Abstract
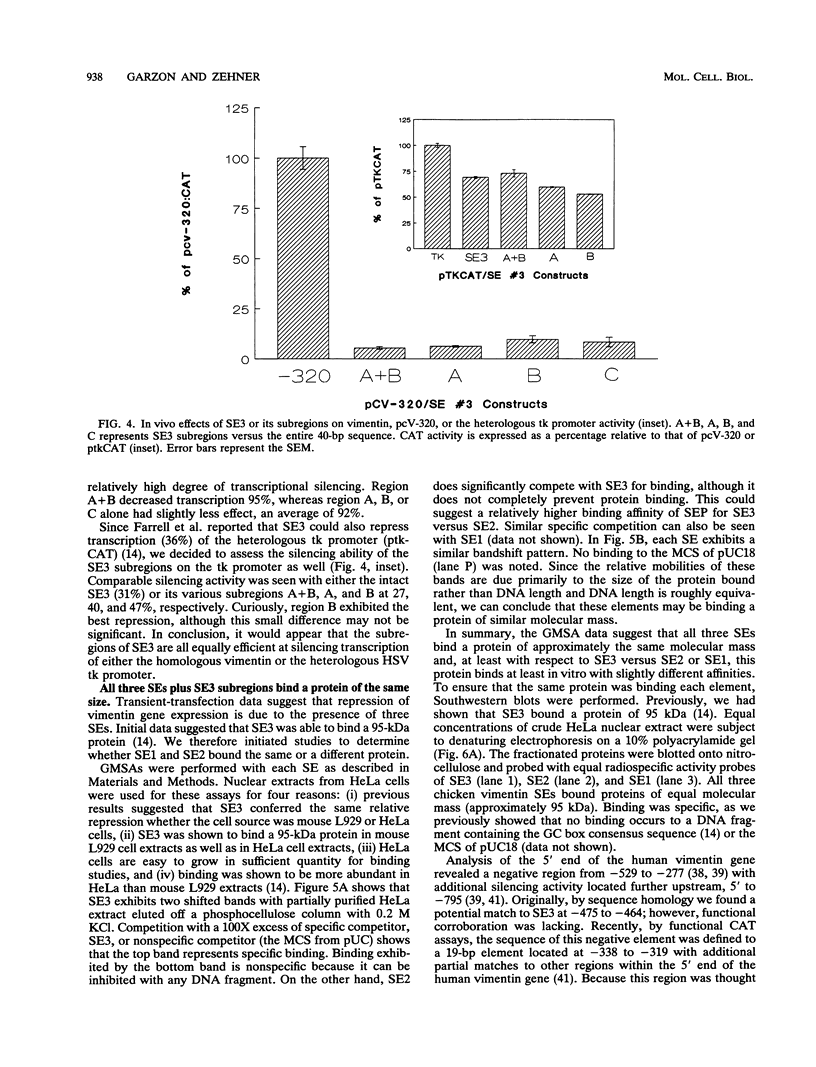
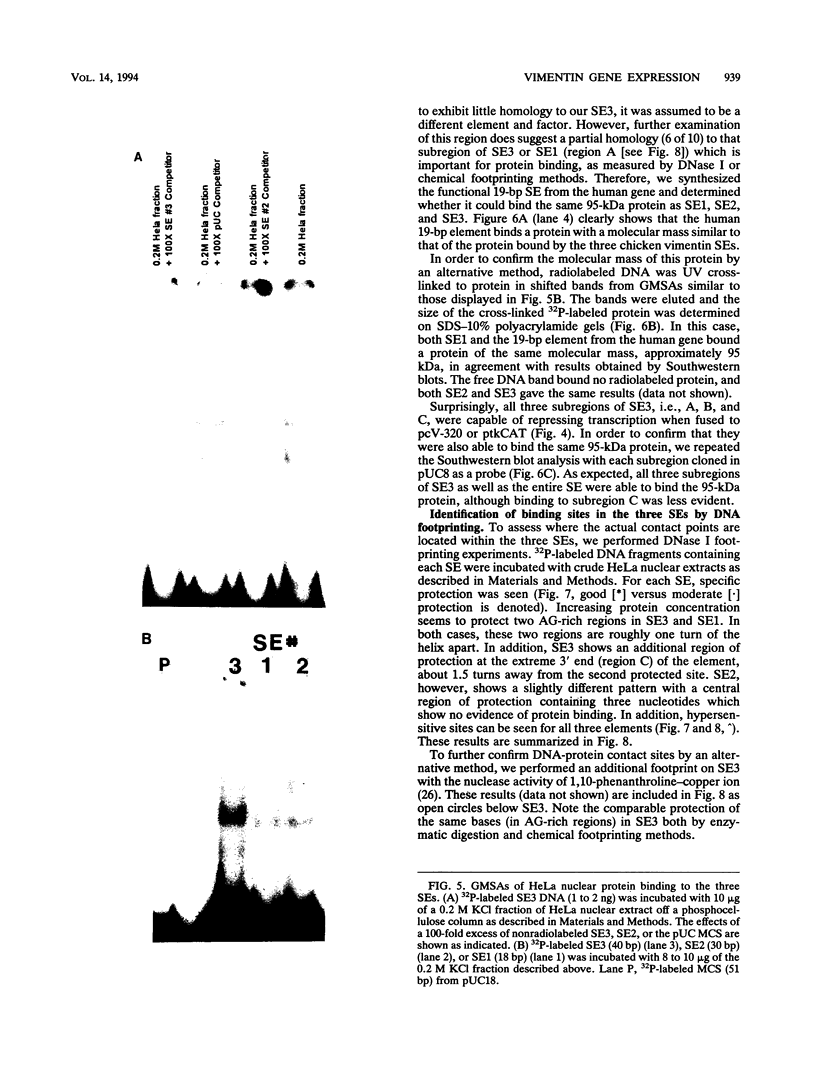
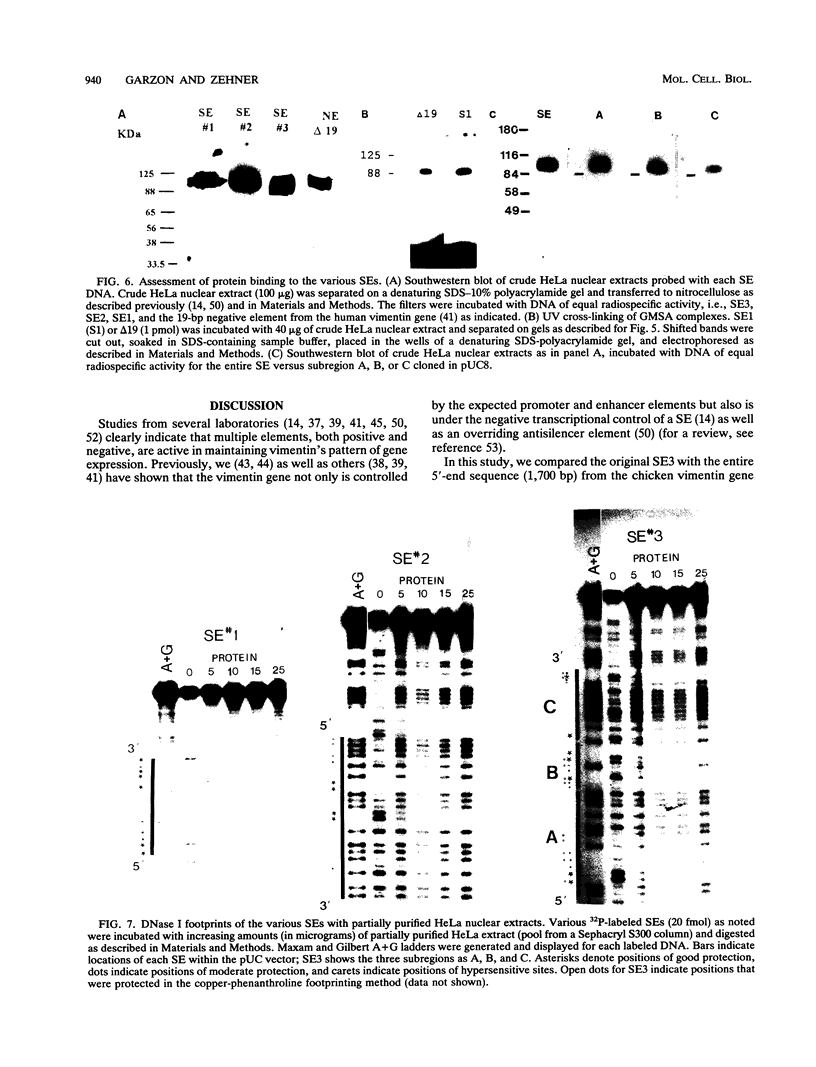
Vimentin, a member of the intermediate filament protein family, exhibits tissue- as well as development-specific expression. Transcription factors that are involved in expression of the chicken vimentin gene have been described and include a cis-acting silencer element (SE3) that is involved in the down-regulation of this gene (F. X. Farrell, C. M. Sax, and Z. E. Zehner, Mol. Cell. Biol. 10:2349-2358, 1990). In this study, we report the identification of two additional silencer elements (SE1 and SE2). We show by transfection analysis that all three silencer elements are functionally active and that optimal silencing occurs when multiple (at least two) silencer elements are present. In addition, the previously identified SE3 can be divided into three subregions, each of which is moderately active alone. By gel mobility shift assays, all three silencer elements plus SE3 subregions bind a protein which by Southwestern (DNA-protein) blot analysis is identical in molecular mass (approximately 95 kDa). DNase I footprinting experiments indicate that this protein binds to purine-rich sites. Therefore, multiple elements appear to be involved in the negative regulation of the chicken vimentin gene, which may be important in the regulation of other genes as well.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baniahmad A., Muller M., Steiner C., Renkawitz R. Activity of two different silencer elements of the chicken lysozyme gene can be compensated by enhancer elements. EMBO J. 1987 Aug;6(8):2297–2303. doi: 10.1002/j.1460-2075.1987.tb02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A., Steiner C., Köhne A. C., Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990 May 4;61(3):505–514. doi: 10.1016/0092-8674(90)90532-j. [DOI] [PubMed] [Google Scholar]
- Baumruker T., Sturm R., Herr W. OBP100 binds remarkably degenerate octamer motifs through specific interactions with flanking sequences. Genes Dev. 1988 Nov;2(11):1400–1413. doi: 10.1101/gad.2.11.1400. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Pieper F. R. Intermediate filaments: known structure, unknown function. Biochim Biophys Acta. 1989 Apr 12;1007(3):245–253. doi: 10.1016/0167-4781(89)90144-9. