomycin sulfate (1 g/mL), and metronidazole (1 g/mL) to reduce microbial load.10
Quantitative Real-Time PCR
Quantitative real-time PCR was performed with the Bio- Rad CFX96 Real-Time System (Biorad, Hercules, CA)6 using the primers listed in Supplementary Table 1. Gene expression was assessed on 2.5% agarose gels using ethidium bromide staining. The expression of following genes as assessed by quantitative RT-PCR was quantified relative to the housekeeping genes β-actin, GAPDH, and RPLO.
Statistical Analysis
Statistical analysis was performed using SPSS 13.0 software (SPSS Inc, Chicago, IL). Analysis of variance was used for comparisons for experiments involving more than 2 experimental groups. Two-tailed Student t test was used for comparison for experiments consisting of 2 experimental groups. For analysis of the severity of NEC, χ2 analysis was performed.
Results
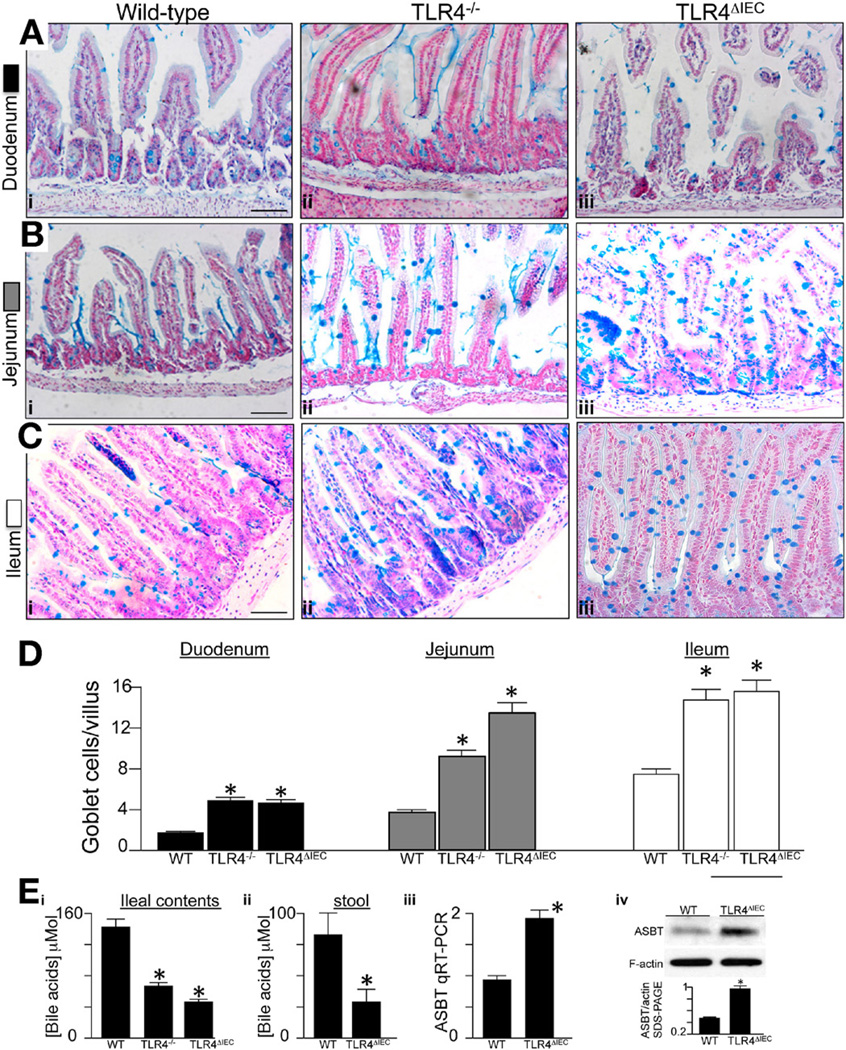
We generated mice lacking TLR4 selectively within the intestinal epithelium (TLR4ΔIEC) or in all cells (TLR4−/−). To do so, we first created a mouse line harboring a floxed allele of TLR4 (Tlr4loxP) and then bred this mouse with transgenic mice expressing the intestinal-specific villincre15 or the global cre “EIIa-cre,”11 respectively (Figure 1). The TLR4ΔIEC mice lacked TLR4 signaling within the intestinal epithelium, as measured by reduced mucosal interleukin (IL)-6 induction in response to systemic administration of LPS (Figure 1). As shown in Figure 2, analysis of the TLR4ΔIEC mice using the mucin stain Alcian blue revealed a significant increase in the frequency of goblet-like cells compared with wild-type mice, which became more apparent along the duodenum-jejunumileum axis (Figure 2). There was a similar increase in goblet cell numbers in the TLR4 global knockout mice as well (see Figure 2Aiii, Biii, Ciii, and D), showing that the effect in the TLR4−/− mice on goblet cell induction could be explained based on effects of TLR4 on the enterocytes.
Figure 2.
Deletion of TLR4 leads to increased goblet cells in the small intestine of mice. (A–C) Alcian blue staining of (Ai–iii) duodenum, (Bi–iii) jejunum, and (Di–iii) ileum of (Ai, Bi, and Ci) wild-type C57/BL-6, (Aii, Bii, and Cii) TLR4−/−, and (Aii, Bii, and Cii) TLR4ΔIEC mice. Bars = 100 µm. (D) Quantification of goblet cells in the villi in more than 100 fields per group, averaged over at least 10 mice per group. (Ei and ii) Bile acid concentration, ileal contents, and stool in the indicated strain averaged over 10 mice/group; *P < .05 versus wild-type (WT); data are expressed as mean ± SEM. (Eiii and iv) Expression of ASBT by (iii) RT-PCR and (iv) sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE) in the indicated strain; *P < .05, 5 mice/group.
