Summary
Innate immunity conferred by the type I interferon is critical for antiviral defense. To date only a limited number of tripartite motif (TRIM) proteins has been implicated in modulation of innate immunity and anti-microbial activity. Here we report the cDNA cloning and systematic analysis of all known 75 human TRIMs. We demonstrate that surprisingly roughly half of the 75 TRIM-family members enhanced the innate immune response, and that they do this at multiple levels in signaling pathways. Moreover, mRNA levels and localization of most of these TRIMs were found to be altered during viral infection, suggesting that their regulatory activities are highly controlled at both pre- and post-transcriptional levels. Taken together, our data demonstrate a very considerable dedication of this large protein family to the positive regulation of the antiviral response, which supports the notion that this family of proteins evolved as a component of innate immunity.
Introduction
The innate immune system forms the first line of defense against invading pathogens. Host cells recognize incoming pathogen-associated molecular patterns (PAMPs) by various pattern-recognition receptors (PRRs), such as the Toll-like receptors (TLRs) and the RIG-I-like receptors (RLR) (Yoneyama and Fujita, 2010). Intracellular signaling pathways down-stream of the PRRs eventually mediate the induction of inflammatory cytokines, chemokines and type I interferons (IFNs), which are critical for anti-microbial activity (Takeuchi and Akira, 2010).
Recently, a few members of the tri-partite motif (TRIM) family have been implicated in regulation of these innate immune pathways (Akira et al., 2006; McNab et al., 2011). The TRIM protein family counts over seventy distinct members in humans. All TRIM proteins share an Nterminal tripartite motif that consists of a combination of a Really Interesting New Gene (RING) domain, one or two B-boxes and a coiled-coil. Some TRIM-like proteins lack one or more of these domains, yet share signature domains in their C-terminal part that are in part shared amongst members of eleven distinct structural and functional TRIM groups (Ozato et al., 2008).
The number of TRIMs has rapidly expanded in vertebrate evolution (Ozato et al., 2008). The expansion of a large proportion of the recently evolved TRIMs shows parallels with the expansion of immune receptors in evolution (Rhodes et al., 2005). We hypothesized that TRIMs may be an integral part of the mechanisms to control immune responses in humans and that more TRIMs regulate innate immune responses than the handful described thus far (Kawai and Akira, 2011). To that end we systematically analyzed all human TRIM proteins for their regulatory roles in efficient initiation and signaling of innate immunity by over-expression and mRNA targeting. Our data indicate that nearly half of all 75 distinct TRIM proteins positively regulate the innate immune system.
Results
TRIM splice forms lack key domains
Several TRIM genes are known to encode for protein isoforms that lack functional domains due to alternative splicing. We first determined whether this heterogeneity is shared by most members of the family. Bio-informatics analysis of full-length TRIM-annotated mRNAs in GenBank using SpliceMiner (Kahn et al., 2007) was performed. This analysis that identifies the exon composition of each TRIM sequence –thus without prediction from gDNA sequences– revealed that almost 90% of all TRIMs have more than one splice variant (Table S1). In the case of TRIM proteins that are being heavily investigated, several of these reported splice variants have been validated and found to be expressed (Cuchet et al., 2011; Gack et al., 2008). Moreover, this analysis showed that 52% of the TRIM splice forms lack potential key domains such as RING or SPRY (Fig. 1a), domains shown to be critical for e.g. RIG-I activation by TRIM25 (Gack et al., 2007), suggesting possible negative regulation of the activity of a given full length TRIM protein by its splice forms. As functionally characterized TRIM proteins have mostly been studied in the context of the longest isoforms, we cloned an isoform of almost every TRIM containing all predicted conserved domains. To facilitate their detection we included an HA-tag or V5-tag (Suppl. Information). In a few cases we were unable to clone the TRIM isoform with all reported domains. In those few instances we cloned a shorter isoform (Suppl. Experimental Procedures). Taken together, these results suggested that various different isoforms of each TRIM may exist. To allow us to study the effects of individual TRIMs on innate immune regulation, single cloned isoforms were exogenously expressed from plasmids in subsequent studies.
Fig. 1. see also Fig. S1 and Table S1. An unprecedented number of TRIMs enhances innate immune responses.
(a) All known TRIM splice variant sequences were mapped using SpliceMiner and the number of distinct proteins they encode determined. These data were used to determine which of previously identified conserved domains they harbor by NCBI CCD. (b) HEK-293T cells were transfected with plasmids encoding individual TRIM proteins. At 48 h p.t. cells were treated with DMSO or lactacystin and subsequently expression of tagged TRIM proteins was analyzed by immunoblot. (c) TRIM21 and TRIM25 were expressed for 50 ng or 500 ng plasmid by transfection in HEK-293T cells in the presence of the limiting amount of 2 ng constitutively active RIG-I(2CARD) plasmid and assayed for their ability to further enhance the IFNβ promoter. Data are represented as mean of triplicates +/− SD. (d) All TRIM proteins were analyzed for their ability to further enhance IFNβ, NF-κB or ISRE promoter activation by a limiting amount of constitutively active RIGI( 2CARD). TRIMs enhancing reporter activity above average were marked as hits and plotted in a heat-map combining the results of all three different promoters in the presence of either 50 ng or 500 ng TRIM plasmid. (e) Overlap and separation of TRIMs enhancing innate immunity in reporter assays at 50 ng and 500 ng plasmid. (f) Distribution of screen hits amongst the TRIM sub-groups as defined by Ozato et al. (Ozato et al., 2008). TRIMs which lack at least one domain in the N-terminal RBCC (RING, B Box, Coiled-coil) domains are shown as unclassified. TRIMs that activate at least one promoter above average are shown in green. TRIM C-terminal domains: ARF, ADP ribosylation factor-like; BR, bromodomain; COS, C-terminal subgroup.
A large proportion of TRIMs is turned over by the proteasome
We first validated expression of the TRIM proteins in HEK-293T cells and anticipated molecular weight. Most TRIM proteins contain a RING domain, which can confer ubiquitin ligase activity and mark proteins for degradation by the proteasome (Fang et al., 2003). Therefore, expression of all constructs was tested both in the presence or absence of the proteasome inhibitor lactacystin. Immunoblot analysis showed a great variation in the expression of the individual TRIMs compared to GST which was used as a control throughout this study (Fig. 1b and Table S1). Moreover, many TRIMs were only detected in the presence of lactacystin, indicating their constant turnover by the proteasome.
Six TRIMs (TRIM24, TRIM42, TRIM43, TRIM59, TRIM60 and TRIM75) were never detectably expressed (Fig. 1b and Table S1). In general, all TRIMs whose expression was detectable by immunoblot ran in SDS-PAGE at their predicted molecular weights, except for TRIM19, TRIM47 and TRIM54, which ran at a slightly higher molecular weight than anticipated. This may have resulted from post-translational modifications such as sumoylation, as has been shown for TRIM19 (Lallemand-Breitenbach et al., 2001). In contrast, the major product from the TRIM62 plasmid ran lower than expected, although a specific less expressed product was detected at the predicted molecular weight (Fig. 1b).
A screening system to identify activators and enhancers of innate immunity
All TRIM clones were tested for their ability to activate the IFNβ promoter, a nuclear factor κB (NFκB) responsive promoter and the IFN-stimulated gene 54 (ISG54) IFN-stimulated response element (ISRE) in reporter assays. In order to identify TRIM proteins that induced the reporters by themselves as well as TRIMs that could enhance antiviral innate immune responses, we established a reporter assay with the respective reporter constructs in the presence of 2 ng of plasmid expressing the two CARD domains of retinoic acid inducible gene I (RIG-I), thereby constitutively inducing the reporters (Yoneyama et al., 1998). The resulting 5–50 fold induction by RIG-I(2CARD) was roughly 1% of the maximum induction and created a window for co-expressed TRIM proteins to further enhance reporter induction by approximately 50- to 100-fold (Fig. 1c and S1a; Fig. 1 demonstrates e.g. a 38-fold window).
