Abstract
Objective:
Previous studies, focusing on the effects of abused drugs, have used mice or rats as the main animal models; the present study tries to introduce a simple animal model. For this propose, we investigated the effects of oral morphine consumption by parents on the development of larvae, pupae and imago in Drosophila Melanogaster (D. Melanogaster).
Materials and Methods:
In this experimental study, twenty male and 20 female D. Melanogaster pupae were housed in test tubes with banana (5 pupae /tube).). Male and female groups each were divided into three experimental group and one control group, which were maintained at 25℃. Morphine (0.2, 0.02, 0.002 mg/ml) was added into the test tubes of the experimental groups. The control group maintained at morphine-free test tube. The male and female groups with the same treatment were coupled and then female fertilization, egg deposit, larval, pupae and imago stages were studied macro and microscopically. The SPSS software (version 9.01) was used for statistical evaluations.
Results:
In the experimental groups, in the larvae stage, both increase and decrease of length and surface area in the pupae stage were observed. The number of larvae pupae, and imago was reduced in the experimental groups.
Conclusion:
The study showed that oral morphine consumption by parents may affect the development of larvae, pupation and imago stages in D. Melanogaster. The results also showed that D. Melanogaster may be a reliable animal model to study on the concerns about abused drugs especially those with opioids.
Keywords: Development, Drosophila Melanogaster, Morphine, Larvae, Pupae
Introduction
Drug addiction and dependence is one of the most important health problems in Iran (1). Existence of addictive people requires us to investigate about drugs in human being body. Numerous studies are being done to comprehend morphine properties as a pain off drug and its tolerance during consumption (2, 3). The advantages and power of Drosophila for leading to compound generation and target identification include not only increased rate of discovery and reduced costs, but being able to follow a system based approach.
On the other hand, preceding researches have always different problems such as financial issues (2, 4) so; one of the reasons of this model usage in the view of bioresearches is that it is cheap and easy to hand. So far, several studies have mainly been done on rat or mouse animal models and rarely with other animals like monkey, cat, dog, rabbit and hamster (5-7 ).
Regarding to various reasons like more proliferation and low price, scientists have focused on insect models. In the present research, we study the effect of oral morphine consumption on the developmentDrosophila Melanogaster (D. Melanogaster).
Drosophila or fruit fly is very valuable in terms of four pairs of chromosomes for genetic researchers and because of high fertility and short-term reproductive and fetal period has been remarkable for embryology and biology. Importantly, a single female fly can lay hundreds of eggs within a few days (8, 9). Also D. Melanogaster has complete metamorphosis. The effect of morphine has been studied on metamorphosis in some flies (8, 10). Researchers have demonstrated that different concentrations of codeine, norcodeine and morphine consumption could alter the larvae, pupae and imago development in Lucilia. The effect of Sericata and morphine on the development acceleration in larvae stage is more than in pupae and imago stages. Totally, development time from egg to imago stage is 21 hours, while time reduction in larvae stage is 29 hours were shown (8, 11). In this regard, however, Kennap and Kramersi revealed that morphine administration does not have teratogenic effect on D. Melanogaster limbs (12).
Larvae stage contains three instar. Larvae have mobility and nourish from first to third instar and grow more. So in the larval stage are more vulnerable than in later stages to teratogen factors. Some substances such as insecticides and alkaloid containing compounds produced by some plants caused disruption in feeding activity and larval mobility (8, 11). After third instar, larval make the cocoon around itself that Indicating the arrival of larvae to pupation stage and was seen surprisingly changes in this stage (13).
After 2 days at this stage, the larva crawls out of the food substrate and develops into an immobile pupa. This stage can identify the insect's sex. During the next 5-7 days, the pupa undergoes a dramatic physical transformation into the adult form when it emerges from the pupal case (eclosion). The adult female is able to mate about 12 hours after eclosion and can lay egg 24 hours after fertilization (8, 11). Due to the importance of finding new animal models for studying morphine effects, this study evaluates effect of different concentrations of oral morphine on the larvae, pupae and imago development in D. Melanogaster. Also breeding powers were studied by the same concentration of oral morphine.
