Abstract
Endothelin plays important roles in various physiological functions including vascular constriction. Recent studies reported that the endothelin receptors ETA and ETB are highly expressed in lung and skin tumor tissues. In contrast, there are few reports on endothelin signalling in the proliferation of head and neck cancer. We found that both ETA and ETB endothelin receptors were overexpressed in tumor cells of tongue cancer samples by immunohistochemistry. ETA and ETB were expressed in cultured lingual and esophageal squamous cell carcinoma (SCCs) cell lines. When both cultured cell lines were treated with an ETA selective antagonist (BQ123) or an ETB selective antagonist (BQ788), inhibition of cell growth was observed. Similar results were observed when SCCs were treated with specific siRNA for the suppression of ETA or ETB. Furthermore, inhibition of the mitogen-activated protein (MAP) kinase pathway by the treatments with ET receptor antagonists and siRNA was also observed. These results indicate that endothelin signalling may, in part, play important roles in cell growth in SCCs through the MAP kinase pathway.
Keywords: squamous cell carcinoma, tongue, cellular proliferation, endothelin receptor, MAP kinase signaling pathway
Introduction
Endothelin (ET) plays important roles on various physiological functions including vascular constriction (1–4). ET family comprises three isoforms, ET-1, ET-2, and ET-3, that bind to two receptor subtypes, endothelin A (ETA) and endothelin B (ETB) receptors (1–4). Recent studies reported that ETA and ETB were highly expressed on lung, colon and skin cancers (5–7). In addition, several reports suggested that ET-1 plays important roles in tumorigenesis, tumor progression, and metastasis (8–10). Thus, the ET receptors and their signalling pathways may be a therapeutic target in cancer therapy (11). However, little is known about the role of ET signalling on tumor cell proliferation of oral squamous cell carcinoma (SCC).
Human SCC is major neoplasm in esophagus or oral cavity and the incidence has recently been increasing (12–14). The optimal treatment for early carcinoma of oral cavity is surgical operation. However, overall survival remains largely unchanged (12–14). In addition, the decrease in quality of life (QOL) after wide excision of tongue is also important issue for patients. Therefore, different therapies are required. In our previous studies, we investigated the whole genome analysis using DNA microarray to find the potential target genes involved in tumor cell growth, and reported the critical role of several important molecules on the cell growth of SCCs (15–19). According to the results of DNA microarray, we found increased expression of ET receptor mRNA in cell lines of oral SCCs and the alteration of expression level on SCC growth (15,18,19). Therefore, we have examined whether ET receptors may be expressed in primary oral SCC tissues, and whether ET receptor-signalling may play a critical role of SCC growth. Our results imply a potentially important and novel role of ET function on SCC growth, and suggest that ET receptor-signalling might be useful target in the therapy of SCCs.
Materials and methods
Tissue samples
All of clinical studies were approved by the Ethics Committee of Osaka University Dental Hospital. Twenty-three samples of squamous cell carcinoma (SCC) located in the tongue were obtained from surgical resection tissue specimens at Osaka University Dental Hospital after informed consent was obtained. The patients, who received no preoperative therapy including chemotherapy and irradiation therapy, were randomly selected (Table I). The age range was 33–92 years (average: 62.0±13.9 years, mean ± SD).
Table I.
Profile of lingual squamous cell carcinoma patients, their histological diagnosis and expression of ETA and ETB in tissue sections.
