Abstract
Previous studies suggest that task-activated fMRI can predict future cognitive decline among healthy older adults. The present fMRI study examined the relative sensitivity of semantic memory (SM) versus episodic memory (EM) activation tasks for predicting cognitive decline. Seventy-eight cognitively intact elders underwent neuropsychological testing at entry and after an 18-month interval, with participants classified as cognitively “Stable” or “Declining” based on ≥1.0 SD decline in performance. Baseline fMRI scanning involved SM (famous name discrimination) and EM (name recognition) tasks. SM and EM fMRI activation, along with APOE ε4 status, served as predictors of cognitive outcome using a logistic regression analysis. Twenty-seven (34.6%) participants were classified as Declining and 51 (65.4%) as Stable. APOE ε4 status alone significantly predicted cognitive decline (R2 = .106; C index = .642). Addition of SM activation significantly improved prediction accuracy (R2 = .285; C index = .787), whereas the addition of EM did not (R2 = .212; C index = .711). In combination with APOE status, SM task activation predicts future cognitive decline better than EM activation. These results have implications for use of fMRI in prevention clinical trials involving the identification of persons at-risk for age-associated memory loss and Alzheimer’s disease.
Keywords: magnetic resonance imaging, aging, Apolipoprotein-E, memory loss, mild cognitive impairment, longitudinal study
Introduction
Genetic risk factors, such as the presence of one or both Apolipoprotein-E (APOE) ε4 alleles, have been associated with increased risk for Alzheimer’s disease (AD) and late-life cognitive decline (Caselli et al., 1999; Farrer et al., 1997; Saunders et al., 1996; Swan, Lessov-Schlaggar, Carmelli, Schellenberg, & La Rue, 2005). Cross-sectional studies suggest that asymptomatic elders with risk factors for AD (i.e., APOE ε4 carriers and persons with a family history of AD) demonstrate different patterns of brain activation on functional magnetic resonance imaging (fMRI) than elders without risk factors [for a review, see (Wierenga & Bondi, 2007)]. Often, these activation changes occur in brain regions critical for memory processes, such as the hippocampus, posterior cingulate, and lateral posterior temporoparietal regions. Such regions are also the earliest to be affected by AD neuropathology (Bassett et al., 2006; Bondi, Houston, Eyler, & Brown, 2005; Johnson et al., 2006; Seidenberg et al., 2009; Sperling et al., 2010).
Aberrant patterns of task-activated fMRI may precede the onset of cognitive symptoms and atrophy on structural MRI in elders at-risk for AD (Seidenberg et al., 2009). Several longitudinal studies suggest that task-activated fMRI may be useful for predicting future cognitive decline in intact elders (Bookheimer et al., 2000; Lind et al., 2006; O’Brien et al., 2010; Persson et al., 2006; Smith et al., 2005; Woodard et al., 2010) and in patients with mild cognitive impairment (MCI), the prodromal condition typically preceding the diagnosis of AD (Miller et al., 2008). The majority of these fMRI prediction studies have used episodic memory (EM) tasks (Bookheimer et al., 2000; Miller et al., 2008; O’Brien et al., 2010; Persson et al., 2006), which is not surprising because EM dysfunction is among the most prominent of cognitive changes in the three-year period preceding a diagnosis of AD (Mickes et al., 2007). EM tasks have been employed widely in cross-sectional fMRI studies to contrast cognitively intact individuals at varying risk for developing AD (Trivedi et al., 2008; Wierenga & Bondi, 2007). However, there exist potential methodological problems in using EM tasks during fMRI. EM performance declines with healthy aging and is accelerated in MCI and AD (Bondi & Kaszniak, 1991). The interpretation of fMRI activation maps is complicated when performance varies across participants. This issue is particularly problematic for blocked trial designs, where the calculated fMRI response is based on the average of both correct and incorrect trials within a block, as the incorporation of incorrect trials may result in activation of unintended, non-memory related processes. In an event-related fMRI design, it is possible to eliminate error trials from the brain maps, but even this procedure may be invalid if performance is close to chance. In addition, EM tasks are inherently difficult for older adults, resulting in potential activation of brain regions associated with increased effort rather than the memory circuits of interest.
Alternatively, semantic memory (SM) fMRI tasks have also successfully predicted future cognitive decline (Lind et al., 2006; Smith et al., 2005; Woodard et al., 2010) and may have some advantages over EM tasks. Performance declines on SM tasks are less severe than EM declines in normal aging and MCI (Hodges & Patterson, 1995). In addition, SM tasks are typically less effortful for older adults than EM tasks. Finally, brain regions recruited in response to SM tasks overlap with the so-called “default mode network” (DMN), a circuit activated during rest and during semantic processing (Binder, Desai, Graves, & Conant, 2009). Regions in this network are susceptible to early AD pathology, including amyloid-beta deposition (Buckner et al., 2005; Pihlajamaki & Sperling, 2009; Raichle et al., 2001; Rombouts & Scheltens, 2005). Thus, SM fMRI tasks may be sensitive to the detection of risk for future cognitive decline and AD.
We published a series of fMRI studies using a famous name discrimination task (FNDT) to probe SM networks in at-risk aging and in MCI (Sugarman et al., 2012). Seidenberg et al. (2009) found greater SM activation in individuals at-risk for AD, especially in the posterior cingulate and lateral posterior temporoparietal regions. Increased SM activation in the same regions was also found in MCI patients as compared to healthy older adults, despite equivalent task performance (Woodard et al., 2009). Recently, Woodard et al. (2010) demonstrated that SM task activation was more effective in predicting future cognitive decline than hippocampal volumes in elders who were cognitively intact at study entry.
In the current study of cognitively intact healthy elders, we compared the accuracy of EM and SM fMRI brain activation patterns in predicting future cognitive decline after an 18-month retest interval. The FNDT served as the SM task; the EM task involved an old-new recognition task involving famous and unfamiliar names. To equate the brain maps derived from the two tasks, we employed an event-related design to eliminate error trials from the image analyses. In addition, a principal components analysis was used to reduce the number of predictors derived from the SM and EM fMRI activation data. We predicted that task performance would be poorer on the EM task relative to the SM task, and that activation during the SM task would be the superior predictor of future cognitive decline.