To determine possible mediators contributing to the increased goblet cells in TLR4-deficient mice, we first used 16S ribosomal RNA gene pyrosequencing to characterize the cecal microbial profiles from wild-type and TLR4-deficient strains. As shown in Supplementary Figure 1 and Supplementary Table 2, no significant difference in community composition was identified between any 2 animal groups at any taxonomic level. Moreover, treatment of newborn mice with a cocktail of 4 antibiotics to reduce the bacterial load of the gastrointestinal tract16 (Supplementary Figure 2A and B), as well as assessment of the intestine at embryonic day 18.5 in an intrauterine environment in which bacteria levels are very low (Supplementary Figure 2C and D), did not affect the increase in goblet cells observed in TLR4-deficient strains (note the large ceca in the antibiotic-treated mice in Supplementary Figure 2B, consistent with earlier reports17). However, both TLR4ΔIEC and TLR4−/− mice showed a significant reduction in bile acids in the ileal lumen and stool compared with wild-type strains (Figure 2Ei and ii). Interestingly, the expression of the apical ileal sodium-dependent bile acid transporter (ASBT)18 was significantly elevated in the ileal mucosa of TLR4ΔIEC compared with TLR4−/− mice (Figure 2Eiii and iv). Given that bile acid concentration in the newborn gut has recently been linked to the regulation of goblet cells,10 these findings raised the possibility that bile acids, and not the microbial flora, could potentially play a role in the increased goblet cells observed in TLR4-deficient mice. TLR4-deficient strains also showed an increase in Paneth cells; however, because the microbial composition was unchanged, the relevance of this finding is currently uncertain.
TLR4 Regulates Notch Signaling Within the Small Intestinal Epithelium
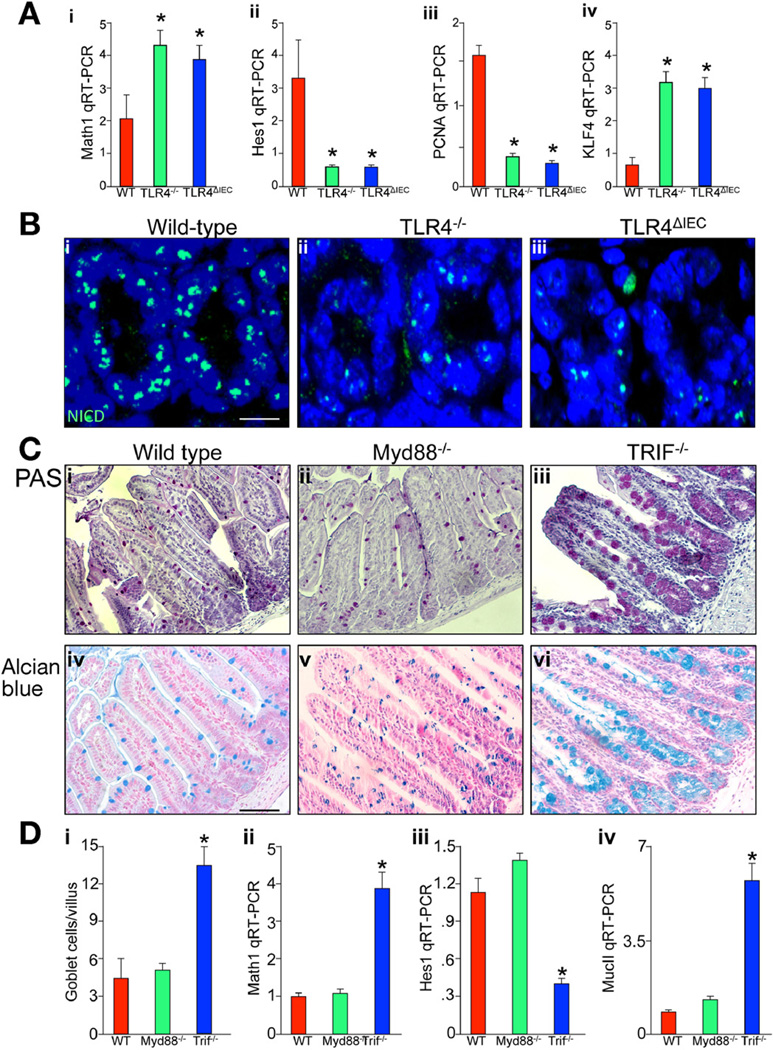
The capacity of the intestinal epithelial progenitor cells to differentiate and their subsequent fate is determined by the activity of the Notch signaling pathway.19 Notch activation has a predilection for the suppression of goblet cell fate through Hes1-mediated repression of the transcription factor Klf4.20 The inhibition of Hes1 leads to the converse phenotype, characterized by the generation of excessive numbers of secretory cells, including goblet cells.21 The ultimate decision to differentiate into the secretory lineages is determined by the activation of Math1, a target gene that is stochastically repressed by Hes1.22 Having determined that the presence or absence of TLR4 within the intestine plays an important role in intestinal epithelial differentiation, we next evaluated whether TLR4 regulates the Notch signaling pathway in the small intestine. To do so, we assessed the effects of TLR expression on Hes1 and Math1 expression and cell proliferation in wild-type, TLR4−/−, and TLR4ΔIEC mice. As shown in Figure 3, compared with wild-type mice, the small intestinal epithelium in TLR4ΔIEC mice showed an increase in Math1 (Figure 3Ai) and a reduction in Hes1 (Figure 3Aii), as well as reduced expression of the proliferation gene PCNA (Figure 3Aiii) and increased expression of the Notch-dependent transcription factor KLF4 (Figure 3Aiv). These findings are consistent with the idea that TLR4 deletion leads to suppressed Notch signaling in the small intestinal epithelium. In further support of this possibility, mice lacking TLR4 both in enterocytes alone or globally showed a reduction in the