The assay was validated using TRIM21 and TRIM25 which were previously reported to enhance IFN induction (Gack et al., 2007; Yang et al., 2009; Yoshimi et al., 2009). We observed that certain highly expressed proteins such as TRIM21 and TRIM25 maximally enhanced the promoter construct when transfecting 50 ng plasmid, whereas with ten times more plasmid the induction was lost (Fig. 1c). Other TRIMs maximally induced the reporter at 500 ng transfected construct (data not shown). Taken together, these results demonstrated successful establishment of a screening assay to identify positive regulators of the type I IFN system.
A large number of TRIMs enhances innate immune responses
Using these conditions, all TRIM constructs were separately screened at 50 ng and 500 ng using the IFNβ, ISRE and NFκB promoter reporters. An unprecedented number of TRIMs activated at least two of the reporters to varying degrees above the GST and RFP controls (Fig. S1b). TRIM19 (PML) and TRIM33 were the only members that consistently down-regulated RIG-I(2CARD)-induced signaling (Fig. S1b).
All TRIMs inducing the transcription of a particular reporter above the average induction in that experiment were classified as a hit. We empirically determined that using the average induction within each assay as a cut-off, effectively eliminated false-positives (both occurring from assay variation and low-level positive feedback loops). Data from all screens were clustered into a heat-map based on the subset of promoters they induced (Fig. 1d). Overall, twelve TRIMs (16% of total) induced two or more reporters exclusively at 50 ng, whereas fifteen TRIMs (20% of total) induced two or more reporters exclusively at 500 ng (Fig. 1e). Ten TRIMs (13% of total) induced two or more reporters at both concentrations (Fig. 1e). TRIMs from all eleven sub-groups were found to modulate innate immunity (Fig. 1f). Importantly, TRIM5, TRIM8, TRIM21, TRIM23, TRIM25, TRIM56 that were previously reported to positively modulate innate immune responses (Arimoto et al., 2010; Gack et al., 2007; Pertel et al., 2011; Toniato et al., 2002; Tsuchida et al., 2010; Yang et al., 2009; Yoshimi et al., 2009) were identified as hits in the screen (Fig. 1d–f and S1b), thus validating the experimental approach.
Most TRIMs are innate immune enhancers rather than direct activators
Since the screen described above was performed in the presence of limited amounts of the innate immune inducer RIG-I(2CARD) –and thus would identify TRIMs directly inducing or enhancing RIG-I(2CARD)-mediated innate immune responses– we next performed experiments to determine the mechanism by which individual hit-TRIMs induce or modulate responses and in what part of the induction pathway they roughly function.
To identify which of the hits would induce IFN signaling without additional stimulation, we expressed the hit-TRIMs from both the 50 and 500 ng groups in the absence of RIG-I(2CARD) plasmid and analyzed their ability to induce the ISRE promoter. None of the hits were able to induce the reporter in the absence of additional stimulus at 50 ng plasmid (Fig. 2a). However, TRIM9 -and to a lesser extent TRIM5, TRIM 50, TRIM66 and TRIM67- induced the ISRE promoter in the absence of a priming stimulus at 500 ng (Fig. 2b). Taken together, these data suggest that most of the TRIM proteins enhance innate immune signaling, rather than confer direct activation. In contrast, TRIM5, TRIM9, TRIM50, TRIM66 and TRIM67 may directly facilitate innate immune activation.
Fig. 2. see also Fig. S2. Most TRIMs are innate immune enhancers and act at different levels of innate immune signaling.
HEK-293T cells were transfected with (a) 50 ng or (b) 500 ng TRIM expression plasmid in the absence of additional stimulus and analyzed for ISRE reporter induction. (c) Key regulatory molecules in type I IFN induction used for subsequent assays. To elucidate the level at which individual TRIMs act as enhancers, HEK-293T cells were transfected with individual TRIMs and limiting amounts of (d) TBK1, (e) IKKε and (f and g) constitutively active IRF-3. Data are represented as mean IFNβ reporter induction relative to GST controls +/− SD. (h) The percentage of TRIMs enhancing a particular stimulus were plotted relative to the total number identified as positive hits at 500 ng TRIM plasmid in the presence of RIG-I(2CARD). (i) Heatmap summary of pathway mapping; TRIMs enhancing particular stimuli above average are marked in red.
Next, we set out to identify at which point in the IFNβ induction pathway individual TRIMs function as enhancers. To this end, we transfected hit-TRIM plasmids in the presence of limiting amounts of the established signaling molecules TBK1, IKKε and IRF3, which function sequentially down-stream of RIG-I in the IFNβ induction pathway (Fig. 2c).
In pilot experiments we determined that only under the conditions where we transfected 500 ng of TRIM plasmid, the experimental window (~50–150 fold enhancement by TRIMs; Fig. S1b and data not shown) allowed for significant and consistent pathway mapping using this method. Hence, nineteen TRIMs identified to enhance limited RIG-I(2CARD) stimulation at 500 ng were investigated for their enhancing effects on the further ‘down-stream’ signaling molecules TBK1, IKKε and IRF3. Indeed, while nineteen TRIMs were shown to enhance RIG-I-mediated signaling under these conditions (Fig. 1d), only eleven and ten TRIMs enhanced TBK1- and IKKε-mediated IFN promoter induction above average, respectively (Fig. 2d and e). This number further decreased to only six TRIMs, when stimulated with the further down-stream signaling molecule IRF3 (Fig. 2f).
The more limited experimental window using only 50 ng of TRIM plasmid prevented us to perform these analyses in the presence of TBK1 or IKKε. However, in the presence of IRF3 the experimental window was sufficient to identify TRIMs that enhanced IFN induction at 50 ng. Similar to the 500 ng conditions, only seven out of nineteen tested TRIMs were still able to enhance the signal at this degree of induction (Fig. 2g). Together, these data show that the number of TRIMs that can enhance inducers, declines the further ‘down’ these stimuli are in the IFN pathway (Fig. 2h). This suggests that individual TRIMs act at various different levels along the IFNβ induction route.
To explore where individual TRIMs function, these data were combined in a heatmap (Fig. 2i). The last stimulus in the pathway at which each individual TRIM still enhances, provides an indication of the point at which each TRIM exerts its function. We thus predict that eight of the tested TRIMs function between RIG-I and TBK1-IKKε (Fig 2i; T3, T13, T23, T24, T37, T38, T45, T55), four TRIMs between TBK1-IKKε and IRF3 (T36, T42, T60, T61) and two TRIMs at the point of IRF3 or downstream of it (T1, T49). Furthermore, five TRIMs (T5, T9, T50, T66, T67) may work as direct inducers, of which we could not determine the rough point of action in the induction pathway using this assay. Unexpected ‘gaps’ found in the pathway mapping for TRIM5 and TRIM50 (Fig. 2i), we attribute to technical assay variations and the cut-off for scoring positives, which are often found to a limited extent in medium-throughput systems such as these.
Overall, these results demonstrate that individual TRIMs function at different stages of the IFNβ induction pathway. Moreover, the ability of TRIMs to enhance the activation of these signaling pathways seems stimulus-specific. This strongly argues that the identified TRIMs in the initial screen with RIG-I as a stimulus, are not mere artifacts.
TRIM-enhanced innate immune signaling confers antiviral cytokine production
The experiments described above were performed using reporter systems. Next, we set out to validate that a selected number of the ‘hit’ TRIMs could also enhance antiviral cytokine production to protect against virus challenge and determine dependence on RING-mediated E3 ligase activity.