Materials and Methods
Drug and administration method
In this experimental study, morphine sulphate purchased from Iran TEMAD Co, was used orally.
Two morphine administration methods were used. The first method was administrated orally and the absorption was performed via digestive system. The second was as a gas form and the absorption was performed via spiracle. The second method, due to lack of oxygen and hypoxia in the laboratory tube contained of morphine powder, can cause some problems like stress and side effects that may interfere with morphine effects. So in this research, morphine was used orally.
In this study, virgin female fruit fly achievement was very important. The female fruit flies due to the existence of spermatheca can conserve sperms in their body for long time, so it is not distinguishable which female fly has been mated by which male fly. Thus, pupae of D. Melanogaster in tube were separated with needle and 50 pupae were transferred into ach tube. After sex determination, 20 male and 20 female flies were located in the tubes containing 1ml water, separately.
The emerged flies from pupae can bear foodless for a couple of time but are very sensitive to lack of water. Because of sensitivity, paupa were grown in tubes containing water, and for confidence about morphine effect, the samples were deprived of feeding. The flies were divided to four groups (3 experimental groups and one control group). Three experimental groups of female and male were fed with three doses of morphine (0.2, 0.02 and 0.002 mg/ml) separately. After mating and egg lying, the larvae, pupae and imago stages were studied in the experimental groups, microscopically and macroscopically. Also number of second progeny was counted in each group. In this study, All experiments were conducted in accordance with standard ethical guidelines approved by the local Ethics Committee [The Baqiyatallah (a.s.) University of Medi- cal Sciences Committee on the Use and Care of Animals, 80/4120, Sep 21, 2000].
Statistical analysis
The results were expressed as the mean ± SEM. Comparison between different groups was carried out by one-way analysis of variance test (ANOVA) followed by TUKEY test. The statistical significance was accepted at a level of p<0.05%.
Results
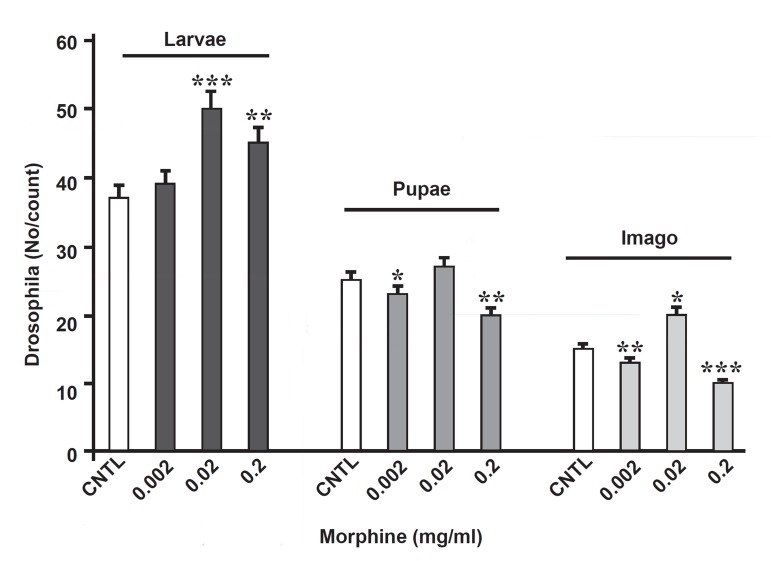
The obtained data indicated that the number of larvae in the experimental groups was more than in the control group (Fig 1). A significant increase was shown in the experimental groups with 0.02 and 0.002 mg/ml doses of morphine.It is interesting the number of pupae and adult flies had decreased in the experimental group comparing to the control group, but surprisingly, the number of pupae and adult flies had increased with 0.02 mg of morphine, indicating the morphine's dose-dependent effect in these flies, probably (Fig 1).
