Abstract
5-Fluorouracil (5-FU) chemotherapy is the first choice treatment for advanced hepatocellular carcinoma (HCC), and resistance is the major obstacle to successful treatment. Recent studies have reported that epithelial-to-mesenchymal transition (EMT) is associated with chemoresistance in cancers. We speculated that EMT and 5-FU metabolism are related to the mechanism of 5-FU resistance. First, two 5-FU-resistant cell lines, HLF-R4 and HLF-R10, were established from the HLF undifferentiated human HCC cell line. Whereas cell growth was similar in the HLF and HLF-R cell lines, HLF-Rs are about 4- and 10-fold more resistant compared with the HLF cells; thus, we named these cell lines HLF-R4 and HLF-R10, respectively. The terminal deoxyribonucleotidyl transferase-mediated dUTP nick end labeling assay also showed a dramatically decreased number of apoptotic cells in the HLF-Rs after treatment with 5-FU. We next assessed the characteristics of the HLF, HLF-R4 and HLF-R10 cells. Consistent with our hypothesis, the HLF-Rs had typical morphologic phenotypes of EMT, loss of cell-cell adhesion, spindle-shaped morphology and increased formation of pseudopodia. Real-time quantitative reverse transcriptase polymerase chain reaction data showed downregulated E-cadherin and upregulated Twist-1 and also indicated that EMT changes occurred in the HLF-Rs. We also found decreased ribonucleotide reductase and increased multidrug resistance protein 5 genes in the HLF-R cells. Our results suggested that the metabolism of EMT and 5-FU has important roles in 5-FU chemoresistance in the HLF-R cells, and that the HLF-R cells would be useful in vitro models for understanding the 5-FU-resistant mechanisms in HCC.
Keywords: 5-fluorouracil, drug resistance, hepatocellular carcinoma, epithelial-to-mesenchymal transition
Introduction
Hepatocellular carcinoma (HCC) is one of the most frequently occurring malignant tumors and a common cause of cancer mortality worldwide (1). The results of surgical treatments for advanced HCC, such as liver resection or liver transplantation, have not been satisfactory. To date, chemotherapy, including transcatheter arterial infusion, is the first choice for advanced HCC (2,3). 5-Fluorouracil (5-FU) is a commonly used chemotherapeutic agent that is effective for treating a wide variety of malignant tumors. However, the effectiveness of the chemotherapy is limited because of acquired or intrinsic drug resistance (4,5).
The development of resistance to 5-FU appears to be a major impediment to the successful chemotherapy of human cancers. 5-FU decreases the biosynthesis of pyrimidine nucleotides by inhibiting thymidylate synthase, which catalyzes the rate-limiting step in DNA synthesis (6–10). Although the mechanism of resistance to 5-FU remains unclear, a possible mechanism is that alterations of plasma membrane proteins reduce the accumulation of 5-FU within tumor cells (11). Two studies also reported that epithelial-to-mesenchymal transition (EMT) was closely related to chemoresistance in the colorectal and pancreatic cancers (12,13).
EMT is initially observed in embryonic development in which cells lose epithelial characteristics and gain mesenchymal properties to increase motility and invasiveness (12,14). Previous research has suggested that EMT is also important in tumor progression, metastasis, and chemoresistance (14,15) and is induced by growth factors, such as hepatocyte growth factor, transforming growth factor β, and epidermal growth factor, implicated in these processes (16).
Tissue culture systems have been established to study the biochemical, physiologic, and genetic bases of alterations that result in development of multidrug resistance. To understand drug resistance well, establishing cultured cell lines resistant to anticancer drugs is necessary. In the current study, we established two 5-FU-resistant cell lines, HLF-R4 and HLF-R10, from an HCC cell line and investigated for the first time the mechanisms of 5-FU resistance including EMT.