As with the reporter assays, first the amount of RIG-I(2CARD) plasmid that would minimally induce antiviral cytokine production was determined. HEK-293T cells were transfected with increasing amounts of RIG-I(2CARD) plasmid (Fig. 3b) and after 24, 36 and 48 h, supernatants were transferred to Vero cells (Fig. 3a). These cells can respond to IFN and confer an antiviral state, yet cannot produce type I IFN themselves. Hence, any antiviral effect observed in these cells must have originated from the cytokines in the provided supernatants.
Fig. 3. TRIM proteins enhance the production of antiviral cytokines in a RING-domaindependent manner.
(a) Schematic overview of assay. (b) HEK-293T cells were transfected with increasing amounts of RIG-I(2CARD). Supernatants were harvest at 24, 36 and 48 h p.t., which were used to incubate Vero cells for 24 h. As a reference universal IFN dilutions were used (right panel). These Vero cells were infected with VSV-GFP (4 PFU/cell) for 7 h and their GFP expression determined using a plate reader. (c) HEK-293T cells were transfected with a limiting amount of RIGI( 2CARD) plasmid (2 ng) and cotransfected with 50 or 150 ng of wildtype or RING point-mutant TRIM plasmid. At 32 h p.t. supernatants were transferred to Vero cells for 24 h, followed by VSVGFP infection for 7 h. GFP expression was determined using a plate reader. Data are represented as mean relative to the background in mock-infected cells +/− SD.
Fifty ng of RIG-I(2CARD) plasmid and higher induced a maximum measurable amount of cytokines conferring antiviral effect in a VSV-GFP infection challenge (Fig. 3b, left panel). The amount of antiviral cytokines was reduced in a dose-dependent manner downwards until approximately 2 ng of 2CARD plasmid. The conferred antiviral activity corresponded with roughly 10–500 IU of IFN in this range (Fig. 3b, right panel). Because 2 ng of 2CARD was just rate-limiting, this amount was used in the subsequent assay.
As expected, antiviral cytokine production was similar upon expression of an irrelevant control protein (GST) or empty vector in the presence of the titrated limited amount of 2 ng RIGI(2CARD) plasmid (Fig. 3c, red bars) and in its absence (orange bars). However, co-expression of 50 ng or 150 ng wildtype TRIM1, TRIM8, TRIM13, TRIM25, TRIM32 or TRIM38 plasmid enhanced the production of antiviral cytokines and reduced VSV-GFP replication to almost undetectable levels (Fig. 3c, bright green bars and adjacent light grey bars). The depicted decrease from ~400% to ~100% relative GFP represents near-complete viral inhibition. In contrast, the same TRIMs with a single cysteine to alanine mutation in their RING domains destroying the zinc-finger, were unable to enhance cytokine production and confer antiviral activity (Fig. 3c, light green and dark grey bars), despite comparable protein expression levels as their wildtype counterparts (data not shown).
These data suggest that these TRIMs enhance antiviral cytokine production by means of E3 ligase-dependent (and hence ubiquitin-like molecule-dependent) mechanisms. Moreover, they confirm and extend the results from the reporter assays by showing that TRIMs can indeed enhance the production of biologically functional, antiviral cytokines.
TRIM proteins are regulated at the transcriptional and post-transcriptional level
Isoform and cell-type specific expression of individual TRIMs suggested that they may be complexly regulated at both the transcriptional and post-transcriptional level. In order to address the complexity of TRIM regulation, we selected 22 members for further evaluation (Fig. S2).
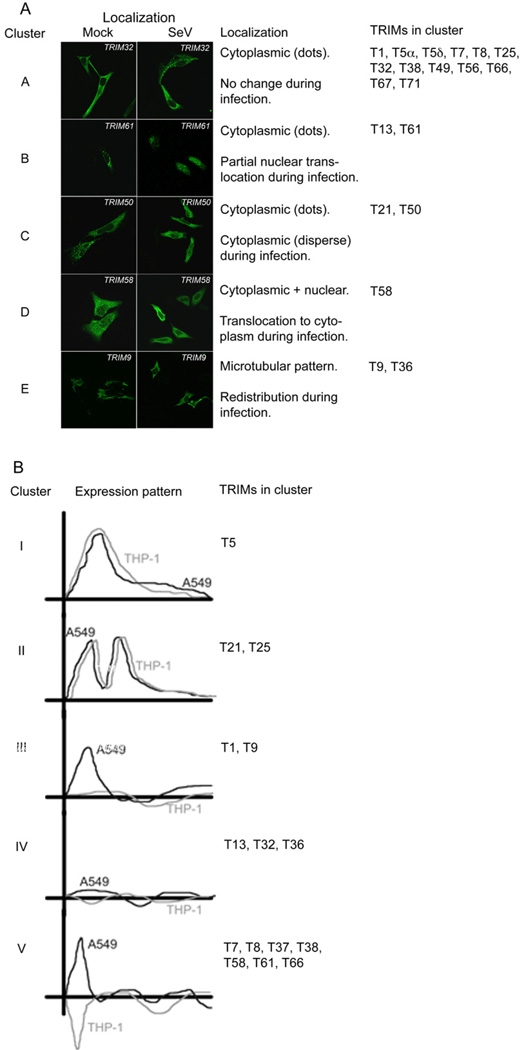
First we identified TRIMs that may require relocalization during infection for their regulation of innate immunity. The selected proteins were expressed in HeLa cells and the majority localized as speckles, microtubule-like fibers or dispersed in the cytoplasm (Fig. 4a and S3-4). A minor fraction of some TRIMs was detected in the nucleus of mock-infected cells (Fig. 4a and S3-4). Upon infection with SeV, seven of the tested TRIMs relocalized compared to mock-infected cells (Fig. 4a and S3-4) as did the positive control IRF-3 (Fig. S4).
Fig. 4. see also Fig. S3-5. TRIMs are complexly regulated at both the transcriptional and posttranscriptional level.
(a) HeLa cells were transfected with tagged TRIM expression plasmids and incubated for 32 h. Subsequently, cells were infected with SeV for 8 h. After fixing, cells were stained using HA and V5 antibodies recognizing the tagged TRIM proteins. The resulting localization data were organized in five distinct clusters by similar localization and relocalization patterns. The depicted immune-fluorescence figures are representative samples for localization patterns in each distinct cluster. (b) Lung-derived A549 and monocyte-derived THP-1 cells were treated with 1000 IU/mL universal IFNβ or infected with SeV. At 3, 6, and 9 h p.t. RNA was isolated and analyzed by real-time RT-PCR for TRIM mRNA expression. The depicted expression figures were drawn by hand to summarize TRIM mRNA expression patterns in each distinct cluster depicted in Fig. S5. TRIM expression regulation was in almost all instances similar upon IFNβ treatment and SeV infection, and thus represented as single lines.
Overall, the redistribution patterns grouped in five TRIM localization clusters (Fig. 4a). Most of the tested TRIMs localized in the cytoplasm and did not relocalize during infection (Fig. 4a, cluster A). However, the ‘strong hits’ TRIM13 and TRIM61 relocalized from the cytoplasm to the nucleus (cluster B), TRIM21 and TRIM50 changed from cytoplasmic dots to a dispersed cytoplasmic localization (cluster C) and the partially nuclear TRIM58 relocalized almost exclusively to the cytoplasm (cluster D). Finally, TRIM9 and TRIM36, which have previously been described to associate with microtubules through their COS-domain (Short and Cox, 2006), relocalized from microtubule-like filaments to a disperse cytoplasmic distribution (Fig. 4a, cluster E). Taken together, these results show that several of the potent innate immune stimulators found in the reporter assays (Fig. 1d) relocalize during infection. Future studies will need to investigate whether relocalization is a cause or effect of innate immune activation. However, it is tempting to speculate that the localization of some TRIMs is required for their regulatory roles.
