Abstract
Objective: The role of staphylococcal enterotoxin B (SEB) in food poisoning is well known, however its role in other diseases remains to be explored. The aim of this study is the molecular screening and characterization of the SEB gene in clinically isolated strains. Materials and Methods: In this experimentally study, 300 Staphylococcus aureus (S. aureus) strains isolated from clinical samples were assayed. The isolated strains were confirmed by conventional bacteriological methods. Polymerase chain reaction (PCR) was used to determine the enterotoxin B (ent B) gene. Assessment of toxin production in all strains that contained the ent B gene was then performed. Finally, using specific antibody against SEB, a Western-blot was applied to confirm detection of enterotoxin B production. Results: Results indicated that only 5% of the 300 clinically isolated S. aureus contained the ent B gene. All strains which contained the ent B gene produced a proteinous enterotoxin B. The results of sequence determination of the PCR product were compared with the gene bank database and 98% similarity was achieved. The results of the Western-blot confirmed that enterotoxin B was produced in strains that contained the ent B gene. Conclusion: The results of this study indicate that 5% of clinically isolated S. aureus strains produce enterotoxin B. Considering that the enterotoxin B is an important superantigen, it is possible that a delay in diagnosis and lack of early proper treatment can cause an incidence of late complications, particularly in staphylococcal chronic infections. For this reason, it is suggested that in addition to detecting bacteria, an enterotoxin B detection test should be performed to control its toxigenicity.
Keywords: Screening, Staphylococcus aureus, Enterotoxin B, PCR, Western-Blot
Introduction
Characteristics of Staphylococcus aureus (S.aureus) enterotoxin B (SEB) are well known (1). SEB is the most studied member of the staphylococcal enterotoxins produced by S.aureus and is responsible for staphylococcal food-poisoning. However, scientific data reveals some variable information. For example, different molecular weights for SEB have been reported (23-29 kDa and 31.4 kDa) (2). All of these reported proteins are heat stable, resistant to proteolytic digestion, and their pH range for tolerance is 4 to 10 (3, 4). SEB is a pyrogenic agent able to incapacitate human beings for up to two weeks with as low as a 0.5 µg/kg dose. Itsinherent stability, high morbidity rate, high intoxication effect, and ease of environmental dissemination were considered as important factors for its toxicity (5, 6). SEB also induces potent cytotoxic activity by intraepithelial lymphocytes (7). SEB has immunomodulatory effects in allergic airway disease and it facilitates sensitization to ovalbumin and consecutive bronchial inflammation with features of allergic asthma (8). SEB exerts direct pro-inflammatory and other pathological effects on human nasal epithelial cells, with induction of chemokine and growth factor release, resulting in the migration and prolonged survival of granulocytes in vitro (9,10). However, SEB acts as a superantigen and exhibits a dramatic proinflammatory impact on epithelial cells, which can be inhibited by the addition of glucocorticoids (11). SEB plays an important role in the treatment of certain cancers and autoimmune diseases (12). It is used as target molecule to find affinity ligands from a solid phase combinatorial peptide library, and then use of such ligands to detect, remove, and purify it by affinity chromatography. The derived peptide ligands can help us understand the interactions of SEB as a superantigen with a major histocompatibility complex class II molecule and T cell receptor (13, 14). Since SEB is an important agent of human disease, access to the methods of controlling diseases caused by SEB are important. Considering the above mentioned reasons, it is necessary to know the frequency of SEB producing strains. Our knowledge about the characteristics of molecular composition, biological properties, and genetics bases is low. The standard strains of the SEB producer S.aureus (for example ATCC 14448) or even a slight amount of this toxin (500µg) are currently not available in Iran and buying them is impossible from international vendors. Therefore, in order to carry out diagnostic techniques, it is necessarry to screen SEB producer strains and determine whether or not native strains that contain the gene of this toxin could be identified and demonstrate their characteristics. This would set up our knowledge base and impart the necessary skills in this important research subject and cater to the community's needs. The precise role of staphylococcal enterotoxins that cause delayed symptoms in different parts of the body is unknown. Thus, identification of enterotoxin producing strains isolated from areas other than the gastrointestinal tract is essential. The aim of this research is the molecular screening of gene encoded SEB in domestic strains and the confirmation of gene expression and enterotoxin B production.