Method
Participants
Participants were 78 healthy older adults (73% female; mean age = 73 years, SD = 4.9 years; mean education = 14.9 years, SD = 2.7 years). They were drawn from a larger sample of 459 community-dwelling adults who were recruited via newspaper advertisements. Following telephone screening, 92 participants met study inclusion and exclusion criteria. Participants were excluded from the study if they had a history or evidence of: 1) neurological illnesses or conditions including dementia and head trauma with a loss of consciousness greater than 30 minutes; 2) medical illnesses or conditions that may affect brain function (such as untreated hypertension or insulin-dependent diabetes mellitus); 3) major psychiatric disturbance meeting DSM-IV Axis I criteria; 4) a Mini-Mental State Exam (MMSE; (Folstein, Folstein, & McHugh, 1975) score less than 28; 5) a Geriatric Depression Scale (Yesavage et al., 1982) score greater than 10; 6) substance abuse meeting DSM-IV Axis I criteria; 7) impairments in activities of daily living as determined by the Lawton Instrumental Activities of Daily Living Scale (ADL(Lawton & Brody, 1969); 8) taking prescribed psychoactive medications, 9) a Hachinski ischemia score above 4; or 10) left-handedness, as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). Of the 92 participants who met study criteria, 81 persons agreed to undergo APOE genotyping from blood samples, a neuropsychological evaluation, and an fMRI scanning session. MRI data were not successfully obtained from three participants, leaving 78 participants for inclusion.
Family history was defined as a report of a clear clinical diagnosis of AD or a reported history of gradual decline in memory and other cognitive functions, confusion, or judgment problems without a formal diagnosis of AD prior to death in a first-degree relative. One participant reported a diagnosis of AD in a second-degree relative, with some mild cognitive changes noted in a parent prior to the parent’s death. Because our study examined the influence of AD risk factors on prediction of cognitive decline, approximately half (51.3%) of the participants were purposely selected because they had a positive family history of AD. In addition, 33.3% of the sample carried at least one APOE ε4 allele. All participants underwent neuropsychological evaluation (see below) and were determined to be cognitively intact at baseline. Informed consent was obtained consistent with the Declaration of Helsinki and institutional guidelines established by the Medical College of Wisconsin Human Subjects Review Committee. All participants received financial compensation for their participation.
Neuropsychological Assessment and APOE genotyping
All participants underwent a baseline and 18-month follow-up neuropsychological battery consisting of the following: MMSE (Folstein et al., 1975), Mattis Dementia Rating Scale-2 (Jurica, 2001; Mattis, 1988), Rey Auditory Verbal Learning Test (RAVLT; (Rey, 1958), Geriatric Depression Scale (Yesavage et al., 1982), and ADL (Lawton & Brody, 1969). Alternate forms of the DRS-2 (Schmidt, 2004; Schmidt, Mattis, Adams, & Nestor, 2005) and RAVLT (Schmidt, 1996) were administered during the follow-up examination. Participant APOE genotype was determined using a polymerase chain reaction method described by Saunders et al. (1996). DNA was isolated with the Gentra Systems Autopure LS for Large Sample Nucleic Acid Purification.
Definition of Cognitive Decline
Membership into the Declining group (n = 27) was defined as a reduction of at least one standard deviation between baseline and follow-up testing on one or more of the following neuropsychological measures: DRS-2 Total Score (DRS-2 Tot), RAVLT Sum of Trials 1-5 (RAVLT-Tot), or RAVLT Delayed Recall (RAVLT-DR). Residualized change scores were computed for each cognitive measure by predicting retest scores using baseline scores. This procedure adjusts for baseline performance, practice effects, and regression to the mean. Participants with standardized residuals of −1.0 or lower on one or more of the three measures were assigned to the cognitively declining group; the remaining participants were classified as cognitively stable.
fMRI Task
All participants were administered SM and EM tasks while undergoing fMRI in the same scanning session. Corrective lenses were provided to participants as needed. The SM task consisted of the presentation of 30 highly recognizable famous names and 30 unfamiliar names. Stimuli were selected from an original pool of 784 names based on the ability of healthy older adults to correctly classify each name as famous or unfamiliar [see (Douville et al., 2005) for details]. The 60 names were randomly interspersed with 20 presentations of a centrally placed fixation crosshair in order to introduce “jitter” into the fMRI time series (inter-stimulus interval = 4 sec). Participants made a right index or right middle finger key press for famous or unfamiliar names, respectively. Accuracy and reaction time were recorded. The imaging run began and ended with 12 sec of fixation and was 5 min and 44 sec in duration.
Following a 20-minute delay, participants were administered an EM recognition task. The stimuli consisted of 60 “old” items (the 30 famous and 30 unfamiliar names from the SM task) randomly intermixed with 60 “new” items (30 famous and 30 unfamiliar names). Participants were asked to indicate by button press if the name was old (right index finger) or new (right middle finger). As in the SM task, name stimuli were presented for 4 sec and presented in a pseudorandom order with 40 presentations of a centrally placed fixation crosshair to introduce “jitter.” The EM task was split into two runs that began and ended with 12 sec of fixation and were each 5 min and 44 sec in duration.
Image Acquisition
Whole-brain, event-related fMRI was conducted on a General Electric (Waukesha, WI) Signa Excite 3.0 Tesla short bore scanner equipped with a quad split quadrature transmit/receive head coil. Echoplanar images were collected using an echoplanar pulse sequence (TE = 25 ms; flip angle = 77 degrees; field of view (FOV) = 24 mm; matrix size = 64 × 64). Thirty-six contiguous axial 4-mm-thick slices were selected to provide coverage of the entire brain (voxel size = 3.75 × 3.75 × 4 mm). The inter-scan interval (TR) was 2 seconds. High-resolution, three-dimensional spoiled gradient-recalled at steady-state (SPGR) anatomic images were acquired (TE = 3.9 ms; TR = 9.5 ms; inversion recovery (IR) preparation time = 450 ms; flip angle = 12 degrees; number of excitations (NEX) = 2; slice thickness = 1.0 mm; FOV = 24 cm; resolution = 256 × 224). Foam padding was used to reduce head movement within the coil.
