Abstract
Aims: The role of endothelium-derived contracting factors (EDCFs) in regulating renovascular function is yet to be elucidated in renovascular hypertension (RH). The current study investigated whether oxidative stress-dependent cyclooxygenase (COX)-2-derived prostaglandin F2α (PGF2α) impairs endothelial function in renal arteries of renovascular hypertensive rats (RHR). Results: Renal hypertension was induced in rats by renal artery stenosis of both kidneys using the 2-kidney 2-clip model. Acute treatment with reactive oxygen species (ROS) scavengers, COX-2 inhibitors, and thromboxane-prostanoid receptor antagonists, but not COX-1 inhibitors, improved endothelium-dependent relaxations and eliminated endothelium-dependent contractions in RHR renal arteries. Five weeks of treatment with celecoxib or tempol reduced blood pressure, increased renal blood flow, and restored endothelial function in RHRs. Increased ROS production in RHR arteries was inhibited by ROS scavengers, but unaffected by COX-2 inhibitors; whereas increased PGF2α release was reduced by both ROS scavengers and COX-2 inhibitors. ROS also induced COX-2-dependent contraction in RHR renal arteries, which was accompanied by the release of COX-2-derived PGF2α. Further, chronic tempol treatment reduced COX-2 and BMP4 upregulation, p38MAPK phosphorylation, and the nitrotyrosine level in RHR renal arteries. Conclusion: These findings demonstrate the functional importance of oxidative stress, which serves as an initiator of increased COX-2 activity, and that COX-2-derived PGF2α plays an important role in mediating endothelial dysfunction in RH. Innovation: The current study, thus, suggests that drugs targeting oxidative stress-dependent COX-2-derived PGF2α may be useful in the prevention and management of RH. Antioxid. Redox Signal. 16, 363–373.
Introduction
Renal artery stenosis is the major cause of renovascular hypertension (RH) and can lead to reduced renal blood flow and end-stage renal damage (36). The role of renal vasculature in RH was established by Goldblatt et al. (15), who demonstrated that partial obstruction of the renal artery increased mean arterial pressure, which has the features of RH in humans. The mechanisms responsible for the development of RH remain largely undefined, but oxidative stress plays an important role (12, 26). Nevertheless, it is unclear how oxidative stress affects renovascular function in RH.
Innovation.
Endothelium-derived contracting factors (EDCFs) participate in the development and maintenance of hypertension. This research reported the functional importance of cyclooxygenase (COX)-2 derived prostaglandin F2α in response to oxidative stress as the major EDCF to impair endothelium-dependent relaxation and enhance endothelium-dependent contraction in renal arteries of renal hypertension induced by renal artery stenosis. Antioxidant or COX-2 inhibition can reduce blood pressure and improve endothelial function in renal hypertensive rats.
Endothelial cell function reflects the condition of vascular health and predicts the severity of future vascular complications (37). Impaired endothelium-dependent relaxations (EDRs) have been reported in aortae (4, 28), superior mesenteric arteries (35), and mesenteric resistance arteries (7, 10) in renovascular hypertensive rats (RHRs). However, little is known about the impact of RH on vascular reactivity of renal arteries and renal blood flow. The mechanisms responsible for reduced EDRs may include activation of the renin-angiotensin-aldosterone system (20, 26), and over-production of superoxide derived from the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (19, 21, 23). In contrast, no study has been directed toward exploring the possible effect of RH on endothelium-dependent contractions (EDCs). The endothelium-derived contracting factors (EDCFs) that contribute to endothelial dysfunction are reported to be either cyclooxygenase (COX)-mediated arachidonic acid-derived prostaglandins (2, 13) or reactive oxygen species (ROS) (44), which act on vascular smooth muscle to cause contractions.
Earlier studies showed that selective COX-1 inhibitors prevent acetylcholine-induced EDCs in aortae of spontaneously hypertensive rats (SHRs) (44) and improve EDRs in mesenteric arteries in angiotensin II-infused mice (39), thus suggesting that COX-1-derived EDCFs impair endothelial function. More recently, COX-2-derived constricting prostanoids have been suggested to attenuate EDRs in SHR mesenteric arteries (40). Indeed, COX-2 inhibitors improve endothelial function in patients with hypertension and coronary heart disease (5, 41). Nevertheless, whether COX-derived prostaglandins contribute to endothelial dysfunction in RH has not been examined.
Although COX-derived prostaglandins and ROS are suggested to be EDCFs, it remains unclear whether ROS can stimulate endothelial COX isoforms to release EDCFs in renal arteries of RHRs. The role of EDCFs in regulating renovascular function and renal blood flow in vivo is yet to be elucidated in RH. Therefore, the current study aims at determining whether ROS regulate COX-derived prostaglandins to cause endothelial dysfunction in RH.
Results
COX-2 mediates impairments in EDRs in renal arteries of RHRs
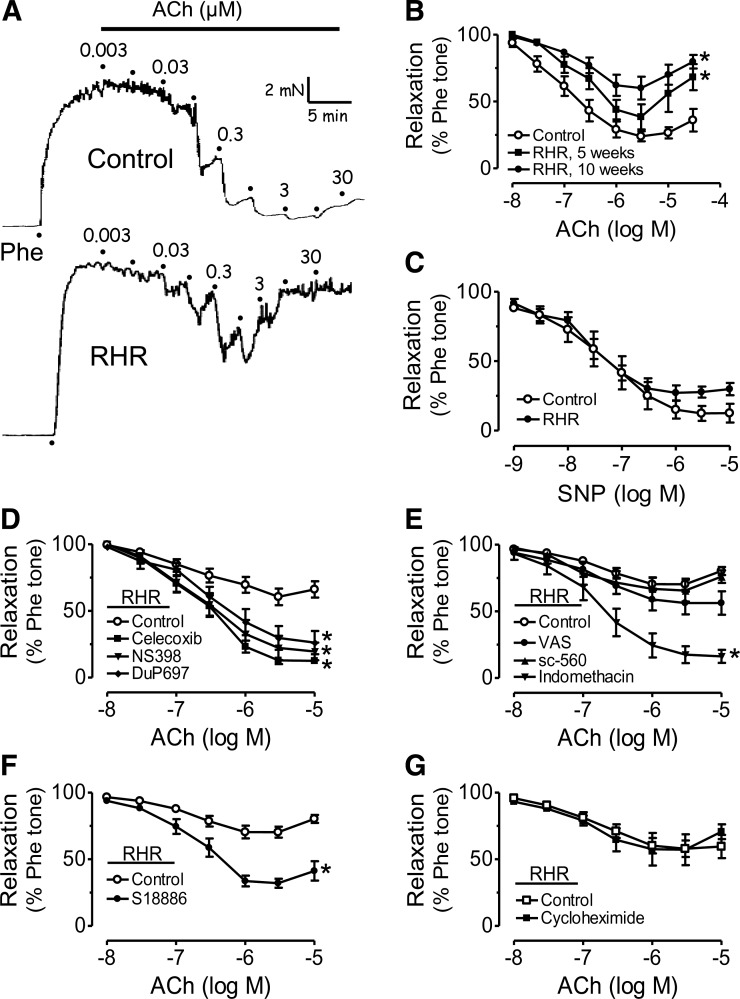
Acetylcholine-induced EDRs were blunted in renal arteries from RHRs 5 weeks after the induction of renal artery stenosis and further reduced 10 weeks after surgery compared with those from sham-operated rats (Fig. 1A, B). Renal arteries from RHRs exhibited a biphasic response to acetylcholine, with an initial relaxation followed by a contraction at concentrations of acetylcholine higher than 1 μM (Fig. 1A, B). Endothelium-independent relaxations in response to sodium nitroprusside (SNP) (Fig. 1C) or to nitroglycerin (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/ars) were similar between the two groups. Thirty minutes of treatment of RHR renal arteries with the nonselective COX inhibitor indomethacin (Fig. 1E), COX-2 inhibitors celecoxib, NS398, DuP697, or the thromboxane prostanoid (TP) receptor antagonist S18886 restored the impaired EDRs (Fig. 1D, F); whereas COX-1 inhibitors SC-560 and valeryl salicylate (VAS) had no effect (Fig. 1E). The protein synthesis inhibitor cycloheximide did not modify EDRs in renal arteries (Fig. 1G).