expression of cytoplasmic “active” Notch1 within the crypts compared with wild-type mice (Figure 3Bi–iii). We next sought to explore in greater detail the potential mechanisms by which TLR4 deletion could lead to an increase in goblet cells. TLR4 is known to signal through either Myd88-dependent or Myd88-independent (ie, TRIF-dependent) pathways.23 Although there was no change in the frequency of goblet cells in Myd88-deficient mice as compared with wild-type controls as determined through the use of both periodic acid–Schiff and Alcian blue staining (Figure 3Ci, Cii, Civ, Cv, and Di), there was a marked increase in the number of goblet cells observed in the ileum from TRIF-deficient mice (Figure 3Ciii, Cvi, and Di). The finding of increased goblet cells in the TRIF-deficient mice was also associated with reduced Notch signaling, as manifest by significantly increased expression of Math1 (Figure 3Dii), reduced expression of Hes1 (Figure 3Diii), and increased expression of MucII (Figure 3Div) within the small intestinal epithelium. Taken together, these findings show that TLR4 deletion in part along the TRIF pathway leads to reduced Notch signaling in the small intestinal epithelium.
Figure 3.
TLR4 regulates Notch signaling within the intestinal epithelium. (Ai–iv) RT-PCR for (i) Math1, (ii) Hes1, (iii) PCNA, and (iv) KLF4 in wild-type (red bars), TLR4−/− (green bars), or TLR4ΔIEC (blue bars) mice. *P < .05 versus wild-type. Data are expressed as mean ± SEM. (Bi–iii) Confocal micrographs of terminal ileum stained for active Notch NICD (Notch intracellular domain). Bar = 10 µm. (Ci–vi) Evaluation of goblet cells in the terminal ileum of (i and iv) wild-type mice, (ii and iv) Myd88−/− mice, and (iii and vi) TRIF−/− mice as stained by (i–iii) periodic acid–Schiff and (iv–vi) Alcian blue; quantification is shown in Di. Bar = 100 µm. Representative of 25 fields, at least 4 mice per field. (Dii–iv) RT-PCR of (ii) Math1, (iii) Hes1, and (iv) MucII in wild-type (red), Myd88−/− (green), and TRIF−/− (blue) mice; *P < .05 versus wild-type. Data are expressed as mean ± SEM.
TLR4 Deletion Leads to Increased Goblet Cell Differentiation in Cultured Enterocytes
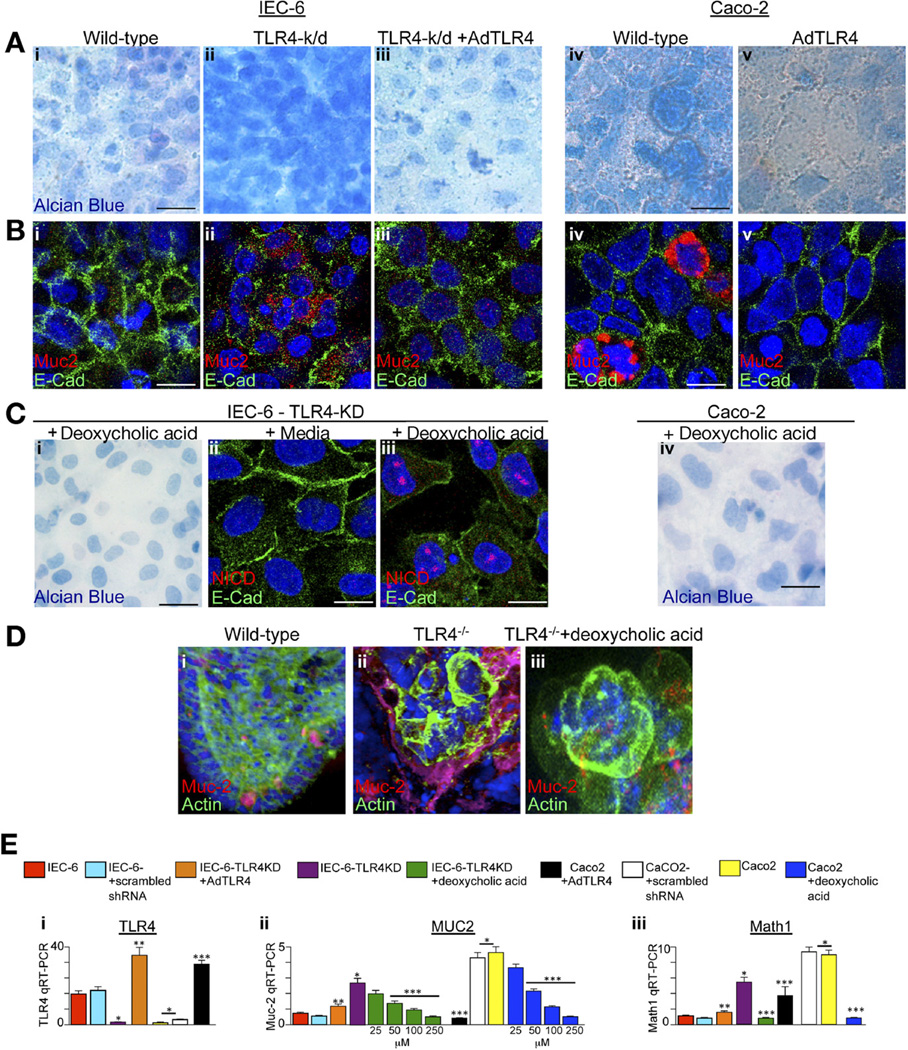
Goblet cells are characterized by the cytosolic accumulation of mucin and the expression of both Muc-2 and Math1, 19 and their lineage is negatively regulated by Notch signaling.22 Given our observation that TLR4 deletion is associated with reduced Notch signaling and increased goblet cell acquisition in vivo, we next considered whether the selective removal of TLR4 could lead to the acquisition of goblet cell–like features in cultured enterocytes. To do so, we examined the behavior of IEC-6 cells, a small intestinal cell line known to express TLR424 that does not form mucin, and Caco-2 cells, a TLR4- deficient colonic epithelial cell line24 that expresses mucin at high levels.25 As shown in Figure 4, the selective removal of TLR4 from IEC-6 cells by transduction with lentiviral expression of TLR4-shRNA resulted in a marked increase in mucin production characteristic of goblet cells, as manifest by increased Alcian blue staining (Figure 4Ai and ii), as well as an increase in Muc-2 by confocal microscopy (Figure 4Bi and ii) and increased Muc-2 and Math1 by RT-PCR (Figure 4Ei–iii, purple vs red bars). There were no effects on these parameters in cells transduced with lentiviruses expressing scrambled shRNA (Figure 4E, cyan and white bars). These goblet cell characteristics