Subsequently, we analyzed the regulation of the selected TRIMs at their mRNA level during infection. Two well-characterized cell lines of different origin were infected with SeV to determine if the selected TRIM proteins are differentially regulated during infection and if differences between cell types exist. Mock- and SeV-infected A549 (lung origin) and THP-1 (monocyte origin) cells were subjected to TRIM-specific real-time RT-PCR during a time course. Detection of IFNβ, ISG15 and ISG54 mRNA confirmed productive infection (Fig. S5a). IFN-stimulated gene (ISG) expression was already measurable at 3 h p.i. in all cell types. IFN is not an ISG and hence was not stimulated during IFN treatment in any of the cell types, validating the specificity of the different treatments (Fig. S5a).
Most of the detectable TRIMs were differentially expressed at 3 h after IFN treatment or infection (Fig. 4b and S5b) and clustered in five different main clusters of regulation (Fig. 4b). In A549 and THP-1 cells, the mRNA of TRIM13, TRIM32 and TRIM36 remained unchanged at all times, whereas TRIM5, TRIM21 and TRIM25 were upregulated as previously reported (Carthagena et al., 2009; Rajsbaum et al., 2008). TRIM1 and TRIM9 mRNA was only upregulated at 3 h p.t. in A549 cells, but not in THP-1s (Fig. 4b and S5b). The remaining TRIMs were upregulated early during treatment in A549s, yet down-regulated in THP-1 cells.
Overall, these kinetics demonstrate that although several TRIMs are regulated comparably in different cell types, most of them differ in their regulation during infection. The difference in mRNA expression of specific TRIMs during infection between A549 and THP-1 cells suggests that the regulatory roles of some TRIMs may differ between epithelial and myeloid cells.
Attenuated TRIM expression in kidney and lung cell lines decreases IFN induction
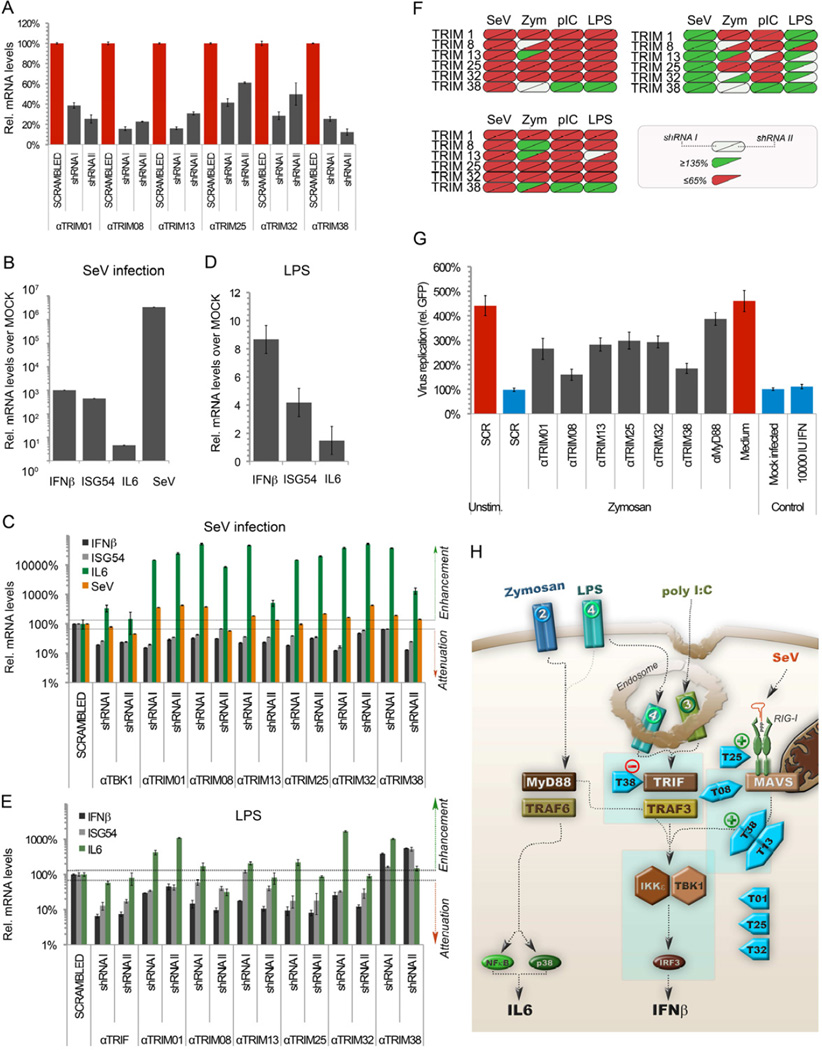
Next, to demonstrate that the TRIM proteins identified by the reporter assays are significant enhancers of the IFN system, polyclonal cell lines stably expressing shRNAs targeting individual TRIMs were made for TRIMs that were detectable by real-time RT-PCR (Fig. S2 and S5). Stable attenuation of gene expression provides the advantage that even moderate attenuation can considerably lower target protein levels. Pilot experiments confirmed only specific attenuation of each targeted TRIM mRNA, while not considerably changing the mRNA expression of the other TRIMs tested (data not shown).
To ensure challenging with a limited amount of stimulus which would allow detecting a phenotype, we titrated the amount of SeV used as an inducer to result in 10–50 fold induction of IFNβ and ISG54 mRNA over mock-infected samples at 4 h p.i. (data not shown). To test if the IFN response was diminished in cells knocked-down for individual TRIMs, all generated cell lines were mock or SeV infected and analyzed by real-time RT-PCR for their ability to upregulate IFNβ and ISG54 mRNA (Fig. 5a).
Fig. 5. Stable mRNA targeting of endogenous TRIMs decreases IFNβ and ISG54 upregulation during SeV infection.
(a) Schematic overview of assay. HEK-293T cells were transduced with lentiviruses expressing shRNAs targeting individual TRIMs. After puromycin selection, transduced polyclonal cells were infected with SeV and analyzed in triplicate by specific real-time RT-PCR for (b) IFN and ISG54 induction in cells expressing a scrambled shRNA, (c) specific TRIM expression in all mRNA targeted cell pools and (d) IFNβ and ISG54 stimulation by SeV in TRIM mRNA targeted cells. Data are represented as mean +/− SD.
In cells expressing a scrambled shRNA, SeV infection stimulated IFNβ and ISG54 mRNA by 20–30 fold (Fig. 5b). Variation amongst different TRIMs in their mRNA targeting efficiency ranged from ~40% to 90% (Fig. 5c). Under those conditions, IFNβ and ISG54 mRNA induction was reduced by more than 50% in TRIM9 and TRIM13 attenuated cells (Fig. 5d). Notably, those TRIMs were amongst the strongest inducers in the reporter assays (Fig. 1d).
Also TRIM36-38, TRIM58 and TRIM66 reduced IFNβ and ISG54 mRNA induction by 20–50% (Fig. 5d). Moreover, attenuation of TRIM21 and TRIM25 expression, both previously shown to be required for efficient IFN induction, diminished IFNβ and ISG54 induction by 40% and 30%, respectively. mRNA targeting of TRIM1, TRIM5, and TRIM32 did not reduce IFNβ induction despite efficient mRNA depletion of all three of these TRIMs (Fig. 5c and d). A549 cells stably expressing the same shRNAs were similarly attenuated in expression of ISGs, with the exception of TRIM36, which was knocked-down, yet did not attenuate ISG induction (data not shown).