| Case | Age/Gender | Differentiation | ETA expression | ETB expression | ||
|---|---|---|---|---|---|---|
| Tumor area | Non-tumor area | Tumor area | Non-tumor area | |||
| 1 | 71/F | Well differentiated SCC | +++ | + | +++ | ++ |
| 2 | 57/M | Well differentiated SCC | + | N/A | ++ | N/A |
| 3 | 69/M | Well differentiated SCC | ++ | N/A | +++ | N/A |
| 4 | 64/M | Well differentiated SCC | +++ | + | +++ | ++ |
| 5 | 46/F | Well differentiated SCC | ++ | + | +++ | + |
| 6 | 61/M | Well differentiated SCC | + | N/A | + | N/A |
| 7 | 48/M | Well differentiated SCC | ++ | N/A | +++ | N/A |
| 8 | 72/M | Well differentiated SCC | ++ | N/A | +++ | N/A |
| 9 | 79/M | Well differentiated SCC | ++ | N/A | ++ | N/A |
| 10 | 68/M | Moderately differentiated SCC | +++ | N/A | +++ | N/A |
| 11 | 64/M | Moderately differentiated SCC | ++ | + | +++ | ++ |
| 12 | 58/F | Moderately differentiated SCC | +++ | N/A | +++ | N/A |
| 13 | 92/F | Moderately differentiated SCC | ++ | − | +++ | − |
| 14 | 86/F | Moderately differentiated SCC | ++ | + | + | + |
| 15 | 57/M | Moderately differentiated SCC | +++ | N/A | +++ | N/A |
| 16 | 62/M | Moderately differentiated SCC | ++ | N/A | +++ | N/A |
| 17 | 52/F | Poor-moderately differentiated SCC | ++ | N/A | ++ | N/A |
| 18 | 38/M | Poorly differentiated SCC | + | − | ++ | + |
| 19 | 51/F | Poorly differentiated SCC | ++ | − | ++ | + |
| 20 | 67/M | Poorly differentiated SCC | ++ | N/A | ++ | N/A |
| 21 | 66/M | Poorly differentiated SCC | ++ | N/A | +++ | N/A |
| 22 | 65/M | Poorly differentiated SCC | ++ | N/A | +++ | N/A |
| 23 | 33/M | Poorly differentiated SCC | ++ | − | +++ | + |
Expression of ETA or ETB by immunohistochemical staining in tumor and non-tumor area is scored and expressed as (−) to (+++). N/A, not applicable.
Chemicals and antibodies
ET receptor specific antagonists, BQ123 for ETA and BQ788 for ETB were purchased from Sigma-Aldrich Japan (Tokyo, Japan). Anti-ETA or ETB polyclonal antibody was from Acris (Acris, Herford, Germany). Antibodies against Focal adhesion kinase (FAK), phosphorylated FAK, phosphorylated MEK1/2, p44/42 MAPK (pErk1/2) phosphorylated p44/42 MAPK and anti-rabbit IgG (HRP-linked) for secondary antibody are from Cell Signalling Technologies (Beverly, MA). Cisplatin was from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Immunohistochemical staining of ETA and ETB
The expression of ETA or ETB in tissues was detected by anti-ETA or ETB specific polyclonal antibody using standard immunohistochemical techniques on formalin-fixed and paraffin-embedded continuous sections. Incubation with anti-ETA or ETB polyclonal antibody was performed at 4°C for 16 h, then the sections were washed out. After the application with secondary antibody, the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) was used with a 3,3′-diaminobenzidine (DAB) substrate kit, according to the manufacturer’s instructions. The staining endpoint was determined when the standard tissue sections were constantly stained to the intensity as described previously (18,19).
The intensity of the immunohistochemical staining with anti-ETA or ETB antibody was evaluated by scoring the staining reaction in four groups: (−), none/weak; (+), weak/moderate; (++), moderate/strong, and (+++), very strong cytoplasmic staining intensity, respectively (18,19). To check the reproducibility of the evaluation system concerning the immunohistochemical staining for the ETA and ETB proteins, another oral surgeon and pathologist who were unaware of the original assessment re-evaluated the results of staining according to the system above. Tumor areas were confirmed by both of the pathologist and surgeon under the microscopy. Non-tumor areas were selected, the comparatively normal areas were separated away from the tumor areas, and confirmed by the pathologist.
Cell culture
We used human oral SCC cell line (SAS) and human esophageal SCC cell line (KYSE70). SAS was established as tongue SCC and KYSE70 was established as esophageal SCC (15,16). SAS was maintained in DMEM containing 10% fetal bovine serum (FBS), and KYSE70 was maintained in DMEM containing 2% FBS at 37°C under 0.5% CO2. For cell growth experiment, cells were trypsinized and replated onto culture dishes (15–19).
Cell survival assay using ET receptor antagonists
SCC cells were treated with ET receptor antagonists, BQ123 for ETA and BQ788 for ETB for 24 and 48 h in culture medium. Then, cell viability was measured 24 and 48 h after the treatment using Countess Automated Cell Counter (Invitrogen, Eugene, OR). The inhibition of cell growth was compared to vehicle-treated control.