Materials and methods
Cell lines
The HLF cell line, derived from undifferentiated human hepatocellular carcinoma, was obtained from the Japanese Cancer Research Resources Bank and maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 100 units/ml penicillin and streptomycin (Gibco). According to previously described methods (17–20), two 5-FU-resistant sublines, HLF-Rs, were established by repeated subcultures in the presence of stepwise-increases in concentrations of 5-FU (5, 7.5, 10 and 20 μM) (Wako, Osaka, Japan). Because the chemoresistance of both cell lines are 4- and 10-fold increased, respectively, the sublines were named HLF-R4 and HLF-R10, and they are fully resistant to 5-FU and can grow exponentially in the presence of 10 and 20 μM of 5-FU. The HLF-Rs showed no loss of resistance even after 2 months of culture in a drug-free medium.
Cell growth
To investigate cell growth in the HLF, HLF-R4, and HLF-R10 cells, we performed a cell proliferation assay. The experiments were carried out for 168 h, and the number of cells were counted every 24 h. The cells in three samples were trypsinized and counted using a hemocytometer at the indicated time points.
Chemosensitivity assay
The cells were seeded at a concentration of 2.0×103 in each well of a 96-well plate in DMEM containing 10% FBS. After 24 h, culture medium was replaced with DMEM with 10% FBS and various concentrations of 5-FU (6.25, 12.5, 25, 50, 100, 200, 400, 800 and 1600 μM). After further incubation for 72 h, a cell viability assay was carried out using the Cell Counting kit-8 according to the manufacturer's instructions (Dojindo, Kumamoto, Japan). Six wells were used for each drug concentration and the experiment was replicated three times. The 50% inhibitory concentration (IC50) was calculated from the survival curves.
TUNEL assay
The cells were seeded at a concentration of 5.0×105 on Lab-TekII chamber slides (Nalge Nunc International, Rochester, NY) in DMEM containing 10% FBS. After incubation for 24 h, the culture medium was replaced with DMEM with 10% FBS and 70 μM of 5-FU. After further incubation for 24 h, the cells on the chamber slides were washed twice with phosphate buffered saline, air dried, and fixed in 4% paraformaldehyde at room temperature for 30 min. The terminal deoxyribonucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was performed using an In Situ Apoptosis Detection kit according to the manufacturer's instructions (Takara, Tokyo, Japan). The cells were viewed and photographed under a fluorescence microscope.
Preparation of cDNA
Total RNA was isolated using an RNeasy Mini kit (Qiagen, Chatsworth, CA) according to the manufacturer's instructions. cDNA was generated from 5 μg of total RNA using an Ominiscript RT kit (Qiagen) and random hexamer (Sigma Genosys, Ishikari, Japan).
mRNA expression analysis
Real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed to evaluate the expression levels of five target genes in HLF and its derivatives. qRT-PCR was carried out with one method using a LightCycler FastStart DNA Master SYBR Green 1 kit (Roche, Mannheim, Germany). The specific primers for the target genes are listed in Table I. Amplified products were analyzed by 3% agarose gel electrophoresis to ascertain size and purity. The PCR reactions using the LightCycler apparatus were performed in a final volume of 20 μl of a reaction mixture comprised of 2 μl of FirstStart DNA Master SYBR Green I mix, 3 mM MgCl2, and l μM of the primers, according to the manufacturer's instructions. The reaction mixture was loaded into glass capillary tubes and subjected to an initial denaturation at 95°C for 10 min, followed by 45 rounds of amplification at 95°C (10 sec) for denaturation, 62°C (10 sec) for annealing, and 72°C (10 sec) for extension, with a temperature slope of 20°C/sec. The transcript amounts for the target genes were estimated from the respective standard curves and normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript amount determined in corresponding samples.
Table I.