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Micklem G., Nasmyth K. A yeast silencer contains sequences that can promote autonomous plasmid replication and transcriptional activation. Cell. 1987 Dec 4;51(5):709–719. doi: 10.1016/0092-8674(87)90094-8. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Cao S. X., Gutman P. D., Dave H. P., Schechter A. N. Identification of a transcriptional silencer in the 5'-flanking region of the human epsilon-globin gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5306–5309. doi: 10.1073/pnas.86.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P., Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991 Nov 1;67(3):569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell F. X., Sax C. M., Zehner Z. E. A negative element involved in vimentin gene expression. Mol Cell Biol. 1990 May;10(5):2349–2358. doi: 10.1128/mcb.10.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Maniatis T. Overlapping positive and negative regulatory domains of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1447–1451. doi: 10.1073/pnas.85.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S. Negative regulation of transcriptional initiation in eukaryotes. Biochim Biophys Acta. 1990 Jun 1;1032(1):53–77. doi: 10.1016/0304-419x(90)90012-p. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi M., Bertuccioli C., Takada R., Wang J., Yamamoto T., Roeder R. G. Transcription factor TFIID induces DNA bending upon binding to the TATA element. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1060–1064. doi: 10.1073/pnas.89.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa M., Osada S., Okuda A., Muramatsu M. Silencer binding proteins function on multiple cis-elements in the glutathione transferase P gene. Nucleic Acids Res. 1991 Jan 11;19(1):5–10. doi: 10.1093/nar/19.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inostroza J. A., Mermelstein F. H., Ha I., Lane W. S., Reinberg D. Dr1, a TATA-binding protein-associated phosphoprotein and inhibitor of class II gene transcription. Cell. 1992 Aug 7;70(3):477–489. doi: 10.1016/0092-8674(92)90172-9. [DOI] [PubMed] [Google Scholar]