RNA interference approach
SAS and KYSE70 were trypsinized and resuspended in DMEM without FBS, and the cells were separated approximately 1×105 cells for each dish. The ETA and ETB-specific siRNA (Stealth siRNA) were purchased from Invitrogen Japan (Tokyo, Japan). The sequence of the sense strand of ETA-siRNA is 5′-UUUGAUGUGGCAUUGAGCAUACAGG-3′, and antisense is 5′-CCUGUAUGCUCAAUGCCACAUCAAA-3′, respectively. The sequence of the sense strand of ETB-siRNA is 5′-UAAUUCUACUCCAAGAAGCAACAGC-3′, and antisense is 5′-GCUGUUGCUUCUUGGAGUAGAAUUA-3′, respectively. For the transfection, ETA, ETB-siRNA (40 nM) or negative control (40 nM Stealth RNAi Negative Control Duplexes, Invitrogen Japan Inc.) solution was added to DMEM medium containing Lipofectamine RNAiMax (Invitrogen Japan) and allowed to incubate for 20 min at room temperature to create the transfection mixture. The transfection mixture was then added to the cells at the indicated final concentration of siRNA. Twenty-four hours after the transfection, the medium was changed to DMEM containing 10% FBS for SAS and 2% FBS for KYSE70. Then, viable cell number was measured 24 and 48 h after the medium change using Countess Automated Cell Counter. The cell growth was expressed as the percentage to that of vehicle control.
Western blot analysis
Adherent or suspended cells were washed in PBS, and cell extracts were prepared by lysing cells in lysis buffer. The proteins were separated by electrophoresis using 10% SDS-PAGE, and transferred to nitrocellulose membrane (Millipore, Bedford, MA). Detection of proteins were performed by each polyclonal antibody and visualized by using the ECL detection kit (Amersham, London, UK) following the manufacturer’s suggested procedure.
Combination of ETA, ETB-siRNA and anti-tumor drug
Com- bined treatment of ETA, ETB-siRNA with anti-tumor drug, cisplatin, was performed. Briefly, after the low concentration of ETA or ETB-siRNA (20 nM) treatment, 2.5 μM of cisplatin that was a concentration slightly effective on cell growth inhibition was treated for 48 h. Cell growth was measured by Countess Automated Cell Counter and expressed as percentage.
Statistical analysis
All results are expressed as mean ± SEM. Statistical comparisons were made using the Student-t test or Scheffe’s method after analysis of variances (ANOVA). The results were considered significantly different at P<0.05.
Results
Lingual SCCs in tumor tissues express ETA and ETB
Lingual SCC primary tissues were stained using anti-ETA or anti-ETB specific antibody, respectively. Positive staining of ETA was observed in tumor area (Fig. 1A, right). In contrast, none of staining of ETA was observed in non-tumor area in the same tissue section (Fig. 1A, left). Similar staining pattern was also observed in other tumor tissue sections (Table I). Statistically significance of the ETA expression between tumor and non-tumor area was observed (Fig. 1B). In addition, positive staining of ETB in tumor area, but not non-tumor area, was also observed in the same tissue section (Fig. 1C). Statistical significance of the ETB expression between tumor and non-tumor areas was also observed (Fig. 1D and Table I). These results are similar to that of ETA. Good correlation between ETA and ETB expression was observed (Fig. 1E).
Figure 1.
Expression of ETA or ETB on tumor cells in lingual tissues. (A) ETA expression on tumor area (right panel) of primary lingual squamous cell carcinoma and non-tumor area (left panel) in the same tissue section (case 13 in Table I) by immunohistochemical observations. The brown color represents positive staining of ETA and the blue represents counterstaining. Scale bar represents 100 μm. (B) Comparison of the expression of ETA between tumor area and non-tumor area. Averaged score of strengthen of ETA expression are expressed from the data in Table I. Each column represents mean ± SEM from 9–23 cases in tumor area or non-tumor area, respectively. **P<0.01. (C) ETB expression on tumor area (right panel) of primary lingual squamous cell carcinoma and non-tumor area (left panel) in the same tissue section (case 13 in Table I) by immunohistochemical observations. The brown color represents positive staining of ETB and the blue represents counterstaining. Scale bar represents 100 μm. (D) Comparison of the expression of ETB between tumor area and non-tumor area. Averaged score of strengthen of ETB expression are expressed from the data in Table I. Each column represents mean ± SEM from 9–23 cases in tumor area or non-tumor area, respectively. **P<0.01. (E) Correlation between ETA and ETB expression. Each point represents each individual.
