Abstract
Innate lymphoid cells (ILCs) are a recently described group of innate immune cells that can regulate immunity, inflammation, and tissue repair in multiple anatomical compartments, particularly the barrier surfaces of the skin, airways, and intestine. Broad categories of ILCs have been defined based on transcription factor expression and the ability to produce distinct patterns of effector molecules. Recent studies have revealed that ILC populations can regulate commensal bacterial communities, contribute to resistance to helminth and bacterial pathogens, promote inflammation, and orchestrate tissue repair and wound healing. This review will examine the phenotype and function of murine and human ILCs and discuss the critical roles these innate immune cells play in health and disease.
Introduction
Innate lymphoid cells (ILCs) represent a heterogeneous group of hematopoietic cells of the innate immune system (Spits and Cupedo, 2012; Spits and Di Santo, 2011). These cells appear to differentiate from a common lymphoid progenitor, and they comprise a small fraction of the total immune cell population in lymphoid organs, at epithelial barrier surfaces, and in other tissues (Spits and Cupedo, 2012; Spits and Di Santo, 2011). While ILCs lack rearranged antigen-specific receptors, these cells express many of the transcription factors and effector molecules expressed by CD4+ T helper (Th) cell populations, suggesting that ILCs may be an evolutionary precursor of cells of the adaptive immune system (Spits and Cupedo, 2012; Spits and Di Santo, 2011). For example, the group 1 ILC population consists of natural killer (NK) cells and potentially other ILCs that express the transcription factor T-bet, produce interferon-γ (IFN-γ), and are associated with cell-mediated immunity, similar to Th1 cells (Spits and Cupedo, 2012; Spits and Di Santo, 2011). The group 2 ILCs are dependent on the transcription factor RORα, express the transcription factor GATA3, produce the Th2-associated cytokines interleukin-5 (IL-5) and IL-13, and promote antihelminth and allergic immune responses, and thus are analogous to GATA3-expressing Th2 cells (Spits and Cupedo, 2012; Spits and Di Santo, 2011). Finally, the group 3 ILC population is composed of fetal lymphoid tissue inducer (LTi) cells that induce lymphoid organogenesis and recently described cells analogous to Th17 cells that are dependent on the transcription factor RORγt, produce IL-17A, IL-17F, and IL-22, and exert inflammatory and protective effects on epithelial cells. This latter group includes LTi-like cells, ILC17s, and NCR22s that express the NK cell cytotoxicity receptor NKp46 (Spits and Cupedo, 2012; Spits and Di Santo, 2011) (Figure 1). While the newly described cell populations that fall within the group 2 and group 3 ILC subsets share some features of LTi and NK cells, these ILCs are distinct from classical LTi and NK cells in their developmental and functional requirements for specific cytokines during homeostasis and inflammation (Spits and Cupedo, 2012; Spits and Di Santo, 2011). As group 1 ILCs and classical LTi cells have been discussed extensively elsewhere (Mebius, 2003; Spits and Cupedo, 2012; Spits and Di Santo, 2011; van de Pavert and Mebius, 2010), this review will focus on the development and function of the GATA3-expressing group 2 ILCs and the LTi-like, RORγt-dependent IL-17- and/or IL-22-expressing group 3 ILCs.
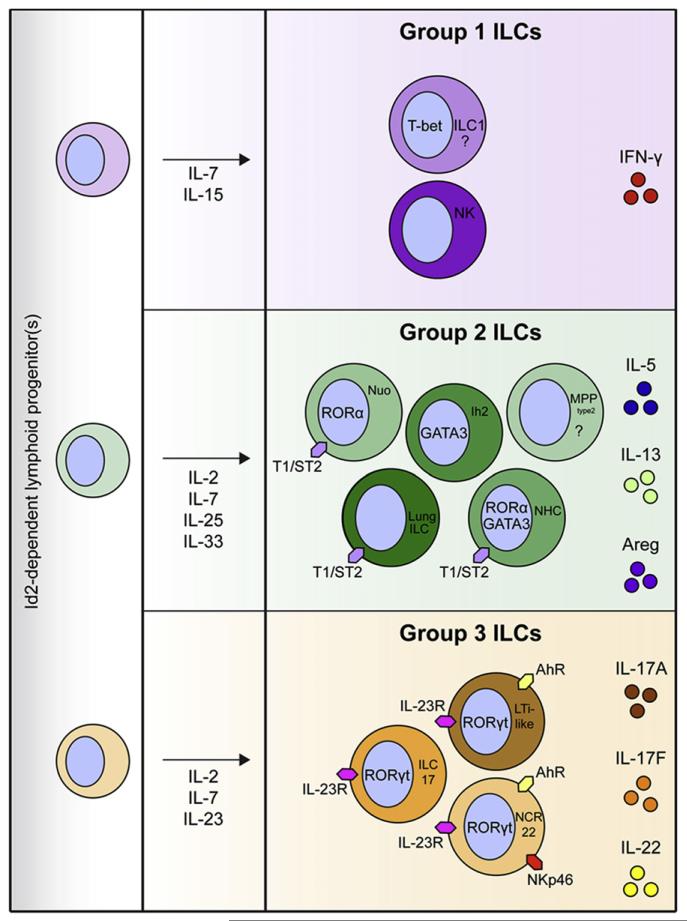
Figure 1. Murine ILC Development and Functional Heterogeneity.
The ILC population can be broadly divided into classical NK cells and potentially T-bet-expressing ILCs (group 1 ILCs); GATA3-expressing ROR-α-dependent ILCs that include NHCs, nuocytes (nuo), Ih2 cells, lung ILCs, and potentially MPPtype2 cells (group 2 ILCs); and RORγt+ ILCs that include LTi-like cells, ILC17s, and NCR22 cells (group 3 ILCs). All ILCs derive from an Id2-dependent lymphoid precursor and respond to γc cytokines, which play important roles in lymphoid cell development and function. Group 1 ILCs produce IFN-γ. Group 2 ILCs express the IL-33R (T1/ST2) and respond to IL-25 and IL-33 to produce IL-5, IL-13, and Areg. RORγt+ group 3 ILCs partially depend on AhR signaling for development and function, and some express NK cell cytotoxicity receptors such as NKp46. These cells express the IL-23R, respond to IL-23, and can produce IL-17A, IL-17F, and IL-22.
Recent seminal studies have revealed critical roles for newly described ILC populations. While the role of LTi cells in fetal lymphoid organogenesis has been appreciated for many years (Cupedo, 2011; Finke, 2009; Mebius, 2003; van de Pavert and Mebius, 2010), recent studies have shown that group 3 ILC populations function after fetal development by maintaining tissue homeostasis at barrier surfaces, particularly the gut, through interactions with commensal bacterial communities (Spits and Cupedo, 2012; Spits and Di Santo, 2011). Other studies have also revealed critical roles for group 2 ILCs in mediating immunity to intestinal helminth parasites and bacterial pathogens (Spits and Cupedo, 2012; Spits and Di Santo, 2011). Additional research has described proinflammatory properties of ILCs associated with immune responses to infection and allergens, and in the context of inflammatory bowel disease (IBD) (Spits and Cupedo, 2012; Spits and Di Santo, 2011). In addition to promoting immunity and inflammation in some settings, recent analyses have highlighted a pivotal role for both group 2 and group 3 ILCs in tissue repair and immune homeostasis, either in the steady state or during the resolution of inflammatory responses (Spits and Cupedo, 2012; Spits and Di Santo, 2011). This review will first describe ILC development and heterogeneity and will then focus on recent insights into how interactions between various ILC subsets and commensal bacterial communities or pathogenic microbes regulate homeostasis and inflammation in the intestine.