Materials and Methods
Collection of bacterial strains
From August 2008 to July 2010 a total 300 strains of S.aureus were isolated from clinical samplesand identified by bacteriological methods (gram stain, coagulase, mannitol fermentation and DNase tests). The isolates were stored on suitable maintenance media in our laboratory. Nontoxigenic strains (ATCC 6538 and PTCC 1435) were used as negative controls. The total protein of coagulase positive strains were measured by the Bradford method.
DNA extraction
DNA was extracted by the modified salting out method (15) as below: 1 ml of the overnight bacterial culture was centrifuged at 3000 × g for 5 minutes at room temperature. The supernatant was then discarded. Then, 500 µl STE buffer (Tris-HCl 10mM, NaCl 10 mM, EDTA 1 mM, pH =7.5) was added to the sediment and suspended by pipetting. After 10 minutes, 200 µl 2% SDS followed by 300 µl of 3M Natrium acetate were added and the tube was gently inverted 10 times. The tube was centrifuged at 5000 × g for 5 minutes and the supernatant was transferred to a new microfuge tube, which was then placed on ice. Cold isopropanol (1 ml) was added and mixed with the contents of the tube by gentle inversion. The tube was then placed in a −20°C freezer for 1 hour or more afterwhich the tube was centrifuged at 15000 × g for 5 minutes at 4°C. The supernatant was removed and 70% ethanol was added to the pellet. After several minutes, the tube was centrifuged for 2 minutes and the ethanol removed. The DNA pellet was allowed to dry for 5-10 minutes. The damp pellet was dissolved in 100 µl of TE buffer (pH= 8.0) and used as a template. DNA concentrations were measured at all stages using Nano-Drap (Thermo Scientific NanoDrop 2000 Spectophotometer USA).
Primer design
The primer was designed on the basis of the reference sequence (S.aureus ATCC 14458 enterotoxin B gene, accession number AY518386.1) and analyzed by primer3 software. Multiple alignment was carried out by DNASIS MAX trial version.
DNA amplification and sequence analysis
For DNA amplification, the mastermix was made in 200 µl microtubes by using a 25 µl reaction mixture that contained the 1 µl DNA template, 2 U of Taq DNA polymerase, 5 µl of 10X PCR buffer II that contained 2 mM of each dNTPs, 2 mM MgCl2 (all reagents were from Fermantase), 10 µM of the primer pair (synthesized by Cinnagen Co., I.R. Iran) and double-distilled water, to a final volumeof 25 µl. All amplifications were carried out in a thermal cycler (Bio-Rad, C1000) with initial denaturation at 94°C for 5 minutes followed by 32 cycles of denaturation at 94°C for 1 minute, primer annealing at 56°C for 1 minute and extension at 68°C for 1 minute, followed by a final extension at 68°C for 7 minutes. The amplified PCR products were electrophoresed in a 1.5% agarose gel, then stained with etidium bromide (0.05 mg/ml; Sigma Aldrich). The gels were photographed under ultraviolet light using Gel Doc (Bio Rad Universal Hood II, USA). Molecular size markers (50 bp) were included in each agarose gel. The PCR product was purified and sent to the Sequencing Laboratory (Hope Generation Medical Foundation, Iran) to determine the sequence.
Western-blotting for confirmation of Staphylococcal enterotoxin B
In this method, SEB antibody (Abcam, Ab 15898, 500; lot 693317) was applied. The immunoblotting method was performed briefly (16) as follows: culture supernatants were concentrated by a centrifugal filter device (Amicom ultracel 10, 30, 50, and 100 kda). Protein samples were separated by SDS-PAGE and then electrophoretically transferred in a semidry transfer apparatus (Trans-Blot SD Electrophoretic Transfer Cell cat. no. 170-3940) to the PVDF membrane. The transferred proteins were bound to the surface of the membrane, which provided access for reaction with immunodetection reagents. All remaining binding sites were blocked by immersing the membrane in a solution that contained 3% bovine serum albumin (BSA) for an hour. The membrane was washed three times with phosphate buffer saline (PBS): 0.23 g NaH2PO41.15 g Na2HPO4, 9.00 g NaCl, to which H2O was added to reach 1000 ml and adjusted to pH=7.2. After washing, the membrane was immersed in a primary antibody solution (1 to 15,000 diluted in BSA 0.5%) overnight. The membrane was then washed three times and the antibody-antigen complex was identified with horseradish peroxidase (HRPO) coupled to the secondary anti-IgG antibody (e.g., rabbit IgG1 was diluted up to 10,000 fold in 0.5% BSA). Chromogenic substrates were then used to visualize the specific bindings.