ET receptor antagonists suppress cell growth of lingual and esophageal SCC
According to the data of ET receptor expression in SCCs, we hypothesized that ET receptor-signalling might play an important role on the cell growth of SCCs. To investigate the hypothesis, we used ET receptor antagonists, BQ123 for ETA and BQ788 for ETB. As shown in Fig. 2A and B, ET receptor antagonists, BQ123 and BQ788 suppressed the cell growth of lingual SCC cell line, SAS. The suppression by the antagonists was concentration- and time-dependent (data not shown).
Figure 2.
Effects of ET receptor antagonists on SCC cell growth. (A and B) Effect of ETA antagonist, BQ123 or ETB antagonist, BQ788 on cell growth of SAS. Typical images (A) and cell growth rate (B). 48 and 72 h after the start of treatment, viable cell number was counted and cell growth rate was expressed. Each column represents the percentage of cell growth (mean ± SEM from 4–5 independent experiments) compared to vehicle control (black column, PBS). Error bars represent standard deviations. **P<0.01, *P<0.05 vs. negative control. (C and D) Effect of ETA antagonist, BQ123 or ETB antagonist, BQ788 on cell growth of KYSE70.
In addition to the results of growth suppression of lingual SCC by the inhibition of ET receptors, both antagonists also suppressed the cell growth of esophageal SCC cell line, KYSE70 (Fig. 2C and D). These results indicate that ET receptor-signalling is required for the growth of SCCs.
ETA and ETB-siRNA suppress cell growth of lingual and esophageal SCC
To clarify the exact function of ET receptors on the growth of SCCs, we used small interfering RNA (siRNA) for ETA and ETB. ETA and ETB-siRNA effectively decreased the ET receptor protein levels in SCCs. The inhibition of cell growth on SAS was clearly observed when ETA or ETB was knocked down by the treatment with siRNA (Fig. 3A and B). Similar suppression of cell growth by the knockdown of ETA or ETB was also observed when esophageal SCC cell line, KYSE70 was treated with siRNA for ETA or ETB (Fig. 3C and D). These results clearly indicate that ET receptor-signalling is required for the growth of SCCs.
Figure 3.
Effects of ET receptor knockdown on the cell growth of SCCs treated with ETA or ETB-siRNA. (A and B) Effects of ETA-siRNA or ETB-siRNA on cell growth of SAS. Typical images (A) and cell growth rate (B). Cells were transfected the siRNA (40 nM) for 24 h and cultured for additional 48 h, followed by viable cell counting. Each value represents the percentage of cell growth compared with vehicle (non-siRNA) control from 4–7 independent experiments. White column represents the growth of cells transfected with ETA (left panel) or ETB (right panel)-siRNA and black column represents that of cells transfected with negative control-siRNA, respectively. Error bars represent standard deviations. **P<0.01, *P<0.05 vs. negative control. (C and D) Effects of ETA-siRNA and ETB-siRNA on cell growth of KYSE70. Typical photos (C) and cell growth rate (D). Cells were transfected the siRNA (40 nM) for 24 h and cultured for additional 48 h, followed by viable cell counting. Each value represents the percentage of cell growth compared with vehicle (non-siRNA) control from 4 independent experiments. White column represents the growth of cells transfected with ETA (left panel) or ETB (right panel)-siRNA and black column represents that of cells transfected with negative control-siRNA, respectively. Error bars represent standard deviations. **P<0.01, *P<0.05 vs. negative control.
Investigation of potential mechanisms
We next investigated the mechanisms of inhibition of cell growth induced by the suppression of ET receptor-signalling. Western blot analysis showed the expression of ETA and ETB proteins on the lingual SCC cell line SAS (Fig. 4A). Although the specific antagonists blocked the ETA or ETB signalling, no alterations of receptor protein expression levels were observed (Fig. 4A). In contrast, blockade of ET receptor-signalling by the treatment with antagonists caused the suppression of phosphorylation of MEK and Erk (mitogen-activated protein kinase), the important members of MAPK pathway (Fig. 4B). In addition, similar suppression of MAPK pathway by knockdown of ET receptors was observed when SAS and KYSE70 were treated with ETA or ETB-siRNA (Fig. 4C and D). These results indicate the involvement of MAPK pathway on the ET receptor-signalling mediated cell growth of SCCs.