Development and Heterogeneity of Murine ILC Populations
Studies in murine model systems have revealed that the transcription factor inhibitor of DNA-binding 2 (Id2) (Cherrier et al., 2012; Eberl et al., 2004; Monticelli et al., 2011; Moro et al., 2010; Satoh-Takayama et al., 2010; Yokota et al., 1999) and signaling through the γc cytokine IL-7, which promotes hematopoietic cell development and proliferation (Moro et al., 2010; Satoh-Takayama et al., 2010), are critical for the development of all murine ILCs. However, group 2 and group 3 ILCs can be distinguished in part by their differential requirements for various factors during development. Some RORγt− group 2 ILCs express GATA3 (Liang et al., 2012; Moro et al., 2010; Price et al., 2010). Others require RORα for development (Halim et al., 2012b; Wong et al., 2012) and derive from a bone marrow-resident lymphoid progenitor that is dependent upon the cytokine receptor fms-like tyrosine kinase receptor 3, which is expressed by hematopoietic progenitors and supports lymphoid hematopoiesis (Yang et al., 2011). In contrast, the RORγt+ group 3 ILCs are critically dependent on RORγt during their development (Eberl et al., 2004; Luci et al., 2009; Sanos et al., 2009; Satoh-Takayama et al., 2008). The aryl hydrocarbon receptor (AhR), a helix-loop-helix family transcription factor (Kiss et al., 2011; Lee et al., 2012; Qiu et al., 2012), and members of the Notch family of signaling molecules (Cherrier et al., 2012; Lee et al., 2012; Possot et al., 2011) also play important roles in influencing the development of group 3 ILCs (Figure 1).
The group 2 and group 3 ILCs can also be distinguished by their phenotype and functional capacity. Murine models of infection or inflammation have been instrumental in interrogating the phenotype and function of heterogeneous populations of ILCs. The group 2 ILCs include cell populations termed natural helper cells (NHCs) (Moro et al., 2010), nuocytes (Neill et al., 2010), and innate helper type 2 (Ih2) cells (Price et al., 2010). While these cells were described independently, they share many similar characteristics and may represent the same cell type found in multiple tissue sites. Group 2 ILCs respond to the epithelial cell-derived cytokines IL-25 and IL-33 through their expression of the IL-25 receptor (IL-17RB) and the IL-33R (T1/ST2) and produce cytokines associated with Th2 cells that induce allergic inflammation, including IL-5 and IL-13, and the epidermal growth factor (EGF) family member amphiregulin (Areg) (Monticelli et al., 2011; Moro et al., 2010; Neill et al., 2010; Price et al., 2010).
Group 3 ILCs that express RORγt resemble LTi cells that promote lymphoid organogenesis (Mebius, 2003), are found in mucosal and lymphoid tissues, respond to the IL-12 family cytokine IL-23 through expression of the IL-23R, and are capable of producing IL-22 and IL-17A (Buonocore et al., 2010; Cella et al., 2009; Kinnebrew et al., 2012; Luci et al., 2009; Sanos et al., 2009; Satoh-Takayama et al., 2008; Takatori et al., 2009). There appears to be significant heterogeneity within the RORγt+ group 3 ILCs (Spits and Cupedo, 2012; Spits and Di Santo, 2011), although, as with the group 2 ILCs, independently identified cell types with slightly different phenotypes in different tissues may represent the same cell type at various stages of differentiation or activation. Some group 3 ILCs are predominantly IL-22 producers and others primarily produce IL-17, while others can produce IFN-γ (although IFN-γ-expressing ILCs may fall into the group 1 ILC subset) (Buonocore et al., 2010; Cella et al., 2009; Kinnebrew et al., 2012; Satoh-Takayama et al., 2008; Takatori et al., 2009; Vonarbourg et al., 2010). In addition, some RORγt+ ILCs, termed NCR22 cells, also express the NK cell cytotoxicity receptor NKp46, which may correlate with the ability to produce IL-22, although the function of this NK cell receptor in RORγt+ ILCs is not known (Cella et al., 2009; Luci et al., 2009; Sanos et al., 2009; Satoh-Takayama et al., 2008, 2009, 2010) (Figure 1).
While the requirement for RORγt, cytokine-producing capacity, and effector function have been used to differentiate group 2 and group 3 ILCs, there will likely be additional factors that are critical for the development and function of one or both populations. For instance, the identification of GATA3-expressing group 2 ILCs suggests that this transcription factor may play an important role in the development or activation of this ILC population (Liang et al., 2012; Moro et al., 2010; Price et al., 2010). Indeed, a recent study has shown that GATA3 is required for IL-13 expression from one type of group 2 ILCs, Ih2 cells (Liang et al., 2012), although the influence of GATA3 on development of group 2 ILCs has yet to be reported. Moreover, ILCs that do not fit into either category (for example, those that do not express GATA3 or RORγt) may comprise a separate population or multiple populations with currently unrecognized functions. Alternatively, these cells may represent an immature precursor pool from which mature RORγt− group 2 ILCs and RORγt+ group 3 ILCs differentiate, although Diefenbach and colleagues have proposed that loss, rather than gain, of RORγt expression accompanies maturation of RORγt+ ILCs (Vonarbourg et al., 2010). Indeed, one distinct population of progenitor-like innate immune cells, designated multipotent progenitor type 2 (MPPtype2) cells, responds to IL-25 and can differentiate into mast cells, basophils, and macrophages that contribute to the development of a Th2 cytokine response (Saenz et al., 2010b). Further studies will be required to more precisely define emerging ILC populations, their functions, and developmental relationships.
Human ILC Populations
While ILC populations in humans are less well-defined than in mice, recent significant advances have been made in the identification and characterization of human ILCs. For example, two independent studies have described cells in human lung, intestine, and nasal tissues that phenotypically and functionally resemble murine group 2 ILCs (Mjösberg et al., 2011; Monticelli et al., 2011). Work by Monticelli et al. showed that a population of cells that did not express markers for known immune cell lineages and did express IL-2Rα (CD25), IL-7Rα (CD127), and IL-33R (ST2) could be identified in healthy human lung tissues and in bronchiolar lavage fluid (BAL) (Monticelli et al., 2011). Another study by Spits and colleagues described various populations of ILCs in the human fetal gut, including CD45intermediate CD127+ RORγt+ group 3-like ILCs and CD45high CD127+ cells that resembled murine group 2-like ILCs (Mjösberg et al., 2011). The CD45high CD127+ cells expressed very low levels of RORγt but strongly expressed chemoattractant receptor-homologous molecule expressed on Th2 lymphocytes (CRTH2) (Mjösberg et al., 2011), a marker associated with Th2 cells, eosinophils, and basophils (Schuligoi et al., 2010). These CD45high CD127+ CRTH2+ cells responded to IL-25 or IL-33 in vitro by producing IL-13, and similar group 2 ILC-like cells could be found in multiple mucosal tissues in humans, including fetal and adult gut and lung tissue (Mjösberg et al., 2011). In addition, Delespesse and colleagues described a human CD34+ progenitor cell in the sputum of patients with asthma that expressed receptors for the epithelial cell-derived cytokines thymic stromal lymphopoietin (TSLP) and IL-33, and could respond to these cytokines to produce IL-5 and IL-13 (Allakhverdi et al., 2009). These cells, similar to MPPtype2 cells in mice (Saenz et al., 2010b), could promote extramedullary hematopoiesis and Th2 cytokine production at tissue sites during allergic inflammation (Allakhverdi et al., 2009) (Figure 2).
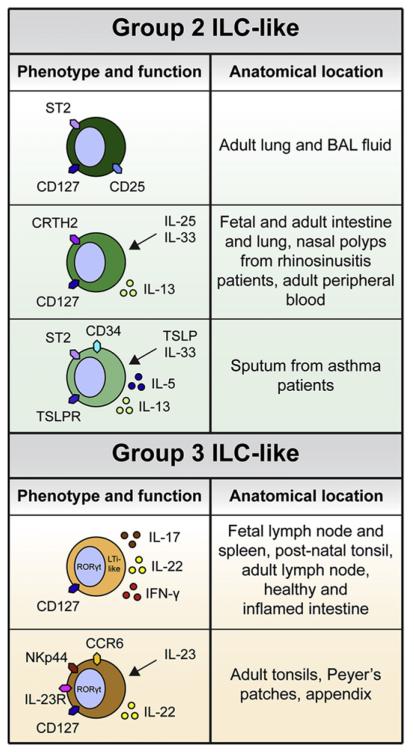
Figure 2. Phenotypic and Functional Heterogeneity of Human ILCs.