FIG. 1.
Cyclooxygenase (COX)-2 mediates the impairment of endothelium-dependent relaxations (EDRs) in renal arteries of renovascular hypertensive rats (RHRs). (A) Representative traces showing blunted acetylcholine (ACh)-induced EDRs of renal arteries from RHRs compared with those from control rats. (B) Concentration-response curves for ACh-induced EDRs in renal arteries from RHRs 5 and 10 weeks after surgery and control rats. (C) Endothelium-independent relaxations induced by sodium nitroprusside (SNP) in renal arteries were comparable in RHRs and control rats. Effects of 30-min exposure to (D) COX-2 inhibitors celecoxib, DuP697, or NS398 (3 μM in each case); (E) COX-1 inhibitors SC-560 (0.3 μM), valeryl salicylate (VAS, 0.3 mM), or nonselective COX inhibitor indomethacin (1 μM); (F) thromboxane prostanoid (TP)-receptor antagonist S18886 (0.1 μM); and (G) protein synthesis inhibitor cycloheximide (10 μM) on EDRs in RHR renal arteries. Results are mean±standard error of the mean (SEM) of six to eight experiments. *p<0.05 versus control in each panel.
COX-2 mediates EDCs in renal arteries of RHRs
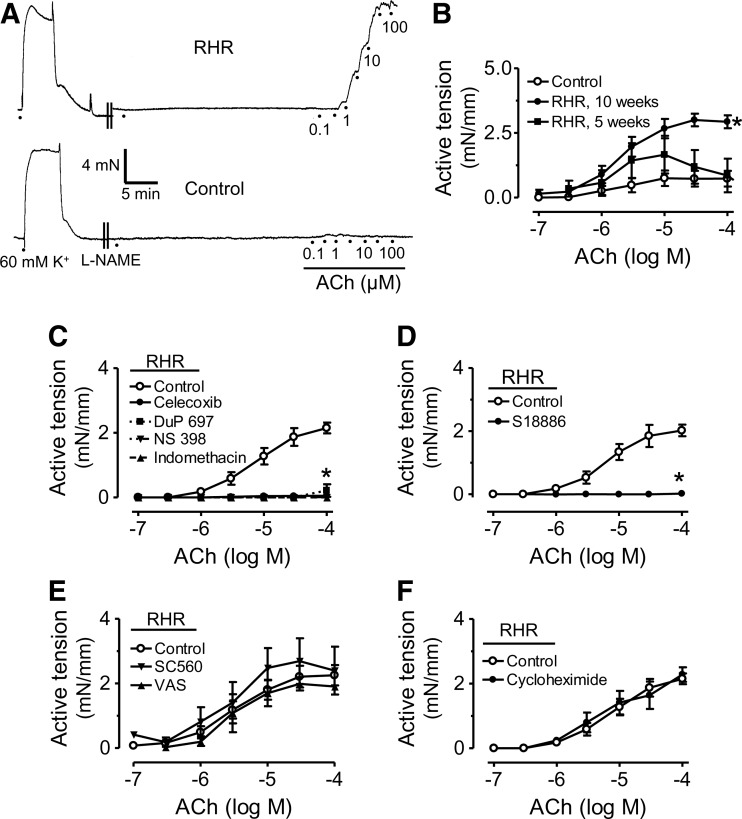
Acetylcholine elicited EDCs in renal arteries from RHRs (5 and 10 weeks after surgery) compared with those from control rats (Fig. 2A, B). Acetylcholine-induced EDCs were only observed in the presence of NG-nitro-L-arginine methyl ester (L-NAME) in rings with endothelium (Supplementary Fig. S2). Indomethacin (Fig. 2C), celecoxib, DuP 697 or NS398 (Fig. 2C), or S18886 (Fig. 2D) abolished EDCs; whereas SC-560, VAS (Fig. 2E), or cycloheximide (Fig. 2F) had no effect.
FIG. 2.
COX-2 mediates endothelium-dependent contractions (EDCs) in renal arteries of RHRs. (A) Representative traces showing the augmented ACh-induced EDCs in RHR renal arteries. (B) Concentration-response curves for ACh-induced EDCs in the presence of NG-nitro-L-arginine methyl ester (L-NAME) 5 and 10 weeks after surgery in RHRs and control rats. Effect of 30-min exposures to (C) indomethacin (1 μM), celecoxib, DuP697, and NS398 (3 μM in each case); (D) S18886 (0.1 μM); (E) VAS (0.3 mM) and SC-560 (0.3 μM); and (F) cycloheximide (10 μM) on EDCs in RHR renal arteries. Results are mean±SEM of six to eight experiments. *p<0.05 versus control in each panel.
Chronic celecoxib or tempol treatment increases renal blood flow, reduces blood pressure, and improves endothelial function in RHRs
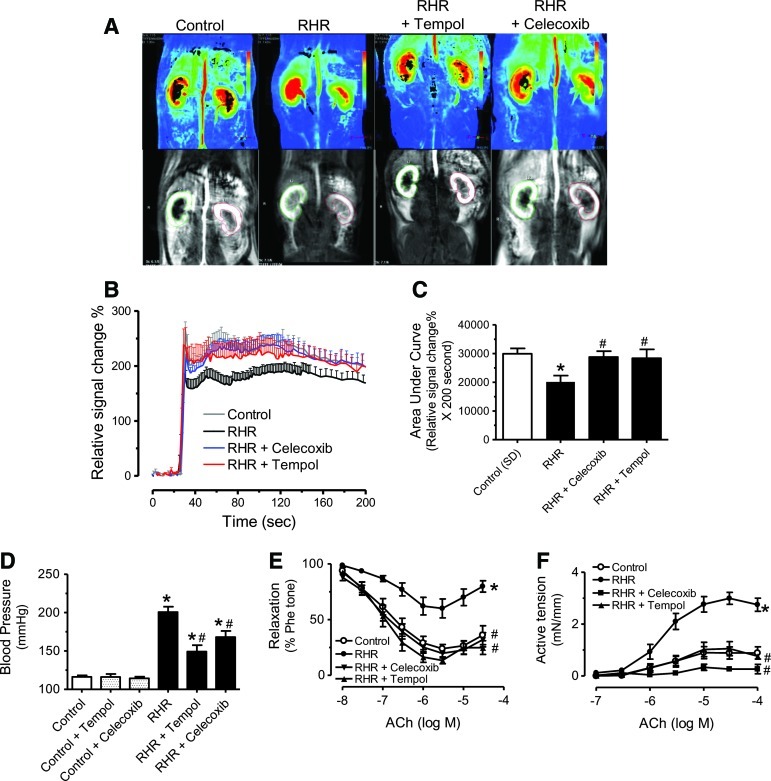
Renal blood flow was measured by magnetic resonance image (MRI) in the renal cortex, which was selected as the region of interest for the intensity analysis of Gd-DOTA-induced signal enhancement. The maximum intensity was observed in the aorta first, followed by the renal cortex ∼20 s after bolus injection of Gd-DOTA into the tail vein, then the outer and inner medulla, and finally, the renal pelvis. Renal blood flow assessed by cortical signal enhancement (Fig. 3A) was reduced in RHRs compared with control rats. The reduced flow was restored after treatment with celecoxib or tempol (Fig. 3B, C), accompanied by a reduction in systolic blood pressure in RHRs; whereas celecoxib or tempol treatment did not affect the blood pressure of control rats (Fig. 3D). After drug treatment, EDRs of RHR renal arteries were improved (Fig. 3E), and EDCs were reduced (Fig. 3F). Acute exposure to the putative NADPH oxidase inhibitor apocynin improved EDRs in RHR renal arteries but did not cause further improvement in celecoxib-treated RHRs (Supplementary Fig. S3).