Figure 4.
Involvement of MAPK pathway on ET receptor-signalling of SCC growth. (A) A Western blot analysis showing ETA or ETB expression in SAS which were pre-treated with ETA antagonist, BQ123, or ETB antagonist, BQ788. GAPDH is standard for equivalent application. No alteration was observed. (B) Suppression of MAPK pathway by ET receptor antagonists. SAS was treated with ETA antagonist, BQ123, or ETB antagonist, BQ788, then the phosphorylation of Mek (p-MEK) or Erk (p-Erk) was detected for Western blot analysis. (C) Western blot analysis for expression of p-MEK and p-Erk by the treatment with ETA or ETB-siRNA on SAS. Cells were treated with ETA or ETB-siRNA (20 and 40 nM), negative control siRNA and vehicle (Veh). Samples were collected for 24 h after the treatment. GAPDH was used to evaluate equivalent loading. (D) Western blot analysis for expression of p-MEK and p-Erk by the treatment with ETA or ETB-siRNA on SAS. Cells were treated with ETA or ETB-siRNA (20 and 40 nM), negative control siRNA and vehicle (Veh). Samples were collected 24 h after the treatment. GAPDH was used to evaluate equivalent loading.
In contrast, no inhibition of phosphorylation of focal adhesion kinase (FAK), a 125 kDa non-receptor tyrosine kinase (20,21), by the suppression of ET receptor signalling was observed (data not shown).
We also investigated the effect of blockade of ET receptor-signalling on expression of integrins such as integrin α5 and β1 (22,23). However, no alterations of integrin α5 and β1 expressions were observed (data not shown). These results suggest that the cell growth suppression of SCCs by the knockdown or blockade of ET receptors is mediated through the direct inhibition of MAPK signalling pathway.
Combination therapy of ETA or ETB-siRNA and anti-tumor drugs
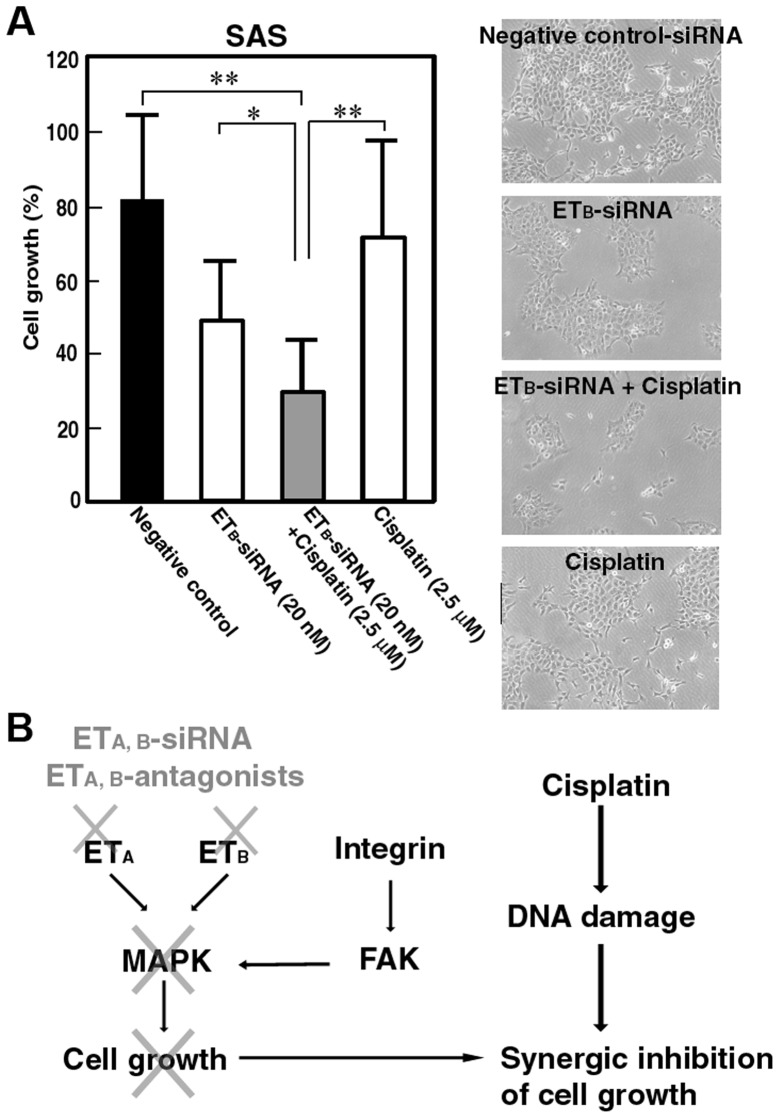
Reduction of dosage of anti-tumor drugs for cancer chemotherapy is clinically important to minimize the side effects, although the complete tumor cell death is required. Combined treatment of ETB-siRNA (20 nM) with anti-tumor drug, cisplatin (2.5 μM), drastically inhibited the cell growth of SAS in comparison to that in each single treatment (Fig. 5A). Similar results were also observed in the combined treatment of ETA-siRNA (20 nM) with cisplatin (data not shown). These results indicate that combination therapy of ETA or ETB-siRNA and ordinal anti-tumor drugs may be a novel and useful therapy for SCCs.
Figure 5.
Combination therapy of ETB-siRNA and anti-tumor drug. (A) Combined treatment of low dose of ETB-siRNA (20 nM) with cisplatin (2.5 μM) was performed on cultured SCC cell line, SAS. Cells were treated with ETB-siRNA, negative control or vehicle for 24 h, then, treated with cisplatin or vehicle for 48 h. Each value represents the percentage of cell growth compared with vehicle (non-siRNA) control from 5 independent experiments. *P<0.05, **P<0.01 vs. negative control, or each single treatment, respectively. (B) Schematic illustration of possible mechanisms on the inhibition of cell growth by the combination of ET receptor-siRNA with cisplatin.