Innate cells that resemble murine group 2 and group 3 ILCs have been identified in various human tissues. Group 2 ILC-like cells that express the IL-2Rα (CD25), the IL-7Rα (CD127), and the IL-33R (ST2) are found in adult lung and BAL fluid. Similar cells that express CD127 and CRTH2 and respond to IL-25 and IL-33 to produce IL-13 have been identified in adult and fetal intestine and lung, inflamed nasal polyps from rhinosinusitis patients, and adult peripheral blood. Progenitor cells that express CD34, a marker of hematopoietic stem cells, TSLPR, and ST2 are found in the sputum of asthma patients and respond to TSLP and IL-33 to produce IL-5 and IL-13. Group 3 ILC-like cells that express CD127 and RORγt, produce IL-17 and IL-22, and contribute to lymphoid organogenesis are found in the fetal and adult lymph node and spleen. Similar cells are found in the postnatal tonsil, adult lymph node, and healthy and inflamed intestine. A group 3 ILC-like NK cell subset in the adult tonsils, Peyer’s patches, and appendix expresses CD127, RORγt, the NK cell marker NKp44, the IL-23R, and the chemokine receptor CCR6, and responds to IL-23 to produce IL-22.
The human counterparts of the murine group 3 ILCs have been more comprehensively described. Spits and colleagues identified human fetal LTi cells in the lymph nodes and spleen that were negative for all lineage cell markers and expressed CD127 and RORγt (Cupedo et al., 2009). Functionally, these cells had the capacity to initiate some of the characteristic early steps of lymphoid organogenesis through interactions with mesenchymal cells that were mediated through the cytokines lymphotoxin and tumor necrosis factor (TNF) (Cupedo et al., 2009). Additionally, these cells could differentiate into mature NK-like cells that retained CD127 surface expression and RORγt expression and upregulated various other NK cell markers, including the cytotoxicity receptors NKp46 and NKp44, and both fetal LTi cells and their CD127+ NK cell progeny expressed transcripts for IL17A and IL22 but not IFNγ (Cupedo et al., 2009). This study also reported the existence of LTi-like cells and CD127+ NK cells in the postnatal tonsil that expressed RORγt and could produce IL-17A and IL-22 after stimulation (Cupedo et al., 2009). IL-22-producing RORγt+ ILCs have also been identified in healthy intestinal tissues from humans and nonhuman primates (Sonnenberg et al., 2012). Moreover, RORγt+ LTi-like cells have been described in noninflamed adult lymph nodes, although compared to their fetal counterparts, these cells did not express high levels of IL-17A or IL-22 (Hoorweg et al., 2012). Colonna and colleagues described a similar population in the tonsil, Peyer’s patches, and appendix as an NK-like cell subset that expressed NKp44 and the chemokine receptor CCR6 and produced IL-22 in response to IL-23 yet did not appear to have cytotoxic capacity (Cella et al., 2009). Indeed, a later study confirmed that the CD127+ RORγt+ LTi-like cells present in the postnatal tonsil represented a distinct lineage from that of classical NK cells (Crellin et al., 2010a). In addition, group 3-like human ILC populations have been identified in inflamed tissues (Geremia et al., 2011). Powrie and colleagues have described CD127+ ILCs that did not express the human NK cell marker CD56 but produced IL-17A and IFN-γ in the intestinal lamina propria of patients with IBD (Geremia et al., 2011). Collectively, these data established the existence of group 3 ILCs in various human fetal and postnatal tissues (Figure 2). However, future studies will be needed to fully elucidate the developmental requirements and functional profiles of both group 2 and group 3 ILCs in healthy versus diseased human tissues.
ILCs Promote Tissue Homeostasis in the Intestine
IL-22 and RORγt+ group 3 ILCs have been implicated in the maintenance of intestinal barrier function and immune homeostasis (Spits and Cupedo, 2012; Spits and Di Santo, 2011). In addition, these cells are critical in the formation and maintenance of lymphoid structures in the gut-associated lymphoid tissue (GALT), including cryptopatches and isolated lymphoid follicles (ILFs), which are thought to be sites of local T and B cell responses (Spits and Cupedo, 2012; Spits and Di Santo, 2011). Although group 2 ILCs are present in the intestine of germ-free (GF) mice that lack live commensal bacterial communities (Monticelli et al., 2011), the nature of their interactions with these bacterial communities is currently unclear. To date, a role for group 2 ILCs in maintaining intestinal homeostasis and mediating GALT formation has not been described; therefore, this section will focus on the role of IL-22-producing group 3 ILCs that interact with commensal bacterial communities to mediate intestinal tissue homeostasis (Figure 3).
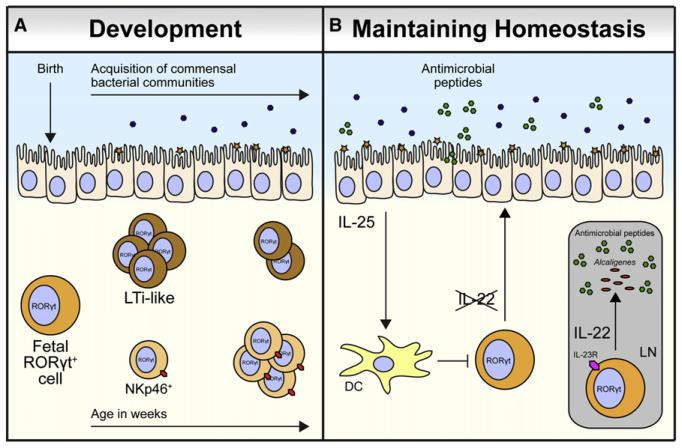
Figure 3. ILC Interactions with Commensal Bacterial Communities.
(A) The population structure of RORγt+ group 3 ILCs changes dramatically after postnatal microbial colonization, with a decrease in the size of the LTi-like cell population and an increase in the size of the NKp46+ ILC population occurring after birth.
(B) Colonization of mice by intestinal commensal bacterial communities promotes IL-25 production from intestinal epithelial cells. IL-25 acts on dendritic cells (DCs) that subsequently limit IL-22 production from RORγt+ group 3 ILCs in the lamina propria, thus decreasing production of IL-22-dependent antimicrobial peptides. In the GALT, IL-23-responsive IL-22-producing LTi-like cells and IL-22-dependent antimicrobial peptides are required to anatomically contain the lymph node (LN)-resident commensal species Alcaligenes xylosoxidans, which can cause systemic inflammation following dissemination.