- Johnson F. B., Krasnow M. A. Differential regulation of transcription preinitiation complex assembly by activator and repressor homeo domain proteins. Genes Dev. 1992 Nov;6(11):2177–2189. doi: 10.1101/gad.6.11.2177. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L., Calabretta B., Baserga R. Expression of cell-cycle-dependent genes in phytohemagglutinin-stimulated human lymphocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5375–5379. doi: 10.1073/pnas.82.16.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Fos-Jun heterodimers and Jun homodimers bend DNA in opposite orientations: implications for transcription factor cooperativity. Cell. 1991 Jul 26;66(2):317–326. doi: 10.1016/0092-8674(91)90621-5. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Inoue J., Davis N., Link E., Baeuerle P. A., Bose H. R., Jr, Verma I. M. The rel-associated pp40 protein prevents DNA binding of Rel and NF-kappa B: relationship with I kappa B beta and regulation by phosphorylation. Genes Dev. 1991 Aug;5(8):1464–1476. doi: 10.1101/gad.5.8.1464. [DOI] [PubMed] [Google Scholar]
- Kuwabara M. D., Sigman D. S. Footprinting DNA-protein complexes in situ following gel retardation assays using 1,10-phenanthroline-copper ion: Escherichia coli RNA polymerase-lac promoter complexes. Biochemistry. 1987 Nov 17;26(23):7234–7238. doi: 10.1021/bi00397a006. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lendahl U., Zimmerman L. B., McKay R. D. CNS stem cells express a new class of intermediate filament protein. Cell. 1990 Feb 23;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Li-Weber M., Eder A., Krafft-Czepa H., Krammer P. H. T cell-specific negative regulation of transcription of the human cytokine IL-4. J Immunol. 1992 Mar 15;148(6):1913–1918. [PubMed] [Google Scholar]
- Meisterernst M., Roeder R. G. Family of proteins that interact with TFIID and regulate promoter activity. Cell. 1991 Nov 1;67(3):557–567. doi: 10.1016/0092-8674(91)90530-c. [DOI] [PubMed] [Google Scholar]
- Meisterernst M., Roy A. L., Lieu H. M., Roeder R. G. Activation of class II gene transcription by regulatory factors is potentiated by a novel activity. Cell. 1991 Sep 6;66(5):981–993. doi: 10.1016/0092-8674(91)90443-3. [DOI] [PubMed] [Google Scholar]