were each reversed in the TLR4-deficient cells after reintroduction of TLR4 by adenoviral transfection, which resulted in an appearance that resembled wild-type cells (Figure 4Ai–iii, Bi–iii, and Ei–iii; orange vs purple bars). Importantly, the administration of bile acids to the TLR4-deficient IEC-6 cells prevented the increase in Alcian blue staining and increased Notch expression, as manifest by increased NICD immunostaining (Figure 4Ci–iii), and reduced Muc-2 and Math1 expression (Figure 4Eii and iii, green vs purple bars; note the dose dependency of the effects on Muc-2), suggesting that bile acids (whose concentrations were reduced in TLR4-deficient mice; Figure 2E) could in part mediate this effect.
Figure 4.
Deletion of TLR4 leads to increased goblet cell differentiation in cultured enterocytes. IEC-6 or Caco-2 cells that were wild-type (Ai and Bi, IEC-6; Aiv and Biv, Caco-2), deficient in TLR4 (Aii and Bii, TLR4-k/d), or deficient in TLR4 were infected with adenoviruses that express wild-type TLR4 (Aiii and Biii, TLR4-k/d + AdTLR4); Av and Bv show Caco-2 + AdTLR4, stained with (Ai–v) Alcian blue or (Bi–v) Muc-2. E-cadherin is shown in green, DAPI in blue, and Muc-2 in red. (C) TLR4-deficient IEC-6 cells cultured for 5 days in (i and iii) deoxycholic acid or (ii) media and then stained for (i) Alcian blue or Notch intracellular signaling domain (red), E-cadherin (green), and DAPI (blue) in ii and iii. (Civ) Alcian blue staining in Caco-2 cells exposed to deoxycholic acid. (Di–iii) Organoids from (i) wild-type, (ii) TLR4−/−, or (iii) TLR4−/− were then cultured in deoxycholic acid for (iii) 5 days and stained for DAPI (blue), actin (green), and Muc-2 (red). (Ei–iii) RT-PCR in wild-type IEC-6 (red bars), IEC-6 cells transduced with scrambled shRNA (cyan bars), IEC-6–TLR4-KD (purple bars), IEC-6-TLR4-KD + AdTLR4 (orange bars), TLR4-KD + deoxycholic acid (green bars, at the indicated dose), wild-type Caco-2 cells (yellow bars), Caco-2 + AdTLR4 (black bars), Caco-2 + scrambled shRNA (white bars), and Caco-2 + deoxycholine (blue bars at the indicated dose). *P <.05 versus control IEC-6, **P <.05 versus IEC-6ΔIEC, ***P <.005 versus wild-type Caco-2. Data are expressed as mean ± SEM. Bar = 10 µm.
Whereas untreated Caco-2 cells (which lack TLR4) showed intense Alcian blue staining and high levels of Muc-2 (Figure 4Aiv and Biv), the overexpression of AdTLR4 in TLR4-deficient Caco-2 cells resulted in a non– goblet-like phenotype characterized by low Alcian blue staining and reduced Math1 and Muc-2 expression (Figure 4Aiv and v, Biv and v, and Ei–iii; black vs yellow bars). The administration of bile acids to Caco-2 cells reduced Alcian blue intensity (Figure 4Civ). These findings suggest that the expression of TLR4 is important in the determination of goblet cell differentiation not only in vivo but also in vitro. To investigate this possibility further, we isolated and maintained in Matrigel culture primary intestinal progenitor cells that were harvested from wildtype and TLR4−/− mice8 for 5 days. Strikingly, the frequency of goblet cells in the cultures obtained from TLR4−/− mice was significantly increased as compared with cultures obtained from wild-type mice, while the addition of bile acids to organoids from TLR4-deficient mice reduced goblet cell differentiation (Figure 4Di–iii). Taken together, these results raise the possibility that the regulation of intestinal epithelial differentiation by TLR4 may at least in part involve effects on bile acids.