Fig 1.
Comparison of the effect of oral morphine on the number of D.Melanogaster in larvae, pupae and adult stages in the experimental and control groups. The number of larvae in the treated-groups with 0.2 and 0.02 mg/ml doses of morphine had increased, whereas significant decrement was observed in number of the treated pupae with 0.2 and 0.002 mg/ml doses of drug. *p<0.05%, **p<0.01%, ***p<0.001%.
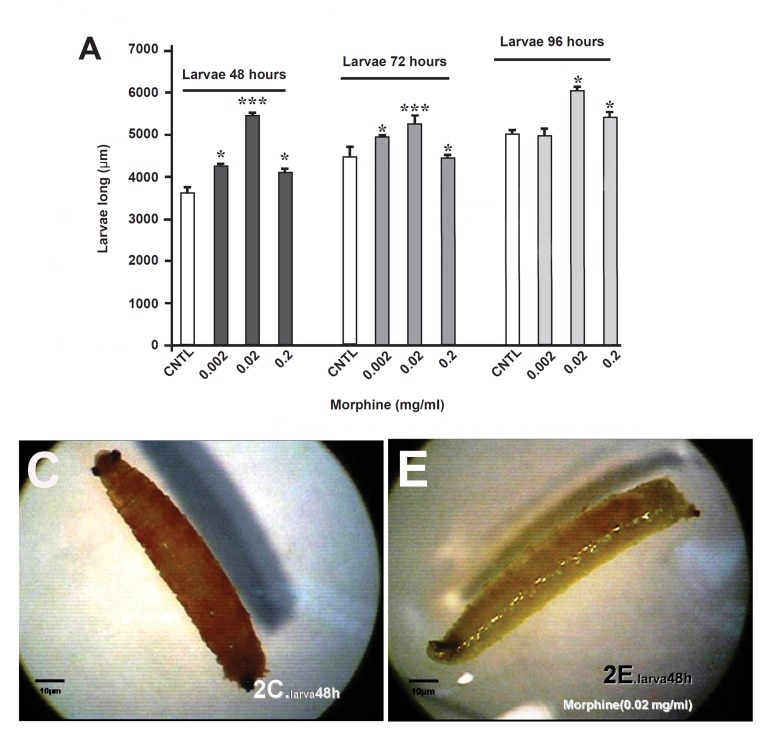
The measurements indicated, that morphine administration resulted in larvae length increment in all the three experimental groups in comparison to the controls (p<0.01%, Fig 2).
Fig 2.
Comparison of the effect of oral morphine on D. Melanogaster's length in the larva stage. Length increment was observable in all of the experimental (E) group to the control (C) group.
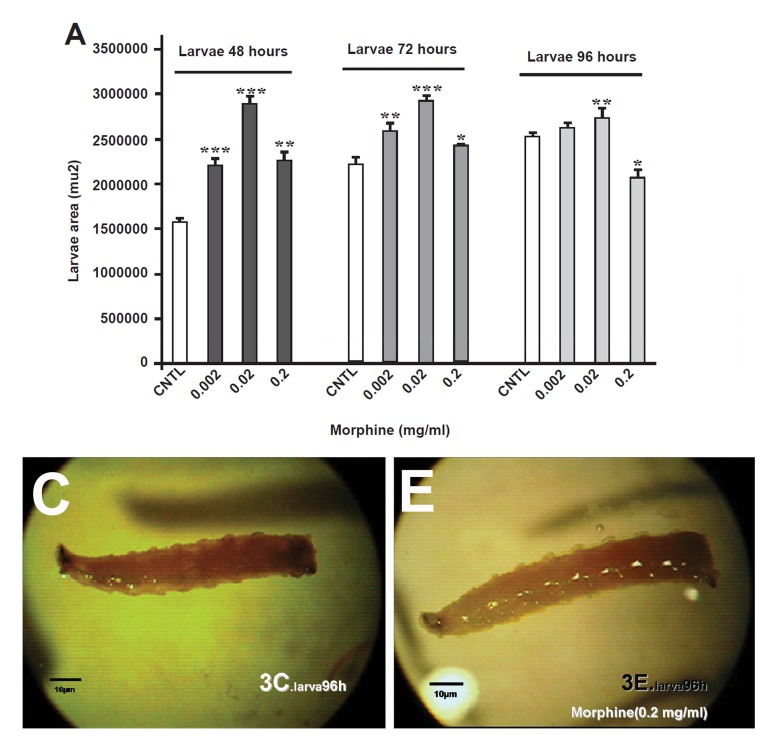
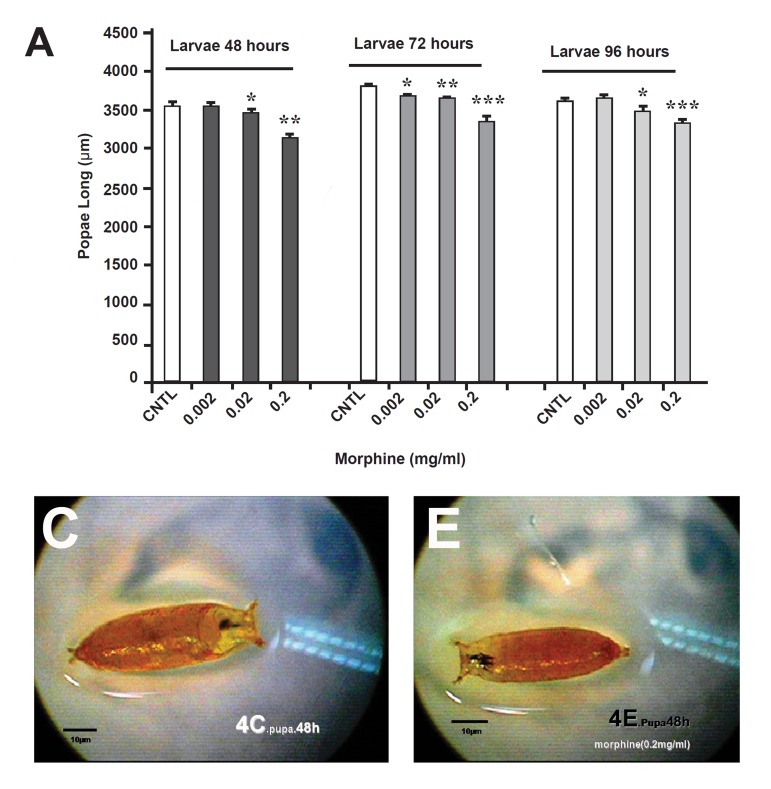
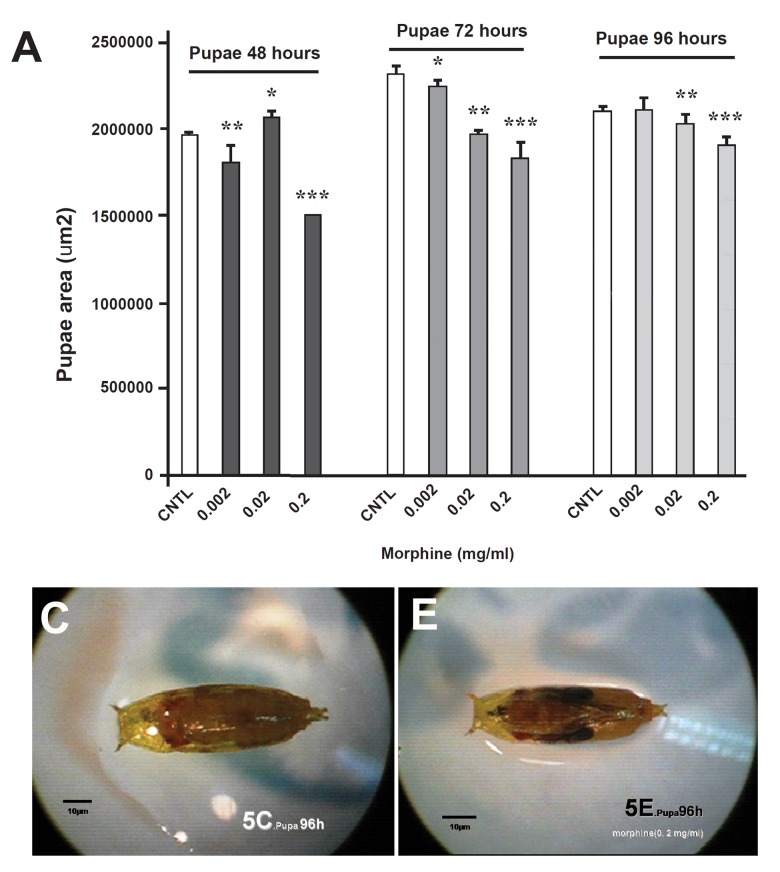