FIG. 3.
Chronic celecoxib or tempol treatment increases renal blood flow, reduces blood pressure and improves endothelial function in RHRs. (A) Representative illustration showing the maximal signal enhancement in the renal cortex after a tail vein injection of Gd-DOTA as measured by magnetic resonance image. Upper panel: colored image; lower panel: black and white image of the same specimen. (B, C) Summarized data showing the relative signal change compared with the time point before the signal intensity increases. (D) Systolic blood pressure measured by a tail-cuff method in tempol- or celecoxib-treated RHRs and control rats. Concentration-response curves for ACh-induced EDRs (E) and EDCs in the presence of L-NAME (F) in renal arteries from control rats, RHRs, and RHRs treated with celecoxib or tempol. Results are mean±SEM of six to eight experiments. *p<0.05 versus control, #p<0.05 versus RHR. (To see this illustration in color the reader is referred to the Web version of this article at www.liebertonline.com/ars).
ROS trigger COX-2-dependent endothelial dysfunction in renal arteries of RHRs
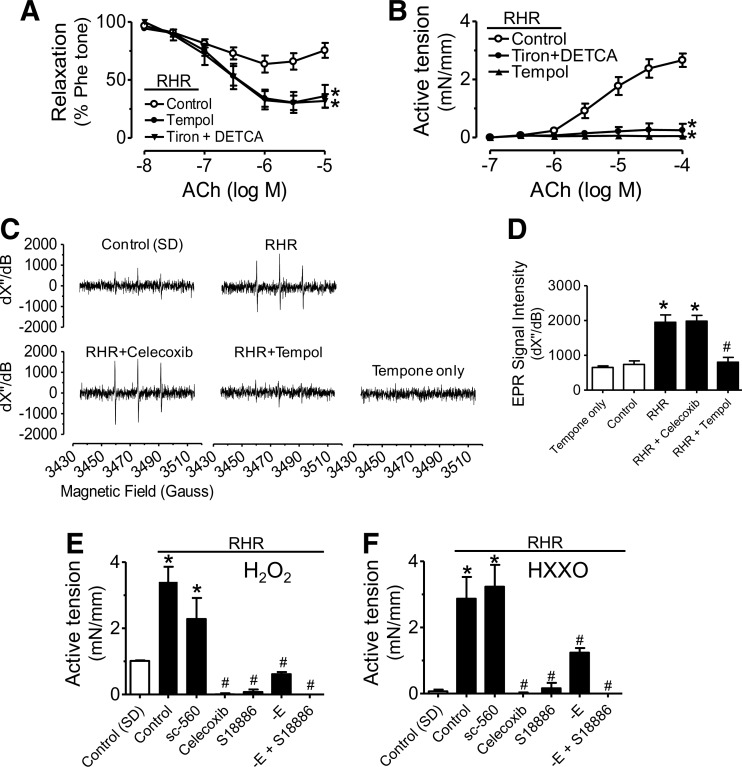
Tiron plus DETCA, or tempol (ROS scavengers) augmented EDRs (Fig. 4A) and abolished EDCs (Fig. 4B) in renal arteries from RHRs, which were unaffected by the xanthine oxidase inhibitor, allopurinol (Supplementary Fig. S4). Electron paramagnetic resonance (EPR) spectra showed an increased generation of superoxide anions (O2•−) in RHR renal arteries on acetylcholine stimulation in the presence of L-NAME (Fig. 4C, D). Renal arteries from tempol-treated RHRs but not from celecoxib-treated RHRs showed a reduction of ROS generation (Fig. 4C, D). Further, H2O2− (Fig. 4E) and hypoxanthine+xanthine oxidase (HX-XO)-induced contractions (Fig. 4F) in RHR renal arteries (3.43±0.56 mN/mm for H2O2 and 2.87±0.66 mN/mm for HX-XO) were greater than those of control rats (1.09±0.13 mN/mm for H2O2 and 0.11±0.03 mN/mm for HX-XO). Both Celecoxib and S18886 prevented H2O2- or HX-XO-induced responses; whereas SC-560 did not (Fig. 4E, F). Removal of the endothelium reduced contractions elicited by H2O2 or HX-XO (Fig. 4E, F). A 4-h period of treatment with H2O2 or HX-XO increased COX-2 expression in renal arteries and in primary aortic endothelial cells from control rats (Supplementary Fig. S5).
FIG. 4.
Reactive oxygen species (ROS) triggers COX-2-dependent endothelial dysfunction in RHRs. Effects of 30-min exposure to tiron (1 mM) plus DETCA (100 μM), or tempol (100 μM) on EDRs (A) and EDCs (B) in RHR renal arteries. (C) Electron paramagnetic resonance (EPR) spectroscopy showing markedly increased amplitude of ROS signal in response to ACh (100 μM) in RHR renal arteries. (D) Summarized data for EPR signal intensity. Effects of SC-560 (0.3 μM), celecoxib (3 μM), S18886 (0.1 μM), and endothelium removal on contractions evoked by H2O2 (100 μM) (E) and HX-XO (100 μM hypoxanthine plus 0.01 units/ml xanthine oxidase (F) in RHR renal arteries. Results are mean±SEM of four to six experiments. *p<0.05 versus control #p<0.05 versus RHR. –E, without endothelium.