Specific primers.
| Forward (5′-3′) | Reverse (5′-3′) | |
|---|---|---|
| RNR-R1 | AGAGAAGGAGAGGAACACAGCAG | AGCAAAGCCTTACCACCTCAAG |
| RNR-R2 | GCCCCTGTTAAGTGGTGAAATC | GCCAGAATAAGACACTGGGTGAC |
| MRP5 | TGAGACAGAAGCTCGATTCACC | AGGGAGGTTTTCTCGGTACCTC |
| E-cadherin | CTCTTCCAGGAACCTCTGTGATG | CCACACTGATGACTCCTGTGTTC |
| Twist-1 | GCCGGAGACCTAGATGTCATTG | CTATCAGAATGCAGAGGTGTGAGG |
| GAPDH | GAGCCAAAAGGGTCATCATCTC | GGTCATGAGTCCTTCCACGATAC |
Statistical analysis
The statistical significance was evaluated using the Mann-Whitney U test. P<0.05 was considered statistically significant.
Results
Cell growth
To obtain 5-FU-resistant cells, the HLF cells were treated with increasing concentrations of 5-FU up to 20 μM, and two clones, designated HLF-R4 and HLF-R10, were established almost 1.5 years later. There were no significant differences in cellular proliferation among the HLF, HLF-R4, and HLF-R10 cells (Fig. 1).
Figure 1.
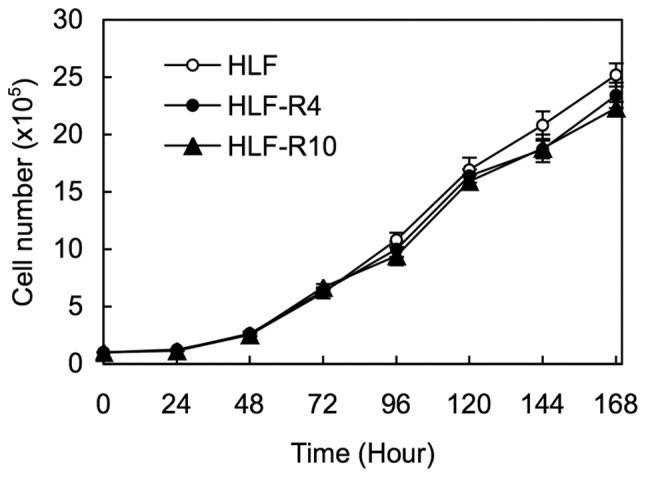
Cell growth assay. The experiments were carried out for 168 h, with counting of the number of cells every 24 h. The proliferation rates of the HLF-R4 (●) and HLF-R10 (▴) cells are similar to that of the HLF cells (○). The results are expressed as the means ± standard error of the mean values from three assays.
Chemosensitivity assay
The IC50 data indicated that the 5-FU-resistance levels of the HLF-R4 cells (IC50, 69.80 μM) and HLF-R10 cells (IC50, 193.47 μM) were 3.9- and 10.8-fold greater than that of the HLF cells (IC50, 17.92 μM) (Fig. 2).
Figure 2.
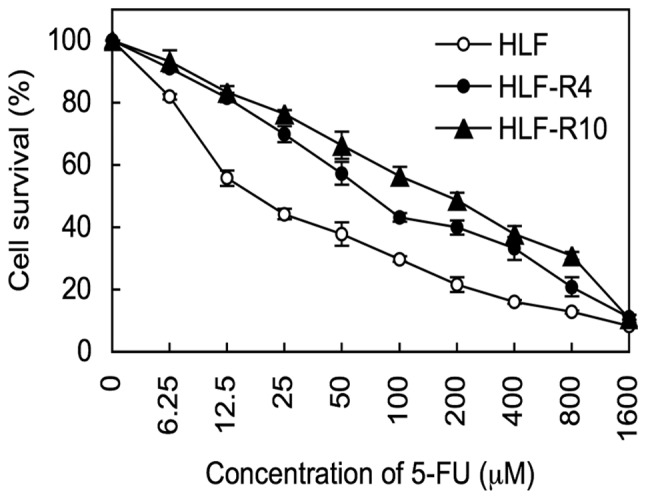
Chemosensitivity assay. IC50 values of 5-FU for the HLF (○), HLF-R4 (●), and HLF-R10 (▴) cells are 17.92 μM, 69.80 μM and 193.47 μM, respectively. Therefore, the 5-FU resistance of the HLF-R4 cells is 3.9-fold, and that of the HLF-R10 cells is 10.8-fold greater than that of the HLF cells. The results are expressed as the means ± standard error of the mean values from three assays.