Image Analysis
Functional images were generated with the Analysis of Functional Neuroimages (AFNI) software package (Cox, 1996). Each image time series was shifted to the beginning of the TR and then spatially registered to reduce the effects of head motion using a rigid body iterative linear least squares method. A deconvolution analysis was used to extract a hemodynamic response function (HRF) for famous and unfamiliar names (SM task) and for novel and previously seen names (EM task) from the time-series. Correct and incorrect trials were modeled separately, and only correct trials were used in the second level analyses. HRFs were modeled for the 0–16 second period post-stimulus onset. Motion parameters were incorporated into the model as nuisance regressors. The HRFs were also transposed so that the value of the HRF at trial onset was zero. Area under the curve (AUC) was calculated by summing the hemodynamic responses at time points 4, 6, and 8 seconds post-trial onset. Individual anatomical and functional scans were transformed into standard stereotaxic Talairach space (Talairach & Tournoux, 1988). To compensate for normal variation in anatomy across participants, functional images were blurred using a 6-mm Gaussian full-width half-maximum filter.
Spatial Extent of Activation Analysis
A voxel-wise analysis was used to determine differences in spatial extent of activation between the stable and declining groups. SM task activation was defined as regions where the AUC for famous names was significantly different from that of unfamiliar names. EM task activation was defined as regions where the AUC for previously shown names was significantly greater than the AUC for novel names. For all voxel-wise analyses, the individual voxel probability threshold was p < .005 with a minimum cluster of 0.731 ml. The statistical threshold was derived from 3,000 Monte Carlo simulations (Forman et al., 1995) and was equivalent to a whole brain family-wise error threshold of p < .05.
Functional Region of Interest Analysis
Using voxel-wise t-tests, SM and EM activations were calculated separately combining all 78 participants. Significant cluster volumes were used to create functional regions of interest (fROI) for each task. The average AUC of all voxels within each fROI was then calculated for each participant. For each task, the data from all fROIs were entered into a principal components analysis (PCA) to further reduce the number of predictors for the logistic regression analysis [see (Woodard et al., 2010) for details].
Data Analysis
Logistic regression analyses were conducted to examine the relative accuracy of SM vs. EM in predicting cognitive decline. Our previous research (Woodard et al., 2010) demonstrated that the combination of APOE ε4 status and SM activation outperformed other predictive models that included combinations of hippocampal volume, a family history of AD, and demographic variables. Therefore, we limited our predictors to only task activation and APOE ε4 status in order to maintain a reasonable number of subjects-to-variables and prevent overfitting the model. We tested four models: APOE ε4 status alone (Model 1), APOE ε4 and SM activation (Model 2), APOE ε4 and EM activation (Model 3), and APOE ε4 and both SM and EM activation (Model 4). Nagelkerke R2 values and the concordance (C) indexes determined the relative fit of each model in predicting participants’ future cognitive decline. Nagelkerke R2 indicates the importance of the predictors in each model relative to a perfectly fitting null model (Nagelkerke, 1991). The C index represents the area under the receiver operating characteristic curve and indicates the proportion of all possible pairs of Stable and Declining subjects in which the Declining subject in the pair had a higher predicted probability of decline than the Stable subject (Harrell, 2001; Woodard et al., 2010). Therefore, greater C index values indicate greater prediction accuracy for a model. For each logistic regression, values of Nagelkerke R2 and C were validated with a bootstrapping analysis using 5000 resamples (Harrell, 2001). Through bootstrapping, holding data out for cross-validation was not required, and each phase of model development was revalidated using repeated resampling from the entire sample (Harrell, 2001; Woodard et al., 2010). The four logistic regression models were compared on the basis of their Bayesian Information Criterion (BIC) values, which account for the number of parameters in each model. Lower BIC values imply a better model for optimally predicting cognitive decline. BIC values can be compared statistically to determine whether one model fits the data significantly better than the other model.
Results
On baseline measures, there were no significant differences between the Stable and Declining groups on the DRS-2 Tot, RALT-Tot, RAVLT-DR, or ADL after controlling for multiple comparisons (Bonferroni adjust alpha level = .0125; 0.04/4 tests; see Table 1). At an 18-month follow-up, 27 of 78 participants (34.6%) demonstrated a reduction in neuropsychological performance of at least 1 SD on one or more of the specified neuropsychological measures, indicating cognitive decline. Of these 27, 22 declined on one measure only (6=DRS-2 Tot, 8=RAVLT-Tot, 8=RAVLT-DR). Four participants declined on two measures (3=RAVLT-Tot & RAVLT-DR, 1=DRS-2 Tot & RAVLT-Tot), and one participant declined on all three measures. A 2 (Group) × 2 (Testing Session) ANOVA revealed a significant interaction for all three neuropsychological measures (RAVLT-Tot, F (1,76) = 14.95, p < .001, partial η2 = .164; RAVLT-DR, F (1,76) = 34.9, p < .001, η2= .315; DRS-2 tot, F (1, 76) = 11.99, p < .001, η2 = .136). Two (7.6%) participants met Petersen criteria for a diagnosis of MCI (Petersen, 2000). No participants reported impairment in ADLs at baseline or follow-up. The presence of one or both APOE ε4 alleles was significantly greater in the declining group (p = 0.02). There were no significant group differences in age, education, sex, test-retest interval, or fMRI task performance between the two groups (see Table 1).
Table 1.