COX-2-derived prostaglandin F2α mediates endothelial dysfunction in renal arteries of RHRs
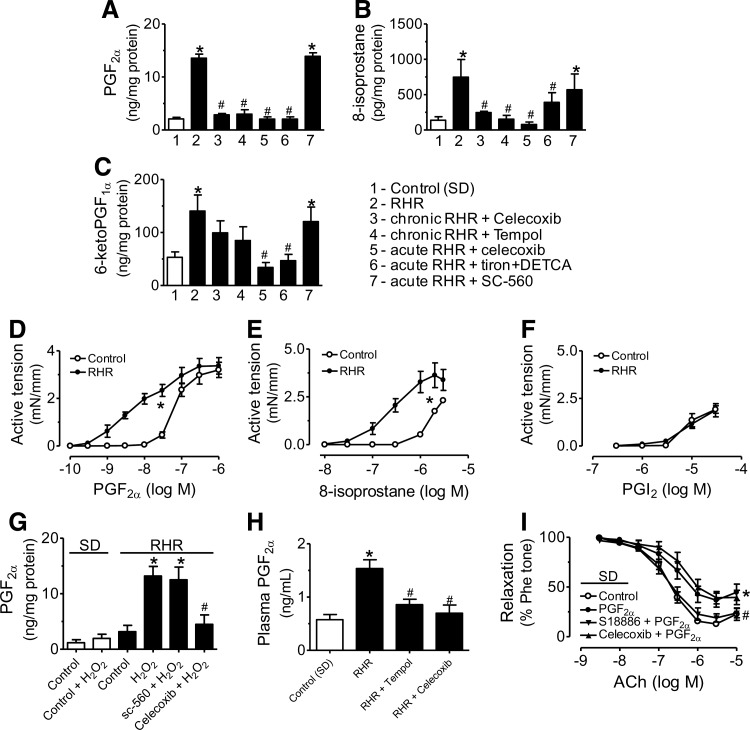
The amount of prostaglandin F2α (PGF2α) (Fig. 5A), 8-isoprostane (Fig. 5B), 6-keto PGF1α (metabolites of prostacyclin [PGI2], Fig. 5C), PGE2 (Supplementary Fig. S6a), PGD2 (Supplementary Fig. S6b), and TXB2 (metabolites of TXA2, Supplementary Fig. S6c) released in response to acetylcholine (100 μM) stimulation was greater in RHR than in control rat renal arteries. Chronic treatment with celecoxib or tempol inhibited acetylcholine-stimulated release of PGF2α, 8-isoprostane, PGE2, and PGD2 but not 6-keto PGF1α and TXB2 (Fig. 5A–C and Supplementary Fig. S5). A 30-min exposure to celecoxib or tiron plus DETCA inhibited the acetylcholine-stimulated release of all six prostaglandins (Fig. 5A–C and Supplementary Fig. S6), whereas SC-560 reduced PGE2, PGD2, and TXB2 (Supplementary Fig. S5) without affecting PGF2α, 8-isoprostane, and 6-keto PGF1α.
FIG. 5.
COX-2 derived prostaglandin F2α (PGF2α) mediates endothelial dysfunction in renal arteries of RHRs. Acetylcholine (100 μM)-stimulated release of PGF2α (A), 8-isoprostane (B), and 6-keto PGF1α (prostacyclin [PGI2]) (C) in control rats, RHRs, and RHRs treated with celecoxib or tempol. Contractions induced by PGF2α (D), 8-isoprostane (E), and PGI2 (F) in renal arteries of RHRs and control rats. (G) Effects of celecoxib and SC-560 on H2O2 (100 μM)-stimulated released of PGF2α in renal arteries. (H) Plasma concentration of PGF2α in control rats, RHRs, and RHRs treated with celecoxib or tempol. (I) Effects of celecoxib and S18886 on PGF2α-induced reduction of EDRs in renal arteries from control rats. Results are mean±SEM of four to eight experiments. *p<0.05 versus control (SD), #p<0.05 versus RHR.
PGF2α and 8-isoprostane produced greater contractions in renal arteries of RHRs than in control arteries (Fig. 5D, E), whereas PGI2 produced comparable responses in both groups (Fig. 5F). When PGF2α was administered to RHR renal artery preparations at a concentration (∼38 nM) comparable to the value measured by enzyme immunoassay (EIA) in acetylcholine-stimulated RHR renal arteries, the induced contractions were similar in magnitude to EDCs (Fig. 5D). In contrast, the EIA-detected amounts of 8-isoprostane at 2.12 nM and PGI2 at 0.38 μM did not cause contraction (Fig. 5E, F). In addition, H2O2 at 100 μM increased the release of PGF2α from RHR renal arteries, which was reduced by celecoxib but not SC-560 (Fig. 5G). Plasma PGF2α concentration was also decreased after chronic celecoxib or tempol administration to RHRs (Fig. 5H). Further, a 30-min exposure to 7 nM PGF2α reduced EDRs in renal arteries from control rats, which was prevented by pretreatment with S18886 (0.1 μM) but not with celecoxib (3 μM) (Fig. 5I).
Increased expression of COX-2 and oxidative stress biomarker proteins in renal arteries of RHRs
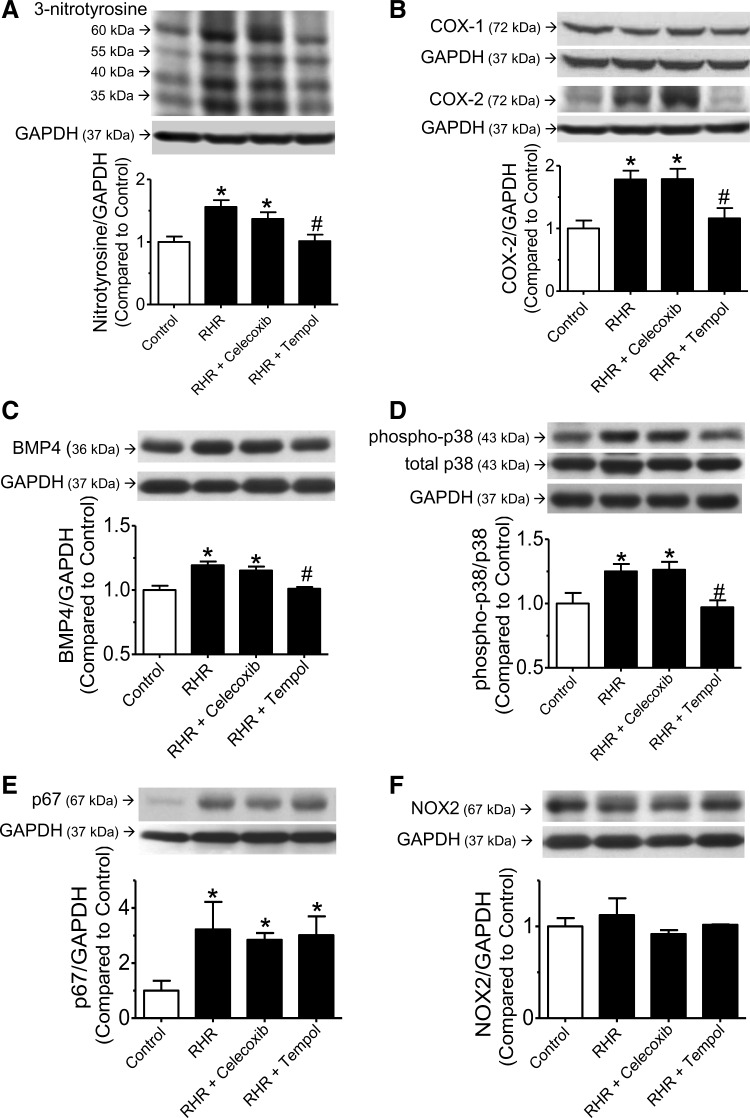
The levels of 3-nitrotyrosine (Fig. 6A), COX-2 (Fig. 6B), BMP4 (Fig. 6C), and the phosphorylated form of p38MAPK (Fig. 6D) were significantly increased in renal arteries of RHRs compared with those from control rats. Multiple bands of nitrotyrosine at ∼60, 55, 40, and 35 kDa were detected in renal arteries (Fig. 6A). Chronic treatment with tempol but not celecoxib inhibited the up-regulation of 3-nitrotyrosine, COX-2, BMP4, and p38 phosphorylation in RHR renal arteries. In contrast, the protein levels of COX-1 (Fig. 6B) and NOX2 (Fig. 6F) were similar among all groups. The expression of p67phox (Fig. 6E) was increased in renal arteries from RHR but remained unaffected after celecoxib or tempol treatment (Fig. 6E).