Interactions between Commensal Bacterial Communities and Group 3 ILCs
Multiple studies have addressed the nature of the interactions between intestinal ILC populations and commensal bacterial communities. In particular, whether ILCs require commensal bacterial-derived signals for development has been an area of intense interest. While NK cells and group 2-like ILCs develop normally in the absence of commensal bacterial communities (Ganal et al., 2012; Monticelli et al., 2011), studies to date have not reached a consensus regarding the role of commensal bacterial communities in the development of group 3 ILCs. Two initial studies indicated that a population of NKp46+ group 3 ILCs in the intestine was significantly reduced in GF mice (Sanos et al., 2009; Satoh-Takayama et al., 2008), although one of these studies also showed that the NKp46− LTi-like group 3 ILC population was not affected (Sanos et al., 2009). Another study showed that the population structure of the group 3 ILCs in the intestine changes dramatically after microbial colonization that occurs postnatally (Sawa et al., 2010). Specifically, Eberl and colleagues have shown that group 3 ILC expression of the NK cell-like marker NKp46 is regulated after birth, suggesting that group 3 ILC populations change postnatally in response to or to accommodate commensal bacterial-derived signals (Sawa et al., 2010). However, the changes in population structure of the group 3 ILCs observed following birth remained intact after antibiotic treatment (Sawa et al., 2010), and subsequent studies have shown that ILC-like populations persist in the intestine in the absence of live commensal bacterial communities (Hamada et al., 2002; Lee et al., 2012; Sanos et al., 2009; Sawa et al., 2010, 2011; Sonnenberg et al., 2012). Moreover, the development of RORγt+ group 3 ILC-like populations in the postnatal intestine is unchanged in GF mice monoassociated with the commensal segmented filamentous bacteria and in mice deficient in various pathogen-associated molecular pattern recognition molecules, including Nod and MyD88 (Sawa et al., 2010). Together, these data suggest that while the development of intestinal group 3 ILCs does not depend on signals derived from commensal bacterial communities, the phenotype, population structure, and functional capacity of these cells in the intestine change to accommodate physiologic alterations in the intestinal environment following microbial colonization at birth (Figure 3A). Further studies will be required to definitively characterize the role of commensal bacterial communities in contributing to the fetal and postnatal development of group 3 ILCs.
The role of commensal bacterial communities in controlling effector cytokine production in ILC populations has also been investigated. RORγt+ group 3 ILCs in mice and humans have been shown to produce effector cytokines following exposure to bacterial products, indicating that these cells may be tuned to directly or indirectly respond to commensal bacterial-derived signals (Crellin et al., 2010b; Kinnebrew et al., 2012; Takatori et al., 2009). Specifically, several studies have examined how signals derived from commensal bacterial communities influence the ability of ILC populations to produce IL-22 (Sanos et al., 2009; Satoh-Takayama et al., 2008.; Sawa et al., 2011; Sonnenberg et al., 2012; Vonarbourg et al., 2010), which plays a role in maintaining intestinal barrier function and homeostasis (Sonnenberg et al., 2011a). RORγt+ group 3 ILCs in the intestine are the predominant source of IL-22, and RORγt-deficient mice lack expression of IL-22 in the intestine (Sawa et al., 2011; Sonnenberg et al., 2012). While one study indicated that IL-22-producing ILC populations were not affected in mice with altered commensal bacterial communities (Sonnenberg et al., 2012), other studies have shown that IL-22-producing RORγt+ NKp46+ ILCs were reduced in GF mice (Sanos et al., 2009; Satoh-Takayama et al., 2008; Vonarbourg et al., 2010), suggesting that interactions with commensal bacterial communities may be important for RORγt expression or IL-22 production from innate cells. However, the presence of commensal bacterial communities actually appears to repress IL-22 production from RORγt+ group 3 ILCs in the intestine, as IL-22 expression was enhanced in fetal mice and in adult GF or antibiotic-treated mice that lack or have altered commensal bacterial communities (Sawa et al., 2011). While the mechanisms of this repression are not fully understood, commensal bacterial communities may promote IL-25 production from epithelial cells, which in turn downregulates IL-23 and IL-22 production (Sawa et al., 2011; Zaph et al., 2008). These studies indicate that interactions between commensal bacterial communities and ILCs may be critical in modulating IL-22 production and the maintenance of intestinal homeostasis (Figure 3B).
Other studies have investigated the influence of RORγt+ group 3 ILCs on intestinal inflammation. Damage to the intestinal epithelium in the dextran sodium sulfate model of intestinal tissue damage and inflammation resulted in a significant population expansion of RORγt+ ILCs and enhanced IL-22 expression in the intestine, indicating that intestinal inflammation can override the suppressive effect of commensal bacterial communities on IL-22 production from RORγt+ ILCs (Sawa et al., 2011). Also, a recent study established a critical role for LTi-like cell-derived IL-22 in preventing systemic inflammation caused by a loss of containment of the commensal species Alcaligenes xylosoxidans (Sonnenberg et al., 2012) (Figure 3B). Critically, Alcaligenes-specific immune responses were detected systemically in patients with chronic inflammatory diseases, including Crohn’s disease (Sonnenberg et al., 2012). These two studies indicate that IL-22 derived from RORγt+ group 3 ILCs may be key in resolving local inflammation in the intestine and preventing systemic inflammation that results from dissemination of commensal bacteria. However, while interactions with commensal bacterial communities clearly influence the development and function of group 3 ILCs in the intestine in the steady state and in the context of inflammation, numerous questions remain. In particular, the potential roles of antigen-presenting cells (APCs) and adaptive immune cells in regulating ILC function and the ability of ILCs to directly sense bacteria-derived products are areas requiring further investigation.
Group 3 ILCs Influence GALT Formation
In addition to their role in controlling lymphoid organogenesis in the fetus (reviewed in Cupedo, 2011; Finke, 2009; Mebius, 2003; van de Pavert and Mebius, 2010), RORγt+ group 3 ILCs also play a critical role in regulating formation of GALT, including cryptopatches and ILFs, in the postnatal intestine (Spits and Cupedo, 2012; Spits and Di Santo, 2011). Cryptopatches were originally described as small lymphoid aggregates in the small and large intestine that developed after birth and were composed primarily of CD127+ Thy1+ cells that expressed c-kit, the receptor for stem cell factor and a marker of hematopoietic progenitors, but did not express markers of T or B cells (Kanamori et al., 1996). These lymphoid structures and the innate cells that composed them were dependent upon CD127, but did not require the presence of commensal bacterial communities (Kanamori et al., 1996). Subsequent studies showed that ILFs, which are distinct from cryptopatches but may derive from them (Bouskra et al., 2008), contain RORγt+ c-kit+ CD127+ LTi-like cells in addition to B cells that are organized into germinal center-like structures that robustly produce IgA (Tsuji et al., 2008). RORγt+ LTi-like cells that resemble group 3 ILCs were important in the formation and organization of cryptopatches and ILFs (Eberl and Littman, 2004) and maintenance of normal IgA levels, suggesting that activities of group 3 ILCs can also support adaptive responses in the intestine that help to maintain tissue homeostasis (Tsuji et al., 2008). As is the case for cryptopatches, ILFs were present in GF mice, although their composition was altered, and the formation of these structures required signaling through CD127 (Hamada et al., 2002). The presence of intestinal IL-22-secreting RORγt+ group 3 ILCs and the formation of cryptopatches and ILFs have also been shown to be dependent on AhR (Kiss et al., 2011; Lee et al., 2012), and partially dependent on Notch signaling (Lee et al., 2012). Together, these studies establish a critical role for RORγt+ ILCs in controlling the formation of intestinal lymphoid structures.
ILCs as Innate Immune Effectors in the Intestine that Mediate Resistance to Pathogens
In addition to the critical role of ILCs in maintaining intestinal homeostasis through interactions with commensal bacterial communities and supporting the formation and function of the GALT, ILCs can act as potent innate immune effector cells that promote resistance to intestinal pathogens (Spits and Cupedo, 2012; Spits and Di Santo, 2011). New tools and approaches have revealed previously unrecognized roles for both group 2 and group 3 ILCs after infection with intestinal helminth and bacterial pathogens (Spits and Cupedo, 2012; Spits and Di Santo, 2011). The following section will describe the recent literature that has contributed to our understanding of the role of ILCs in promoting immune responses to pathogens that infect the intestine (Figure 4).