Sample characteristics and fMRI behavioral data for stable and declining groups.
| Stable (n=51) Mean (SD) | Declining (n=27) Mean (SD) | Between Groups p | η2 | |
|---|---|---|---|---|
| Sample characteristics | ||||
| Age (yrs) | 72.7 (5.1) | 73.7 (4.7) | 0.41 | 0.01 |
| Education (yrs) | 15.1 (2.5) | 14.6 (3.2) | 0.40 | 0.01 |
| Sex | 38F/13M | 19F/8M | 0.79 | - |
| Possession of APOE ε4 allele | 12 (23.5%) | 14 (51.9%) | 0.02 | - |
| Retest interval in days | 551.7 (43.5) | 560.6 (47.0) | 0.41 | 0.01 |
| SM task performance | ||||
| % correct hits (famous names) | 93.1 (6.8) | 90.4 (7.4) | 0.10 | 0.04 |
| % correct rejections (unfamiliar names) | 96.9 (4.6) | 95.2 (8.8) | 0.27 | 0.02 |
| d′ | 3.4 (0.63) | 3.1 (0.64) | 0.10 | 0.04 |
| EM task performance | ||||
| % correct hits (old names) | 64.0 (13.2) | 61.3 (12.3) | 0.38 | 0.01 |
| % correct rejections (new names) | 88.0 (10.1) | 82.7 (15.9) | 0.08 | 0.04 |
| d′ | 1.7 (0.56) | 1.4 (0.74) | 0.12 | 0.03 |
| Neuropsychological measures | ||||
| DRS-2 Total | ||||
| baseline | 140.7 (3.2) | 139.7 (3.8) | 0.28 | 0.02 |
| follow-up | 139.5 (2.1) | 135.6 (5.0)* | <0.01 | 0.21 |
| RAVLT Trials 1-5 | ||||
| baseline | 50.6 (8.8) | 46.8 (8.1) | 0.06 | 0.05 |
| follow-up | 49.5 (7.5) | 40.1 (7.1)* | <0.01 | 0.29 |
| RAVLT Delayed Recall | ||||
| baseline | 10.1 (2.6) | 9 (2.8) | 0.09 | 0.04 |
| follow-up | 10.2 (2.4) | 6.0 (2.3)* | <0.01 | 0.44 |
| Lawton ADL | ||||
| baseline | 5.0 (0.0) | 5.0 (0.0) | NS | - |
| follow-up | 4.9 (.1) | 4.9 (.2) | 0.68 | <0.01 |
| GDS Total | ||||
| baseline | 2.7 (2.5) | 1.4 (1.8) | 0.01 | 0.07 |
| follow-up | 2.6 (2.6) | 1.3 (1.8) | 0.01 | 0.09 |
| MMSE | ||||
| baseline | 29.4 (0.8) | 28.9 (1.2) | 0.04 | 0.06 |
| follow-up | 29.5 (1.1) | 29.0 (1.2) | 0.07 | 0.05 |
Note: F = female; M=male; SM = semantic memory; EM = episodic memory; DRS-2 = Mattis Dementia Rating Scale-2; RAVLT = Rey Auditory Verbal Learning Test; ADL=Activities of Daily Living; GDS=Geriatric Depression Scale; MMSE=Mini-Mental State Exam;
significant decrease (p < .05) from baseline to follow-up.
Baseline fMRI
The Declining group demonstrated less overall activation on both the SM and EM tasks as compared to the Stable group (see Figure 1). As described previously in Woodard et al. (2010), a voxelwise analysis of the SM task resulted in eight fROIs (see Table 2). The EM voxelwise analysis resulted in 19 fROIs (see Table 3).
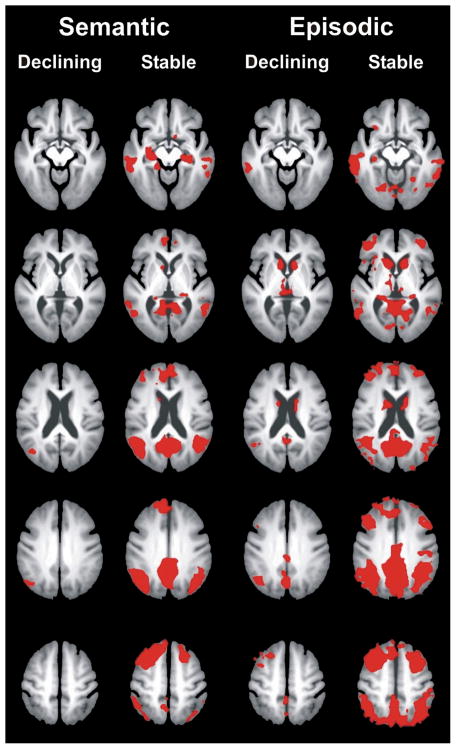
Figure 1.
Comparison of semantic and episodic task activation between the declining and stable groups. The declining group demonstrated less activation on both the semantic (famous > unfamiliar names) and episodic (previously seen > novel names) memory tasks compared to the non-declining group.
Table 2.
PCA components derived from semantic memory fROIs for stable versus declining groups (from (Woodard et al., 2010).
| Region# | Region | Stable Group Component Volume (ml) | Declining Group Component Volume (ml) | X | Y | Z | Cortical Component Loading | Hippocampal Component Loadings |
|---|---|---|---|---|---|---|---|---|
| 1 | Bilateral Posterior Cingulate Cortex, Precuneus | 115.92 | 1.44 | −1 | −52 | 25 | 0.884 | 0.257 |
| 2 | Left Angular Gyrus | −45 | −56 | 24 | 0.889 | 0.266 | ||
| 3 | Left Superior Frontal Gyrus | −18 | 30 | 41 | 0.805 | 0.018 | ||
| 4 | Right Angular Gyrus | 46 | −60 | 27 | 0.815 | 0.275 | ||
| 5 | Right Superior, Middle Frontal Gyrus | 23 | 17 | 47 | 0.839 | 0.009 | ||
|
| ||||||||
| 6 | Left Parahippocampal Gyrus, Hippocampus | 3.15 | - | −22 | −21 | −11 | 0.269 | 0.898 |
| 7 | Right Parahippocampal Gyrus, Hippocampus | 24 | −23 | −12 | 0.053 | 0.946 | ||
|
| ||||||||
| 8 | Right Cerebellum | 1.03 | - | 11 | −75 | −22 | 0.425 | 0.268 |
Table 3.