TUNEL assay
TUNEL staining showed a dramatic increase in the number of cells that were stained green, which indicated that apoptosis occurred in HLF cells exposed to 5-FU, whereas 5-FU-induced apoptosis was prevented in the HLF-R4 and HLF-R10 cells (Fig. 3).
Figure 3.
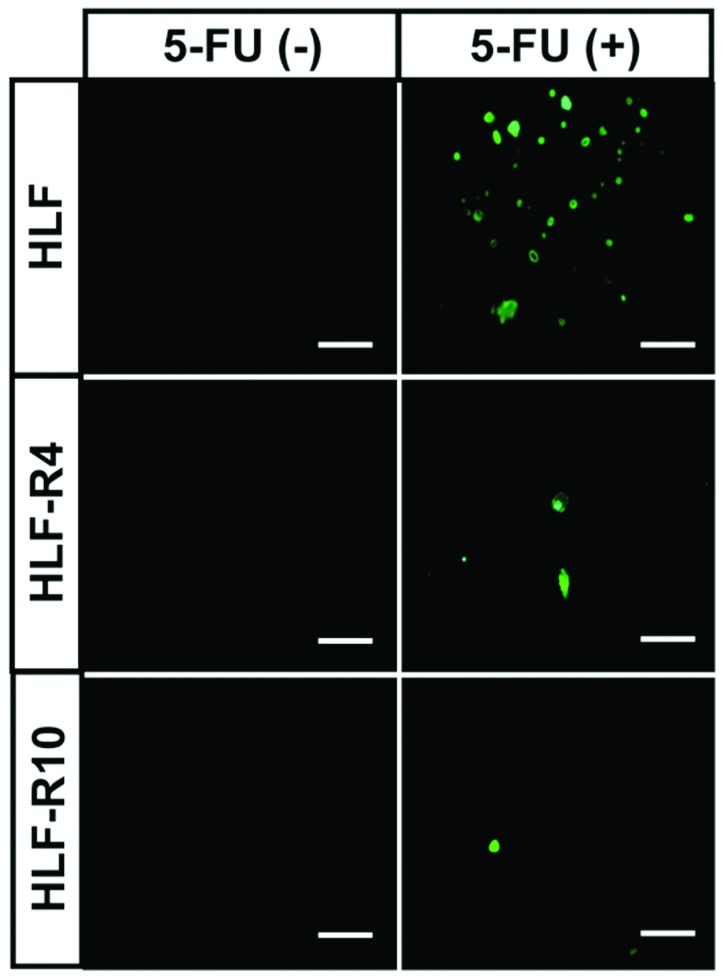
TUNEL assay. To evaluate the effect of 5-FU on the HLF, HLF-R4, and HLF-R10 cells, a TUNEL assay was used to confirm the apoptotic changes. TUNEL staining shows a dramatic increase in the number of cells that were stained green, which indicates that apoptosis occurred in HLF cells exposed to 5-FU. 5-FU-induced apoptosis is inhibited in the HLF-R4 and HLF-R10 cells. The experiments were repeated three times with similar results. Scale bar = 50 μm.
Cell morphology
The 5-FU-resistant cells, HLF-R4 and HLF-R10, were morphologically distinct from their parental cell line (Fig. 4). The resistant cells had loss of cell-cell adhesion, spindle-shaped morphology, and increased formation of pseudopodia. These changes are typical phenotypes of EMT.
Figure 4.
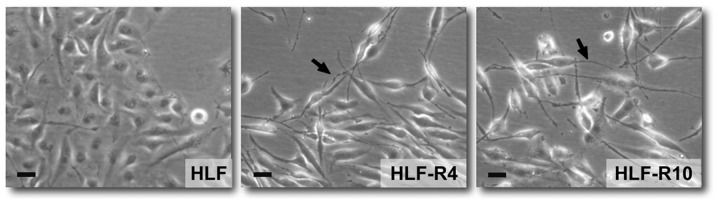
Cell morphology. 5-FU-resistant cells (HLF-R4 and HLF-R10) are morphologically distinct from their parental cell line, HLF. The resistant cells have loss of cell-cell adhesion, spindle-shaped morphology, and increased formation of pseudopodia (arrow). Scale bar, 20 μm.