FIG. 6.
Protein expression in renal arteries of RHRs. Western blots showing the expression of (A) 3-nitrotyrosine (60, 55, 40, and 35 kDa); (B) COX-2 (72 kDa) and COX-1 (72 kDa); (C) BMP4 (36 kDa); (D) phospho-p38MAPK and total p38MAPK (43 kDa); (E) p67phox (67 kDa); and (F) NOX2 (67 kDa) in renal arteries from control rats, RHRs, and RHRs treated with celecoxib or tempol. GAPDH (37 kDa) was used as a house-keeping protein. Results represent mean±SEM of four experiments. *p<0.05 versus control, #p<0.05 versus RHR.
Discussion
The current study provides evidence for a crucial role of ROS-dependent COX-2-derived PGF2α in endothelial dysfunction in RH. The major findings are as follows: (i) the impaired EDRs and the augmented EDCs in renal arteries of RHRs are restored by treatment with COX-2 inhibitors, TP receptor antagonists and ROS scavengers; (ii) chronic treatment with celecoxib or tempol increases renal blood flow, reduces blood pressure, and improves endothelial function in RHRs; and (iii) ROS are a prerequisite for the occurrence of endothelial dysfunction through the generation of COX-2-derived PGF2α, suggesting that ROS activates COX-2 to release PGF2α, which impairs EDRs and induces EDCs in RHR renal arteries.
ROS contribute to the development of endothelial dysfunction in renal vasculature during the development of RH. We showed that reduced EDRs in RHR renal arteries were reversed by ROS scavengers, under both in vitro and in vivo conditions. In addition, the p67phox level increased, and NADPH oxidase inhibitor apocynin improved EDRs in RHR renal arteries (Supplementary Fig. S2). ROS production from NADPH oxidase is a major source of oxidative stress in endothelial dysfunction associated with hypertension (17, 18, 23, 30). In aortae of renovascular hypertensive mice, the blunted EDRs were prevented by genetic deletion of the NADPH oxidase subunit gp91phox (23). Recent studies also demonstrated that antioxidant treatment is able to lower blood pressure and improve EDRs in RHRs (4). The current results show that tempol treatment improved EDRs in renal arteries, reduced blood pressure (Supplementary Fig. S7), and increased renal blood flow in vivo in RHRs.
An earlier report indicated that nonselective COX inhibition normalizes the blunted EDRs in small mesenteric arteries in RHRs (10). To the best of our knowledge, there is no report about the potential impact of RH on EDC responses. The current study demonstrates that COX-2, rather than COX-1, is the key enzyme mediating the effect that reduces EDRs and augments EDC in RHR renal arteries. In addition, up-regulation of COX-2 rather than COX-1 was observed in RHR renal arteries. It was reported that COX-1, rather than COX-2, mediates EDCs in aortae from SHR (45). However, COX-2-derived constricting prostanoids have been recently suggested to impair EDRs in mesenteric arteries of SHR (40). Taken together, these observations suggest that there could be a significant difference in the involvement of COX isoforms depending on the etiology of hypertension and/or the specific vascular bed chosen for examination.
ROS might function as EDCFs in the canine basilar artery (25), and ROS facilitated EDC in aortae of SHR (45). In addition, COX-derived prostaglandins were suggested to be one major source of EDCF apart from superoxide anions (1, 14, 39, 46). The current study indicates that EDCFs derived from COX-2 were released in response to ROS stimulation based on the following findings. First, ROS scavengers prevented EDCs. Second, acetylcholine-stimulated ROS production was greater in RHR renal arteries and ROS-generating agents, such as H2O2 and HX-XO, could elicit celecoxib-inhibitable contractions and upregulate COX-2 expression. Moreover, the increase in peroxynitrite-induced protein nitration (48) observed in RHR renal arteries was inhibited after tempol treatment but was unaffected by celecoxib. On the basis of our previous report, it is worth noting that ROS is also an upstream regulator of COX-2 expression and activity that induces endothelial dysfunction in mouse arteries (42).
The current study shows that BMP4, an upstream mediator of endothelial dysfunction, was upregulated in RHR renal arteries, which is consistent with our previous finding of increased BMP4 expression in renal arteries from patients with hypertension and SHRs (42). Of importance, treatment with the antioxidant tempol but not celecoxib inhibited BMP4 upregulation. Although BMP4 is an upstream regulator of the expression of pro-inflammatory genes in endothelial cells in response to oxidative stress (29, 31, 33), BMP4 expression can also be regulated by oxidative stress (6, 33). We also found that p38 phosphorylation in RHR renal arteries was reduced after tempol treatment, thus suggesting that p38 was involved in ROS-induced endothelial dysfunction in RHRs (42).
The current study extends the understanding of arachidonic acid derivatives as EDCFs, which was supported by measurements of prostaglandin release by EIA and use of exogenously applied prostaglandins to induce vasoconstriction. The EIA results showed increased release of all types of prostaglandins in RHR renal arteries in response to acetylcholine, whereas only PGF2α, 6-keto PGF1α (the stable metabolite of PGI2), and 8-isoprostane were indicated to be COX-2-derived EDCF candidates. To verify this, exogenous PGI2, PGF2α, or 8-isoprostane were applied to determine whether they caused contractions in renal arteries. Only PGF2α produced contraction at a concentration near to that determined by EIA upon acetylcholine stimulation. Although 8-isoprostane and PGI2 contracted renal arteries, the threshold concentration for both prostaglandins to evoke contraction was much higher than that detected by EIA in response to acetylcholine. The involvement of PGD2, PGE2, or TXA2 was unlikely, because their release was prevented by COX-1 inhibitors that did not block EDCs. These results support the conclusion that PGF2α is the most likely EDCF in RHR renal arteries. Previous studies on oxidative stress-dependent COX-2 induction were mainly focused on endothelial cell inflammation and apoptosis, and PGE2 was considered the major contributor to the effect of COX-2 induction (3, 8, 24, 34). However, PGF2α was less studied (3, 22). The current study provides new information about the critical role of COX-2 and PGF2α in renal vascular hypertension. It is also noted that the pathological role of PGF2α has been recently revealed, as this prostaglandin increases blood pressure and promotes atherogenesis in mice (47). The current results also show that COX-2 inhibition, either in vivo or ex vivo, can reduce the production of the PGI2 metabolite 6-keto-PGF1α, which is unaffected by COX-1 inhibitors, thus suggesting that COX-2 is the major enzyme responsible for PGI2 synthesis in renal vasculature. This finding is different from that of a previous study concluding that COX-1 is the major enzyme of PGI2 synthesis (9). The relative importance of COX-2-derived PGI2 in the development of renal hypertension is yet to be examined.
