Abstract
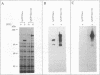
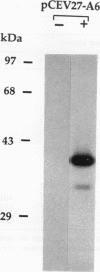
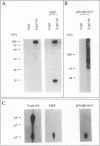
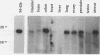
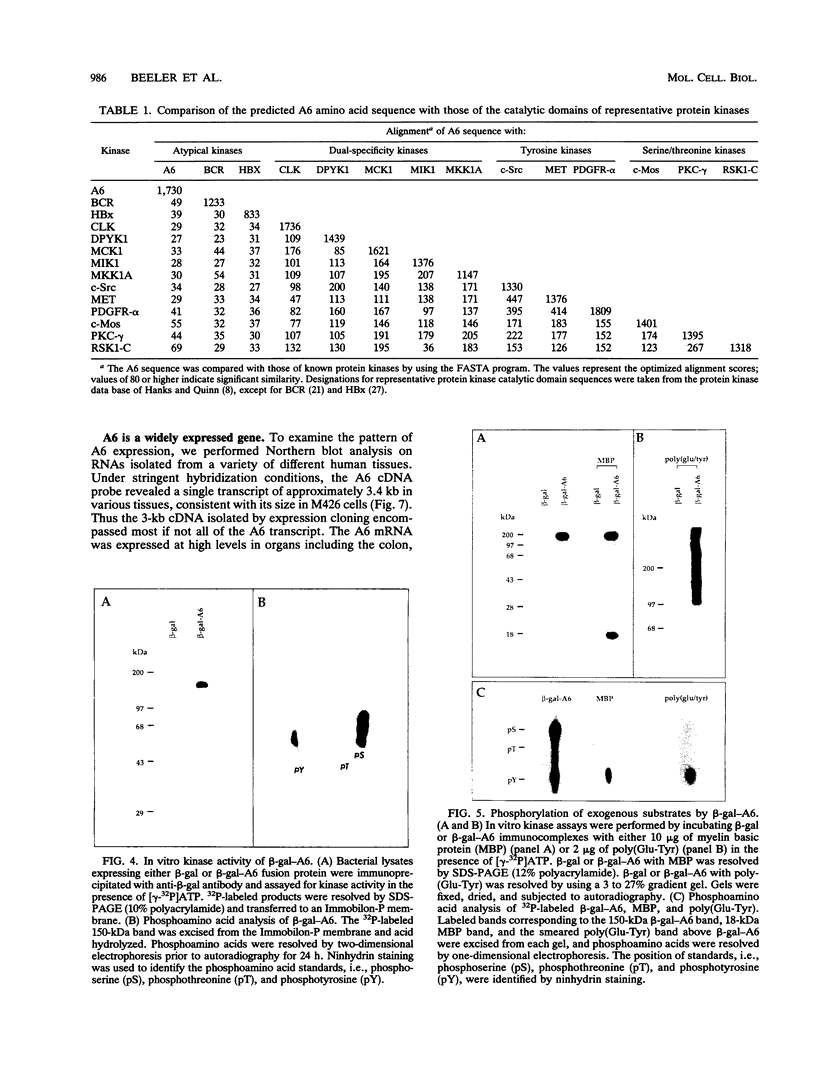
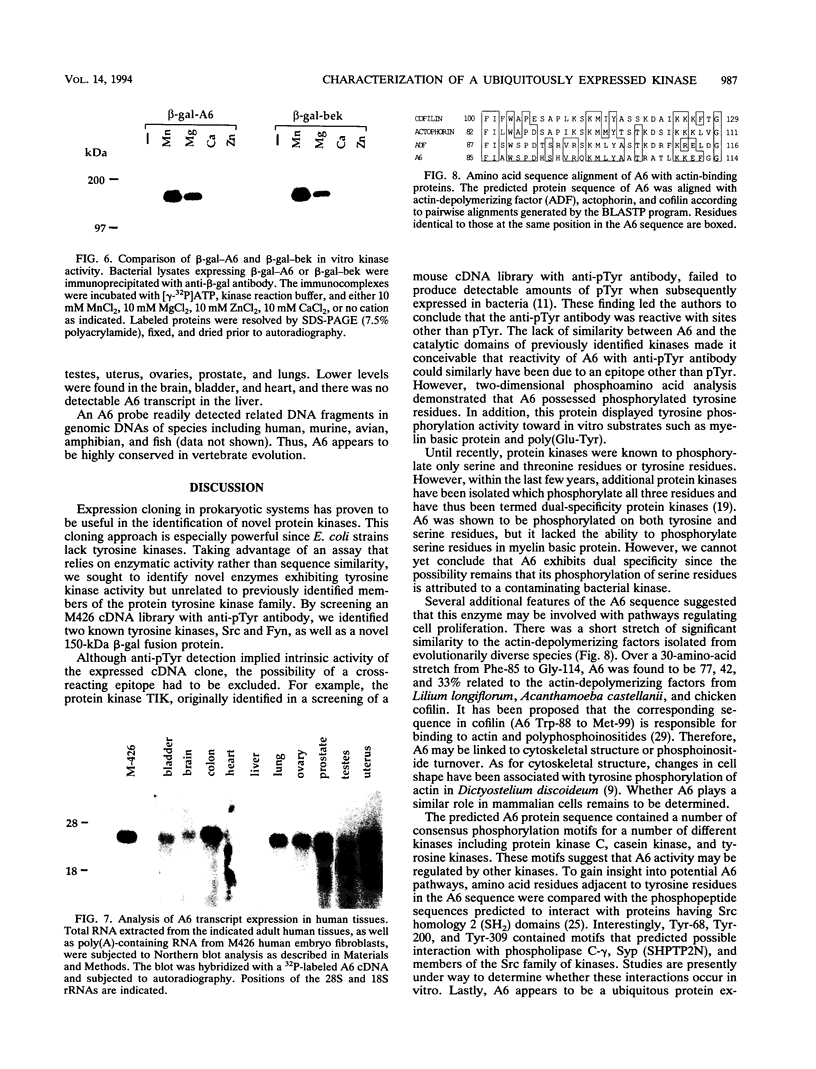
Screening of a human embryonic lung fibroblast cDNA expression library with antiphosphotyrosine antibodies led to isolation of a novel protein kinase. A clone, designated A6, contained a 3-kb cDNA insert with a predicted open reading frame of 350 amino acids. DNA sequence analysis failed to reveal any detectable similarity with previously known genes, and the predicted A6 protein lacked any of the motifs commonly conserved in the catalytic domains of protein kinases. However, the bacterially expressed beta-galactosidase-A6 fusion protein demonstrated both tyrosine and serine phosphorylation in an in vitro kinase assay and phosphorylated exogenous substrates including myelin basic protein specifically on tyrosine residues. The enzyme also displayed biochemical properties analogous to those of other protein tyrosine kinases. The A6 gene was found to be expressed widely at the transcript level in normal tissues and was evolutionarily conserved. Thus, A6 represents a novel tyrosine kinase which is highly divergent from previously described members of this important class of regulatory molecules.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Growth factors and cancer. Science. 1991 Nov 22;254(5035):1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ben-David Y., Letwin K., Tannock L., Bernstein A., Pawson T. A mammalian protein kinase with potential for serine/threonine and tyrosine phosphorylation is related to cell cycle regulators. EMBO J. 1991 Feb;10(2):317–325. doi: 10.1002/j.1460-2075.1991.tb07952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douville E. M., Afar D. E., Howell B. W., Letwin K., Tannock L., Ben-David Y., Pawson T., Bell J. C. Multiple cDNAs encoding the esk kinase predict transmembrane and intracellular enzyme isoforms. Mol Cell Biol. 1992 Jun;12(6):2681–2689. doi: 10.1128/mcb.12.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Haring H. U., Kasuga M., White M. F., Crettaz M., Kahn C. R. Phosphorylation and dephosphorylation of the insulin receptor: evidence against an intrinsic phosphatase activity. Biochemistry. 1984 Jul 3;23(14):3298–3306. doi: 10.1021/bi00309a028. [DOI] [PubMed] [Google Scholar]