Evaluation of the expression of genes putatively related to 5-FU resistance and EMT
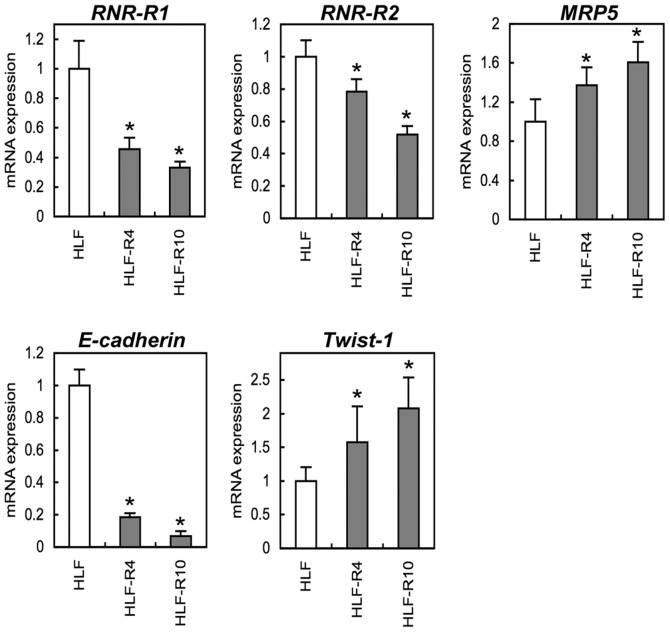
Consistent with our hypothesis that EMT occurs in 5-FU-resistant cells, qRT-PCR showed decreased E-cadherin and increased Twist-1 by in the HLF-Rs. Among the genes associated with 5-FU metabolism, we also found that mRNA expression of ribonucleotide reductases (RNR-R1, RNR-R2) was down-regulated and multi-drug resistance protein 5 (MRP5) was up-regulated in the HLF-Rs cells in a 5-FU-chemoresistant level-dependent manner (Fig. 5) (p<0.05).
Figure 5.
mRNA expression analysis. qRT-PCR was performed to investigate mRNA levels of RNR-R1, RNR-R2, MRP5, E-cadherin, and Twist-1. PCR shows up-regulation of MRP5 and Twist-1 and down-regulation of RNR-R1, RNR-R2, and E-cadherin dependent on the level of 5-FU chemoresistance. Data are expressed as the means ± standard error of the mean values from three assays (*p<0.05 by the Mann-Whitney U test).
Discussion
5-FU is key anticancer chemotherapy used to treat solid tumors, such as gastric, colorectal, pancreas, breast, and lung carcinomas (21–25). 5-FU also has been preferentially used alone or combined with other chemotherapeutic drugs for HCCs (2,3). Although several mechanisms of 5-FU resistance have been investigated, no one mechanism completely explains the clinical response to 5-FU chemotherapy. Since it is extremely important to understand the resistance mechanism in order to develop better treatments, establishing 5-FU-resistant cells was indispensable to this type of study. Cell lines that are resistant to 5-FU have been established from several cancers (6,26–28). However, to date, only the Bel7402/5-FU cell line from HCC has been reported to be resistant to 5-FU (29). To obtain further detailed information on 5-FU resistance in HCC, in the current study we evaluated two new 5-FU-resistant cell lines, HLF-R4 and HLF-R10, from an undifferentiated HCC cell line that showed different resistance levels to 5-FU.
