Abstract
Enhanced oxidative stress and inflammation contribute to telomere erosion. Friedreich's ataxia is a neurodegenerative disorder caused by a reduction in frataxin expression that results in mitochondrial dysfunction and oxidative damage. Furthermore, frataxin deficiency induces a strong activation of inflammatory genes and neuronal death. We investigated telomere length (TL) in peripheral blood leukocytes of 37 patients with Friedreich's ataxia and 36 controls. We noted a significant telomere shortening in patients with Friedreich's ataxia compared to healthy controls (p=0.03). We also found a correlation between TL and disease duration (p=0.001). Our observations lead to the hypothesis that the TL of human peripheral blood leukocytes may serve as a biomarker of Friedreich's ataxia that could be used as an outcome measure in clinical trials. Antioxid. Redox Signal. 18, 1303–1306.
Telomere Length and Diseases
Telomeres are protein–DNA complexes localized at chromosomal ends. They consist of highly conserved hexanucleotide repeats (TTAGGG) and play an essential role in maintenance of genomic stability. Telomere length (TL) shortens in each somatic cell division, because the 3′-DNA replication strand is incomplete while a ribonucleoprotein enzyme called telomerase reverse transcriptase mediates the synthesis of telomere repeats, balancing the natural chromosome erosion. The balance between these two contrasting forces controls telomere size. Aberration of the telomere complex does not allow the protection of the chromosome ends, leading to cellular senescence or apoptosis (9).
TL seems to be a variable trait with a genetic component, displaying high variations at birth among different populations. The telomere is longer in women than in men and undergoes progressive shortening with age. Several nongenetic factors such as obesity, smoking, and sedentary living also seem to play a role in TL (9). Oxidative stress has been proposed to be an important modulator of TL (8).
Innovation.
Many findings have established a clear role for telomere dysfunction in different chronic diseases, and a close correlation between oxidative stress and telomere shortening has been suggested. Mitochondrial dysfunction and oxidative damage are the causes of Friedreich's Ataxia (FRDA). Until now, no data about telomere length (TL) in patients with FRDA were available. In the present study, we observed that telomere shortening was correlated with the FRDA duration. To the best of our knowledge, this is the first study demonstrating this association. The novelty of present study is that TL measurement could be used in monitoring disease progression and therapy outcomes.
In humans, shortened TL is associated with rare monogenic diseases, including dyskeratosis congenita, aplastic anemia, idiopathic pulmonary fibrosis, and ataxia telangiectasia (1). Many studies have also reported the association between TL (with or without modulation of telomerase activity) and complex diseases such as type 1 and 2 diabetes, hypertension, cardiovascular disease, psychiatric disorders, and cancer (9). TL shortening has also been found in several age-related neurological diseases (Alzheimer, Parkinson, cerebral autosomal-dominant arteriopathy with subcortical infarcts, and leucoencephalopathy (CADASIL) and dementia after stroke) (9,6).
Friedreich's ataxia (FRDA; OMIM# 229300), the most common inherited ataxia, is an autosomal recessive disease that generally begins at puberty and leads to death in the fifth or sixth decade of life. FRDA is caused by a GAA-triplet repeat expansion in the first intron of the frataxin gene. This expansion impairs the transcription of the gene, leading to deficiency of frataxin. This protein is a ubiquitous mitochondrial protein involved in mitochondrial iron transport and/or in the assembly of iron–sulfur cluster enzymes (4). Frataxin deficiency is associated with iron dysregulation and with a severe disruption of iron–sulfur cluster proteins. Lack of iron detoxification and the increase in bioavailable iron are likely to enhance cellular sensitivity to oxidative stress (4) and activate inflammatory pathways (3). Therefore, we hypothesized that TL might be shortened in patients with FRDA.
Does TL of Peripheral Blood Leukocytes Correlate with FRDA?