Results
The results of identified strains showed that 28 strains (~ 9.3%) were coagulase negative and 272 strains (~ 90.7%) were coagulase positive. The 300 S.aureus strains were derived from blood (63 strains), CSF (51 strains), synovial fluid (9 strains),throat swabs (63 strains), wounds (81 strains), stool (22 strains) and the genital tract (11 strains).
The results of DNA extraction by the salting out method represented relative purity and concentration. Results of samples analyzed by the Nano-Drap method revealed an absorption ratio of 260/280 nm, which was above 1.8 and represented the desired purity of extracted DNA.
Agarose gel electrophoresis of DNA extracted in this method showed favorable and sharp bands. As shown in figure 1, the results of different primers designing were as; F1-TGTATGTATGGTGGTGTAAC and R1- ATAGTGACGAGTTAGGTA; and F2-GAGAGTCAACCAGATCCTAAACCAGA and R2-TCACTTTTTCTTTGTCGTAAG which were amplified at 165bp and 720bp fragments as PCR products, respectively. In Fig 1, the result of 1.5% and 1% agarose gel electrophoresis of PCR products from primer 1 and 2 are shown in parts Aand B, respectively.
Fig 1.
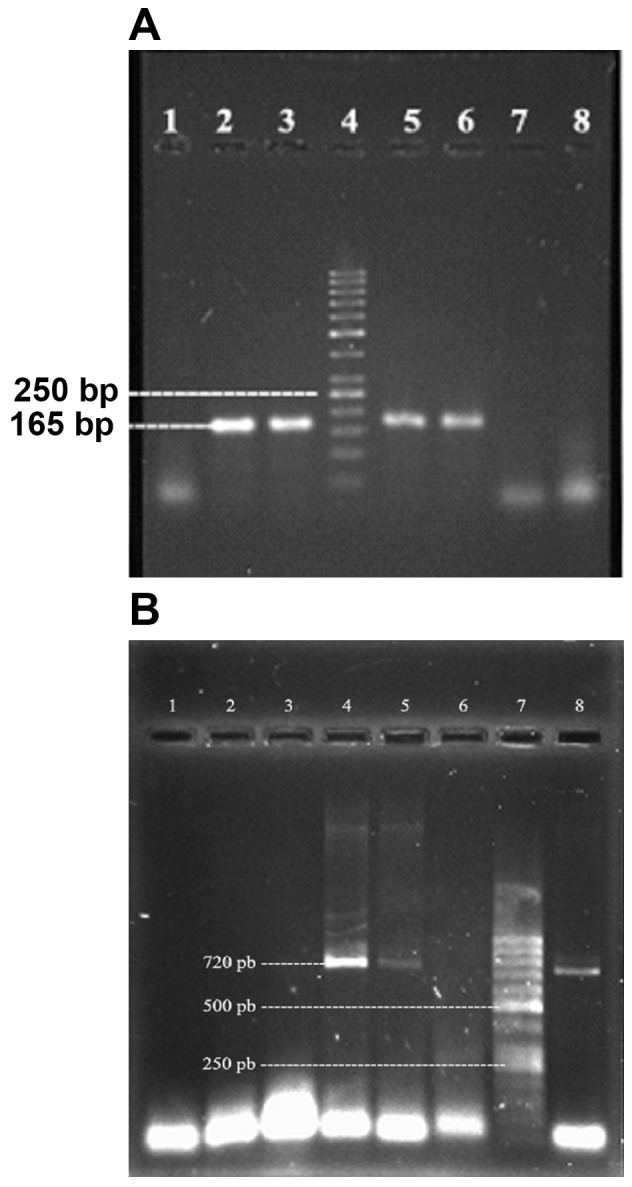
A. lanes 2, 3, 5 and 6 are 165 bp PCR product, lane 4 is a 50 bp MW standard and lanes 1, 7 and 8 are negative controls. B. lane 7 is a 50 bp MW standard, lanes 4, 5 and 8 are 720 bp and lanes 1, 2, 3 and 6 are negative controls
The results indicated that the PCR method was optimized for the detection of the SEB gene. All 300 isolated strains were studied, and the primer pairs which were amplified for the 165 bp fragment as well as the primer pairs which were amplified for the 720 bp fragment were only detected in 15 out of the 300 strains of the S.aureus SEB Gene. The distribution of 15 S.aureus strains which carried the SEB gene were isolated from blood (1 strain), synovial fluid (3 strains), throat swabs (4 strains), wounds (3 strains), and stool (4 strains).