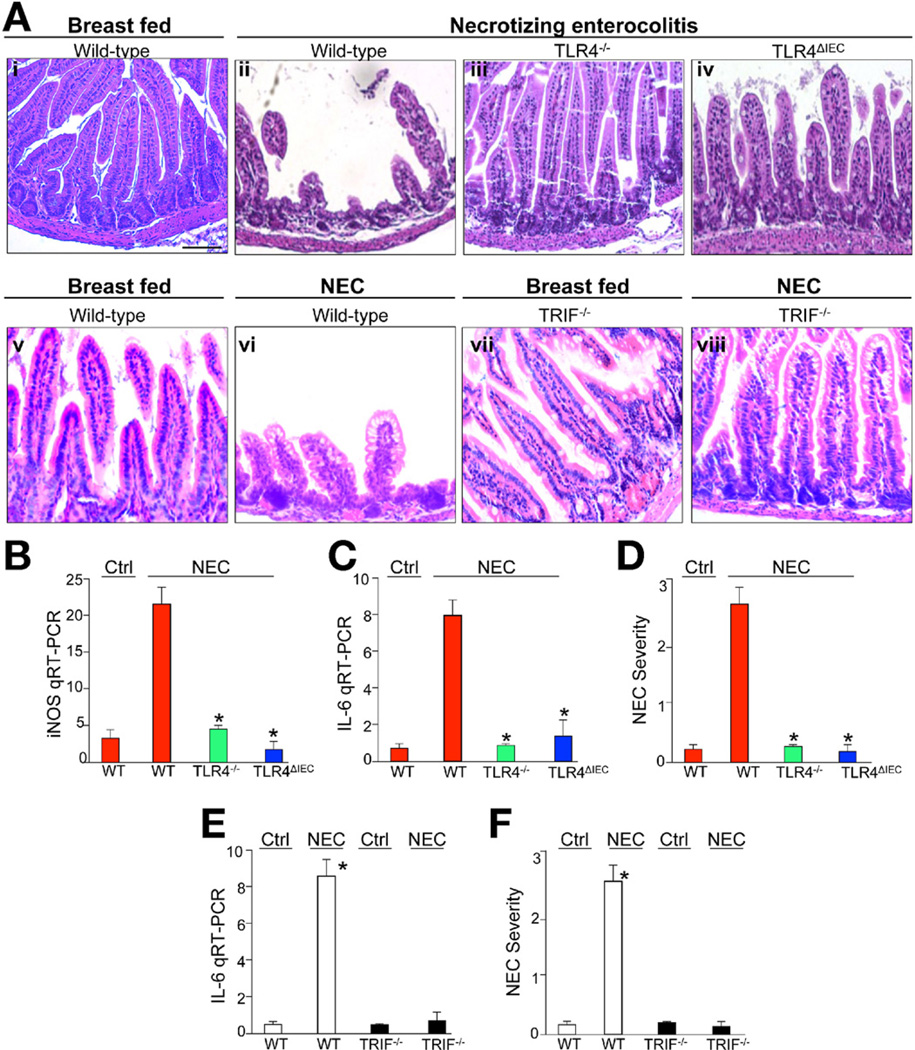
TLR4 in the Intestinal Epithelium Is Required for the Development of NEC
Exaggerated bacterial-enterocyte signaling is believed to play a causative role in the pathogenesis of NEC, a disease characterized by the acute development of inflammation and necrosis of the intestine of premature infants, who by definition interact with bacteria at a time of incomplete intestinal development.4, 26 Although we6 and others5 have recently established a role for TLR4 in disease development, the precise cellular location of the TLR4 signal required for NEC (intestinal epithelial vs other cells) remains unknown, and a link between the role of TLR4 in intestinal differentiation and in the development of NEC remains unexplored. We therefore sought to evaluate the effects of deleting TLR4 from the intestinal epithelium on the pathogenesis of NEC in an established model of experimental NEC that involves 4 days of gavage feeds and hypoxia.7, 12, 13 Compared with untreated mice, wild-type littermates showed a marked disruption in mucosal architecture after exposure to the NEC model that was accompanied by an up-regulation of the proinflammatory cytokines IL-6 and inducible nitric oxide synthase (Figure 5Ai, Aii, B, and C). By contrast, the induction of NEC in TLR4−/− mice showed preservation of the small intestinal mucosa and minimal cytokine elevation (Figure 5Aiii and B–D), similar to that of breastfed wild-type mice. Strikingly, the induction of NEC in TLR4ΔIEC mice showed markedly reduced NEC severity, comparable to that seen in the TLR4−/− strain (Figure 5Aiv and B–D). These findings are consistent with the idea that TLR4 in the intestinal epithelial cells, but not in other cells, is responsible for the induction of NEC. Further, consistent with our finding in Figure 3C and D that TRIF is important in mediating the effects of TLR4 on goblet cell differentiation in the small intestine, TRIF-deficient mice were protected from NEC to a similar degree as the TLR4-deficient strains (Figure 5Av–viii, E, and F). Importantly, a role for microbial signaling in the pathogenesis of NEC is also important, because newborn mice that were administered antibiotics to decrease bacterial load were protected from development of NEC (Supplementary Figure 3).