Discussion
There have been several reports on the expression of ET receptors in various human cancers (5–7), and it is considered to be the relationship between ET receptor-signalling and tumor cell growth. There are, however, few reports on the evaluation and investigation of the exact role of ET receptor-signalling using human SCC tissues and cultured cell lines of oral and esophageal carcinomas.
In the present study, using an immunohistochemical method, we demonstrated significantly higher levels of expression of ETA and ETB protein in human lingual cancer tissues than in non-tumor areas in the same tissue samples. Similar results were also observed on the cultured SCC cell lines such as SAS, lingual SCC, and KYSE70, esophageal SCC. These results indicate the involvement of ET receptor-signalling on SCC growth. Furthermore, we showed that the suppression of ET receptor protein by siRNA or the blockade by antagonists caused the inhibition of SCC growth. In our experimental conditions, both the treatment with ETA and ETB antagonists and siRNA strongly inhibited the cell growth of SCCs. These results strongly suggest the important role for ET receptor-signalling in SCC cell survival. In fact, recent reports strongly indicated the involvement of ET and its receptor on oral cancer (24,25). In addition, it was also reported that suppression of endothelin-converting enzyme-1 caused the inhibition of SCC proliferation (26). Our results, together with those reports, strongly suggest the importance of ET synthesis and its receptor-signalling pathway on oral SCC proliferation.
It is reported that phosphorylation of FAK is involved in the inhibition of apoptosis and promote cell growth in SCC cell lines (15,18). FAK is a 125 kDa non-receptor tyrosine kinase and an important regulator of cell survival, invasion, migration, and cell cycle progression (15,18,20,21). FAK is functionally important in transducing intracellular messages that are associated with growth factor signalling (15,18,20,21,27). The intracellular messages link p-FAK at Tyr925 to signalling pathways that activate MAPK cascades. In our present study, however, the inhibition of phosphorylation of FAK in SCCs treated with ET antagonists and siRNAs was not observed. In contrast, the inhibition of the phosphorylation of MEK and Erk by the treatment with ET antagonists and siRNAs was clearly observed. These results indicate that the inhibition of MAPK pathway by the suppression of ET receptor-signalling is due to the direct inhibition of MAPK pathway, but not through FAK pathway (Fig. 5B). Several reports have indicated the coupling of ET receptor-signalling and MAPK pathway (28,29). Our results agree with those reports and indicate that the mechanisms of the inhibition of cell growth by ET receptor-siRNAs and antagonists are, in part, due to the inhibition of MAPK pathway.