To answer to this question, we have investigated TL in peripheral leukocytes from patients with FRDA and healthy controls. Sample details are shown in Table 1. There were more men than women in the control group; however, this difference was not statistically significant.
Table 1.
Demographic and Clinical Data
| Variables | Controls | Patients |
|---|---|---|
| Number of subject (%) | 36 (49.3%) | 37 (50.7%) |
| Men (%) | 27 (65%) | 19 (51%) |
| Age (years) | 33.2±9.4 SE | 30.6±10.9 SE |
| Age at onset (years) | 15.9±1.96 SE | |
| GAA1a | 661.65±36.02 SE | |
| GAA2a | 870.8±31.35 SE | |
| Cardiomyopathy | 15 (40.5%) | |
| Diabetes | 4 (10.8%) | |
| Disease duration (years)b | 14.2±9.4 SE |
Alleles 1 and 2 of GAA trinucleotide repeat causing Friedreich's ataxia.
This variable was calculated by deducting the age at onset from the age at sampling.
SE, standard error.
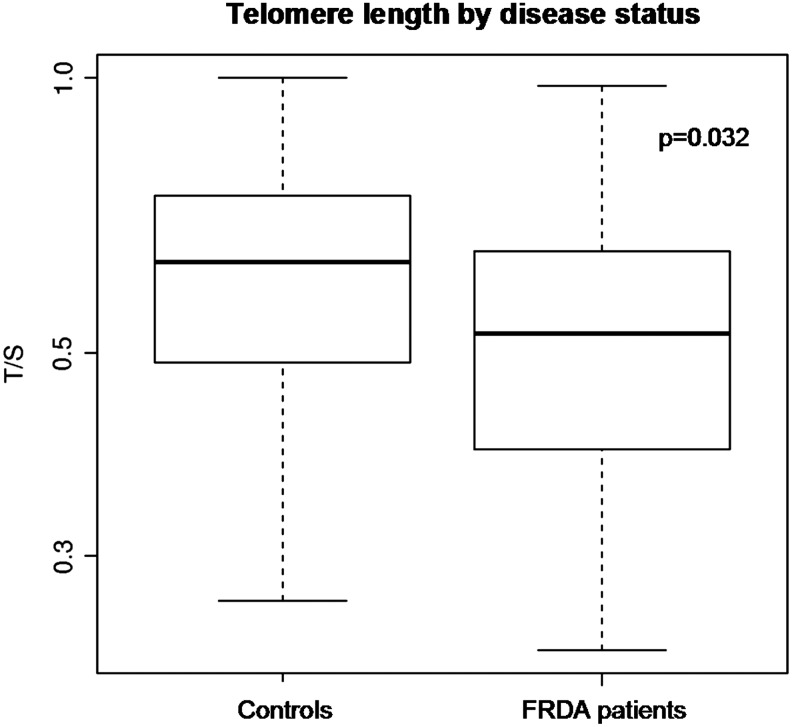
We found significantly shortened TL in patients compared with the controls (p=0.032, Fig. 1). Since TL is also influenced by age and gender, we employed a binary logistic regression model to take into account the role of these confounding variables. The difference in TL means remained statistically significant (p=0.012). In a subsample of patients, we also investigated the correlation between specific clinical features and TL. To this aim, we employed the Pearson test between TL and age, gender, GAA repeats, age of onset, and disease duration. As expected, there was a significant correlation between TL and age; TL was longer in younger patients (r=− 0.286, p=0.014). Gender was not associated with TL in leucocytes in either the healthy controls or the patients. There was no correlation between TL and the smaller expanded allele GAA1 and the larger expanded allele GAA2 repeats. Interestingly, we found a significant correlation between TL and disease duration. The patients with longer disease duration had shorter TLs (r=− 0.594, p=0.001) (Fig. 2). This finding remained significant after correction for age (p=0.012) or for age of onset (p=0.001) of FRDA. We were unable to analyze the possible relationship between TL and cardiomyopathy and/or diabetes because of the small size of these specific subsets of patients that were available.