PCA components derived from episodic memory fROIs for stable versus declining groups.
| Region # | Region | Stable Group Component Volume (ml) | Declining Group Component Volume (ml) | X | Y | Z | Subcortical Component Loadings | Parietal/Temporal Component Loadings | Frontal Component Loadings |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Left Caudate | 16.52 | 7.18 | −11 | 7 | 12 | 0.850 | 0.234 | 0.273 |
| 2 | Right Caudate | 13 | 6 | 13 | 0.850 | 0.260 | 0.279 | ||
| 3 | Left Thalamus | −7 | −17 | 9 | 0.839 | 0.281 | 0.304 | ||
| 4 | Right Thalamus | 10 | −16 | 10 | 0.827 | 0.322 | 0.289 | ||
|
| |||||||||
| 5 | Right Middle Temporal Gyrus | 153.24 | 11.72 | 46 | −52 | 16 | 0.253 | 0.742 | 0.408 |
| 6 | Left Angular Gyrus | −41 | −60 | 34 | 0.212 | 0.718 | 0.446 | ||
| 7 | Right Hippocampus | 28 | −29 | −1 | 0.435 | 0.718 | 0.024 | ||
| 8 | Left Precuneus | 0 | −62 | 30 | 0.458 | 0.684 | 0.420 | ||
| 9 | Left Linual Gyrus | −4 | −74 | −11 | 0.358 | 0.671 | 0.290 | ||
| 10 | Left Hippocampus | −21 | −31 | 2 | .548 | 0.584 | 0.044 | ||
| 11 | Left Cingulate | −0 | −37 | 36 | 0.485 | 0.584 | 0.375 | ||
| 12 | Left Middle Temporal Gyrus | −59 | −33 | −7 | 0.43 | 0.526 | 0.388 | ||
|
| |||||||||
| 13 | Left Superior Medial Gyrus | 68.60 | 2.72 | −1 | 50 | 18 | 0.271 | 0.279 | 0.762 |
| 14 | Right Middle Frontal Gyrus | 34 | 11 | 47 | .0233 | 0.185 | 0.752 | ||
| 15 | Left Inferior Frontal Gyrus | −32 | 36 | 8 | 0.028 | 0.339 | 0.692 | ||
| 16 | Left Superior Medial Gyrus | −2 | 34 | 38 | 0.342 | 0.049 | 0.672 | ||
| 17 | Left Middle Frontal Gyrus | −31 | 13 | 44 | 0.380 | 0.316 | 0.662 | ||
| 18 | Right Middle Frontal Gyrus | 31 | 46 | 22 | 0.392 | 0.376 | 0.649 | ||
|
| |||||||||
| 19 | Left Cerebellum | 4.82 | - | −39 | −45 | −50 | 0.150 | −0.073 | −0.114 |
A PCA conducted on the eight SM fROIs resulted in two components: “cortical” and “hippocampal” (see Table 2, Figure 1). The cortical component included significant loadings of fROIs in the bilateral posterior cingulate, left and right angular gyrus, left superior frontal gyrus, and the right superior middle frontal gyrus. The hippocampal component included significant loadings for both the left and right parahippocampal/hippocampal fROIs.
Three components resulted from the PCA of the 19 EM fROIs: “subcortical,” “frontal,” and “parietal/temporal” (see Table 3; Figure 2). The subcortical component consisted of the left and right caudate and left and right thalamic fROIs. The frontal component included the left superior medial gyrus, the left and right middle frontal gyrus, and left inferior frontal gyrus fROIs. The parietal/temporal component included left and right middle temporal gyrus, left angular gyrus, left and right hippocampus, left lingual gyrus, left cingulate, and left precuneus fROIs.
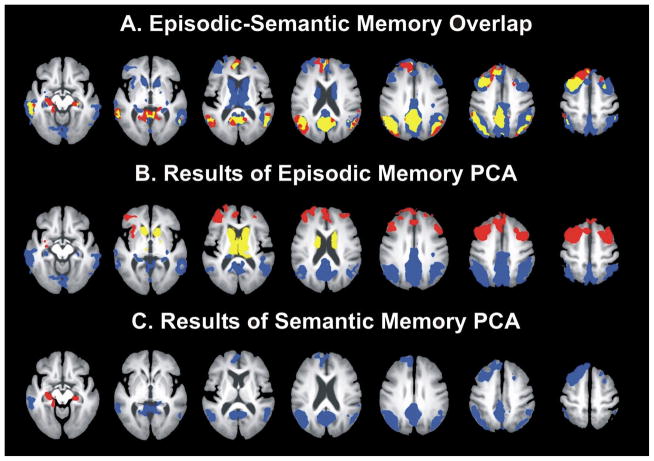
Figure 2.
Semantic and episodic activation fROI analysis. A) The top row displays the overlap of semantic and episodic activation (yellow), regions that activated only during the semantic task (red), and regions that activated only during the episodic task (blue). A principal components analysis of task-related activation for each condition resulted in the following factor loadings: B) The episodic model consisted of subcortical (yellow), frontal (red), and parietal/temporal (blue) components. C) The semantic activation model consisted of cortical (blue) and hippocampal (red) components.
Cerebellar activation was observed during both the SM and EM tasks. However, this activation did not demonstrate significant loadings in either the SM or EM PCA and was thus excluded from logistic regression analyses.
Logistic Regression Analysis
APOE ε4 allele status was found to be a significant predictor of cognitive decline in our previous study using the SM task (Woodard et al., 2010) and was therefore included in the logistic regression analyses comparing SM and EM task activation models in the current study.
Four logistic regression models were evaluated (see Table 4). Model 1 (APOE ε4 alone) was the poorest fitting model (Nagelkerke R2 = .106; C = .642). Model 2 (SM task + APOE) fit the data much better (Nagelkerke R2 = .285; C = .787), with significant contributions from APOE (p = .003) and both cortical (p = .01) and hippocampal (p = .03) fMRI components. Model 3 (EM task + APOE) was not a significantly better fit than Model 2 (Nagelkerke R2 = .212; C = .711) with none of the EM fMRI components significantly predicting decline. The significance of APOE ε4 as a predictor was reduced to marginally significant level (p = .051) in Model 3, likely due to a significant relationship between frontal activation during the episodic task and APOE ε4 inheritance; the frontal component became significant when ε4 was removed from the model (p = .039). Model 4 (APOE, SM, and EM) demonstrated the greatest predictive accuracy of all four models (R2 = .422; C = .837). However, an examination of BIC values indicated that Model 4 most likely overfit the data due to the large number of variables relative to number of participants (see below). Interestingly, APOE ε4 and both SM fMRI components were significant predictors in this model, but the only significant EM predictor was the parietal-temporal component.