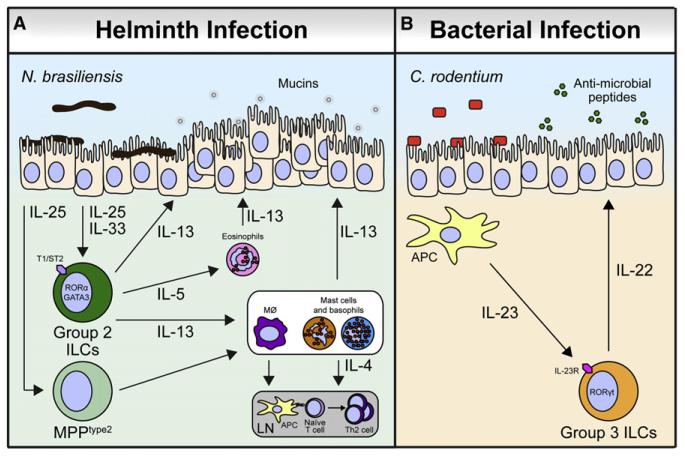
Figure 4. ILCs Mediate Immune Response to Pathogens in the Intestine.
(A) During infection with the helminth parasite N. brasiliensis, epithelial cells secrete IL-25 and IL-33 that promote the accumulation of group 2 ILCs that express the IL-33R (T1/ST2) and produce IL-5 and IL-13 to promote responses from IL-4-producing mast cells and basophils. IL-4 derived from these sources and others supports the development and maintenance of Th2 cells in the draining lymph node (LN). IL-5 also recruits eosinophils that produce IL-13. IL-13 from group 2 ILCs, mast cells, basophils, and eosinophils promotes gut epithelial cell hyperplasia and mucin secretion. In addition, MPPtype2 cells respond to IL-25 from intestinal epithelial cells to induce extramedullary hematopoiesis and differentiation of macrophages, mast cells, and basophils, which support Th2 cytokine responses. Together, these mechanisms lead to helminth expulsion.
(B) During infection with the enteric bacterial pathogen C. rodentium, APCs in the intestine produce IL-23. RORγt+ group 3 ILCs express the IL-23R and respond to IL-23 by secreting IL-22, which acts on epithelial cells to support production of antimicrobial peptides that limit bacterial replication.
Group 2 ILCs Promote Immunity to Intestinal Helminth Infection
In 2010, four independent publications identified and characterized various innate cell populations (NHCs [Moro et al., 2010], nuocytes [Neill et al., 2010], Ih2 cells [Price et al., 2010], and MPPtype2 cells [Saenz et al., 2010b]) that responded to epithelial-derived cytokines and were important in promoting immunity to intestinal helminth parasites in mice. Initial studies had suggested that innate cell populations might respond to the epithelial cell-derived cytokines IL-25 and IL-33 (Schmitz et al., 2005; Tulin et al., 2001). IL-25 induced production of Th2-associated cytokines from an innate cell population that contributed to pathological changes in multiple organs, including the intestine (Fort et al., 2001; Hurst et al., 2002). Subsequent studies indicated that IL-25 signaling promoted the development of a nonB/nonT c-kit+ FcεRI− innate immune cell population that was required for resistance to the gastrointestinal nematode Nippostrongylus brasiliensis (Fallon et al., 2006) and showed that IL-25 was required for immunity to the helminth parasite Trichuris muris (Owyang et al., 2006). IL-33-IL-33R (T1/ST2) interactions were initially implicated in the promotion of Th2 cytokine responses and associated allergic inflammation (Coyle et al., 1999; Löhning et al., 1998; Oshikawa et al., 2001, 2002; Saenz et al., 2010a; Schmitz et al., 2005). Subsequent studies showed that IL-33 was important in the generation of adaptive immunity to T. muris (Humphreys et al., 2008) and could also promote T cell-independent pathology in the intestine and airways (Kondo et al., 2008). Together, these studies set the stage for investigation into the roles of IL-25 and IL-33 in promoting innate cell responses during helminth infection.
NHCs were the first group 2 ILC population described that responded to epithelial cell-derived cytokines and contributed to Th2 cytokine-associated inflammation (Moro et al., 2010). In elegant studies by Koyasu and colleagues, NHCs were characterized as c-kit+ Sca-1+ cells that lacked expression of other immune cell lineage markers and were present in the fat-associated lymphoid tissue of mice (Moro et al., 2010). These cells responded to IL-33, were elicited during N. brasiliensis infection, and produced IL-5 and IL-13. Rag2−/−/γc−/− mice, which lack all adaptive immune cells and NHCs, were susceptible to N. brasiliensis infection and exhibited delayed worm clearance (Moro et al., 2010). Critically, adoptive transfer of NHCs into Rag2−/−/γc−/− mice was sufficient to promote IL-13 production and goblet cell hyperplasia in the intestine during N. brasiliensis infection, which are both important in worm clearance (Moro et al., 2010). In a subsequent study, IL-33-dependent IL-5 and IL-13 responses were shown to be associated with the population expansion of NHC-like cells and eosinophilia in the lung of mice infected with the helminth Strongyloides venezuelensis (Yasuda et al., 2012) (Figure 4A).
Shortly after NHCs were described, other group 2 ILC populations that responded to IL-25 or IL-33 were identified and characterized (Neill et al., 2010; Price et al., 2010; Saenz et al., 2010b). A cell population termed “nuocytes” was first identified by McKenzie and colleagues in the context of N. brasiliensis infection using an IL-13 reporter mouse model (Neill et al., 2010). A population of cells that did not express lineage markers for known cell types, but did express the IL-25R (IL-17RB) and the IL-33R (T1/ST2), were the predominant producers of IL-13 in the mesenteric lymph nodes after N. brasiliensis infection (Neill et al., 2010). The transfer of IL-13-sufficient but not IL-13-deficient nuocytes into susceptible Il17br−/− mice could promote worm expulsion, indicating that nuocyte-derived IL-13 can mediate protective immunity to N. brasiliensis (Neill et al., 2010). In addition, nuocytes were required for the development of optimal Th2 responses, suggesting that these cells are also important in promoting effective adaptive immune responses (Neill et al., 2010). A follow-up study indicated that the signaling adaptor molecule Act1 expressed by epithelial cells plays an important role in promoting nuocyte responses in this model (Kang et al., 2012). Another group 2 ILC population referred to as Ih2 cells were identified by Locksley and colleagues using another IL-13 reporter mouse model (Price et al., 2010). In agreement with the findings of Neill et al., Ih2 cells that did not express markers of known immune cell lineages were the primary producers of IL-13 after exposure to IL-25 or during N. brasiliensis infection (Price et al., 2010). When Ih2 cells were transferred to Rag2−/−/γc−/− mice in the context of recombinant IL-25 treatment, these cells were able to mediate worm expulsion (Price et al., 2010). An additional study using this IL-13 reporter mouse has recently indicated that GATA3 plays a critical role in the ability of Ih2 cells to mount protective IL-13 responses during N. brasiliensis infection (Liang et al., 2012) (Figure 4A).
Consistent with a role for IL-25 in promoting innate cell responses during helminth infection, IL-25 has also been shown to induce myelopoiesis that supports the generation of Th2 cytokine responses during helminth infection (Saenz et al., 2010b). Saenz et al. described a nonB/nonT c-kitintermediate innate immune cell population that was elicited by IL-25-IL-25R (IL-17RB) interactions and could differentiate into mast cells, basophils, and macrophages (Saenz et al., 2010b). Based on this multipotent capacity, these cells were designated MPPtype2 cells (Saenz et al., 2010b). The progeny of MPPtype2 cells promoted robust Th2 cytokine responses, and transfer of MPPtype2 cells into normally susceptible IL-25-deficient mice led to worm expulsion in the context of T. muris infection, providing the first report that a multipotent innate cell population could respond to epithelial cell-derived cytokines to mediate immunity to a parasite (Saenz et al., 2010b). Therefore, IL-25 appears to elicit both ILC and myeloid progenitor responses, although it is currently unclear whether or how these two cell populations are related (Saenz et al., 2010a). Taken together, the studies describing NHCs, nuocytes, Ih2 cells, and MPPtype2 cells indicate that various types of group 2 ILCs respond to epithelial cell-derived cytokines and are pivotal players in the immune response to helminth parasites (Figure 4A).