Consistent with previous reports that showed that 5-FU metabolism and activity of 5-FU transport were closely related to 5-FU resistance (30,31), the current data showed down-regulated RNRs and up-regulated MRP5 genes. RNR, a key enzyme in 5-FU metabolism, catalyzed 5-fluorouridine diphosphate to 5-fluorodeoxyuridine diphosphate, the main precursor of 5-fluorodeoxyuridine monophosphate (FdUMP) (32). Decreased RNR activity leads to a low FdUMP level followed by inhibited thymidylate synthase cellular activity, indicating that acquired resistance to 5-FU occurs in the cells (6–10). MRP5, an energy-dependent ATP-binding cassette transporter protein (33), mediates the ATP-dependent transport of various substrates, such as monophosphate metabolites (30), across biologic membranes (34) and is responsible for broad-spectrum resistance to chemotherapy including 5-FU. 5-FU metabolism may contribute to a basic understanding of the molecular mechanisms of chemoresistance. Further study is necessary to identify other mechanisms.
In addition to typical morphologic phenotypes of EMT in the HLF-Rs cells, we found decreased E-cadherin expression caused by Twist-1 expression, which is consistent with the results of Matsuo et al (35). These findings reflected an important process by which cancer cells may potentially become chemoresistant. Even though the relationship between EMT and chemoresistance remains unclear, induction of EMT in 5-FU-chemoresistant HCC cells might represent a new potentially exciting research area into 5-FU-resistance mechanisms. Thus, EMT is likely to be a potential therapeutic target for development of anticancer drugs in HCCs. Our data should eventually benefit patients with advanced HCC or 5-FU-chemoresistant HCCs for whom there currently are few effective treatment options.
In conclusion, we found that alterations of enzymes affecting 5-FU transport and metabolism as well as EMT were observed in the HLF-Rs. Nevertheless, it seems likely that multiple mechanisms may lead to 5-FU resistance. To the best of our knowledge, the Bel7402/5-FU cell line showed no EMT changes (29), 5-FU-resistant cell line associated with EMT is clearly required in HCC. Therefore, the HLF-Rs cells might be useful in vitro models for understanding 5-FU-resistant mechanisms in HCCs. Further, the HLF-R4 and HLF-R10 cells have different degrees of 5-FU resistance, so there are advantages to investigating the process of 5-FU resistance.
Acknowledgements
We thank Dr Mamoru Nukatsuka (Taiho Pharmaceutical Co., Ltd.) for helpful discussion and Yuhki Suzuki, Chisato Kitayama, and Rina Ito (Department of Drug Information and Communication, Graduate School of Pharmaceutical Sciences, Chiba University) for their considerable contributions to cell culture in the process of establishment of the HLF-Rs. We also thank Lynda C. Charters for editing this manuscript.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Wang XN, Han X, Xu LN, Yin LH, Xu YW, Qi Y, Peng JY. Enhancement of apoptosis of human hepatocellular carcinoma SMMC-7721 cells through synergy of berberine and evodiamine. Phytomedicine. 2008;15:1062–1068. doi: 10.1016/j.phymed.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Ming ZJ, Hu Y, Qiu YH, Cao L, Zhang XG. Synergistic effects of beta-aescin and 5-fluorouracil in human hepatocellular carcinoma SMMC-7721 cells. Phytomedicine. 2010;17:575–580. doi: 10.1016/j.phymed.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Higuchi E, Oridate N, Furuta Y, Suzuki S, Hatakeyama H, Sawa H, Sunayashiki-Kusuzaki K, Yamazaki K, Inuyama Y, Fukuda S. Differentially expressed genes associated with CIS-diamminedichloroplatinum (II) resistance in head and neck cancer using differential display and CDNA microarray. Head Neck. 2003;25:187–193. doi: 10.1002/hed.10204. [DOI] [PubMed] [Google Scholar]