Overall, these results demonstrate that individual mRNA targeting of eight out of eleven selected TRIMs reduced IFNβ and ISG54 mRNA induction during SeV infection. This corroborates the initial results from the reporter assays and strongly supports regulatory roles in antiviral innate immune responses for many TRIM proteins.
Attenuated TRIM expression in primary MDDCs reduces LPS-mediated cytokine expression
To ensure that these TRIMs indeed have a biological function in relevant primary innate immune cells, the same shRNA-expressing lentiviruses were used to transduce primary human monocytes from four independent donors. These cells were subsequently differentiated into monocyte-derived dendritic cells (MDDCs) and challenged with LPS. Since TLR and RIG-I pathways converge downstream and share several key regulators, we expected that LPS stimulation would also be regulated by several of the TRIMs identified in our study as positive regulators of RIG-I mediated innate immunity.
As in the previous experiments using cell lines, the amount of LPS and time of stimulation were carefully titrated to result in a limited IFNβ, TNFα, IL-6 and IL-8 induction, potentially increasing the window to observe phenotypes. As a consequence of choosing the optimal time point for analyzing IFNβ and other ‘immediate early’ cytokines (2 h p.t.), no ISGs were induced yet at the time of analysis (data not shown).
IFNβ mRNA expression was induced 20–200 fold by LPS compared to mock-treated samples (Fig. 6a). Other cytokines were induced roughly 10–1000 fold (Fig. S6). Cytokine induction by LPS was similar in transduced and non-transduced cells, indicating that the transduction procedure per se had no significant effect on the cells (data not shown). As expected from human samples –especially analyzing such a substantial number of conditions and cytokines– some inter-donor variation was observed, Both at the amount of cytokine induction by LPS, as well as in the effect of individual TRIM mRNA targeting on this induction (Fig. 6a).
Fig. 6. see also Fig. S6. mRNA targeting of endogenous TRIMs in MDDCs attenuates cytokine expression upon LPS stimulation.
MDDCs transduced with lentiviruses expressing TRIM-specific shRNAs were stimulated with LPS. Each condition was repeated in four different donors, with 2-4 wells per condition per donor. (a) At 2 h p.t. cells were harvested and their IFNβ mRNA levels determined by RT-qPCR and plotted as fold induction over mock-induced samples. (b) Similarly, TNFα, IL-6 and IL-8 mRNA levels were determined and plotted as a heat map representing the fraction of donors in which expression of a particular cytokine was >10% affected compared to the scrambled shRNA control. Red-orange colors: 50% or more of the donors exhibited consistent attenuation of the cytokine response upon TRIM mRNA targeting without an equal number of donors with the opposite phenotype. Green colors: 50% or more of the donors consistently upregulated cytokine responses during TRIM mRNA targeting without an equal number of donors with the opposite phenotype. (c) MDDCs from donor 3 and 4 expressing TRIM5 or TRIM38 specific shRNAs, were infected with SeV. At 4 h p.i. their cytokine mRNA levels were determined by RT-qPCR and plotted as fold induction over mock-induced samples. Numbers in parentheses indicate TRIM mRNA targeting levels (higher numbers represent better mRNA targeting); n.d. indicates that mRNA targeting could not be reliably measured resulting from low TRIM mRNA levels. Data are represented as mean +/− SD.
Despite these inter-donor variations, decreased expression of TRIM8 and TRIM32 consistently reduced IFNβ induction by at least 10% in all four donors. mRNA targeting of TRIM1 and TRIM36 also attenuated IFNβ expression in most of the donors. With a few exceptions, TRIM mRNA targeting in the lower IFN inducing donors consistently and most-potently attenuated IFN and ISG induction. Interestingly, both TRIM1 and TRIM32 did not attenuate IFNβ expression in SeV-challenged cell lines (Fig. 5d), yet attenuated induction in LPS stimulated MDDCs (Fig. 6a), suggesting that cell type- and, or stimulation-specific differences exist. Both TRIM13 and TRIM25 mRNA targeting only attenuated IFNβ expression in two of the four donors. However, for both of these TRIMs one of the non-attenuated donors lacked significant TRIM mRNA targeting (TRIM13, donor 3; TRIM25, donor 2), which could explain the lack of phenotype in those particular donors. As observed before in the cell lines (Fig. 5c and d), TRIM5 shRNA expression reduced TRIM5 mRNA expression in all donors, yet did not reduce IFNβ mRNA expression (Fig. 6a). Finally, TRIM38 mRNA targeting in LPS-stimulated MDDCs consistently increased IFNβ expression in all four donors, which is an opposite phenotype compared to SeV-stimulation in the cell lines (Fig. 5d).
Together, these data suggest that TRIM1, 8, 13, 25, 32 and 36 are enhancers of the IFNβ induction pathway in MDDCs. The phenotypic differences between TRIM1 and TRIM32 mRNA targeting in LPS-stimulated MDDCs and SeV-stimulated cell lines underscores that some TRIMs may play only a significant role in some cell types or in certain induction pathways of the type I IFN response. In support of this notion, TRIM38 mRNA targeting even yielded a completely opposite phenotype during the two different stimulations.
In the same samples, the effect of TRIM mRNA targeting on expression of TNFα, IL-6 and IL-8 was measured. All three cytokines were induced in all donors, yet were differentially attenuated depending on the TRIM that was knocked-down (Fig. S6). To allow analysis of the cytokine profiles upon different TRIM mRNA targeting conditions, the attenuation of all four measured cytokines amongst different donors was plotted in a heat map (Fig. 6b). The predominant effect of mRNA targeting of a particular TRIM on a particular cytokine was plotted as red-orange (indicating downregulation of the cytokine response and thus mRNA targeting of an enhancer) or green (to represent increased cytokine expression, indicating attenuation of a repressor).
As described above, TRIM5 and TRIM38 mRNA targeting overall increased, rather than attenuated, expression of all cytokines. Although this phenotype was similar to TRIM5 mRNA targeting in cell lines (Fig. 5c), TRIM38 attenuation resulted in the complete opposite upon LPS stimulation. To test if the inverted phenotype upon TRIM38 mRNA targeting resulted from different cell types or different stimuli, we also infected MDDCs (expressing either αTRIM5 or αTRIM38 shRNAs) from donor 3 and 4 with SeV and analyzed their cytokine profiles by RT-qPCR.
Levels of the RIG-I-inducing SeV defective-interfering (DI) RNA (Baum et al., 2010) were not different between samples (data not shown). In addition, IFNβ mRNA levels had already reached maximum induction at the time of analysis and hence did not show any significant differences upon TRIM mRNA targeting (data not shown). However, TNFα, ISG15 and ISG54 had not reached maximum induction yet and were analyzed for attenuation upon TRIM mRNA targeting. As before, TRIM5 mRNA targeting did not attenuate cytokine expression or expression of ISGs (Fig. 6c), whereas TRIM38 –in contrast to LPS stimulation- now attenuated expression of TNFα and the two ISGs tested in both donors (Fig. 6c). IL-6 and IL-8 were not consistently changed in both donors (data not shown).
Taken together, these results show that the majority of the tested TRIMs are positive regulators of cytokine expression and this effect may be cell-type specific. However, the opposite phenotypes observed for TRIM38 mRNA targeting show that the effect of some TRIMs is induction pathway specific.
TRIM proteins regulate innate immune signaling originating from different PRRs
The results of the pathway mapping by exogenous TRIM expression (Fig. 2) indicated that TRIM proteins act at different levels of the IFN induction cascade. We set out to complement these results by mapping the effects of TRIM mRNA targeting on cytokine expression in the context of biologically relevant and more complex stimuli.