Figure 5.
TLR4 in the intestinal epithelium is required for the development of NEC. Terminal ileum from newborn mice and evaluated by H&E after (Ai, v, and vii) breastfeeding or (Aii–iv, vi, viii) induction of NEC in either TLR4 or TRIF-deficient strains. (B, C, and E) RT-PCR in intestinal mucosa of wild-type (red bars or white bars), TLR4−/− (green bars), TLR4ΔIEC (blue bars), and TRIF−/− (black bars) for (B) inducible nitric oxide synthase or (C and E) IL-6 as shown. (D and F) Severity of NEC. Mean ± SEM; **P < .05 versus wild-type (WT) NEC; *P <.05 versus WT control. Representative of >100 fields, >10 mice/group. Bar = 100 µm.
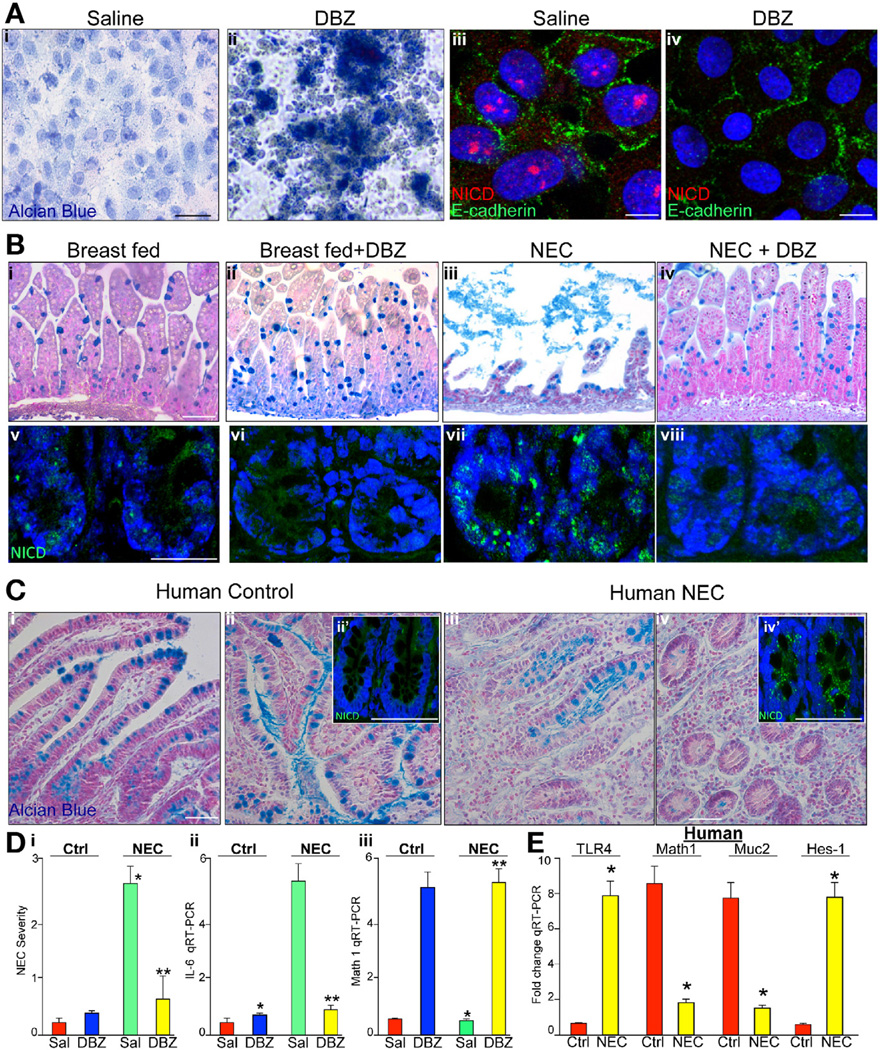
Inhibition of Notch Signaling Leads to Increased Goblet Cells and Reduced Severity of Experimental NEC
Having observed that TLR4 deficiency protects against the development of NEC and inhibits Notch signaling, we next considered whether pharmacologic inhibition of Notch using the γ-secretase inhibitor DBZ could alter goblet cell differentiation and NEC severity.27 As shown in Figure 6, treatment of IEC-6 enterocytes with DBZ markedly increased Alcian blue staining (Figure 6Ai and ii) and decreased the expression of activated Notch (Figure 6Aiii and iv), confirming the inhibition of Notch signaling by DBZ in enterocytes. The administration of DBZ to newborn mice resulted in a significant increase in goblet cells (Figure 6Bi and ii) that was associated with reduced Notch1 cytoplasmic expression (Figure 6Bv and vi), confirming that DBZ inhibited Notch in the newborn intestine. Importantly, animals receiving DBZ showed reduced NEC severity compared with saline-treated mice with NEC as shown histologically (Figure 6Biii and iv) and quantitatively (Figure 6Di, yellow vs green bars) and by the observation of reduced mucosal cytokine expression (Figure 6Dii; yellow vs green bars), which was associated with reduced Notch activation (Figure 6Bvii and viii), and an increase in Math1 expression (Figure 6Diii; yellow vs green bars). These findings have relevance to human NEC, because in sections obtained at laparotomy from human infants with NEC, we note an increase in TLR4 in the small intestine (Figure 6E, consistent with our previous findings6), as well as reduced goblet cells (Figure 6Ci–iv), consistent with previous reports,28 and increased Notch staining in crypts (Figure 6Cii′ and 6Civ′), which was associated with markedly reduced Math1 expression, reduced Muc-2 expression, and increased Hes1 expression (Figure 6E; yellow vs red bars). Taken together, these findings show that TLR4 signaling in the intestine regulates intestinal differentiation and Notch signaling in the pathogenesis of NEC.