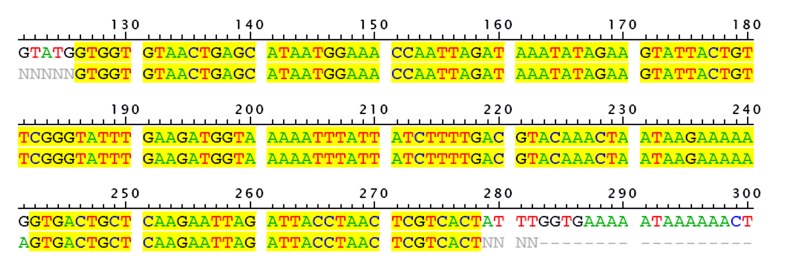
These strains were maintained on culture media that contained 15% glycerol in a −80°C freezer. DNA sequencing of the amplicon 165 bp fragment SEB gene as a PCR product was carried out by the Iranian company sequencer (Hope Generation Medical Foundation, Iran). The result of the multiple alignment of the reference gene (S.aureus strain ATCC 14458 enterotoxin B gene, accession number AY518386.1) with sequenced PCR product is shown in Figure 2. In order to prevent contamination, each of the samples were analysed separately.
Fig 2.
The 165 bp fragment amplified (purified PCR product) in this study was sequenced and aligned with the reference SEB gene ATCC 14458 (accession number AY518386.1). The first row sequence is related to the reference gene and the second row is the PCR product sequence derived from this research.
The result of total protein measurment in the 24 hour culture supernatant of all strains that contained the SEB gene were 735 µg/ml to 7.9 mg/ ml. The result of using the centrifugal filter device (Amicom ultracel 10, 30, 50 and 100 kda) revealed that the supernatants were 10 times more concentrated than initial.
SDS PAGE electophoresis of 15 µl of concentrated culture supernatant from each of the samples revealed a protein band in the area marked 28 kda and 56 kda (Fig 3A). In order to perform the Western-blotting test, an alternative SDS PAGE electrophoresis was carried out and the unstained gel (protein band) was electrophoretically transfered to PVDF (Millipore Immobilon P) paper. After that, Western-blots with specific antibody against SEB (Abcam, Ab 15898, 500; lot 693317) were carried out. The result is shown in Figure 3.
Fig 3.
A. Lanes 2,3,7, and 9 SDS PAGE of the concentrated extracts of supernatant from four samples. B. Lanes 2,3,7 and 8 show Western-blotting of the same samples.
As shown in Figure 3A, ultrafiltration (centrifugal solution over 10 kda and below 30 kda filter device) product electophoresis has made a weak 28 kda band and a strong 56 kda band. The presence of SEB in both areas was approved by Western- blot (Fig 3B).
Discussion
S.aureus is a major human pathogen and several health problems caused by this pathogen have been reported worldwide. Thus, different aspects of its pathogenicity and invasiveness have been investigated by researchers. Studies have been done on antibiotic resistance (17), infection control (18), diagnostic aspects, and pathogenicity (19). However, the role of staphylococcal enterotoxins have not yet received the adequate attention of investigators and still require more research.
In this study, both the presence of the enterotoxin B gene of S.aureus in clinical specimens and also its ability for toxin production was examined. The results revealed that out of 300 S.aureus strains isolated from clinical samples, only 15 strains (5%) contained the ent B gene and had the ability to produce related enterotoxin in vitro. This finding is based on the PCR and Western-blot methods used in this study.
Previous studies have reported variation in the prevalence of S.sureus production of enterotoxin B. For example, the SEB producer among S.aureus isolated from basket handlers was 12%, nasal swabs of supermarket workers 2%, and clinical specimens 4% (20). This study revealed that 5% of the strains carried the ent B gene and were able to produce enterotoxin B. However, the pathogenesis of this toxin in organs other than the gastrointestinal tract remains unclear.
In addition, the characteristics listed for the SEB are increasing importance. For example, SEB is a powerful superantigen and may have synergistic effects with gram-negative bacterial endotoxins(21). In addition, SEB is a potent mitogen that elicits life threatening and T- cell proliferation, and cytokine production (22). Hence, a diagnostic microbiology laboratory should be equipped to detect enterotoxigenicity in addition to bacterial identification. In this way, new methods can be designed to treat infections better, prevent undue use of antibiotics, and prevent the emergence of antibiotic resistance. S.aureus is a major pathogen responsible for both nosocomial and community acquired infections ranging from localized skin infections to severe systemic diseases such as septicemia (23).