To this end, monocyte-like cells (THP-1) were transduced with two distinct, individual shRNAs targeting six individual TRIMs (Fig. S7a). Significant TRIM mRNA targeting was confirmed by specific RT-qPCR (Fig. 7a). These cells were subsequently stimulated with four different stimuli mediating cytokine expression via distinct receptors and signaling pathways: i) SeV (through RIG-I and MAVS), zymosan (through TLR2 and MyD88), poly I:C (through TLR3 and TRIF) and LPS (through TLR4, TRIF and MyD88). This experimental setup allowed i) confirming attenuation of cytokine expression by TRIM mRNA targeting in an additional cell type, ii) ruling out shRNA off-target effects by using multiple distinct shRNA sequences, and iii) mapping in which innate immune signaling pathways individual TRIM proteins exert their activity.
Fig. 7. see also Fig. S7 and Table S2. TRIM proteins differentially regulate various innate signaling pathways.
THP1 monocyte-like cells were transduced with lentiviruses expressing a scrambled control shRNA or two individual, different shRNAs targeting the indicated TRIMs. (a) After puromycin selection, mRNA targeting of individual TRIMs was confirmed in polyclonal cell populations by RT-qPCR. Subsequently, these cells were stimulated with (b–c) SeV (3 h; RIG-IMAVS axis), or (d–e) LPS (3 h; TLR4-MyD88-TRIF axis). At the indicated times, total RNA was isolated and IFNβ, ISG54 and IL6 mRNA induction determined by RT-qPCR. Data are represented as mean +/− SD. (f) Heatmap summary of results from cytokine induction in mRNA targeted cells. Half of each oval unit represents each of the two different shRNAs. Samples with ≥ 35% attenuation in cytokine induction are marked in red, or inversely marked green upon ≥ 35% enhancement of cytokine induction. (g) Vero cells were incubated for 24 h with supernatants from THP1 mRNA targeted cells stimulated for 24 h with zymosan. Subsequently, these Vero cells were infected for 7 h with VSV-GFP (4 PFU/cell). Data are represented as mean +/− SD. (h) Model-overview of predicted levels of action of the tested subset of TRIMs.
SeV infection induced IFNβ mRNA at 3 h p.i approximately by 1000-fold compared to mock-infected cells (Fig. 7b), which increased further up to 10,000-fold by 6 h p.i. (data not shown). This induction was paralled by both ISG54 and IL6 mRNA, albeit to a lesser absolute extent. In contrast, viral RNA replication was already maximal at 3 h p.i. At this time point mRNA targeting of the six tested TRIMs (T1, T8, T13, T25, T32, T38) attenuated IFNβ and ISG54 expression for all shRNAs tested by ~2–8 fold compared to the scrambled shRNA control (Fig. 7c: black and gray bars). This reduction did not result from reduced SeV replication, since SeV RNA levels were similar or even higher than in the scrambled control (Fig. 7c, orange bars).
In contrast to IFNβ and ISG54, TRIM mRNA targeting resulted in elevated pro-inflammatory IL6 expression (Fig. 7c, green bars). Thus, TRIM mRNA targeting with different shRNA sequences attenuated IFNβ and ISG54 expression in THP1 cells, confirming our findings in HEK-293T and MDDCs (Fig. 5 and 6) that the tested TRIMs act as positive regulators in the RIG-I-mediated IFN induction pathway.
Subsequently, cells were stimulated in similar experiments with zymosan (TLR2; Fig. S7b-c), poly I:C (TLR3, Fig. S7d-e) or LPS (TLR4, Fig. 7d–e) to determine pathway specificity of TRIM function. The results from all stimulations were summarized in a heatmap to facilitate comparison (Fig. 7f). All three stimuli increased IFNβ and ISG54 expression (Fig. 7d, S7b and S7d). IL6 induction was less pronounced in the case of poly I:C (~ 3 fold) and absent upon LPS stimulation. Similar to the results found during SeV infection, mRNA targeting of TRIM1, TRIM8, TRIM13, TRIM25 and TRIM32 attenuated poly I:C- and LPS-induced IFNβ and ISG54 mRNA induction (Fig. 7e and S7e). However, TRIM38 mRNA targeting enhanced both IFNβ, ISG54 and IL6 expression (Fig. 7e and S7e), suggesting that it may act as a negative regulator in the TRIF-dependent pathways. These results are in agreement with the enhanced cytokine expression observed in LPS-stimulated MDDCs with reduced levels of TRIM38 (Fig. 6a–b).
Zymosan and TLR2-induced cytokine expression was also reduced compared to the scrambled shRNA control in the presence of reduced TRIM1, TRIM25 and TRIM32 levels (Fig. S7c). In general, the attenuation seemed less pronounced, which may have in part resulted from the later time point of analysis (6 h p.t. compared to 3–4 h p.t. for SeV, pIC, LPS). Throughout the zymosan experiment, cells expressing shRNA I against TRIM13 for unknown reasons had increased cytokine levels, which conflicted with results from shRNA II in the same experiment and results from the other tested stimuli. However, a third distinct shRNA targeting TRIM13 also attenuated zymosan-mediated cytokine induction (data not shown), suggesting that TRIM13 knockdown indeed attenuates IFN and ISG induction.
TRIM8 and TRIM38 mRNA targeting did not attenuate IFNβ induction, suggesting that these two TRIMs play no substantial role in the TLR2-mediated IFNβ induction (Fig. S7c). Similarly, TRIM38 mRNA targeting did not consistently change ISG54 induction. In contrast, TRIM8 mRNA targeting consistently enhanced ISG54 expression, suggesting that under physiological conditions TRIM8 may be a regulator of type I IFN signaling, but not IFN induction upon TLR2 stimulation. A role for TRIM8 in IFN regulation has in fact been previously described (Toniato et al., 2002). We speculate that we only observed this effect during the longer zymosan treatment, because under those conditions ISG54 production may have been in part through IFNβ secretion and IFNαR engagement. With the other, shorter stimuli, ISG54 induction was likely predominantly induced directly through IRF3, which would not allow observing any attenuation of the IFN signaling pathway by ISG54 mRNA levels. However, future experiments will be required to determine whether this is the case.
To confirm the results found during zymosan stimulation and address biological relevance, TRIM mRNA targeted THP1 cells were stimulated with zymosan and their supernatants analyzed for antiviral cytokine levels in a VSV-GFP infection challenge on Vero cells (Fig. S7f). As expected, mock-stimulated or zymosan-containing medium did not protect Vero cells from infection (Fig. 7g, red bars), whereas IFN treatment or supernatant from zymosan-stimulated control cells protected against viral replication (blue bars). In line with a minimal or non-existing role for TRIM8 and TRIM38 in TLR2 mediated IFN production, their mRNA targeting only minimally attenuated antiviral cytokine production (Fig. 7g). In contrast, mRNA targeting of TRIM1, TRIM13, TRIM25 and TRIM32 attenuated antiviral cytokine production, thus allowing considerably higher viral replication. These data thus confirm the requirement of these four TRIM proteins in TLR2-mediated antiviral cytokine synthesis.
Overall, TRIM mRNA targeting most considerably affected IFNβ and ISG54 expression, while having less pronounced and more variable impact on pro-inflammatory cytokine IL6 synthesis (Fig. 7f). We thus conclude that TRIM proteins have a more prominent role in the type I IFN system, while limited or indirect effects on pro-inflammatory cytokine synthesis (Fig. 7f and h). Importantly, we demonstrate that TRIM knockdown resulted in consistent effects on IFNβ induction throughout different experiments, although certain TRIMs regulated cytokine expression in a cell-type dependent or stimulus-dependent manner (Table S2).