FIG. 1.
Box-plot and whisker diagram displaying the telomere length distributions in controls and patients with Friedreich's Ataxia (FRDA). Telomere length (TL) is expressed as a relative telomere length (T/S, see NOTES). Bold lines in the boxes indicate the median T/S values, and the lower and upper boundaries of the boxes are the first and third quartiles, respectively (p=0.032). The horizontal lines (whiskers) indicate the minimum and maximum T/S values.
FIG. 2.
Scatter plot showing the negative correlation between T/S and disease duration in 27 patients with FRDA (r=− 0.594, p=0.001).
Could TL Be a Blood Biomarker in FRDA?
Strategies for FRDA treatment are currently under evaluation and several of them (antioxidant and iron chelators) are already in phases II and III of the process. To assess the efficacy of these therapeutic agents, different clinical and biochemical markers are used (4).
ICARS (International Cooperative Ataxia Rating Scale) and FARS (Friedreich Ataxia Rating Scale) are the most used clinical markers. It should be underlined that although these scales are useful for monitoring disease progression, they are not always appropriate for clinical trials (7). Biomarkers of oxidative stress have been discovered in urine (8-hydroxy-2′-deoxyguanosine) and blood (malondialdehyde) samples from patients with FRDA, but some conflicting results have been obtained, probably because of technical difficulties (4).
As already mentioned, FRDA is caused by decreased frataxin in mitochondria, which in turn results in mitochondrial dysfunction and oxidative damage in patients. The exact pathophysiology of FRDA is still unknown; however, a decrease in frataxin increases the amount of reactive oxygen species, making the cells more sensitive to oxidative damage (4). Frataxin deficiency has been reported previously to induce inflammatory genes (3).
Oxidative stress and inflammation are widely considered to be two main mediators of chronic diseases and are reported to increase the rate of leukocyte telomere attrition (9). How oxidative stress influences TL has not yet been fully elucidated. It has been suggested that because of the high guanine content in their sequences, telomeres are remarkably sensitive to damage by oxidative stress. Thus, enhanced oxidative stress and neuroinflammation could synergize telomere shortening in FRDA.
Here we report the first study of TL changes in patients with FRDA. The TL in patients with FRDA was significantly shorter than in the controls. We did not find any significant correlation between TL, GAA expansion, and age of onset. Interestingly, we did find a correlation between TL and disease duration. Patients affected by FRDA for a longer time showed more shortened TL also after adjustment by age. This finding may suggest that TL could be considered a biomarker of disease progression rather than disease severity.
The pathogenesis of TL shortening in FRDA needs to be further explored. It is possible to speculate that this phenomenon could depend on the increased oxidative stress and inflammation observed in patients with FRDA.
Limitations of the Study
A limitation of our study was the relatively small sample size, which reduced the statistical power needed to analyze comorbidities such as diabetes and cardiomyopathy. FRDA is a rare disease, and larger cohorts in cooperative studies will be needed to address this issue. These larger cohorts could also allow the study of other FRDA subphenotypes, such as late-onset and retained reflexes in patients.
Conclusions
Up to now, there is no effective treatment and no well-defined clinical or biochemical marker for FRDA. However, several trials are currently active. The improved understanding of the role of frataxin has led to the common use of antioxidants in FRDA clinical practice. It has been demonstrated that antioxidants decrease telomere shortening (4). Telomere measurement is increasingly used in other diseases as a reliable method to predict disease prognosis (5). Our data suggest that telomere size evaluation could be integrated with other clinical and biochemical markers to evaluate both disease progression and possible efficacy of antioxidant therapies.