- Howard P. K., Sefton B. M., Firtel R. A. Tyrosine phosphorylation of actin in Dictyostelium associated with cell-shape changes. Science. 1993 Jan 8;259(5092):241–244. doi: 10.1126/science.7678470. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Icely P. L., Gros P., Bergeron J. J., Devault A., Afar D. E., Bell J. C. TIK, a novel serine/threonine kinase, is recognized by antibodies directed against phosphotyrosine. J Biol Chem. 1991 Aug 25;266(24):16073–16077. [PubMed] [Google Scholar]
- Ishibashi T., Bottaro D. P., Chan A., Miki T., Aaronson S. A. Expression cloning of a human dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12170–12174. doi: 10.1073/pnas.89.24.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp D. J., Plank D. W., Bowdin L. J., Stueland C. S., Chung T., LaPorte D. C. Nucleotide sequence of aceK, the gene encoding isocitrate dehydrogenase kinase/phosphatase. J Bacteriol. 1988 Jun;170(6):2763–2769. doi: 10.1128/jb.170.6.2763-2769.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S., Paulson K. E., Hanafusa H. Novel tyrosine kinase identified by phosphotyrosine antibody screening of cDNA libraries. Mol Cell Biol. 1988 Dec;8(12):5541–5544. doi: 10.1128/mcb.8.12.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M. H., Aaronson S. A. Detection and isolation of novel protein-tyrosine kinase genes employing reduced stringency hybridization. Methods Enzymol. 1991;200:546–556. doi: 10.1016/0076-6879(91)00170-2. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. The phosphorylation of proteins: a major mechanism for biological regulation. Fourteenth Sir Frederick Gowland Hopkins memorial lecture. Biochem Soc Trans. 1985 Oct;13(5):813–820. doi: 10.1042/bst0130813. [DOI] [PubMed] [Google Scholar]