This increment was detectable in 0.02 mg morphine. The surface measurement in larvae also confirmed this increment in the morphine-treated groups for 48 and 72 hours , but surprisingly a sever decrement was observed in 0.002 mg morphine for 96 hours, as compared with the control group (p<0.01%, Fig 3). Both surface area and lengths in the morphine-treated pupae's were decreased, and in dose of 0.002 mg morphine was statistically sig- nificant (p<0.01%, Fig 4, Fig 5).
Fig 3.
Comparison of the effect of oral morphine on D.Melanogaster's area in the larva stage. Surface increment was observable in all of the experimental (E) group to the control (C) group.
Fig 4.
Comparison of the effect of oral morphine on D.Melanogaster's length in the pupa stage. Significant decrement was observed in all of the experimental (E) groups to the control (C)groups.
Fig 5.
Comparison of the effect of oral morphine on D. Melanogaster's surface in the pupa stage. Surface decrement was observable in all of the experimental (E) groups to the controls (C) groups.
Discussion
Previous reports have shown no specific effects for morphine on the mutagenic changes induction in D. Melanogaster (12). Rather, they found opioid binding sites in the D. Melanogaster's nervous system (13) However, it is indicated that some insecticidal alkaloids have similar structure with opioids (14), which may explain morphine effectiveness on this fly. Our data showed that fruit fly development was affected by morphine administration and this effect was dose-dependent. So, one can concluded that the flies development may affected by morphine by stimulation of opioid receptors (15, 16).
In this research, different stages of D. Melanogaster development were assessed. Previous studies had demonstrated that the effectiveness of materials on the life cycle of flies resulted in change in these stages (15-20 ). Our results showed that the numbers of eggs in high morphine concentrations (0.2 and 0.02 mg) was increased in accordance with the control group. These data support that morphine has positive effect on the ability of lying eggs. However, previous researches had shown that morphine can reduce reproductive ability in balb/c mice as well as in the other mammals (18, 21). These data suggest that the effect of morphine in insects may differ from mammalians (22, 24).