Reduction of dosage of anti-tumor drugs for cancer chemotherapy is clinically important to minimize the side effects, although the complete tumor cell death is required. Combined treatment of low concentration of ET receptor-siRNA (20 nM) with low concentration of anti-tumor drug, cisplatin (2.5 μM), drastically inhibited the cell growth of SAS in comparison to that in each single treatment. Cisplatin is extensively characterized as DNA damaging agent and the cytotoxicity of cisplatin is attributed to the ability to form inter and intra-strand nuclear DNA crosslinks (30,31). In contrast, inhibition of cell growth by ET receptor-siRNAs presented in our study was mainly due to the direct inhibition of MAPK pathway. Therefore, those two pathways on growth inhibition are different. This difference of mechanisms between ET receptor-siRNA and cisplatin may lead to show synergistic effect on the inhibition of tumor cell growth (Fig. 5A and B). Our results indicate that the decrease in ET receptor levels in SCCs that strongly express ET receptors increases the sensitivity against chemotherapy, and that the siRNA for ET receptors combined with anti-tumor drugs might be a useful therapy to reduce the dosage of anti-tumor drugs.
In summary, we showed the overexpression of ETA and ETB in tumor cells of human primary lingual SCC tissues and cultured SCC cell lines, and suggest a potentially important role for ET receptor-signalling on the cell growth of human SCCs.
Acknowledgements
This study was supported in part by grants (21592357 to K.W., 20390471 to Y.K., and 21390535 to M.K.) from the Japanese Society for the Promotion of Science, and was supported by The Osaka Medical Research foundation for Intractable Diseases.
References
- 1.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 2.Inoue A, Yanagisawa M, Kimura S, et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport AP. International union of pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol Rev. 2002;54:219–226. doi: 10.1124/pr.54.2.219. [DOI] [PubMed] [Google Scholar]
- 4.Kusserow H, Unger T. Vasoacitive peptides, their receptors and drug development. Basic Clin Pharmacol Toxicol. 2004;94:5–12. [PubMed] [Google Scholar]
- 5.Ahmed SI, Thompson J, Coulson JM, Woll PJ. Studies on the expression of endothelin, its receptor subtypes, and converting enzymes in lung cancer and in human bronchial epithelium. Am J Respir Cell Mol Biol. 2000;22:422–431. doi: 10.1165/ajrcmb.22.4.3795. [DOI] [PubMed] [Google Scholar]
- 6.Asham E, Shankar A, Loizidou M, et al. Increased endothelin-1 in colorectal cancer and reduction of tumor growth by ET(A) receptor antagonism. Br J Cancer. 2001;85:1759–1763. doi: 10.1054/bjoc.2001.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Tang L, Su M, et al. Expression of endothelines and their receptors in nonmelanoma skin cancers. J Cutan Med Surg. 2006;10:269–276. doi: 10.2310/7750.2006.00062. [DOI] [PubMed] [Google Scholar]
- 8.Spinella F, Rosano L, DiCastro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1 alpha in ovarian carcinoma cells. J Biol Chem. 2002;277:27850–27855. doi: 10.1074/jbc.M202421200. [DOI] [PubMed] [Google Scholar]
- 9.Wulfing P, Kersting C, Tio J, et al. Endothelin-1-, endothelin-A-, and endothelin-B-receptor expression is correlated with vascular endothelial growth factor expression and angiogenesis in breast cancer. Clin Cancer Res. 2004;10:2393–2400. doi: 10.1158/1078-0432.ccr-03-0115. [DOI] [PubMed] [Google Scholar]
- 10.Boldrin L, Gisfredi S, Ursino S, et al. Expression of endothelin-1 is related to poor prognosis in non-small cell lung carcinoma. Eur J Cancer. 2005;41:2828–2835. doi: 10.1016/j.ejca.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Kandalaft LE, Facciabene A, Buckanovich RJ, Coukos G. Endothelin B receptor, a new target in cancer immune therapy. Clin Cancer Res. 2009;15:4521–4528. doi: 10.1158/1078-0432.CCR-08-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goepfert H. Squamous cell carcinoma of the head and neck: past progress and future promise. CA Cancer J Clin. 1998;48:195–198. doi: 10.3322/canjclin.48.4.195. [DOI] [PubMed] [Google Scholar]