The current study also shows that ROS do not cause contraction directly in RHR renal arteries. A more likely explanation is that the increased production of ROS stimulates endothelial COX-2 to release PGF2α, which activates TP receptors to cause contraction of vascular smooth muscle cells under renovascular hypertensive conditions. This was supported by the following observations: (i) Exogenous ROS (H2O2 or HX-XO) produced greater contractions in renal arteries from RHRs than control; (ii) ROS-induced contractions were abolished by celecoxib and S18886; (iii) ROS-induced contractions were reduced in arteries without endothelium; (iv) H2O2 stimulated PGF2α release was prevented by celecoxib; (v) The acetylcholine-stimulated release of PGF2α was inhibited by ROS scavengers in RHR arteries; (vi) PGF2α attenuated EDRs in renal arteries from control rats, which was prevented by S18886 but not by a COX-2 inhibitor. Moreover, chronic treatment with tempol, but not celecoxib, attenuated the up-regulation of both nitrotyrosine and COX-2 in RHR arteries. Taken together, the current results support the role of ROS as a trigger in the acetylcholine-stimulated release of endothelial COX-2-derived PGF2α in RHR renal arteries. However, there are other potential factors that might also be involved in the enhanced contraction in RHR renal arteries including diminished nitric oxide (NO) production, oxidative stress per se, and angiotensin II (11, 32).
In summary, the current study demonstrates that both celecoxib and tempol reduce blood pressure, increase renal blood flow, and improve endothelial function in RHRs. In RHR renal arteries, ROS is the initiator that activates endothelial COX-2 to release PGF2α, the most likely EDCF to participate in endothelial dysfunction in RHRs. Drugs targeting this ROS-COX-TP cascade may be useful in the prevention and management of RH.
Materials and Methods
Chemicals
Cycloheximide was purchased from Calbiochem, EMD Biosciences. VAS, PGF2α and PGI2 were from Cayman Chemical. Indomethacin, 5-bromo-2-(4-fluorophenyl)-3-[4-(methylsulfonyl)phenyl]-thiophene (DuP-697) and N-[2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide (NS-398), indomethacin, and 8-isoprostane were from Tocris (Avonmouth). SC-560 and 3-[(6-amino-(4-chlorobenzensulphonyl)-2-methyl-5,6,7,8-tetrahydronapht]-1-yl) propionic acid (S18886) were kind gifts from Institut de Recherches Servier. Nitroglycerin was from Schwarz Pharma. All others were purchased from Sigma-Aldrich Chemical Co. Acetylcholine, L-NAME, phenylephrine, SNP, nitroglycerin, PGI2, tiron, and tempol were prepared in distilled water; all other drugs were dissolved in dimethylsulfoxide (Sigma-Aldrich).
Induction of RH and drug treatment
The experimental protocol approved by the institutional animal care and use committee was consistent with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. RH was induced by a surgical bilateral renal artery stenosis procedure in male Sprague-Dawley rats. The rats (80–100 g) were anaesthetized with ketamine (35 mg kg−1) plus xylazine (7 mg kg−1). After performing a midline laparotomy, renal arteries of both kidneys were carefully separated from adjacent adhering tissue and then individually occluded by a silver clip (internal diameter: 0.2 mm). Sham-operated rats (Control) were subjected to laparotomy and renal artery separation only. All rats had free access to rat chow and tap water, and were maintained in their cages in a controlled environment at 23°C±1°C with a 12-h dark/light cycle. Five weeks later, RHRs with systolic blood pressure over 180 mmHg were randomly divided into three groups: RHRs receiving vehicle (RHR); RHRs receiving orally administered celecoxib at a dose of 10 mg.kg−1.day−1 for 5 weeks (RHR+Celecoxib); or RHRs receiving tempol (RHR+Tempol) at a dose of 10 mg.kg−1.day−1 for 5 weeks. Some sham-operated control rats were also orally treated with 10 mg.kg−1.day−1 celecoxib (Control+Celecoxib) or 10 mg.kg−1.day−1 tempol (Control+Tempol). Systolic blood pressure was measured by a tail-cuff method.
Vascular reactivity
Vasoreactivity of the interlobar renal arteries were measured as described (27). The effects of various compounds on EDRs and EDCs were tested. The EDRs occurred in response to the cumulative addition (0.01–30 μM) of acetylcholine in arteries that had been precontracted by phenylephrine (1 μM), and the EDCs were elicited by acetylcholine (0.1–100 μM) after pretreatment with 100 μM L-NAME, which was used to eliminate the interference of endothelium-derived NO. These effects were tested using 30-min incubations with the following compounds: SC-560 (0.3 μM; COX-1 inhibitor); VAS (300 μM; COX-1 inhibitor); celecoxib, NS398, DuP697 (3 μM; COX-2 inhibitor); indomethacin (1 μM; nonselective COX inhibitor); tiron [1 mM; superoxide dismutase mimetic] plus diethyldithiocarbamate acid [DETCA, 100 μM; hydroxyl radical (HO•) scavenger]; tempol (100 μM); apocynin (100 μM, NADPH oxidase inhibitor); allopurinol (100 μM xanthine oxidase inhibitor); S18886 (0.1 μM; thromboxane receptor antagonist); and cycloheximide (10 μM, protein synthesis inhibitor) (45). Endothelium-independent relaxations to SNP or nitroglycerin were studied in arteries without endothelium. Hydrogen peroxide (H2O2, 100 μM), PGF2α, 8-isoprostane, PGI2, and a combination of hypoxanthine (HX, 100 μM) and xanthine oxidase (XO, 0.01 unit/ml), were used to induce contraction in the presence of 100 μM L-NAME.
EPR spectroscopy
The formation of ROS was measured by a reaction with the spin trap agent TEMPONE-H using EPR spectroscopy in rat renal arteries. The detailed method is included in the Supplementary Materials.
Measurement of prostaglandins by EIA
The amounts of individual prostaglandins were measured with EIA kits (Cayman Chemical) in rat renal arteries. The detailed method is included in the Supplementary Materials.
Measurement of renal blood flow by MRI acquisition
MRI studies were performed using a 3 T clinical whole-body imaging system (Achieva; Philips Healthcare) as previously described (16). The detailed method is presented in the Supplementary Materials.
Protein extraction and western blotting
Renal arteries were isolated and frozen in liquid nitrogen and homogenized in RIPA lysis buffer. Proteins were extracted from the vessels as previously described (43). Western blot analyses were performed with appropriate specific primary antibodies including COX-2 or COX-1 (1:1000; Cayman Chemical), 3-nitrotyrosine (1:1000; Upstate Biotechnology), BMP4 (1:1000; Sigma), phospho- and total p38MAPK, p67phox (1:1000; Cell Signaling), and NOX2 (1:1000; Abcam).
Data analysis
Results are given as mean±standard error of the mean of n experiments in arteries from different rats. Contractions are expressed as active tension (force recorded/[2×length of ring]) (38). Western blots were analyzed using Quantity One (Bio-Rad). The protein expression is normalized to the level of GAPDH and is expressed relative to the control. Student's t-test was used for statistical comparison between two groups, whereas ANOVA was used for comparisons involving more than two groups. The concentration-response curves were analyzed by one-way ANOVA followed by Bonferroni's post hoc test. Probability values of less than 0.05 indicate statistically significant differences between treatments.