- 5.Scotto KW, Bertino JR. The Molecular Basis of Cancer. 2nd edition. W. B. Sanders; Foster City: 2001. Natural and acquired resistance to chemotherapeutic agents; pp. 407–422. [Google Scholar]
- 6.Johnston PG, Drake JC, Trepel J, Allegra CJ. Immunological quantitation of thymidylate synthase using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive and -resistant human cancer cell lines. Cancer Res. 1992;52:4306–4312. [PubMed] [Google Scholar]
- 7.Aschele C, Sobrero A, Faderan MA, Bertino JR. Novel mechanism(s) of resistance to 5-fluorouracil in human colon cancer (HCT-8) sublines following exposure to two different clinically relevant dose schedules. Cancer Res. 1992;52:1855–1864. [PubMed] [Google Scholar]
- 8.Copur S, Aiba K, Drake JC, Allegra CJ, Chu E. Thymidylate synthase gene amplification in human colon cancer cell lines resistant to 5-fluorouracil. Biochem Pharmacol. 1995;49:1419–1426. doi: 10.1016/0006-2952(95)00067-a. [DOI] [PubMed] [Google Scholar]
- 9.Berger SH, Jenh CH, Johnson LF, Berger FG. Thymidylate synthase overproduction and gene amplification in fluorodeoxyuridine-resistant human cells. Mol Pharmacol. 1985;28:461–467. [PubMed] [Google Scholar]
- 10.Berger SH, Berger FG. Thymidylate synthase as a determinant of 5-fluoro-2′-deoxyuridine response in human colonic tumor cell lines. Mol Pharmacol. 1988;34:474–479. [PubMed] [Google Scholar]
- 11.Jin S, Scotto KW. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol Cell Biol. 1998;18:4377–4384. doi: 10.1128/mcb.18.7.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, Gray MJ, Cheng H, Hoff PM, Ellis LM. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12:4147–4153. doi: 10.1158/1078-0432.CCR-06-0038. [DOI] [PubMed] [Google Scholar]
- 13.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 14.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 15.Thiery JP, Chopin D. Epithelial cell plasticity in development and tumor progression. Cancer Metastasis Rev. 1999;18:31–42. doi: 10.1023/a:1006256219004. [DOI] [PubMed] [Google Scholar]
- 16.Elliott BE, Hung WL, Boag AH, Tuck AB. The role of hepatocyte growth factor (scatter factor) in epithelial-mesenchymal transition and breast cancer. Can J Physiol Pharmacol. 2002;80:91–102. doi: 10.1139/y02-010. [DOI] [PubMed] [Google Scholar]
- 17.Chung YM, Park S, Park JK, Kim Y, Kang Y, Yoo YD. Establishment and characterization of 5-fluorouracil-resistant gastric cancer cells. Cancer Lett. 2000;159:95–101. doi: 10.1016/s0304-3835(00)00535-8. [DOI] [PubMed] [Google Scholar]
- 18.Negoro K, Yamano Y, Fushimi K, Saito K, Nakatani K, Shiiba M, Yokoe H, Bukawa H, Uzawa K, Wada T, Tanzawa H, Fujita S. Establishment and characterization of a cisplatin-resistant cell line, KB-R, derived from oral carcinoma cell line, KB. Int J Oncol. 2007;30:1325–1332. doi: 10.3892/ijo.30.6.1325. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M, Nakatani K, Uzawa K, Ono K, Uesugi H, Ogawara K, Shiiba M, Bukawa H, Yokoe H, Wada T, Fujita S, Tanzawa H. Establishment and characterization of a cisplatin-resistant oral squamous cell carcinoma cell line, H-1R. Oncol Rep. 2005;14:1281–1286. [PubMed] [Google Scholar]
- 20.Nakatani K, Nakamura M, Uzawa K, Wada T, Seki N, Tanzawa H, Fujita S. Establishment and gene analysis of a cisplatin-resistant cell line, Sa-3R, derived from oral squamous cell carcinoma. Oncol Rep. 2005;13:709–714. [PubMed] [Google Scholar]