The combined data from expression and mRNA targeting experiments (Fig. 2, 5, 6, 7 and Table S2) support the notion that TRIM38 is a positive regulator of the RIG-I pathway, while being a negative regulator of the TRIF-dependent pathways, with no or minimal importance in MyD88-dependent signaling (Fig. 7f and h). This is substantiated by the fact that three different publications reported similar conclusions while our manuscript was under revision (Xue et al., 2012; Zhao et al., 2012a; Zhao et al., 2012b)).
Moreover, our results indicate that TRIM25 may also play a role down-stream of RIG-I (Fig. 7h). Since TRIM1, TRIM13 and TRIM32 are seemingly involved in enhancement of all tested pathways, we predict them to act in a part of the pathway down-stream of the distinct adaptor proteins MAVS-TRIF-MyD88, which is shared by all three induction routes (Fig. 7h), between TBK1-IKKε and IRF3. Many regulatory molecules have been reported to act at this part of the signaling pathway and could thus be potential targets for individual TRIMs.
Exogenous TRIM expression mapping suggested that TRIM13 may act upstream of TBK1-IKKε, whereas TRIM1 may act at the level of IRF3 (Fig. 2i). TRIM8 seemingly plays only a minimal role in TLR2 induced signaling, suggesting that it may have a target shared between the TRIF- and MAVS-controlled pathways (Fig. 7h). Thus, with the resolution to be expected from medium through put analyses, we predict most of the TRIMs studied (both by expression and mRNA targeting) to act at a level shared between different induction pathways (TBK1-IKKε to IRF3) or just upstream between the adaptors TRIF-MAVS and TBK1-IKKε.
Discussion
We report here the cDNA cloning of all known TRIM-encoding genes and systematic analysis of their respective proteins in innate immune activation. Although some TRIMs had previously been implicated in innate immune activation, here we demonstrate that an unprecedented large number of TRIMs modulates innate immunity and that their transcriptional and post-transcriptional regulation is complex. It supports the notion that many human TRIM proteins rapidly evolved and expanded as part of the innate immune system.
The majority of the studied TRIM proteins contain a RING domain, which has been shown to facilitate ubiquitin E3 ligase activity in several TRIM members (Napolitano et al., 2011). Also our data from expression experiments using RING mutants demonstrated that all of the six tested TRIMs required an intact RING domain for efficient antiviral cytokine production. However, although all of these tested TRIM required their RING for function, we speculate that this is not the case for all innate immune enhancing TRIMs since two TRIM-like proteins which have no RING domain (TRIM14 and TRIM66), still potently enhanced immune induction.
TRIMs have been classified in sub-groups in part according to their C-terminal domains. Interestingly, TRIMs from all sub-groups were found to modulate innate immunity. Partially as a result of low numbers of TRIMs in certain groups, none of them contained more modulatory members than others. However, except for TRIM46, all five other members of sub-group I–characterized by C-terminal COS-FN3 and PRY-SPRY domains (Short and Cox, 2006)– (TRIM1, TRIM9, TRIM18, TRIM36 and TRIM67) strongly enhanced at least two promoters in the reporter assays above average. This correlation is even more striking for all the TRIMs containing a COS domain (subgroup I, II and III). Nine out of ten TRIMs containing the COS domain activated at least one promoter above average, suggesting that their COS domain may be important for innate immune function.
TRIM5 mRNA targeting did not negatively affect cytokine expression in our hands, irrespective of the inducer used. This contrasts with a recent report demonstrating that TRIM5 positively regulates NFκB signaling, AP-1 activation and the expression of several pro-inflammatory cytokines (Pertel et al., 2011). Nevertheless, overexpression of both the alpha and delta isoforms of TRIM5 strongly enhanced the innate immune reporters in our assays, which is in line with the observations by Pertel et al. The precise reason for a lack of attenuation in our hands upon TRIM5 mRNA targeting remains currently unclear. We consistently knocked-down TRIM5 mRNA levels by 50–90%, yet Pertel et al. reported a much more efficient TRIM5 mRNA ablation of 10–50 fold. Although our mRNA targeting levels are sufficient for observing phenotypes for other TRIMs tested, in the case of TRIM5 the decrease in functional TRIM5 in our hands may be insufficient for observing a phenotype. Whether this is the predominant factor underlying the observed differences remains to be determined in the future.
A recent manuscript reported the role of a limited set of TRIMs in NFκB regulation, and to a lesser extent in IFN regulation (Uchil et al., 2012). The limited number of TRIMs that the authors identify as important for IFN regulation in their screen largely overlaps with the group we report here and have investigated in more detail by knockdown in various cell types. However, our approach using an activating stimulus allowed the identification of many additional TRIMs that were not recognized previously. This underpins the fact that most TRIMs act as enhancers rather that direct activators of innate immune pathways.
In conclusion, our data demonstrate that many TRIM proteins are important regulators of the innate immune response. Each of these regulatory TRIMs is likely to act at different steps during the induction of IFN and pro-inflammatory cytokines. Our data open up the field for many follow-up studies addressing the importance of TRIMs in different cell types, signaling pathways and immune disorders in the future. It is feasible that our findings have only scratched the surface of the complexity of TRIM regulation. Based on the unique nature of the dedication of such a large protein family to regulation of innate immunity, future studies hold great promise to expose novel mechanisms and even paradigms in protein regulation and interplay during signal transduction.
Experimental procedures
Isolation, transduction and stimulation of MDDCs
All human research protocols for this work have been reviewed and approved by the Institutional Review Board of the Mount Sinai School of Medicine. Peripheral blood mononuclear cells were isolated from buffy coats of healthy human donors by Ficoll density gradient centrifugation (Histopaque, Sigma Aldrich) as previously described (Fernandez-Sesma et al., 2006; Haye et al., 2009). Plated monocytes were transduced as previously described (Berger et al., 2011). Equal amounts of each virus were added, just sufficient to transduce >95% of the cells. At 5 d post-transduction, MDDCs were either induced with a final concentration of 0.4 ng/mL LPS (Alexis Biochemicals; 581-008-L002) for 2 h or infected with 2 HAU of SeV in a M96 well for 4 h.
Cell lines with stable attenuation of gene expression
HEK-293T, A549 and THP-1 cells were seeded in 12-well clusters. Sixteen hrs later, medium was replaced with complete growth medium containing polybrene (Sigma) and 2.5 × 105 TU of lentiviruses expressing specific anti-TRIM shRNAs (Santa Cruz Biotechnology). Cells were selected for puromycin resistance and polyclonal pools of stable mRNA targeted cells were maintained in growth medium containing puromycin for a maximum of 10 passages. THP-1 cells were differentiated into a macrophage-like phenotype by PMA treatment (for SeV and zymosan inductions) or left in their monocyte-like phenotype (for poly I:C and LPS stimulations).
IFN antiviral activity assay by VSV-GFP infection
HEK-293T, which were previously selected for increased protein expression (293T-HiEx), were transfected in triplicate in M24 clusters with per well: 2 ng RIG-I(2CARD) plasmid in the presence of either 48 ng or 148 ng TRIM plasmid. Alternatively, THP-1 cells differentiated to their macrophagelike phenotype were washed and stimulated with 10 µg/mL zymosan. At 32 h p.t. supernatants were harvested and analyzed in an IFN bio-assay on Vero cells.
Supplementary Material
Highlights.
Nearly half of all 75 TRIM protein family members are innate immune enhancers.
TRIM mRNA expression and protein localization are complexly regulated during infection.
TRIMs confer differential regulation in distinct innate immune signaling pathways.