Sources of Funding
This study was supported by Hong Kong General Research Fund (465308, 466110, and 465611), UGC Direct Grant (2041450), National Basic Research Program of China (2012CB517805), Focused Investment Scheme from the Chinese University of Hong Kong, and the National Institutes of Health (RC2HL103400, 1U01HL100397).
Supplementary Material
Abbreviations Used
- ACh
acetylcholine
- COX
cyclooxygenase
- DTPA
diethylenetriaminepentaacetic acid
- EDC
endothelium-dependent contraction
- EDCF
endothelium-derived contracting factor
- EDR
endothelium-dependent relaxation
- EIA
enzyme immunoassay
- EPR
electron paramagnetic resonance
- HX-XO
hypoxanthine+xanthine oxidase
- L-NAME
NG-nitro-L-arginine methyl ester
- NADPH
nicotinamide adenine dinucleotide phosphate
- MRI
magnetic resonance image
- NO
nitric oxide
- PGF2α
prostaglandin F2α
- PGI2
prostacyclin
- RHR
renovascular hypertensive rat
- ROI
region of interest
- ROS
reactive oxygen species
- SEM
standard error of the mean
- SHR
spontaneously hypertensive rat
- SNP
sodium nitroprusside
- TE
time to echo
- TP
thromboxane prostanoid
- TR
repetition time
- VAS
valeryl salicylate
- 3D
three-dimensional
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adeagbo AS. Zhang X. Patel D. Joshua IG. Wang Y. Sun X. Igbo IN. Oriowo MA. Cyclo-oxygenase-2, endothelium and aortic reactivity during deoxycorticosterone acetate salt-induced hypertension. J Hypertens. 2005;23:1025–1036. doi: 10.1097/01.hjh.0000166844.42227.5c. [DOI] [PubMed] [Google Scholar]
- 2.Auch-Schwelk W. Katusic ZS. Vanhoutte PM. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- 3.Camacho M. Lopez-Belmonte J. Vila L. Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and prostaglandin I synthase activity. Circ Res. 1998;83:353–365. doi: 10.1161/01.res.83.4.353. [DOI] [PubMed] [Google Scholar]
- 4.Castro MM. Rizzi E. Rodrigues GJ. Ceron CS. Bendhack LM. Gerlach RF. Tanus-Santos JE. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic Biol Med. 2009;46:1298–1307. doi: 10.1016/j.freeradbiomed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Chenevard R. Hurlimann D. Bechir M. Enseleit F. Spieker L. Hermann M. Riesen W. Gay S. Gay RE. Neidhart M. Michel B. Luscher TF. Noll G. Ruschitzka F. Selective COX-2 inhibition improves endothelial function in coronary artery disease. Circulation. 2003;107:405–409. doi: 10.1161/01.cir.0000051361.69808.3a. [DOI] [PubMed] [Google Scholar]
- 6.Csiszar A. Smith KE. Koller A. Kaley G. Edwards JG. Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- 7.Dohi Y. Criscione L. Luscher TF. Renovascular hypertension impairs formation of endothelium-derived relaxing factors and sensitivity to endothelin-1 in resistance arteries. Br J Pharmacol. 1991;104:349–354. doi: 10.1111/j.1476-5381.1991.tb12434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eligini S. Barbieri SS. Cavalca V. Camera M. Brambilla M. De Franceschi M. Tremoli E. Colli S. Diversity and similarity in signaling events leading to rapid Cox-2 induction by tumor necrosis factor-alpha and phorbol ester in human endothelial cells. Cardiovasc Res. 2005;65:683–693. doi: 10.1016/j.cardiores.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Flavahan NA. Balancing prostanoid activity in the human vascular system. Trends Pharmacol Sci. 2007;28:106–110. doi: 10.1016/j.tips.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Fortes ZB. Costa SG. Nigro D. Scivoletto R. de Oliveira MA. de Carvalho MH. Effect of indomethacin on the microvessel reactivity of two-kidney, one-clip hypertensive rats. Arch Int Pharmacodyn Ther. 1992;316:75–89. [PubMed] [Google Scholar]
- 11.Galli SM. Phillips MI. Angiotensin II AT(1A) receptor antisense lowers blood pressure in acute 2-kidney, 1-clip hypertension. Hypertension. 2001;38:674–678. doi: 10.1161/hy09t1.095207. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Saura MF. Galisteo M. Villar IC. Bermejo A. Zarzuelo A. Vargas F. Duarte J. Effects of chronic quercetin treatment in experimental renovascular hypertension. Mol Cell Biochem. 2005;270:147–155. doi: 10.1007/s11010-005-4503-0. [DOI] [PubMed] [Google Scholar]
- 13.Ge T. Hughes H. Junquero DC. Wu KK. Vanhoutte PM. Boulanger CM. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ Res. 1995;76:1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- 14.Gluais P. Lonchampt M. Morrow JD. Vanhoutte PM. Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol. 2005;146:834–845. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldblatt H. Lynch J. Hanzal RF. Summerville WW. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffith JF. Wang YX. Zhou H. Kwong WH. Wong WT. Sun YL. Huang Y. Yeung DK. Qin L. Ahuja AT. Reduced bone perfusion in osteoporosis: likely causes in an ovariectomy rat model. Radiology. 2010;254:739–746. doi: 10.1148/radiol.09090608. [DOI] [PubMed] [Google Scholar]
- 17.Grote K. Ortmann M. Salguero G. Doerries C. Landmesser U. Luchtefeld M. Brandes RP. Gwinner W. Tschernig T. Brabant EG. Klos A. Schaefer A. Drexler H. Schieffer B. Critical role for p47phox in renin-angiotensin system activation and blood pressure regulation. Cardiovasc Res. 2006;71:596–605. doi: 10.1016/j.cardiores.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Hanna IR. Taniyama Y. Szocs K. Rocic P. Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal. 2002;4:899–914. doi: 10.1089/152308602762197443. [DOI] [PubMed] [Google Scholar]
- 19.Heitzer T. Wenzel U. Hink U. Krollner D. Skatchkov M. Stahl RA. MacHarzina R. Brasen JH. Meinertz T. Munzel T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int. 1999;55:252–260. doi: 10.1046/j.1523-1755.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- 20.Higashi Y. Oshima T. Sasaki S. Nakano Y. Kambe M. Matsuura H. Kajiyama G. Angiotensin-converting enzyme inhibition, but not calcium antagonism, improves a response of the renal vasculature to L-arginine in patients with essential hypertension. Hypertension. 1998;32:16–24. doi: 10.1161/01.hyp.32.1.16. [DOI] [PubMed] [Google Scholar]
- 21.Higashi Y. Sasaki S. Nakagawa K. Matsuura H. Oshima T. Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med. 2002;346:1954–1962. doi: 10.1056/NEJMoa013591. [DOI] [PubMed] [Google Scholar]
- 22.Hirao A. Kondo K. Takeuchi K. Inui N. Umemura K. Ohashi K. Watanabe H. Cyclooxygenase-dependent vasoconstricting factor(s) in remodelled rat femoral arteries. Cardiovasc Res. 2008;79:161–168. doi: 10.1093/cvr/cvn111. [DOI] [PubMed] [Google Scholar]