On the other hand, in experimental groups (0.2and 0.02 mg/ml), the larvae size was greater than control group. It is to be noted that the larva stage is of great important in the development of D. Melanogaster(17). Microscopic studies in larva stage also showed that the length and surface area in these groups have increased in comparison to the control group, indicating the effect of morphine on the larva development. The results of present research are consistent with the previous data suggesting the effect of morphine on cell proliferation in the early stages of embryonic growth (18). This issue confirms the existence of morphine - dependent mechanisms in flies' development and is consistent with the results of past data. It also indicates that morphine effect appears to be due to provoking more cell proliferation in the larval stage. On the other hand, the teratogens may by more dangerous in this stage (10, 12). In the present research, developments of larval stages were assessed in 48 hours (first instar), 72 hours (second instar) and 96 hours (third instar) with different concentrations of morphine. Our results demonstrated significant differences in time and dose-dependent morphine effectiveness. Likewise, a significant difference was observed in codeine-treated larval weight in previous research (10). Since, the sites of opioids action in D. Melanogaster have been identified (13) it appears that one of the actions of these areas is likely to influence the evolution of fruit flies' larvae. Our results also showed that in the pupa stage, not only the number of pupae is decreased by increment of morphine concentrations, but also larvae (0.2 and 0.02 mg/ml) ,in spite of their larger body size, reached to the third instar later than the control group. Also microscopic studies showed that the surface area and length in the pupa stage were lower than in the control group. The highest decrement was seen in the 48 hours pupa with 0.2 mg/ml morphine. This result shows that morphine has positive effect in the larval stage, but it has negative effect in the pupa stage. Thereby, larval development and entrance to the pupa stage occurred with some delay in the experimental groups as compared with the controls. Also a significant decrement was observed in the congestion of adult flies population in the experimental groups. Probably, morphine delays pupa metamorphosis development and causes pupa immaturities. Also increase in mortality rate can be one of the reasons of decrement in the adult flies' population congestion.
Conclusion
Morphine showed destructive effect on fruit flies developmental trace. However, unfortunately, in this study morphine effects were not assessed in cellular and molecular levels. Also morphine effects on transformation in next progeny of D. Melanogaster were not analyzed. The results of this paper showed that D. Melanogaster may be served as an interchangeable animal model to study morphine ,opioid drugs and teratogenic substances effects.
Acknowledgments
We would like to thank Dr. Hashemi Madani and Mrs. Ramezani. The fund of this project was provided by the Neuroscience Research Center, Baqyiatallah (a.s.) University of Medical Sciences. There is no conflict of interest in this article.
References
- 1.Kazemi M, Sahraei H, Azarnia M, Bahadoran H, Salehy M. Oral morphine consumption delayed choroid plexus and ventricle 4th development in fourteen Wistar rats embryos. Gom University of Medical Sciences Journal. 2010;4:3–90. [Google Scholar]
- 2.Bickel WK, DeGrandpre RJ, Hughes JR, Higgins ST. Behavioral economics of drug self-administration. II. A unitprice analysis of cigarette smoking . J Exp Anal Behav. 1991;55(2):145–154. doi: 10.1901/jeab.1991.55-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bickel WK, Madden GJ, Petry NM. The price of change: The behavioral economics of drug dependence. Behav Ther. 1998;29(4):545–565. [Google Scholar]
- 4.Hursh SR. Economic concepts for the analysis of behavior. J Exp Anal Behav. 1980;34(2):219–238. doi: 10.1901/jeab.1980.34-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McSweeney FK, Swindell S. Behavioral economics and within-session changes in responding. J Exp Anal Behav. 1999;72(3):355–371. doi: 10.1901/jeab.1999.72-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hursh SR, Galuska CM, Winger G, Woods JH. The economics of drug abuse: a quantitative assessment of drug demand. Mol Interv. 2005;5(1):20–28. doi: 10.1124/mi.5.1.6. [DOI] [PubMed] [Google Scholar]