RORγt+ Group 3 ILCs Play a Critical Role in Immunity to Gram-Negative Bacterial Infection
Initial studies in murine models of bacterial infection highlighted the importance of IL-22 in promoting resistance to extracellular bacterial pathogens at barrier surfaces. A study conducted by Kolls and colleagues utilized a model of Klebsiella pneumoniae infection to explore the role of IL-22 during bacterial infection in the lung (Aujla et al., 2008). K. pneumoniae is an extracellular, Gram-negative bacterial pathogen that provides a robust model of infection at the pulmonary mucosal barrier (Ye et al., 2001). In this model, IL-23-dependent IL-22 production was required for antimicrobial responses in the lung epithelium, optimal bacterial clearance, and resistance to infection (Aujla et al., 2008). The authors concluded that T cells were the primary source of IL-22, based on the lack of IL-22 production in Rag2−/−/γc−/− mice (Aujla et al., 2008). However, mice that lack the gc, a receptor subunit essential for the signaling of several cytokines, are also deficient in ILCs; thus, retrospectively, these data suggest a potential role for IL-22-producing gc-dependent innate cells and highlight the critical importance of group 3 ILC-derived products in controlling pulmonary bacterial infections (Aujla et al., 2008). Indeed, a recent study has shown that an innate IFN-γ- and IL-17-producing cell population in the lungs of mice subjected to a Bacillus Calmette-Guérin (BCG) vaccination protocol was associated with enhanced protection against challenge with Mycobacterium tuberculosis (Pitt et al., 2012).
Further studies described an innate source of IL-22 in the context of infection with Citrobacter rodentium (Cella et al., 2009; Satoh-Takayama et al., 2008, 2009; Sonnenberg et al., 2011b; Zheng et al., 2008), a Gram-negative attaching and effacing enteric bacterium that naturally infects mice (Frankel et al., 1996). IL-22 production was maintained in C. rodentium-infected Rag2−/− mice, suggesting that innate cells were a potent source of IL-22 in this model (Zheng et al., 2008). A subsequent study by Di Santo and colleagues showed that innate cells that expressed NKp46 and RORγt were important IL-22 producers during infection with C. rodentium, since Rag2−/−/Il2rb−/− mice, which lacked T and classical NK cells but retained NKp46+ innate cells, mounted IL-22 responses and were resistant to infection (Satoh-Takayama et al., 2008). Expression of the marker NKp46 was later shown to be dispensable for IL-22 production and protection from C. rodentium infection (Satoh-Takayama et al., 2009). In addition, NK cell-like innate cells in the intestine were shown to robustly produce IL-22 during C. rodentium infection, but whether these cells are the same or different from NKp46+ ILCs is unclear (Cella et al., 2009). Finally, a recent study identified gut-resident CD4+ LTi-like cells as critical IL-22 producers that mediate resistance to C. rodentium and orchestrate immune responses that upregulate antibacterial effector mechanisms (Sonnenberg et al., 2011b), including production of antimicrobial peptides (Liang et al., 2006). Notably, this study showed that innate IL-22-producing LTi-like cells were required for resistance to C. rodentium in lymphocyte-deficient and lymphocyte-replete mice (Sonnenberg et al., 2011b). The finding that group 3 ILCs are active in lymphocyte-replete mice supports other studies that indicate that group 3 ILCs promote adaptive immune responses. Group 3-like ILCs express the costimulatory molecules CD30L and OX40L, allowing them to interact with T cells (Kim et al., 2003, 2005). In addition, group 3-like ILCs contribute to the maintenance of CD4+ T cell memory responses after immunization and infection with the intracellular bacterium Listeria monocytogenes (Withers et al., 2009, 2012). However, the potential role of group 3 ILCs in generating adaptive responses after Gram-negative bacterial infection in the intestine remains unknown. Together, these data suggest that IL-22-producing RORγt+ group 3 ILCs are critical cells in promoting resistance to infection with Gram-negative extracellular bacteria at barrier surfaces, including the lung and intestine (Figure 4B).
ILCs and the Balance of Inflammation and Tissue Repair
In addition to their role as innate immune effectors during immune responses to pathogens in the intestine and lung, ILCs have also been shown to contribute to infection-induced inflammation and other noninfectious inflammatory conditions, including IBD and colitis (Spits and Cupedo, 2012; Spits and Di Santo, 2011). Conversely, emerging studies suggest that ILCs may have an important role in limiting inflammation and supporting tissue repair and wound healing mechanisms during the resolution phase of immune responses against certain pathogens and insults (Spits and Cupedo, 2012; Spits and Di Santo, 2011). The following section will discuss the context-dependent roles of ILCs in promoting inflammatory conditions and in participating in the resolution of inflammation (Figure 5).
Figure 5. Pro- and Anti-inflammatory Activities of ILCs.
(A) In response to various allergens and the influenza A virus subtype H3N1, lung epithelial cells produce IL-25 and IL-33 that promote IL-5 and IL-13 production from lung-resident group 2 ILCs that express the IL-33R (T1/ST2). IL-5 recruits eosinophils to the tissue, and IL-13 induces pathogenic epithelial cell proliferation and contraction of smooth muscle tissue, causing allergic airway inflammation and AHR. In the intestine, in a model of H. hepaticus-induced colitis, group 3 ILCs express the IL-23R and respond to IL-23 to produce IL-17A and IFN-γ that together contribute to colitis.
(B) In contrast, during infection with influenza virus A subtype H1N1, IL-33 derived from epithelial cells and macrophages promotes Areg expression from group 2 ILCs in the lung that express the IL-33R (T1/ST2), which support reparative epithelial cell proliferation. Additionally, in a model of OVA-induced AHR, lung resident RORγt+ group 3 ILCs produce IL-22 that limits the production of IL-5 and IL-13 that contributes to AHR.
ILCs Can Contribute to Inflammation at Barrier Surfaces
Group 2 ILCs have been shown to contribute to inflammation in murine models of allergic inflammation in the lung and influenza-induced airway hyperresponsiveness (AHR) (Barlow et al., 2012; Bartemes et al., 2012; Chang et al., 2011; Halim et al., 2012a, 2012b; Kim et al., 2012; Wolterink et al., 2012). IL-33 and IL-33R (T1/ST2) were initially shown to contribute to Th2 cell-associated cytokine-mediated pathology and allergic airway inflammation and AHR (Coyle et al., 1999; Schmitz et al., 2005) in the absence of adaptive immune responses, suggesting that innate cells respond to IL-33 to promote AHR (Kondo et al., 2008). More-recent work has shown that group 2 RORα-dependent or GATA3-expressing ILCs that produce IL-5 and IL-13 in response to IL-25, IL-33, a fungal allergen, glycolipid antigens, or the protease papain can promote AHR and/or allergic airway inflammation (Barlow et al., 2012; Bartemes et al., 2012; Halim et al., 2012a, 2012b; Kim et al., 2012; Wolterink et al., 2012). In an influenza A virus infection-induced model of AHR using the H3N1 influenza virus subtype, Umetsu and colleagues reported that IL-13-producing group 2-like ILCs that responded to IL-33 signals were sufficient to induce AHR after viral infection (Chang et al., 2011) (Figure 5A). Critically, these cells were also associated with allergic disease in human patients, as the nasal polyps isolated from patients with chronic rhinosinusitis were enriched for CD127+ CRTH2+ CD161+ ILCs compared to uninflamed nasal tissues from nonallergic control patients (Mjösberg et al., 2011) (Figure 2). While the contribution of group 2 ILCs to other allergic diseases, including those that affect the skin, nasopharynx, and in particular the intestine, have not yet been studied, the ability of these cells to produce IL-5 and IL-13 suggest that they may play a role in the development of wide array of allergic disorders.