Recent genetic advances have established several methods which have enabled identification of many pathogenic factors and characterization of clinical isolates (24). However, detection and amplification reaction tests are still improving and fu- ture developments coupled with other techniques might provide more broadly usable tools.
Symptoms of toxic shock syndrome (TSS) were documented for the first time by the famous Greek physician Hippocrates (c. 460-377 B.C.). From that time onward, S.aureus has remained a major health problem. This indicates two important facts: one is the diversity of pathogenic factors related to S.aureus and the other the lack of properly designed methods for diagnosis and effective protection. Thus, there is need for extensive studies on the staphylococcal toxin and other pathogenic factors related to it.
In this study, we found multiple alignments of the amplified fragment (165 bp) with reference S.aureus strain ATCC 14458 enterotoxin B gene (GeneBank: AY518386.1) which were highly matached (max score 98%). It began at the 126 nucleotide position in the reference S.aureus strain ATCC 14458 enterotoxin B gene. As shown in Fig 2, there is only one nucleotide difference in position 141, code AGT for amino acid serine instead of code GGT for amino acid glycine. Blast homology analysis of the 165 bp fragment showed 98% similarity to all SEB sequences in the gene bank data base. Despite the fact that the enterotoxin B gene in native strains has a one amino acid difference with the reference strain, it reacted well with the specific antibody (Abcam, Ab 15898, 500; lot 693317).
Conclusion
The results in this study conclude that the presence of the SEB gene among clinical isolates is 5%, which is noticeable. In addition, all strains containing SEB gene have produced detectable amounts of enterotoxin B in cultured media. Thus,it is important to note that in the clinical cases with transient staphylococcal infection, patients may have a small amount of enterotoxin B secreted in their body. This can lead to serious consequences such as the enhancement of the lethality of the endotoxin, which can promote fatal diseases such as TSS. These complications caused by enterotoxin B can be prevented by comprehensive and sensitive detection techniques combined with the conventional identification of S.aureus used routinely in microbiology laboratories.
Acknowledgments
The authors would like to thank the Head of Baqiyatallah Research Institute, Dr Mostafa Ghanei for financial support of this research (Grant no:88-554), Dr. Mohsen Reza Haidari for editing the manuscript, and Dr. Mehdi Kamali for technical assistance. The authors have no conflict of interest.
References
- 1.Harteveld JL, Nieuwenhuizen MS, Wils ER. Detection of Staphylococcal enterotoxin B employing a piezoelectric crystal immunosensor. Biosens Bioelectron. 1997;12(7):661–667. doi: 10.1016/s0956-5663(97)00024-9. [DOI] [PubMed] [Google Scholar]
- 2.Jones CL, Khan SA. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panneerseelan L, Muriana PM. An immunomagnetic PCR signal amplification assay for sensitive detection of Staphylococcus aureus enterotoxins in foods. J Food Prot. 2009;72(12):2538–2546. doi: 10.4315/0362-028x-72.12.2538. [DOI] [PubMed] [Google Scholar]
- 4.Chen CP, Wang NL, Tai FH, Chung JS. Studies on staphylococcal enterotoxin B. III. Purification with absorbents. Zhonghua Min Guo Wei Sheng Wu Xue Za Zhi. 1979;12(2):65–69. [PubMed] [Google Scholar]
- 5.Giezendanner N, Meyer B, Gort M, Muller P, Zweifel C. Raw milk-associated Staphylococcus aureus intoxication in children. Schweiz Arch Tierheilkd. 2009;151(7):329–331. doi: 10.1024/0036-7281.151.7.329. [DOI] [PubMed] [Google Scholar]
- 6.Taub DD, Lin YS, Hu SC, Rogers TJ. Immunomodulatory activity of staphylococcal enterotoxin-B. The induction of an I-J-restricted suppressor factor. J Immunol. 1989;143(3):813–820. [PubMed] [Google Scholar]