Table 4.
Results of the logistic regressions for the relative accuracy of semantic and episodic memory activation in predicting cognitive decline.
| Nagelkerke R2 | C Index | Variables | Coeff | SE | p-value | BIC | |
|---|---|---|---|---|---|---|---|
| Model 1 | 0.089 | 0.637 | APOE ε4 | 1.253 | 0.507 | 0.014 | 103.09 |
|
| |||||||
| Model 2 | 0.285 | 0.787 | APOE ε4 | 1.846 | 0.612 | 0.003 | 96.28 |
| SM Cortical | −0.874 | 0.309 | 0.013 | ||||
| SM Hippocampal | −0.699 | 0.323 | 0.031 | ||||
|
| |||||||
| Model 3 | 0.109 | 0.656 | APOE ε4 | 1.057 | 0.451 | 0.051 | 109.38 |
| EM Subcortical | 0.451 | 0.284 | 0.112 | ||||
| EM Parietal/Temporal | −0.410 | 0.270 | 0.129 | ||||
| EM Frontal | −0.420 | 0.277 | 0.130 | ||||
|
| |||||||
| Model 4 | 0.298 | 0.786 | APOE ε4 | 1.836 | 0.675 | 0.007 | 102.68 |
| SM Cortical | −0.953 | 0.381 | 0.012 | ||||
| SM Hippocampal | −0.801 | 0.337 | 0.018 | ||||
| EM Parietal/Temporal | −0.645 | 0.336 | 0.055 | ||||
| EM Subcortical | 0.466 | 0.310 | 0.133 | ||||
| EM Frontal | −0.228 | 0.340 | 0.502 | ||||
Note: SM = semantic memory; EM = episodic memory. Nagelkerke R2 and C Index values have been corrected for optimism using bootstrapping (see text for additional description).
When comparing the models, BIC values favored the SM model over the combined SM + EM model (p < .036) and the APOE ε4 only model (p < .001) (Table 4). The APOE ε4 model demonstrated a significantly lower BIC than the EM model (p < .034). Thus, the SM+APOE model fit the data significantly better and more parsimoniously than either the EM+APOE model or the APOE only model.
Discussion
In our previous study, we demonstrated that two factors, baseline semantic memory fMRI activation and APOE ε4 allele status, predicted future cognitive decline in healthy participants after an 18-month retest interval (Woodard et al., 2010). The current study extends these findings by focusing on the type of fMRI activation task used to predict cognitive decline. The current results indicate that in combination with APOE ε4 status, fMRI brain activation patterns derived from a SM activation task were superior to an EM activation task for predicting cognitive decline after an 18-month interval. These results suggest the type of activation task used in fMRI studies may play an important role in accurately identifying healthy elders at risk for developing future cognitive decline.
Previous research reported that EM fMRI activation may also be effective in predicting future cognitive decline (Bookheimer et al., 2000; O’Brien et al., 2010). In contrast, we found that when SM and EM predictors were combined in the same model, the EM components did not increase the overall predictive ability of the model relative to APOE status alone. Only the parietal-temporal EM component significantly contributed to prediction of cognitive decline. Whereas there was extensive overlap in fMRI activation between the SM and EM tasks (Figure 2), the greater sensitivity of the SM task may be due to its activation of a more spatially-localized network than the multiple activation networks associated with the EM task (Figure 2, Tables 2 and 3). Specifically, the SM task (famous name discrimination) has been previously shown to activate AD-vulnerable regions such as the hippocampus and parahippocampal gyrus (Douville et al., 2005) and the posterior cingulate (Woodard et al., 2007). These regions overlaps with the resting state default mode network (DMN), which includes the posterior cingulate, medial prefrontal cortex, medial temporal, and angular gyrus (Raichle et al., 2001). Recent studies have indicated that the DMN is disrupted in patients with a diagnosis of MCI and AD (Pihlajamaki & Sperling, 2009; Rombouts & Scheltens, 2005). In a recent review, Binder and colleagues (2009) point out that the DMN overlaps with regions involved in task-activated fMRI experiments involving semantic processing. Because it appears to selectively activate brain regions associated with the DMN, task-activated fMRI that engages the semantic processing may be more sensitive to future cognitive decline than episodic memory tasks.
The more diffuse pattern of activation observed with the EM task may be associated with task difficulty. Importantly, EM task accuracy was worse than SM task accuracy, which is not surprising because episodic memory declines with age (Cansino, 2009), while semantic memory abilities remain relatively intact (Nilsson, 2003). The EM task activated more regions than the semantic task, especially those associated with effort and error detection (e.g., frontostriatal, anterior cingulate). Thus, SM activation may be superior to EM activation for predicting cognitive decline due to its ability to effectively stress AD-vulnerable regions, while minimizing activation of networks associated with task performance and effort.
Our study found that a greater magnitude of fMRI activation at baseline was associated with preserved cognitive performance regardless of task (Figure 1). Bookheimer et al.’s (2000) study found the opposite results, namely that increased baseline activation was associated with future cognitive decline. Our findings, however, are consistent with those of a more recent study conducted by Lind et al. (2006) demonstrating that decreased activity predicted future cognitive decline. The precise reasons for the divergent results are unclear. It should be noted, however, that greater task-induced activation is consistently observed in asymptomatic APOE ε4-positive individuals relative to non-carriers (Bondi et al., 2005; Kukolja, Thiel, Eggermann, Zerres, & Fink, 2010; Seidenberg et al., 2009; Trivedi et al., 2008). In addition, increased activation in fMRI studies is frequently observed in older compared to younger subjects (Cabeza, 2002; Nielson et al., 2006; Nielson, Langenecker, & Garavan, 2002).