Figure 6.
Inhibition of Notch leads to increased goblet cells and reduced severity of NEC. (Ai and ii) IEC-6 cells in (i) absence or (ii) presence of DBZ stained by Alcian blue. (Aiii and iv) Confocal micrographs of IEC-6 cells stained for E-cadherin (green) or active Notch1, that is, NICD (red in iii and iv). (Bi–viii) Alcian blue (i–iv) or confocal for active Notch (NICD, green) and DAPI (blue) in terminal ileum of newborn mice (v–viii). Bar = 100 µm; representative of more than 10 mice/group in 3 separate experiments. (Ci–iv) Terminal ileum of human infants undergoing surgical resection of the intestine for (iii and iv) NEC or (i and ii) controls; ii? and iv?: NICD. Representative of more than 10 patients/group. (Di) Severity of NEC. (Dii and iii) RT-PCR for (ii) IL-6 and (iii) Math1. For Di–iii: *P < .05 versus saline breastfed; **P < .05 versus saline NEC. *P < .05, yellow versus red. Bar = 100 µm. Data are expressed as mean ± SEM. (E) RT-PCR for TLR4, Math1, Muc-2, and hes1 as indicated in human ileum from control patients (red) and patients with NEC (yellow); *P < .05 versus control; 15 patients per group.
Discussion
Although an expanding body of data has recently defined the intestinal stem cells and the signaling pathways that regulate the differentiation of the intestinal epithelium, the upstream mechanisms that initiate intestinal differentiation remain largely unknown.29 We now provide evidence for the importance of TLR4 in the normal differentiation of the small intestine, because mice lacking TLR4 showed a marked increase in goblet cells. In mechanistic studies, we now determine that deletion of TLR4 was associated with a decrease in Notch signaling and that this phenotype can be reversed by overexpression of TLR4 in vitro. These findings are consistent with earlier studies performed in macrophages, indicating that Notch signaling may be activated by TLR stimulation.30 The effects of TLR4 on goblet cell differentiation were likely not due to effects of the microbiota, because 16S pyrosequencing revealed no differences in microbial communities between wild-type and TLR4-deficient strains, and an increase in goblet cells in TLR4-deficient strains was also observed in conditions of antibiotic treatment to reduce microbial load. It is noteworthy that exposure of bile acids to TLR4-deficient IEC-6 cells as well as to crypts from TLR4-deficient mice reversed the goblet cell phenotype, a finding that is particularly relevant given that bile acids can activate Notch in the gastrointestinal epithelium31, 32 and that increased bile acids were associated with reduced goblet cells in newborn rats and increased NEC severity.10 These findings together indicate that TLR4 can regulate goblet cell differentiation and suggest the possibility that bile acids may serve as intermediates in the regulation of Notch.
We readily acknowledge that several questions remain regarding the precise mechanisms by which the levels of bile acids in the intestinal lumen may be regulated by TLR4. The luminal concentration of bile acids reflects the net balance between production and secretion of bile acids into the bile by the liver and their subsequent absorption by the ileal enterocytes and recirculation into the portal circulation.33 We now show that the expression of the predominant bile acid transporter found along the apical membrane of enterocytes in the ileum is increased in TLR4ΔIEC compared with wild-type mice, suggesting that increased ileal absorption may explain in part the reduced concentration of bile acids in the ileal contents in TLR4-deficient mice, and consistent with the finding that bile acids are also reduced in the stool. However, the effects of TLR4 on other bile acid transporters, including the basolateral Ostα-β transporter that regulates absorption into the portal circulation,34 as well as the production and export from the hepatocyte, remain unknown. It is also unclear as to whether increased ASBT expression coincides with increased uptake of bile acids and whether such an increase can occur in the differentiated enterocytes as well as undifferentiated crypt cells in which changes in bile acids may be expected to alter Notch signaling and epithelial differentiation. Further studies will be required to sort out these important unanswered questions.
In seeking to understand the potential teleological explanation for the regulation of intestinal differentiation by an innate immune receptor, it is apparent that TLR4 activation in the regulation of intestinal differentiation is likely distinct from its signature role in host defense, because the intestinal epithelium develops in utero in a relatively bacteria-free environment. Importantly, we and others have shown that the expression of TLR4 in the developing intestinal lining rises during embryonic development and then falls precipitously at birth.14, 35 We now surmise that in the postnatal environment to which the premature infant is exposed, in which the expression of intestinal TLR4 remains persistently elevated, the developmental role for TLR4 switches to a proinflammatory role on its interaction with colonizing microbes, leading to development of NEC. The results of the current experiments suggest that a separate role for TRIF and MyD88 in regulating goblet cell differentiation may exist; the future identification of molecules that can activate TRIF and not MyD88 may reveal important clues into the developmental role for TLR4 within the developing gut.