The role of group 3 ILCs in intestinal inflammation has also been investigated, and inflammatory roles have been described for RORγt+ group 3 ILCs in the context of IBD (Buonocore et al., 2010; Geremia et al., 2011). One study reported that IL-23 signaling in a murine model of Helicobacter hepaticus-mediated colitis was associated with the accumulation of RORγt+ ILCs in the colon that produced IL-17A and IFN-γ and that depletion of these cells prevented colitis (Buonocore et al., 2010) (Figure 5A). Of note, while the cells described above are capable of producing IL-22, in the context of T cell-mediated colitis IL-22 production has been reported to have an anti-inflammatory effect (Zenewicz et al., 2008); thus, the relative contribution of ILC-derived IL-22, IL-17A, and IFN-γ in colitis remains to be determined. As IL-17 and IL-22 can be either pathogenic or protective in murine models of inflammation in the airway or intestine (Ahern et al., 2008; Monteleone et al., 2012; Sonnenberg et al., 2010), the role of IL-17- or IL-22-producing group 3 ILCs is likely to be context dependent.
One recent report from Powrie and colleagues has highlighted a potential role for LTi-like cells that resemble murine group 3 ILCs in a human disease state. A population of innate cells that did not express markers of T cells isolated from the intestinal lamina propria of patients with IBD expressed higher IL17A and IL17F transcript levels than those isolated from the lamina propria of noninflamed control patients, suggesting that an innate source of IL-17 in the inflamed colon may contribute to chronic inflammatory disease (Geremia et al., 2011). Further examination indicated that patients with Crohn’s disease showed an accumulation of CD127+ CD56− ILCs in the colon and ileum compared to control patients, and that these cells produced IL-17A and IFN-γ (Geremia et al., 2011). While this study did not directly assess whether this ILC population was RORγt+, the surface phenotype and cytokine production profile of these cells suggest that they express RORγt (Geremia et al., 2011). Thus, these results indicate that RORγt+ ILCs may be part of a complex immune cell infiltrate that contributes to the development of chronic intestinal inflammation during IBD (Geremia et al., 2011). However, additional studies will be required to determine whether these cells play a role in the initiation or maintenance of disease, and whether ILC interactions with commensal or pathogenic bacteria influence intestinal inflammation during IBD in patients.
ILCs Can Limit Inflammation and Mediate Tissue Repair and Wound Healing
In addition to the established role of ILCs in promoting inflammation, ILCs can also limit inflammation and promote wound healing and tissue repair (Spits and Cupedo, 2012; Spits and Di Santo, 2011). Recent reports have shown that group 2 ILCs can participate in tissue repair in the lung. In a study by Monticelli et al., expansion of group 2-like ILCs was observed in the lung of mice infected with influenza A virus (H1N1 subtype), and depletion of these cells in Rag1−/− mice was associated with more rapid host mortality due to decreased lung function and a loss of lung epithelial barrier cell integrity (Monticelli et al., 2011). Lung ILCs had a transcriptional profile enriched for genes that promoted tissue repair and wound healing, including the gene that encodes the EGF family member Areg (Monticelli et al., 2011). In influenza A virus-infected mice depleted of lung ILCs, treatment with recombinant Areg was sufficient to rescue epithelial cell barrier integrity and lung function, indicating that Areg derived from lung ILCs is critical in tissue repair after acute injury induced by influenza A virus infection (Monticelli et al., 2011) (Figure 5B). These findings differ from those described by Chang et al. showing that ILCs were pathogenic and contributed to AHR after H3N1 influenza virus infection (Chang et al., 2011) (Figure 5A). While the basis for the different findings in these two studies remains unclear, the different subtypes of virus used could play a role. Regardless, these findings further illustrate the context-dependent effects of ILC populations and the need for further investigation of the tissue repair functions of various types of ILCs at different barrier surfaces. Of note, tissue repair functions have not yet been described for group 2 ILCs in the intestine, although this is an area that requires further study, as the tissue repair mechanisms ascribed to group 2 ILCs in the lung may also be critical in tissue repair and wound healing in the intestine or other barrier surfaces.
In addition, group 3 and group 3-like ILCs have also been shown to limit inflammation and contribute to tissue repair (Kumar et al., 2012; Sawa et al., 2011; Scandella et al., 2008; Taube et al., 2011). Scandella and colleagues reported that destruction of normal lymphoid architecture and loss of the stromal cell network occurs in the spleen in response to lymphocytic choriomeningitis virus (LCMV) infection (Scandella et al., 2008). Using a bone marrow chimera approach in which wild-type mice were reconstituted with Rorc−/− cells to generate mice that were deficient in RORγt+ ILCs, the authors showed that LCMV-induced loss of stromal cell architecture in the spleen was repaired by a population of RORγt+-dependent ILCs (Scandella et al., 2008). The ability of RORγt+ ILCs to aid in the regeneration of normal lymphoid architecture after LCMV infection is in line with the requirement for LTi cells in the generation and organization of secondary lymphoid structure in the fetus (Mebius, 2003). However, LTi-like cells did not appear to participate in the resolution of damage caused by other viral infections, indicating that this may be a context-specific phenomenon (Scandella et al., 2008). Additional studies will be required to assess whether RORγt+ group 3 ILCs participate in the reconstruction of lymphoid structures after reorganization and damage that occurs during other infections, particularly those that affect the intestine and GALT.
Production of IL-22 by RORγt+ group 3 ILCs has also been shown to limit inflammation in the lung. In a model of ovalbumin (OVA)-induced airway inflammation, RORγt+ ILCs were a predominant source of IL-22 in the inflamed lung (Taube et al., 2011). Il22−/− mice had increased AHR symptoms, and administration of recombinant IL-22 to Il22−/− mice ameliorated AHR, suggesting that ILC-derived IL-22 is anti-inflammatory during allergic airway inflammation (Taube et al., 2011) (Figure 5B). A new study has also described an IL-22-producing NK cell-like population that mediates tracheal epithelial cell regeneration and repair after influenza virus subtype H1N1 infection (Kumar et al., 2012). Together, these reports suggest that ILCs can limit inflammation and promote tissue repair in the lung. To date, the role of group 3 ILCs in tissue repair after intestinal inflammation has not been comprehensively investigated, although one study has shown that IL-22-producing group 3 ILCs were protective in a model of dextran sodium sulfate-induced colitis (Sawa et al., 2011). As IL-22 can be tissue-protective (Sonnenberg et al., 2011a), the capacity of the group 3 ILCs to produce this factor suggests that these cells may contribute to the resolution of inflammation in the intestine.
Summary and Future Perspectives
While recent studies have revealed considerable new information regarding the development, phenotype, and role of ILCs in maintaining homeostasis and during infection, inflammation, and tissue repair, there is still much to be learned. Whether various subpopulations of similar ILCs represent the same cell type in various states of activation in heterogeneous tissue sites remains to be clarified. In addition, many questions still remain regarding the development and functionality of ILC subsets. In particular, the identity of the cell-intrinsic and -extrinsic factors that govern ILC development, the location of ILC differentiation, and the mechanisms that control trafficking of ILC precursors and mature ILCs are not fully understood. In addition, whether the RORγt-, RORα- and GATA3-dependent group 2 ILCs and the RORγt+ group 3 ILCs represent fixed cell fates or there is plasticity in their functional potential is unclear. While there is evidence for functional plasticity in human ILCs (Crellin et al., 2010b; Spits and Di Santo, 2011), whether this is true in mice, and whether this functional plasticity is biologically relevant, remains to be determined. For all ILC subsets, the ability to dynamically regulate cytokine producing capacity and function in response to the local cytokine environment may allow these cells to more effectively participate in diverse inflammatory and immune responses.