Acknowledgements
This research is partially supported by NIAID grants U54 AI57158 (North East Biodefense Center) (to AG-S), R01DA033733 (to AG-S), U19AI83025, (to AG-S and JJ) and P01AI090935 (to AG-S and AF-S). We thank J. Ayllon for kindly providing the ISG54 ISRE-luciferase reporter plasmid and D. Littman for providing plasmid pSIV3+. We thank A. Ballabio, .P. Bieniasz and F. Naya for kindly providing the TRIM expression plasmids specified in the supplementary information. We are grateful to M. Ooms for technical assistance related to lentivirus production and to R. Cadagan and O. Lizardo for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
GAV, RR, AFS, JJ and AG-S designed the study. GAV, RR and AG-S wrote the paper. GAV and RR performed bio-informatics analysis, experiments and analyzed data. MTS-A performed confocal microscopy. GAV, JV, MS and K-SI cloned TRIM expression constructs. AMM performed lentivirus transductions of dendritic cells. All authors discussed the results and commented on the manuscript. GAV and RR contributed equally to this work.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Arimoto K, Funami K, Saeki Y, Tanaka K, Okawa K, Takeuchi O, Akira S, Murakami Y, Shimotohno K. Polyubiquitin conjugation to NEMO by triparite motif protein 23 (TRIM23) is critical in antiviral defense. Proc Natl Acad Sci U S A. 2010;107:15856–15861. doi: 10.1073/pnas.1004621107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A, Sachidanandam R, Garcia-Sastre A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc Natl Acad Sci U S A. 2010;107:16303–16308. doi: 10.1073/pnas.1005077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger G, Durand S, Goujon C, Nguyen XN, Cordeil S, Darlix JL, Cimarelli A. A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1-derived lentiviral vectors. Nat Protoc. 2011;6:806–816. doi: 10.1038/nprot.2011.327. [DOI] [PubMed] [Google Scholar]
- Carthagena L, Bergamaschi A, Luna JM, David A, Uchil PD, Margottin-Goguet F, Mothes W, Hazan U, Transy C, Pancino G, Nisole S. Human TRIM gene expression in response to interferons. PLoS One. 2009;4:e4894. doi: 10.1371/journal.pone.0004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchet D, Sykes A, Nicolas A, Orr A, Murray J, Sirma H, Heeren J, Bartelt A, Everett RD. PML isoforms I and II participate in PML-dependent restriction of HSV-1 replication. J Cell Sci. 2011;124:280–291. doi: 10.1242/jcs.075390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Lorick KL, Jensen JP, Weissman AM. RING finger ubiquitin protein ligases: implications for tumorigenesis, metastasis and for molecular targets in cancer. Semin Cancer Biol. 2003;13:5–14. doi: 10.1016/s1044-579x(02)00095-0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sesma A, Marukian S, Ebersole BJ, Kaminski D, Park MS, Yuen T, Sealfon SC, Garcia-Sastre A, Moran TM. Influenza virus evades innate and adaptive immunity via the NS1 protein. J Virol. 2006;80:6295–6304. doi: 10.1128/JVI.02381-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Kirchhofer A, Shin YC, Inn KS, Liang C, Cui S, Myong S, Ha T, Hopfner KP, Jung JU. Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction. Proc Natl Acad Sci U S A. 2008;105:16743–16748. doi: 10.1073/pnas.0804947105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Haye K, Burmakina S, Moran T, Garcia-Sastre A, Fernandez-Sesma A. The NS1 protein of a human influenza virus inhibits type I interferon production and the induction of antiviral responses in primary human dendritic and respiratory epithelial cells. J Virol. 2009;83:6849–6862. doi: 10.1128/JVI.02323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn AB, Ryan MC, Liu H, Zeeberg BR, Jamison DC, Weinstein JN. SpliceMiner: a high-throughput database implementation of the NCBI Evidence Viewer for microarray splice variant analysis. BMC Bioinformatics. 2007;8:75. doi: 10.1186/1471-2105-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Regulation of innate immune signalling pathways by the tripartite motif (TRIM) family proteins. EMBO Mol Med. 2011;3:513–527. doi: 10.1002/emmm.201100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand-Breitenbach V, Zhu J, Puvion F, Koken M, Honore N, Doubeikovsky A, Duprez E, Pandolfi PP, Puvion E, Freemont P, de The H. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J Exp Med. 2001;193:1361–1371. doi: 10.1084/jem.193.12.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab FW, Rajsbaum R, Stoye JP, O'Garra A. Tripartite-motif proteins and innate immune regulation. Curr Opin Immunol. 2011;23:46–56. doi: 10.1016/j.coi.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Napolitano LM, Jaffray EG, Hay RT, Meroni G. Functional interactions between ubiquitin E2 enzymes and TRIM proteins. Biochem J. 2011;434:309–319. doi: 10.1042/BJ20101487. [DOI] [PubMed] [Google Scholar]
- Ozato K, Shin DM, Chang TH, Morse HC., 3rd TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol. 2008;8:849–860. doi: 10.1038/nri2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertel T, Hausmann S, Morger D, Zuger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature. 2011;472:361–365. doi: 10.1038/nature09976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajsbaum R, Stoye JP, O'Garra A. Type I interferon-dependent and -independent expression of tripartite motif proteins in immune cells. Eur J Immunol. 2008;38:619–630. doi: 10.1002/eji.200737916. [DOI] [PubMed] [Google Scholar]
- Rhodes DA, de Bono B, Trowsdale J. Relationship between SPRY and B30.2 protein domains. Evolution of a component of immune defence? Immunology. 2005;116:411–417. doi: 10.1111/j.1365-2567.2005.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KM, Cox TC. Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J Biol Chem. 2006;281:8970–8980. doi: 10.1074/jbc.M512755200. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Toniato E, Chen XP, Losman J, Flati V, Donahue L, Rothman P. TRIM8/GERP RING finger protein interacts with SOCS-1. J Biol Chem. 2002;277:37315–37322. doi: 10.1074/jbc.M205900200. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Zou J, Saitoh T, Kumar H, Abe T, Matsuura Y, Kawai T, Akira S. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity. 2010;33:765–776. doi: 10.1016/j.immuni.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Uchil PD, Hinz A, Siegel S, Coenen-Stass A, Pertel T, Luban J, Mothes W. TRIM protein mediated regulation of inflammatory and innate immune signaling and its association with antiretroviral activity. J Virol. 2012 doi: 10.1128/JVI.01804-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Zhou Z, Lei X, Liu X, He B, Wang J, Hung T. TRIM38 Negatively Regulates TLR3-Mediated IFN-beta Signaling by Targeting TRIF for Degradation. PLoS One. 2012;7:e46825. doi: 10.1371/journal.pone.0046825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Shi HX, Liu XY, Shan YF, Wei B, Chen S, Wang C. TRIM21 is essential to sustain IFN regulatory factor 3 activation during antiviral response. J Immunol. 2009;182:3782–3792. doi: 10.4049/jimmunol.0803126. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. Recognition of viral nucleic acids in innate immunity. Rev Med Virol. 2010;20:4–22. doi: 10.1002/rmv.633. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi R, Chang TH, Wang H, Atsumi T, Morse HC, 3rd, Ozato K. Gene disruption study reveals a nonredundant role for TRIM21/Ro52 in NF-kappaB-dependent cytokine expression in fibroblasts. J Immunol. 2009;182:7527–7538. doi: 10.4049/jimmunol.0804121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Wang L, Zhang M, Wang P, Yuan C, Qi J, Meng H, Gao C. Tripartite motif-containing protein 38 negatively regulates TLR3/4- and RIG-I-mediated IFN-beta production and antiviral response by targeting NAP1. J Immunol. 2012a;188:5311–5318. doi: 10.4049/jimmunol.1103506. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang L, Zhang M, Yuan C, Gao C. E3 ubiquitin ligase tripartite motif 38 negatively regulates TLR-mediated immune responses by proteasomal degradation of TNF receptor-associated factor 6 in macrophages. J Immunol. 2012b;188:2567–2574. doi: 10.4049/jimmunol.1103255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.