- Merino A., Madden K. R., Lane W. S., Champoux J. J., Reinberg D. DNA topoisomerase I is involved in both repression and activation of transcription. Nature. 1993 Sep 16;365(6443):227–232. doi: 10.1038/365227a0. [DOI] [PubMed] [Google Scholar]
- Miksicek R., Heber A., Schmid W., Danesch U., Posseckert G., Beato M., Schütz G. Glucocorticoid responsiveness of the transcriptional enhancer of Moloney murine sarcoma virus. Cell. 1986 Jul 18;46(2):283–290. doi: 10.1016/0092-8674(86)90745-2. [DOI] [PubMed] [Google Scholar]
- Miller A. M., Nasmyth K. A. Role of DNA replication in the repression of silent mating type loci in yeast. Nature. 1984 Nov 15;312(5991):247–251. doi: 10.1038/312247a0. [DOI] [PubMed] [Google Scholar]
- Paulin D. Expression de gènes codant pour les protéines des filaments intermédiaires chez l'homme. Pathol Biol (Paris) 1989 Apr;37(4):277–282. [PubMed] [Google Scholar]
- Rittling S. R., Baserga R. Functional analysis and growth factor regulation of the human vimentin promoter. Mol Cell Biol. 1987 Nov;7(11):3908–3915. doi: 10.1128/mcb.7.11.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittling S. R., Coutinho L., Amram T., Kolbe M. AP-1/jun binding sites mediate serum inducibility of the human vimentin promoter. Nucleic Acids Res. 1989 Feb 25;17(4):1619–1633. doi: 10.1093/nar/17.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S. G., Ha I., Maldonado E., Reinberg D., Green M. R. Interaction between an acidic activator and transcription factor TFIIB is required for transcriptional activation. Nature. 1993 Jun 24;363(6431):741–744. doi: 10.1038/363741a0. [DOI] [PubMed] [Google Scholar]
- Salvetti A., Lilienbaum A., Li Z., Paulin D., Gazzolo L. Identification of a negative element in the human vimentin promoter: modulation by the human T-cell leukemia virus type I Tax protein. Mol Cell Biol. 1993 Jan;13(1):89–97. doi: 10.1128/mcb.13.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savagner P., Miyashita T., Yamada Y. Two silencers regulate the tissue-specific expression of the collagen II gene. J Biol Chem. 1990 Apr 25;265(12):6669–6674. [PubMed] [Google Scholar]