- 23.Jung O. Schreiber JG. Geiger H. Pedrazzini T. Busse R. Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109:1795–1801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 24.Karim S. Habib A. Levy-Toledano S. Maclouf J. Cyclooxygenase-1 and − 2 of endothelial cells utilize exogenous or endogenous arachidonic acid for transcellular production of thromboxane. J Biol Chem. 1996;271:12042–12048. doi: 10.1074/jbc.271.20.12042. [DOI] [PubMed] [Google Scholar]
- 25.Katusic ZS. Schugel J. Cosentino F. Vanhoutte PM. Endothelium-dependent contractions to oxygen-derived free radicals in the canine basilar artery. Am J Physiol. 1993;264:H859–H864. doi: 10.1152/ajpheart.1993.264.3.H859. [DOI] [PubMed] [Google Scholar]
- 26.Lerman LO. Nath KA. Rodriguez-Porcel M. Krier JD. Schwartz RS. Napoli C. Romero JC. Increased oxidative stress in experimental renovascular hypertension. Hypertension. 2001;37:541–546. doi: 10.1161/01.hyp.37.2.541. [DOI] [PubMed] [Google Scholar]
- 27.Leung FP. Yao X. Lau CW. Ko WH. Lu L. Huang Y. Raloxifene relaxes rat intrarenal arteries by inhibiting Ca2+ influx. Am J Physiol Renal Physiol. 2005;289:F137–F144. doi: 10.1152/ajprenal.00353.2004. [DOI] [PubMed] [Google Scholar]
- 28.Lockette W. Otsuka Y. Carretero O. The loss of endothelium-dependent vascular relaxation in hypertension. Hypertension. 1986;8:II61–II66. doi: 10.1161/01.hyp.8.6_pt_2.ii61. [DOI] [PubMed] [Google Scholar]
- 29.Maloney E. Sweet IR. Hockenbery DM. Pham M. Rizzo NO. Tateya S. Handa P. Schwartz MW. Kim F. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol. 2009;29:1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller FJ., Jr. Gutterman DD. Rios CD. Heistad DD. Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 31.Miriyala S. Gongora Nieto MC. Mingone C. Smith D. Dikalov S. Harrison DG. Jo H. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation. 2006;113:2818–2825. doi: 10.1161/CIRCULATIONAHA.106.611822. [DOI] [PubMed] [Google Scholar]
- 32.Rees D. Ben-Ishay D. Moncada S. Nitric oxide and the regulation of blood pressure in the hypertension-prone and hypertension-resistant Sabra rat. Hypertension. 1996;28:367–371. doi: 10.1161/01.hyp.28.3.367. [DOI] [PubMed] [Google Scholar]
- 33.San Martin A. Du P. Dikalova A. Lassegue B. Aleman M. Gongora MC. Brown K. Joseph G. Harrison DG. Taylor WR. Jo H. Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H2073–H2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 34.Sheu ML. Chiang CK. Tsai KS. Ho FM. Weng TI. Wu HY. Liu SH. Inhibition of NADPH oxidase-related oxidative stress-triggered signaling by honokiol suppresses high glucose-induced human endothelial cell apoptosis. Free Radic Biol Med. 2008;44:2043–2050. doi: 10.1016/j.freeradbiomed.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Stankevicius E. Martinez AC. Mulvany MJ. Simonsen U. Blunted acetylcholine relaxation and nitric oxide release in arteries from renal hypertensive rats. J Hypertens. 2002;20:1571–1579. doi: 10.1097/00004872-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Textor SC. Wilcox CS. Renal artery stenosis: a common, treatable cause of renal failure? Annu Rev Med. 2001;52:421–442. doi: 10.1146/annurev.med.52.1.421. [DOI] [PubMed] [Google Scholar]
- 37.Thomas SR. Witting PK. Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 38.Tian J. Wong WT. Tian XY. Zhang P. Huang Y. Wang N. Rosiglitazone attenuates endothelin-1-induced vasoconstriction by upregulating endothelial expression of endothelin B receptor. Hypertension. 2010;56:129–135. doi: 10.1161/HYPERTENSIONAHA.110.150375. [DOI] [PubMed] [Google Scholar]
- 39.Virdis A. Colucci R. Fornai M. Duranti E. Giannarelli C. Bernardini N. Segnani C. Ippolito C. Antonioli L. Blandizzi C. Taddei S. Salvetti A. Del Tacca M. Cyclooxygenase-1 is involved in endothelial dysfunction of mesenteric small arteries from angiotensin II-infused mice. Hypertension. 2007;49:679–686. doi: 10.1161/01.HYP.0000253085.56217.11. [DOI] [PubMed] [Google Scholar]
- 40.Virdis A. Colucci R. Versari D. Ghisu N. Fornai M. Antonioli L. Duranti E. Daghini E. Giannarelli C. Blandizzi C. Taddei S. Del Tacca M. Atorvastatin prevents endothelial dysfunction in mesenteric arteries from spontaneously hypertensive rats: role of cyclooxygenase 2-derived contracting prostanoids. Hypertension. 2009;53:1008–1016. doi: 10.1161/HYPERTENSIONAHA.109.132258. [DOI] [PubMed] [Google Scholar]
- 41.Widlansky ME. Price DT. Gokce N. Eberhardt RT. Duffy SJ. Holbrook M. Maxwell C. Palmisano J. Keaney JF., Jr. Morrow JD. Vita JA. Short- and long-term COX-2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension. 2003;42:310–315. doi: 10.1161/01.HYP.0000084603.93510.28. [DOI] [PubMed] [Google Scholar]
- 42.Wong WT. Tian XY. Chen Y. Leung FP. Liu L. Lee HK. Ng CF. Xu A. Yao X. Vanhoutte PM. Tipoe GL. Huang Y. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: implications on hypertension. Circ Res. 2010;107:984–991. doi: 10.1161/CIRCRESAHA.110.222794. [DOI] [PubMed] [Google Scholar]
- 43.Wong WT. Tian XY. Xu A. Ng CF. Lee HK. Chen ZY. Au CL. Yao X. Huang Y. Angiotensin II type 1 receptor-dependent oxidative stress mediates endothelial dysfunction in type 2 diabetic mice. Antioxid Redox Signal. 2010;13:757–768. doi: 10.1089/ars.2009.2831. [DOI] [PubMed] [Google Scholar]
- 44.Yang D. Feletou M. Boulanger CM. Wu HF. Levens N. Zhang JN. Vanhoutte PM. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang D. Levens N. Zhang JN. Vanhoutte PM. Feletou M. Specific potentiation of endothelium-dependent contractions in SHR by tetrahydrobiopterin. Hypertension. 2003;41:136–142. doi: 10.1161/01.hyp.0000047669.93078.a7. [DOI] [PubMed] [Google Scholar]
- 46.Yogi A. Callera GE. Hipolito UV. Silva CR. Touyz RM. Tirapelli CR. Ethanol-induced vasoconstriction is mediated via redox-sensitive cyclo-oxygenase-dependent mechanisms. Clin Sci (Lond) 2010;118:657–668. doi: 10.1042/CS20090352. [DOI] [PubMed] [Google Scholar]
- 47.Yu Y. Lucitt MB. Stubbe J. Cheng Y. Friis UG. Hansen PB. Jensen BL. Smyth EM. FitzGerald GA. Prostaglandin F2alpha elevates blood pressure and promotes atherosclerosis. Proc Natl Acad Sci U S A. 2009;106:7985–7990. doi: 10.1073/pnas.0811834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou MH. Shi C. Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51:198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.