Other questions remain in terms of the functions of group 2 and group 3 ILCs in maintaining intestinal homeostasis and during immune responses to pathogens and inflammation. While recent studies have highlighted the role of ILCs in maintaining intestinal homeostasis and barrier function, there is still more to be learned regarding the complex interactions between commensal bacterial communities, ILCs, and the intestinal epithelium. Similarly, very little is known about the signaling pathways that induce effector cytokine production from group 2 and group 3 ILCs during immune responses to pathogens, inflammation, or tissue repair, and further study will be required to assess the mechanisms that regulate the context-dependent activities of ILCs. In addition to cytokine production, genome-wide profiling studies have identified that both group 2 and group 3 ILCs express a wide array of effector molecules, and future studies will be required to interrogate the arsenal of ILC effector functions and their capacity to regulate adaptive immune responses. More broadly, how group 2 and group 3 ILCs participate in immune responses at various tissue sites, including the intestine, against a wide array of pathogens and during various inflammatory states has not been thoroughly investigated. Perhaps most importantly, the phenotype and function of ILCs in the context of human infectious disease and in various inflammatory and autoimmune diseases has not been reported. In addition to more-elaborate analysis of human ILCs, new murine models and genetic approaches will allow further definition of the phenotype, function, and regulation of ILCs. These new insights will hopefully serve to inform the development of new therapeutic approaches to manipulate ILC responses in the context of multiple infectious and inflammatory diseases.
ACKNOWLEDGMENTS
We thank members of the Artis laboratory for discussions and critical reading of the manuscript. Research in the Artis laboratory is supported by the National Institutes of Health (AI061570, AI087990, AI074878, AI083480, AI095466, AI095608, and AI097333 to D.A.), grants T32-AI060516 and F32-AI098365 (to E.D.T.W.), and the Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease Award (to D.A.).
REFERENCES
- Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol. Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, Liu YJ, Howie KJ, Denburg JA, Gauvreau GM, Delespesse G. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J. Allergy Clin. Immunol. 2009;123:472–478. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 2012;129:191–198. e1–e4. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J. Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier M, Sawa S, Eberl G. Notch, Id2, and RORγt sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J. Exp. Med. 2012;209:729–740. doi: 10.1084/jem.20111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J. Exp. Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J. Exp. Med. 2010a;207:281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010b;33:752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Cupedo T. Human lymph node development: An inflammatory interaction. Immunol. Lett. 2011;138:4–6. doi: 10.1016/j.imlet.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+CD127+ natural killer-like cells. Nat. Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke D. Induction of intestinal lymphoid tissue formation by intrinsic and extrinsic signals. Semin. Immunopathol. 2009;31:151–169. doi: 10.1007/s00281-009-0163-6. [DOI] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Frankel G, Phillips AD, Novakova M, Field H, Candy DC, Schauer DB, Douce G, Dougan G. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect. Immun. 1996;64:5315–5325. doi: 10.1128/iai.64.12.5315-5325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, Diefenbach A. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37:171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Geremia A, Arancibia-Cárcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012a;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Halim TYF, Maclaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-Acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012b;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- Hoorweg K, Peters CP, Cornelissen F, Aparicio-Domingo P, Papazian N, Kazemier G, Mjösberg JM, Spits H, Cupedo T. Functional Differences between Human NKp44(−) and NKp44(+) RORC(+) Innate Lymphoid Cells. Front Immunol. 2012;3:72. doi: 10.3389/fimmu.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J. Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J. Exp. Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z, Swaidani S, Yin W, Wang C, Barlow JL, Gulen MF, Bulek K, Do JS, Aronica M, McKenzie AN, et al. Epithelial cell-specific Act1 adaptor mediates interleukin-25-dependent helminth expulsion through expansion of Lin(−)c-Kit(+) innate cell population. Immunity. 2012;36:821–833. doi: 10.1016/j.immuni.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+) CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–654. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- Kim MY, Anderson G, White A, Jenkinson E, Arlt W, Martensson IL, Erlandsson L, Lane PJ. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3− inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J. Immunol. 2005;174:6686–6691. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, Savage PB, McKenzie AN, Smith DE, Rottman JB, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J. Allergy Clin. Immunol. 2012;129:216–227. e1–e6. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, Hoshino T, Fujimoto J, Nakanishi K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.49. Published online June 27, 2012. http://dx.doi.org/10.1038/mi.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, Colonna M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat. Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. USA. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- Mebius RE. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Sarra M, Pallone F, Monteleone G. Th17-related cytokines in inflammatory bowel diseases: friends or foes? Curr. Mol. Med. 2012;12:592–597. doi: 10.2174/156652412800620066. [DOI] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, Tominaga SI, Sugiyama Y. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am. J. Respir. Crit. Care Med. 2001;164:277–281. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. ST2 protein induced by inflammatory stimuli can modulate acute lung inflammation. Biochem. Biophys. Res. Commun. 2002;299:18–24. doi: 10.1016/s0006-291x(02)02578-0. [DOI] [PubMed] [Google Scholar]
- Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, O’Garra A. Blockade of IL-10 Signaling during Bacillus Calmette-Guerin Vaccination Enhances and Sustains Th1, Th17, and Innate Lymphoid IFN-γ and IL-17 Responses and Increases Protection to Mycobacterium tuberculosis Infection. J. Immunol. 2012 doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, Golub R. Notch signaling is necessary for adult, but not fetal, development of RORγt(+) innate lymphoid cells. Nat. Immunol. 2011;12:949–958. doi: 10.1038/ni.2105. [DOI] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010a;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr., Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010b;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N, Dumoutier L, Lesjean-Pottier S, Ribeiro VS, Mandelboim O, Renauld JC, Vosshenrich CA, Di Santo JP. The natural cytotoxicity receptor NKp46 is dispensable for IL-22-mediated innate intestinal immune defense against Citrobacter rodentium. J. Immunol. 2009;183:6579–6587. doi: 10.4049/jimmunol.0901935. [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3−NKp46+ cell subsets from Id2-dependent precursors. J. Exp. Med. 2010;207:273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Bérard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat. Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat. Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Schuligoi R, Sturm E, Luschnig P, Konya V, Philipose S, Sedej M, Waldhoer M, Peskar BA, Heinemann A. CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology. 2010;85:372–382. doi: 10.1159/000313836. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011a;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011b;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube C, Tertilt C, Gyülveszi G, Dehzad N, Kreymborg K, Schneeweiss K, Michel E, Reuter S, Renauld JC, Arnold-Schild D, et al. IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS ONE. 2011;6:e21799. doi: 10.1371/journal.pone.0021799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Tulin EE, Onoda N, Nakata Y, Maeda M, Hasegawa M, Nomura H, Kitamura T. SF20/IL-25, a novel bone marrow stroma-derived growth factor that binds to mouse thymic shared antigen-1 and supports lymphoid cell proliferation. J. Immunol. 2001;167:6338–6347. doi: 10.4049/jimmunol.167.11.6338. [DOI] [PubMed] [Google Scholar]
- van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Hölscher C, et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers DR, Jaensson E, Gaspal F, McConnell FM, Eksteen B, Anderson G, Agace WW, Lane PJ. The survival of memory CD4+ T cells within the gut lamina propria requires OX40 and CD30 signals. J. Immunol. 2009;183:5079–5084. doi: 10.4049/jimmunol.0901514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers DR, Gaspal FM, Mackley EC, Marriott CL, Ross EA, Desanti GE, Roberts NA, White AJ, Flores-Langarica A, McConnell FM, et al. Cutting Edge: Lymphoid Tissue Inducer Cells Maintain Memory CD4 T Cells within Secondary Lymphoid Tissue. J. Immunol. 2012;189:2094–2098. doi: 10.4049/jimmunol.1201639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, Hendriks RW. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur. J. Immunol. 2012;42:1106–1116. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, et al. Transcription factor RORα is critical for nuocyte development. Nat. Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. J. Immunol. 2011;187:5505–5509. doi: 10.4049/jimmunol.1102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K, Muto T, Kawagoe T, Matsumoto M, Sasaki Y, Matsushita K, Taki Y, Futatsugi-Yumikura S, Tsutsui H, Ishii KJ, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc. Natl. Acad. Sci. USA. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J. Exp. Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]