Whether increased activation is helpful or harmful for the individual is an important question. Park and Reuter-Lorenz (2009) proposed the Scaffolding Theory of Aging and Cognition (STAC) to suggest that increased activation in cognitively intact elders represents a compensatory response to neural changes associated with normal aging and/or the impact of emerging disease processes. The increased activity serves to preserve intact levels of cognitive performance. Conversely, once cognitive symptoms emerge, brain activity diminishes since the “scaffold” is no longer successful in preserving normal performance levels (Han, Bangen, & Bondi, 2009). In the context of this study, cognitively intact elders with the ε4 gene would be expected to experience more brain activity than cognitively intact non-carriers presumably because at least some at-risk individuals have begun to experience the early stages of AD-related pathology. In support of this hypothesis, longitudinal fMRI studies suggest a progression of hyperactivation to hypoactivation over the course of AD (cf. Machulda et al., 2003; O’Brien et al., 2010).
The broader literature has demonstrated that comprehensive neuropsychological testing is predictive of impending cognitive decline (cf. Twamley, Ropacki, & Bondi, 2006). Thus, some might question the value of using fMRI for prognostic purposes. The goal of the current study was to use functional MRI as a surrogate for such testing because recent studies suggest it might reveal specific patterns that could eventually serve as better or earlier predictors of cognitive decline. In the current study, the neuropsychological measures used, specifically the DRS-2 and the RAVLT, served as criterion variables for assessing cognitive decline, thereby serving to determine group membership. Thus, they could not be used also as predictors of cognitive in our models and no other measures were available. However, the role of cognitive performance in predicting decline could be examined in this study by using the semantic and episodic tasks given in the scanner. When including the d′ score for semantic and episodic performance in the model with the other significant predictors (i.e., adding these behavioral factors to Model 2), the bootstrapped C index was .80 compared with .79 without them, and neither of the cognitive performance factors offered significant prediction (semantic d′ p = .16; episodic d′ p = .77). While behavioral performance may provide adequate prediction of impending decline, in this context where it was examined directly in conjunction with fMRI activation, fMRI was superior. One limitation of this interpretation is that the semantic task (FNDT) was specifically designed to produce > 90% accuracy in performance. The value of the design was to limit task difficulty contributions to activation, but a consequence of it is a limited ability to discern behavioral differences between groups.
There are several other limitations of the current study worth noting. Participant performance on the EM task was poorer than performance on the SM task. Although only correct trials were used in the brain imaging analysis, an easier EM task may have resulted in improved sensitivity for predicting cognitive decline. Similarly, EM task performance was dependent upon encoding during the semantic task. During the EM task, participants in both groups sometimes judged novel famous names as previously seen due to familiarity effects with these stimuli, adding to task difficulty. Furthermore, both the SM and EM tasks used in this study relied on retrieval in a forced choice-recognition format. Activation maps based on encoding evoke different brain systems and may possess different degrees of accuracy in predicting cognitive decline (Bondi, 1999; Wolk, 2011). Finally, results of the current study require replication using larger sample sizes and a longer follow-up interval in order to draw more definitive conclusions on the ability of fMRI task activation to predict conversion to MCI or AD.
In summary, our findings suggest that fMRI activation during a semantic memory task is more accurate in predicting future cognitive decline in asymptomatic older adults than activation during an episodic memory task. Future studies are required to determine the relative sensitivity of task-activated fMRI in comparison to other biomarkers (structural MRI, resting state functional connectivity MRI, amyloid PET scanning, CSF and blood analyses) in identifying individuals at-risk for future cognitive decline or the development of MCI/AD. Results of this study suggest that semantic memory activation in combination with APOE ε4 status holds promise for identifying asymptomatic at-risk individuals for inclusion in primary prevention randomized clinical trials of interventions designed to prevent or delay cognitive decline.
References
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL. Familial risk for Alzheimer’s disease alters fMRI activation patterns. Brain. 2006;129(Pt 5):1229–1239. doi: 10.1093/brain/awl089. 129/5/1229 [pii], [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. bhp055 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. 64/3/501 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Kaszniak AW. Implicit and explicit memory in Alzheimer’s disease and Parkinson’s disease. Journal of Clinical and Experimental Neuropsychology. 1991;13(2):339–358. doi: 10.1080/01688639108401048. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Glasako D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E genotype in preclinical detection of Alzheimer’s disease. Psychology and Aging. 1999;14(2):195–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. New England Journal of Medicine. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. 25/34/7709 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cansino S. Episodic memory decay along the adult lifespan: a review of behavioral and neurophysiological evidence. International Journal of Psychophysiology. 2009;71(1):64–69. doi: 10.1016/j.ijpsycho.2008.07.005. S0167-8760(08)00756-3 [pii] [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Graff-Radford NR, Reiman EM, Weaver A, Osborne D, Lucas J, Thibodeau SN. Preclinical memory decline in cognitively normal apolipoprotein E-epsilon4 homozygotes. Neurology. 1999;53(1):201–207. doi: 10.1212/wnl.53.1.201. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. S0010480996900142 [pii] [DOI] [PubMed] [Google Scholar]