Notes
Subjects and methods
This study comprised 37 patients with FRDA and 36 healthy controls. Patients with FRDA were recruited from among the patients referred to the Department of Neurology, “Federico II” Medical School, Naples. The following data were collected for each patient: age, gender, age of onset, comorbidities, and disease duration (age at the time of sampling minus age at onset). Age at onset was available only for 27 patients. The patients were not treated with idebenone or other antioxidant drugs. Molecular diagnosis showed that all patients were homozygotes for the GAA-repeat expansion (7); the smaller-expanded allele was named GAA1, and the larger was named GAA2. The Local Ethics Committee approved the study, and all participants signed an informed consent form.
Genomic DNA was extracted directly from peripheral blood cells using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI). All samples were treated in the same way. TL was examined using a real-time PCR method described previously (2) in a Step One Plus Real Time PCR system (Applied Biosystems, Foster City, CA). Briefly, this method consists of measuring the telomeric region (T) with specific designed primers, and normalizing with a single copy gene (S) (the 36B4 gene, which encodes the acid ribosomal phosphoprotein PO). The relative TL is calculated as ratio of telomere-repeat copy number (T) to single copy gene (S). The T/S ratio data of the study subjects were normalized by an arbitrarily chosen reference DNA sample (2).
Statistical analysis
The relative T/S ratio was log-transformed to approximate normal distribution. The data were expressed as mean and standard error. Comparisons between frequencies were assessed by chi-square tests, while comparisons between means were by the Student's t-tests. All the relationships between TL and other variables were calculated by multiple regression analysis to control the role of confounding variables. For all the statistics, a p-value smaller than 0.05 was considered as significant. Statistical analyses were performed with SPSS for Windows, version 15.0.
Abbreviations Used
- FRDA
Friedreich's Ataxia
- GAA1
the smaller-expanded allele of the GAA repeats
- GAA2
the larger-expanded allele of the GAA repeats
- TL
telomere length
Acknowledgments
This study was supported by the Monte dei Paschi di Siena Foundation (Italy) (Grant number 15509). We thank Dr. Tiziana Parisi for technical advice.
References
- 1.Armanios M. Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu C. Schoenfeld R. Shan Y. Tsai HJ. Hammock B. Cortopassi G. Frataxin deficiency induces Schwann cell inflammation and death. Biochim Biophys Acta. 2009;1792:1052–1061. doi: 10.1016/j.bbadis.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marmolino D. Friedreich's ataxia: past, present and future. Brain Res Rev. 2011;67:311–330. doi: 10.1016/j.brainresrev.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Pavesi E. Avondo F. Aspesi A. Quarello P. Rocci A. Vimercati C. Pigullo S. Dufour C. Ramenghi U. Dianzani I. Analysis of telomeres in peripheral blood cells from patients with bone marrow failure. Pediatr Blood Cancer. 2009;53:411–416. doi: 10.1002/pbc.22107. [DOI] [PubMed] [Google Scholar]
- 6.Ragno M. Pianese L. Pinelli M. Silvestri S. Cacchiò G. Di Marzio F. Scarcella M. Coretti F. Altamura F. Monticelli A. Castaldo I. Shorter telomeres in patients with cerebral autosomal dominant arteriopathy and leukoencephalopathy (CADASIL) Neurogenetics. 2011;12:337–343. doi: 10.1007/s10048-011-0298-1. [DOI] [PubMed] [Google Scholar]
- 7.Schulz JB. Boesch S. Bürk K. Dürr A. Giunti P. Mariotti C. Pousset F. Schöls L. Vankan P. Pandolfo M. Diagnosis and treatment of Friedreich ataxia: a European perspective. Nat Rev Neurol. 2009;5:222–234. doi: 10.1038/nrneurol.2009.26. [DOI] [PubMed] [Google Scholar]
- 8.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhu H. Belcher M. van der Harst P. Healthy aging and disease: role for telomere biology? Clin Sci (Lond) 2011;120:427–440. doi: 10.1042/CS20100385. [DOI] [PMC free article] [PubMed] [Google Scholar]