- 7.Roberts AL, Blumberg RS, Christ AD, Brolin RE, Ebert EC. Staphylococcal enterotoxin B induces potent cytotoxic activity by intraepithelial lymphocytes. Immunology. 2000;101(2):185–190. doi: 10.1046/j.1365-2567.2000.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huvenne W, Callebaut I, Plantinga M, Vanoirbeek JA, Krysko O, Bullens DM, et al. Staphylococcus aureus enterotoxin B facilitates allergic sensitization in experimental asthma. Clin Exp Allergy. 2010;40(7):1079–1090. doi: 10.1111/j.1365-2222.2010.03464.x. [DOI] [PubMed] [Google Scholar]
- 9.Huvenne W, Callebaut I, Reekmans k, Hens g, Bobic s, Jorissen M, et al. Staphylococcus aureus enterotoxin B augments granulocyte migration and survival via airway epithelial cell activation. Allergy. 2010;65(8):1013–1020. doi: 10.1111/j.1398-9995.2009.02313.x. [DOI] [PubMed] [Google Scholar]
- 10.Li HY, Yao YM, Shi ZG, Dong N, Yu Y, Lu LR, et al. The potential role of Staphylococcal enterotoxin B in rats with postburn Staphylococcus aureus sepsis. Shock. 2003;20(3):257–263. doi: 10.1097/00024382-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Yu RL, Zhu DD, Dong Z. [Proinflammatory effects of Staphylococcus aureus enterotoxin B on human nasal epithelial cells] Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;42(3):202–206. [PubMed] [Google Scholar]
- 12.Balint J Jr, Totorica C, Stewart J, Cochran S. Detection,isolation and characterization of staphylococcal enterotoxin B in protein A preparations purified by immunoglobulin G affinity chromatography . J Immunol Methods. 1989;116(1):37–43. doi: 10.1016/0022-1759(89)90310-4. [DOI] [PubMed] [Google Scholar]
- 13.Swaminathan S, Furey W, Pletcher J, Sax M. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature. 1992;359(6398):801–806. doi: 10.1038/359801a0. [DOI] [PubMed] [Google Scholar]
- 14.Kerouanton A, Hennekinne JA, Letertre C, Petit L, Chesneau O, Brisabois A, et al. Characterization of Staphylococcus aureus strains associated with food poisoning outbreaks in France. Int J Food Microbiol. 2007;115(3):369–375. doi: 10.1016/j.ijfoodmicro.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J, Russel DW. Molecular Cloning: a laboratory manual. 3rd ed. New York: Coldspring Harbour Laboratory Press; 2001. pp. 417–421. [Google Scholar]
- 16.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, et al. Current Protocols in Molecular Biology. John Wiley & Sons Inc; USA: 2004. pp. 1357–1380. [Google Scholar]
- 17.Fatholahzadeh B, Emaneini M, Aligholi M, Gilbert G, Taherikalani M, Jonaidi N, et al. Molecular Characterization of Methicillin- Resistant Staphylococcus aureus clones from a teaching hospital in Tehran. Jpn J Infect Dis. 2009;62(4):309–311. [PubMed] [Google Scholar]
- 18.Ghasemi S, Giri A, Rostami H, Beigi H, Hamoon HZR. The Investigation on Effectivity of Curcumin- Nanosilver on Staphylococcus aureus Growth. Yakhteh. 2011;12(suppl 1):P123–P123. [Google Scholar]
- 19.Ataee RA, Mehrabi-Tavana A, Izadi M, Hosseini SMJ, Ataee MH. Bacterial Meningitis: a new risk factor. JRMS. 2011;16(2):207–210. [PMC free article] [PubMed] [Google Scholar]
- 20.Mizumachi E, Kato F, Hisatsune J, Tsuruda K, Uehera Y, Seo H, et al. Clonal distribution of enterotoxigenic Staphylococcus aureus on handles of handheld shopping baskets in supermarkets. J Appl Microbiol. 2011;110(2):562–567. doi: 10.1111/j.1365-2672.2010.04910.x. [DOI] [PubMed] [Google Scholar]
- 21.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13(1):16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook E, Wang X, Robiou N, Fries BC. Measurement of staphylococcal enterotoxin B in serum and culture supernatant with a capture enzyme-linked immunosorbent assay. Clin Vaccine Immunol. 2007;14(9):1094–1101. doi: 10.1128/CVI.00183-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazariwala A, Sanders Q, Hudson CR, Hofacre C, Thayer SG, Maurer JJ. Distribution of staphylococcal enterotoxin genes among Staphylococcus aureus isolates from poultry and humans with invasive staphylococcal disease. Avian Dis. 2002;46(1):132–136. doi: 10.1637/0005-2086(2002)046[0132:DOSEGA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Tenover FC, Gay EA, Frye S, Eells SJ, Healy M, McGowan JE Jr. Comparison of typing results obtained for methicillin-resistant Staphylococcus aureus isolates with the DiversiLab system and pulsed-field gel electrophoresis. J Clin Microbiol. 2009;47(8):2452–2457. doi: 10.1128/JCM.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]