- 7.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade . Addict Biol. 2007;12(3-4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 8.Nichols CD. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther. 2006;112(3):677–700. doi: 10.1016/j.pharmthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Riddiford LM. Broad specifies pupal development and mediates the 'status quo' action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development. 2002;129(9):2259–2269. doi: 10.1242/dev.129.9.2259. [DOI] [PubMed] [Google Scholar]
- 10.Kharbouche H, Augsburger M, Cherix D, Sporkert F, Giroud C, Wyss C, et al. Codeine accumulation and elimination in larvae, pupae, and imago of the blowfly Lucilia sericata and effects on its development. Int J Legal Med. 2008;122(3):205–211. doi: 10.1007/s00414-007-0217-z. [DOI] [PubMed] [Google Scholar]
- 11.Schachtner J, Trosowski B, DHanis W, Stubner S, Homberg U. Development and steroid regulation of RF amide immunoreactivity in antennal-lobe neurons of the sphinx moth Manduca sexta. J Exp Biol. 2004;207(14):2389–2400. doi: 10.1242/jeb.01036. [DOI] [PubMed] [Google Scholar]
- 12.Knaap AG, Kramers PG. Absence of mutagenic effects of morphine in Drosophila. Mutat Res. 1976;40(2):97–100. doi: 10.1016/0165-1218(76)90003-3. [DOI] [PubMed] [Google Scholar]
- 13.Santoro C, Hall LM, Zukin RS. Characterization of two classes of opioid binding sites in Drosophila melanogaster head membranes . J Neurochem. 1990;54(1):164–170. doi: 10.1111/j.1471-4159.1990.tb13297.x. [DOI] [PubMed] [Google Scholar]
- 14.Miyazawa M, Yoshio K, Ishikawaand Y, Kameoka H. Insecticidal alkaloids against Drosophila melanogaster from nuphar japonicum DC. J Agric Food Chem. 1998;46(3):1059–1063. [Google Scholar]
- 15.Frankfort BJ, Pepple KL, Mamlouk M, Rose MF, Mardon G. Senseless is required for pupal retinal development in Drosophila. Genesis. 2004;38(4):182–194. doi: 10.1002/gene.20018. [DOI] [PubMed] [Google Scholar]
- 16.Goff ML, Brown WA, Omori AI. Preliminary observations of the effect of methamphetamine in decomposing tissues on the development of Parasarcophaga ruficornis (Diptera: Sarcophagidae) and implications of this effect on the estimations of postmortem intervals . J Forensic Sci. 1992;37(3):867–872. [PubMed] [Google Scholar]
- 17.Kintz P, Godelar B, Tracqui A, Mangin P, Lugnier AA, Chaumont AJ. Fly larvae: a new toxicological method of investigation in forensic medicine. J Forensic Sci. 1990;35(1):204–207. [PubMed] [Google Scholar]
- 18.Goff ML, Brown WA, Hewadikaram KA, Omori AL. Effect of heroin in decomposing tissues on the development rate of Boettcherisca peregrina (Diptera, Sarcophagidae) and implications of this effect on estimation of postmortem intervals using arthropod development patterns. J Forensic Sci. 1991;36(2):537–542. [PubMed] [Google Scholar]
- 19.Bourel B, Hédouin V, Martin-Bouyer L, Bécart A, Tournel G, Deveaux M, et al. Effects of morphine in decomposing bodies on the development of Lucilia sericata (Diptera: Calliphoridae) J Forensic Sci. 1999;44(2):354–358. [PubMed] [Google Scholar]
- 20.O'Brien C, Turner B. Impact of paracetamol on Calliphora vicina larval development. Int J Legal Med. 2004;118(4):188–189. doi: 10.1007/s00414-004-0440-9. [DOI] [PubMed] [Google Scholar]
- 21.Sahraei H, Kaka GH, Ghoshooni H, Shams Lahijani M, Ramazani M. Effect of oral morphine administration on fertility of blab/c mice. J Reprod Infertil. 2002;3(3):4–10. [Google Scholar]
- 22.Kazemi M, Sahraei H, Azarnia M, Dehghani L, Bahadoran H. Effect of oral morphine consumption in female rats on development of brain cavities, central canal and choroid plexus of their embryos. Cell Journal (Yakhteh) 2011;12(4):489–494. [Google Scholar]
- 23.Kazemi M, Sahraei H, Azarnia M, Dehghani L, Bahadoran H, Tekieh E. The effect of morphine consump- tion on plasma corticosteron concentration and placenta development in pregnant rats. Iran J Reprod Med. 2011;9(2):71–76. [PMC free article] [PubMed] [Google Scholar]
- 24.Glasel JA. The effects of morphine on cell proliferation. Prog Drug Res. 2000;55:33–80. doi: 10.1007/978-3-0348-8385-6_2. [DOI] [PubMed] [Google Scholar]