- Douville K, Woodard JL, Seidenberg M, Miller SK, Leveroni CL, Nielson KA, Rao SM. Medial temporal lobe activity for recognition of recent and remote famous names: an event-related fMRI study. Neuropsychologia. 2005;43(5):693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. S0028-3932(04)00238-6 [pii] [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. The Journal of the American Medical Association. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. 0022-3956(75)90026-6 [pii] [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Han SD, Bangen KJ, Bondi MW. Functional magnetic resonance imaging of compensatory neural recruitment in aging and risk for Alzheimer’s disease: review and recommendations. Dementia and Geriatric Cognitive Disorders. 2009;27(1):1–10. doi: 10.1159/000182420. 000182420 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer’s disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33(4):441–459. doi: 10.1016/0028-3932(94)00127-b. 0028-3932(94)00127-B [pii] [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, Sager MA. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. The Journal of Neuroscience. 2006;26(22):6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. 26/22/6069 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2 professional manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- Kukolja J, Thiel CM, Eggermann T, Zerres K, Fink GR. Medial temporal lobe dysfunction during encoding and retrieval of episodic memory in non-demented APOE epsilon4 carriers. Neuroscience. 2010;168(2):487–497. doi: 10.1016/j.neuroscience.2010.03.044. S0306-4522(10)00437-9 [pii] [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Lind J, Ingvar M, Persson J, Sleegers K, Van Broeckhoven C, Adolfsson R, Nyberg L. Parietal cortex activation predicts memory decline in apolipoprotein E-epsilon4 carriers. Neuroreport. 2006;17(16):1683–1686. doi: 10.1097/01.wnr.0000239954.60695.c6. 00001756-200611060-00005 [pii] [DOI] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O’Brien PC, Jack CR., Jr Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61(4):500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale professional manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Mickes L, Wixted JT, Fennema-Notestine C, Galasko D, Bondi MW, Thal LJ, Salmon DP. Progressive impairment on neuropsychological tasks in a longitudinal study of preclinical Alzheimer’s disease. Neuropsychology. 2007;21(6):696–705. doi: 10.1037/0894-4105.21.6.696. 2007-15625-007 [pii] [DOI] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. Journal of Neurolology, Neurosurgery & Psychiatry. 2008;79(6):630–635. doi: 10.1136/jnnp.2007.124149. jnnp.2007.124149 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. [Google Scholar]
- Nielson KA, Douville KL, Seidenberg M, Woodard JL, Miller SK, Franczak M, Rao SM. Age-related functional recruitment for famous name recognition: an event-related fMRI study. Neurobiology of Aging. 2006;27(10):1494–1504. doi: 10.1016/j.neurobiolaging.2005.08.022. S0197-4580(05)00226-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychology and Aging. 2002;17(1):56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Nilsson LG. Memory function in normal aging. Acta Neurologica Scandinavica Supplementum. 2003;179:7–13. doi: 10.1034/j.1600-0404.107.s179.5.x. [DOI] [PubMed] [Google Scholar]
- O’Brien JL, O’Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, Sperling RA. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74(24):1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. WNL.0b013e3181e3966e [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–111. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Structure-function correlates of cognitive decline in aging. Cerebral Cortex. 2006;16(7):907–915. doi: 10.1093/cercor/bhj036. bhj036 [pii] [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia. 2000;15(3):93–101. [PubMed] [Google Scholar]
- Pihlajamaki M, Sperling RA. Functional MRI assessment of task-induced deactivation of the default mode network in Alzheimer’s disease and at-risk older individuals. Behavioural Neurology. 2009;21(1):77–91. doi: 10.3233/BEN-2009-0231. R6331X54T779523W [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. 98/2/676 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1958. [Google Scholar]
- Rombouts S, Scheltens P. Functional connectivity in elderly controls and AD patients using resting state fMRI: a pilot study. Current Alzheimer Research. 2005;2(2):115–116. doi: 10.2174/1567205053585783. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Hulette O, Welsh-Bohmer KA, Schmechel DE, Crain B, Burke JR, Rosenberg C. Specificity, sensitivity, and predictive value of apolipoprotein-E genotyping for sporadic Alzheimer’s disease. Lancet. 1996;348(9020):90–93. doi: 10.1016/s0140-6736(96)01251-2. S0140673696012512 [pii] [DOI] [PubMed] [Google Scholar]
- Schmidt KS. DRS-2: Alternate form professional manual. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- Schmidt KS, Mattis PJ, Adams J, Nestor P. Alternate-form reliability of the Dementia Rating Scale-2. Archives of Clinical Neuropsychology. 2005;20(4):435–441. doi: 10.1016/j.acn.2004.09.011. S0887-6177(04)00145-3 [pii] [DOI] [PubMed] [Google Scholar]
- Schmidt M. Rey Auditory and Verbal Learning Test: A handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- Seidenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Antuono P, Rao SM. Semantic memory activation in individuals at risk for developing Alzheimer disease. Neurology. 2009;73(8):612–620. doi: 10.1212/WNL.0b013e3181b389ad. 73/8/612 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Kryscio RJ, Schmitt FA, Lovell MA, Blonder LX, Rayens WS, Andersen AH. Longitudinal functional alterations in asymptomatic women at risk for Alzheimer’s disease. Journal of Neuroimaging. 2005;15(3):271–277. doi: 10.1177/1051228405277340. 15/3/271 [pii] [DOI] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Johnson KA. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Medicine. 2010;12(1):27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman MA, Woodard JL, Nielson KA, Seidenberg M, Smith JC, Durgerian S, Rao SM. Functional magnetic resonance imaging of semantic memory as a presymptomatic biomarker of Alzheimer’s disease is. Biochimica et Biophysica Acta. 2012;1822(3):442–456. doi: 10.1016/j.bbadis.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN, Carmelli D, Schellenberg GD, La Rue A. Apolipoprotein E epsilon4 and change in cognitive functioning in community-dwelling older adults. Journal of Geriatric Psychiatry and Neurology. 2005;18(4):196–201. doi: 10.1177/0891988705281864. 18/4/196 [pii] [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Hess TM, Fitzgerald ME, Atwood CS, Johnson SC. fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: risk factors for Alzheimer’s disease. Neuropsychologia. 2008;46(6):1667–1678. doi: 10.1016/j.neuropsychologia.2007.11.035. S0028-3932(07)00429-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SA, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer’s disease. Journal of the International Neuropsychological Association. 2006;12(5):707–735. doi: 10.1017/S1355617706060863. S1355617706060863 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Bondi MW. Use of functional magnetic resonance imaging in the early identification of Alzheimer’s disease. Neuropsychology Review. 2007;17(2):127–143. doi: 10.1007/s11065-007-9025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC Alzheimer’s Disease Neuroimaging Initiative. Fractioning verbal episodic memory in Alzheimer’s disease. Neuroimage. 2011;54(2):1530–1539. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Antuono P, Guidotti L, Durgerian S, Rao SM. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132(Pt 8):2068–2078. doi: 10.1093/brain/awp157. awp157 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Miller SK, Franczak M, Antuono P, Rao SM. Temporally graded activation of neocortical regions in response to memories of different ages. Journal of Cognitive Neuroscience. 2007;19(7):1113–1124. doi: 10.1162/jocn.2007.19.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Smith JC, Antuono P, Durgerian S, Rao SM. Prediction of cognitive decline in healthy older adults using fMRI. Journal of Alzheimers Disease. 2010;21(3):871–885. doi: 10.3233/JAD-2010-091693. M27677251202372G [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]