- 13.Okura M, Hiranuma T, Adachi T, et al. Induction chemotherapy is associated with an increase in the incidence of locoregional recurrence in patients with carcinoma of the oral cavity: results from a single institution. Cancer. 1998;82:804–815. [PubMed] [Google Scholar]
- 14.Prince S, Bailey BM. Squamous carcinoma of the tongue: review. Br J Oral Maxillofac Surg. 1999;37:164–174. doi: 10.1054/bjom.1999.0031. [DOI] [PubMed] [Google Scholar]
- 15.Masuda T, Wada K, Nakajima A, et al. Critical role of peroxisome proliferator-activated receptor γ on anoikis and invasion of squamous cell carcinomas. Clin Cancer Res. 2005;11:4012–4021. doi: 10.1158/1078-0432.CCR-05-0087. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi H, Fujita K, Fujisawa T, et al. Inhibition of peroxisome proliferator-activated receptor gamma activity in esophageal carcinoma cells results in a drastic decrease of invasive properties. Cancer Sci. 2006;97:854–860. doi: 10.1111/j.1349-7006.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida H, Wada K, Masuda T, et al. Critical role of estrogen receptor on anoikis and invasion of squamous cell carcinoma. Cancer Sci. 2007;98:636–643. doi: 10.1111/j.1349-7006.2007.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata M, Wada K, Nakajima A, et al. Role of myeloid cell leukemia-1 on cell growth of squamous cell carcinoma. J Pharmacol Sci. 2009;110:344–353. doi: 10.1254/jphs.08339fp. [DOI] [PubMed] [Google Scholar]
- 19.Kusayama M, Wada K, Nagata M, et al. Critical role of aquaporin 3 on growth of human esophageal and oral squamous cell carcinoma. Cancer Sci. 2011;102:1128–1136. doi: 10.1111/j.1349-7006.2011.01927.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- 21.Sieg DJ, Hauck CR, Ilic D, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol. 1997;9:76–85. doi: 10.1016/s0955-0674(97)80155-x. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman RR, Yurgel LS, Campos MM. Endothelins and their receptors as biological markers for oral cancer. Oral Oncol. 2010;46:644–647. doi: 10.1016/j.oraloncology.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman RR, Yurgel LS, Campos MM. Evaluation of salivary endothelin-1 levels in oral squamous cell carcinoma and oral leukoplakia. Regulatory Peptide. 2011;166:55–58. doi: 10.1016/j.regpep.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Awano S, Dawson LA, Hunter AR, Turner AJ, Usmani BA. Endothelin system in oral squamous carcinoma cells: Specific siRNA targeting of ECE-1 blocks cell proliferation. Int J Cancer. 2006;118:1645–1652. doi: 10.1002/ijc.21525. [DOI] [PubMed] [Google Scholar]
- 27.Frish SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Boil. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogoyevitch MA, Glennon PE, Andersson MB, et al. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase signalling cascade in cardiac myocytes. J Biol Chem. 1994;269:1110–1119. [PubMed] [Google Scholar]
- 29.Cramer H, Schmenger K, Heinrich K, et al. Coupling of endothelin receptors to the ERK/MAP kinase pathway. Eur J Biochem. 2001;268:5449–5459. doi: 10.1046/j.0014-2956.2001.02486.x. [DOI] [PubMed] [Google Scholar]
- 30.Meyers M, Hwang A, Wagner MW, Boothman DA. Role of DNA mismatch repair in apoptotic responses to therapeutic agents. Environ Mol Mutagen. 2004;44:249–264. doi: 10.1002/em.20056. [DOI] [PubMed] [Google Scholar]
- 31.Psyrri A, Fountzilas G. Advances in the treatment of locally advanced non-nasopharyngeal squamous cell carcinoma of the head and neck region. Med Oncol. 2006;23:1–15. doi: 10.1385/MO:23:1:1. [DOI] [PubMed] [Google Scholar]