- 21.Lee JH, Kim MC, Oh SY, Kwon HC, Kim SH, Kwon KA, Lee S, Jeong JS, Choi SR, Kim HJ. Predictive value of in vitro adeno-sine triphosphate-based chemotherapy response assay in advanced gastric cancer patients who received oral 5-Fluorouracil after curative resection. Cancer Res Treat. 2011;43:117–123. doi: 10.4143/crt.2011.43.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, Maillart PJ, Goudier MJ, Burtin PC, Delva RG, Lortholary AH, Gesta PH, Larra FG. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer. 1996;77:441–451. doi: 10.1002/(SICI)1097-0142(19960201)77:3<441::AID-CNCR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Ohge H, Sueda T. Impact of adjuvant gemcitabine plus S-1 chemotherapy after surgical resection for adenocarcinoma of the body or tail of the pancreas. J Gastrointest Surg. 2009;13:85–92. doi: 10.1007/s11605-008-0650-4. [DOI] [PubMed] [Google Scholar]
- 24.Uday CK, Devarayalu BVSK, Mahmood S. Overall survival rate in breast cancer patients treated with 5-fluorouracil: a review of randomized clinical trials. Int J Pharmaceut Biomed Res. 2010;1:132–135. [Google Scholar]
- 25.Huang CL, Yokomise H, Kobayashi S, Fukushima M, Hitomi S, Wada H. Intratumoral expression of thymidylate synthase and dihydropyrimidine dehydrogenase in non-small cell lung cancer patients treated with 5-FU-based chemotherapy. Int J Oncol. 2000;17:47–54. [PubMed] [Google Scholar]
- 26.Plasencia C, Rooney PH, Taron M, Martinez-Balibrea E, McLeod HL, Abad A. Chromosomal imbalance maps of human 5FU-resistant colorectal cancer cell lines: implications in the analysis of 5FU-acquired resistance mechanisms. Int J Oncol. 2003;22:945–953. doi: 10.3892/ijo.22.5.945. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka T, Bai T, Toujima S. Establishment and characterization of monoclonal 5-fluorouracil-resistant cell lines derived from human endometrial adenocarcinoma. Int J Oncol. 2010;37:731–736. doi: 10.3892/ijo_00000722. [DOI] [PubMed] [Google Scholar]
- 28.Wen J, Zheng B, Hu Y, Zhang X, Yang H, Luo KJ, Li YF, Fu JH. Establishment and biological analysis of the EC109/CDDP multidrug-resistant esophageal squamous cell carcinoma cell line. Oncol Rep. 2009;22:65–71. doi: 10.3892/or_00000407. [DOI] [PubMed] [Google Scholar]
- 29.Huang M, Liu G. The study of innate drug resistance of human hepatocellular carcinoma Bel7402 cell line. Cancer Lett. 1999;135:97–105. doi: 10.1016/s0304-3835(98)00280-8. [DOI] [PubMed] [Google Scholar]
- 30.Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W, III, Dantzig AH. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther. 2005;4:855–863. doi: 10.1158/1535-7163.MCT-04-0291. [DOI] [PubMed] [Google Scholar]
- 31.Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T, Kohgo Y. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96:457–463. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukushima M, Fujioka A, Uchida J, Nakagawa F, Takechi T. Thymidylate synthase (TS) and ribonucleotide reductase (RNR) may be involved in acquired resistance to 5-fluorouracil (5-FU) in human cancer xenografts in vivo. Eur J Cancer. 2001;37:1681–1687. doi: 10.1016/s0959-8049(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 33.Hinoshita E, Uchiumi T, Taguchi K, Kinukawa N, Tsuneyoshi M, Maehara Y, Sugimachi K, Kuwano M. Increased expression of an ATP-binding cassette superfamily transporter, multidrug resistance protein 2, in human colorectal carcinomas. Clin Cancer Res. 2000;6:2401–2407. [PubMed] [Google Scholar]
- 34.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 35.Matsuo N, Shiraha H, Fujikawa T, Takaoka N, Ueda N, Tanaka S, Nishina S, Nakanishi Y, Uemura M, Takaki A, Nakamura S, Kobayashi Y, Nouso K, Yagi T, Yamamoto K. Twist expression promotes migration and invasion in hepatocellular carcinoma. BMC Cancer. 2009;9:240–251. doi: 10.1186/1471-2407-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]