In summary, we now identify a novel role for TLR4 in normal small intestinal epithelial differentiation and suggest that further exploration of the TLR4-Notch signaling pathway, and in particular the role that bile acids may play as intermediates in regulating Notch activation in response to TLR4, may offer novel therapeutic approaches to NEC by seeking to reverse the effects of prematurity on the newborn intestine.
Supplementary Material
Acknowledgments
Funding
Supported by The Hartwell Foundation (Memphis, TN) and R01GM078238 and RO1DK08752 from the National Institutes of Health. D.J.H. and T.R.B. are supported by P50GM053789 from the National Institutes of Health, and G.K.G. is supported by RO1-1DK083541 from the National Institutes of Health.
Abbreviations used in this paper
- ASBT
apical ileal sodium-dependent bile acid transporter
- DAPI
4′, 6-diamidino-2-phenylindole
- DBZ
dibenzapine
- GFP
green fluorescent protein
- IL
interleukin
- LPS
lipopolysaccharide
- NEC
necrotizing enterocolitis
- PCNA
proliferating cell nuclear antigen
- RT-PCR
reverse-transcription polymerase chain reaction
- shRNA
small hairpin RNA
- TLR
Toll-like receptor
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2012.05.053.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 2.Neal MD, Leaphart C, Levy R, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176:3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 3.Cario E, Rosenberg IM, Brandwein SL, et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 4.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;363:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jilling T, Simon D, Lu J, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leaphart CL, Cavallo JC, Gribar SC, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 7.Sodhi CP, Shi XH, Richardson WM, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology. 2010;138:185–196. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 9.Afrazi A, Sodhi CP, Good M, et al. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J Immunol. 2012;188:4543–4557. doi: 10.4049/jimmunol.1103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin NA, Mount Patrick SK, Estrada TE, et al. Active transport of bile acids decreases mucin 2 in neonatal ileum: implications for development of necrotizing enterocolitis. PLoS One. 2011;6:e27191. doi: 10.1371/journal.pone.0027191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzenberger M, Lenzner C, Leneuve P, et al. Cre-mediated germline mosaicism: a method allowing rapid generation of several alleles of a target gene. Nucleic Acids Res. 2000;28:E92. doi: 10.1093/nar/28.21.e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leaphart CL, Qureshi F, Cetin S, et al. Interferon-γ inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology. 2007;132:2395–2411. doi: 10.1053/j.gastro.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Richardson WM, Sodhi CP, Russo A, et al. Nucleotide-binding oligomerization domain-2 inhibits Toll like receptor-4 signaling in the intestinal epithelium. Gastroenterology. 2010;139:904–917. doi: 10.1053/j.gastro.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gribar SC, Sodhi CP, Richardson WM, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol. 2009;182:636–646. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto D, Robine S, Jaisser F, et al. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem. 1999;274:6476–6482. doi: 10.1074/jbc.274.10.6476. [DOI] [PubMed] [Google Scholar]
- 16.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Thompson GR, Trexler PC. Gastrointestinal structure and function in germ-free or gnotobiotic animals. Gut. 1971;12:230–235. doi: 10.1136/gut.12.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong MH, Oelkers P, Craddock AL, et al. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem. 1994;269:1340–1347. [PubMed] [Google Scholar]
- 19.Fre S, Huyghe M, Mourikis P, et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 20.Pellegrinet L, Rodilla V, Liu Z, et al. Dll1-and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240 e7. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen J, Pedersen EE, Galante P, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 22.Yang Q, Bermingham NA, Finegold MJ, et al. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 23.Kenny EF, O’Neill LA. Signalling adaptors used by Toll-like receptors: an update. Cytokine. 2008;43:342–349. doi: 10.1016/j.cyto.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi FG, Leaphart C, Cetin S, et al. Increased expression and function of integrins in enterocytes by endotoxin impairs epithelial restitution. Gastroenterology. 2005;128:1012–1022. doi: 10.1053/j.gastro.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 25.Casali A, Batlle E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 2009;4:124–127. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Afrazi A, Sodhi CP, Richardson W, et al. New insights into the pathogenesis and treatment of necrotizing enterocolitis: Toll-like receptors and beyond. Pediatr Res. 2011;69:183–188. doi: 10.1203/PDR.0b013e3182093280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milano J, McKay J, Dagenais C, et al. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 28.Clark JA, Doelle SM, Halpern MD, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291:G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 29.Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 30.Palaga T, Buranaruk C, Rengpipat S, et al. Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur J Immunol. 2008;38:174–183. doi: 10.1002/eji.200636999. [DOI] [PubMed] [Google Scholar]
- 31.Su Y, Buchler P, Gazdhar A, et al. Pancreatic regeneration in chronic pancreatitis requires activation of the notch signaling pathway. J Gastrointest Surg. 2006;10:1230–1241. doi: 10.1016/j.gassur.2006.08.017. discussion 1242. [DOI] [PubMed] [Google Scholar]
- 32.Quante M, Bhagat G, Abrams JA, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012;21:36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis RA, Attie AD. Deletion of the ileal basolateral bile acid transporter identifies the cellular sentinels that regulate the bile acid pool. Proc Natl Acad Sci U S A. 2008;105:4965–4966. doi: 10.1073/pnas.0801194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao A, Haywood J, Craddock AL, et al. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A. 2008;105:3891–3896. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfs TG, Derikx JP, Vanderlocht J, et al. Localization of the lipopolysaccharide recognition complex in the human healthy and inflamed premature and adult gut. Inflamm Bowel Dis. 2010;16:68–75. doi: 10.1002/ibd.20995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.