- Letwin K., Yee S. P., Pawson T. Novel protein-tyrosine kinase cDNAs related to fps/fes and eph cloned using anti-phosphotyrosine antibody. Oncogene. 1988 Dec;3(6):621–627. [PubMed] [Google Scholar]
- Lindberg R. A., Quinn A. M., Hunter T. Dual-specificity protein kinases: will any hydroxyl do? Trends Biochem Sci. 1992 Mar;17(3):114–119. doi: 10.1016/0968-0004(92)90248-8. [DOI] [PubMed] [Google Scholar]
- Lindberg R. A., Thompson D. P., Hunter T. Identification of cDNA clones that code for protein-tyrosine kinases by screening expression libraries with antibodies against phosphotyrosine. Oncogene. 1988 Dec;3(6):629–633. [PubMed] [Google Scholar]
- Maru Y., Witte O. N. The BCR gene encodes a novel serine/threonine kinase activity within a single exon. Cell. 1991 Nov 1;67(3):459–468. doi: 10.1016/0092-8674(91)90521-y. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Schmandt R., McGill M., Amendola A., Hill M., Jacobs K., May C., Rodricks A. M., Campbell S., Hogg D. Expression of TTK, a novel human protein kinase, is associated with cell proliferation. J Biol Chem. 1992 Aug 5;267(22):16000–16006. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Stern D. F., Zheng P., Beidler D. R., Zerillo C. Spk1, a new kinase from Saccharomyces cerevisiae, phosphorylates proteins on serine, threonine, and tyrosine. Mol Cell Biol. 1991 Feb;11(2):987–1001. doi: 10.1128/mcb.11.2.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A. F. Cloning members of protein-tyrosine kinase family using polymerase chain reaction. Methods Enzymol. 1991;200:533–546. doi: 10.1016/0076-6879(91)00169-w. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Zhou Z. Y., Judd A., Cartwright C. A., Robinson W. S. The hepatitis B virus-encoded transcriptional trans-activator hbx appears to be a novel protein serine/threonine kinase. Cell. 1990 Nov 16;63(4):687–695. doi: 10.1016/0092-8674(90)90135-2. [DOI] [PubMed] [Google Scholar]
- Yonezawa N., Homma Y., Yahara I., Sakai H., Nishida E. A short sequence responsible for both phosphoinositide binding and actin binding activities of cofilin. J Biol Chem. 1991 Sep 15;266(26):17218–17221. [PubMed] [Google Scholar]