- Sax C. M., Farrell F. X., Tobian J. A., Zehner Z. E. Multiple elements are required for expression of an intermediate filament gene. Nucleic Acids Res. 1988 Aug 25;16(16):8057–8076. doi: 10.1093/nar/16.16.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax C. M., Farrell F. X., Zehner Z. E. Down-regulation of vimentin gene expression during myogenesis is controlled by a 5'-flanking sequence. Gene. 1989 May 30;78(2):235–242. doi: 10.1016/0378-1119(89)90226-6. [DOI] [PubMed] [Google Scholar]
- Sax C. M., Farrell F. X., Zehner Z. E., Piatigorsky J. Regulation of vimentin gene expression in the ocular lens. Dev Biol. 1990 May;139(1):56–64. doi: 10.1016/0012-1606(90)90278-q. [DOI] [PubMed] [Google Scholar]
- Schultz S. C., Shields G. C., Steitz T. A. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science. 1991 Aug 30;253(5023):1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Liem R. K. Intermediate filament dynamics. Cell. 1990 Feb 23;60(4):521–523. doi: 10.1016/0092-8674(90)90651-t. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Stover D. M., Zehner Z. E. Identification of a cis-acting DNA antisilencer element which modulates vimentin gene expression. Mol Cell Biol. 1992 May;12(5):2230–2240. doi: 10.1128/mcb.12.5.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto M., Quinn J. P., Farina A. R., Staudt L. M., Levens D. fos/jun and octamer-binding protein interact with a common site in a negative element of the human c-myc gene. J Biol Chem. 1989 May 25;264(15):8992–8999. [PubMed] [Google Scholar]
- Zehner Z. E., Li Y., Roe B. A., Paterson B. M., Sax C. M. The chicken vimentin gene. Nucleotide sequence, regulatory elements, and comparison to the hamster gene. J Biol Chem. 1987 Jun 15;262(17):8112–8120. [PubMed] [Google Scholar]
- Zehner Z. E. Regulation of intermediate filament gene expression. Curr Opin Cell Biol. 1991 Feb;3(1):67–74. doi: 10.1016/0955-0674(91)90167-w. [DOI] [PubMed] [Google Scholar]
- Zwieb C., Kim J., Adhya S. DNA bending by negative regulatory proteins: Gal and Lac repressors. Genes Dev. 1989 May;3(5):606–611. doi: 10.1101/gad.3.5.606. [DOI] [PubMed] [Google Scholar]
- van de Klundert F. A., van Eldik G. J., Pieper F. R., Jansen H. J., Bloemendal H. Identification of two silencers flanking an AP-1 enhancer in the vimentin promoter. Gene. 1992 Dec 15;122(2):337–343. doi: 10.1016/0378-1119(92)90223-c. [DOI] [PubMed] [Google Scholar]