Introduction
Vestibular schwannomas (VS) are benign, but potentially devastating tumors arising from Schwann cells encapsulating the vestibular branch of the eighth cranial nerve. VS tumors are mostly sporadic with 4% being attributed to Neurofibromatosis Type 2 (NF2), an autosomal dominant disorder characterized by bilateral VS and other cranial nerve schwannomas.1,2 “Watchful waiting” has been advocated for small VS tumors with no detectable growth.3 Large tumors, however, can cause brainstem compression, leading to hydrocephalus, herniation, and even death.4 When VS are large or bilateral, as in Neurofibromatosis 2 (NF2), they present a significant management challenge. Current treatment modalities in these cases include microsurgical excision and stereotactic radiosurgery. Both modalities can incur significant risks to the patient, including total loss of hearing and/or vestibular function, facial nerve deficits, other cranial neuropathies, cerebrospinal fluid leak, meningitis, persistent headaches, hydrocephalus seizures, cerebellar ataxia or death.5
It is imperative to develop pharmacologic therapies to add to the arsenal against VS, particularly in NF2 patients. Pharmacologic agents targeted against the ErbB family, or the intracellular pathways that mediate their effects, could slow clinical progression of VS in patients where other modalities carry a high risk to benefit ratio. The ErbB family of receptor tyrosine kinases consist of four members: EGFR (ErbB1), ErbB2 (HER2), ErbB3 (HER3), and ErbB4 (HER4). ErbB members have extracellular ligand binding domains and intracellular tyrosine kinase domains; although ErbB2 does not have a known ligand and ErbB3 does not have tyrosine kinase activity.6,7
Recent evidence suggests that growth and/or proliferation signaling in VS offer opportunities to identify molecular therapeutic targets. Primary human VS tumors from both sporadic and NF2 patients have been demonstrated by immunohistochemistry and co-cultures to express EGFR, ErbB2, and ErbB3.8 Furthermore, the activated, phosphorylated form of ErbB2 is expressed in VS.8 Activation of ErbB receptors requires dimerization, and all four members of the ErbB family (EGFR, ErbB2, ErbB3, and ErbB4) are able to form heterodimers when specific ligands bind to the extracellular (ligand-binding) domain, inducing a conformational change in the transmembrane region that results in receptor dimerization and simultaneous activation of the intracellular kinase domain. The activated tyrosine kinase domain then transphosphorylates and, in some cases (e.g., ErbB2), autophosphorylates the intracellular tyrosine residue at a consensus phosphorylation site. This site then recruits second messengers and rapidly induces a cascade of intracellular signaling involving both the PI3K/AKT and Ras/ERK/MAPK pathways, culminating in gene transcription for growth, proliferation, and survival. 7, 8
In this study, we examined the effect of small molecule inhibitors on VS. We first investigated ErbB dimerization in VS tumor samples. Here, we demonstrate that human VS express EGFR/ErbB2 heterodimers in an activated state. ErbB3 receptor expression is also demonstrated and identified in heterodimers with both EGFR and ErbB2, but the predominant ErbB heterodimer pairs in VS appear to be comprised of EGFR and ErbB2. The effect of Lapatinib and AG825, a selective small molecule inhibitor of ErbB2 on cell cycle and apoptosis was investigated. We performed Annexin-V cell death assays, and cell cycle assays. We also studied VS proliferation. We found a robust, dose-dependent inhibition of cellular growth and proliferation with Lapatinib. AG825 also inhibits growth, but to a lesser extent. These findings compliment our lab’s results from co-immunoprecipitation assays, which predominantly demonstrate EGFR and ErbB2 heterodimer pairs in VS.
Material and Methods
The human subjects study protocol was approved by the Institutional Review Boards of the University of California, San Diego (#070135) and Kaiser Permanente San Diego Medical Center (#5172), and all participants provided written, informed consent.
Primary VS Cell Culture
Following excision, VS specimens were transported to the laboratory in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA) on ice. VS specimens were minced into 1-mm3 fragments with a # 10 blade then digested with 0.1 % trypsin and 0.125 % collagenase (Sigma Aldrich, St Louis, MO) for two hours at 37 °C. During digestion, specimens were mechanically dissociated with micropipette trituration every 40 minutes. Following centrifugation at 2000g for 5 minutes, the supernatant was discarded, cells were rinsed twice with PBS, and then Cells were resuspended and plated in sterile tissue culture dishes (Corning Lifesciences; Lowell, MA). Primary VS cultures were maintained in DMEM supplemented with 10% fetal bovine serum, 1% human Schwann cell supplement (ScienCell, Carlsbad, CA) and 2% penicillin/streptomycin (Invitrogen), at 37°C in an atmosphere of 5% carbon dioxide until desired confluence reached. Twenty-four hours prior to experimental manipulation, cells were maintained in DMEM with reduced serum (2% FBS).
Treatment with ErbB Inhibitors
Cultures were treated with Lapatinib (0.01, 0.1, 0.2, 1, and 10 µM) or AG825 (0.01, 0.1, 0.2, 1, and 10 µM) via media changes every 24 hours for 72 hours and treated with 10 nM EGF or DMSO alone (control, final concentration less than 0.05%)one hour prior to the experiment, where indicated.
Co-Immunoprecipitation
In order to determine what ErbB family heterodimer pairs are present, co-immunoprecipitation for ErbB family members was performed on 18 VS tumors. Following excision, VS specimens were flash frozen in liquid nitrogen and crushed in tissue grinders (VWR International, West Chester, PA) while still in liquid nitrogen. Specimens were lysed in MTG lysis buffer (50 mM Tris, 100 mM NaCl, 1% nonidet-P40, 10% Glycerol, to final pH 8.0), with the addition of 0.1% phosphatase and 0.01% protease inhibitor cocktail (Roche, Indianapolis, IN). Cellular debris was pelleted by centrifugation at 9300g for 30 minutes in the cold room. 1 mg of VS lysates were pre-cleared by 1 hour incubation with non-specific IgG antibodies and protein A/G plus agarose beads (San Cruz Biotechnology, CA), and then centrifuged at 2000g for 1 min. The supernatant was incubated with gentle rocking at 4°C overnight in primary antibody anti-EGFR, ErbB2, or ErbB3 (Capralogics; Hardwick, MA; Abcam; Cambridge, MA; and Santa Cruz Biotechnology; CA, respectively). Then, 20 µL of Protein A/G plus agarose beads was added for 24 hours to collect the immune complexes, which were resuspended in electrophoresis sample buffer and kept in −20 °C, per manufacturer’s instructions. The immunoprecipitate was then subjected to NuPAGE electrophoresis to separate protein bands as described below under Immunoblotting.
Immunoblotting
after washing with phosphate buffered-saline (PBS), cells were lysed in 1÷ cell lysis buffer (Cell Signaling, Danvers, MA) supplemented with 0.1% phosphatase inhibitor and 0.01% protease inhibitor cocktail (Roche; Indianapolis, IN). Lysates were obtained with a cell scraper and sonicated in 2.0 ml Eppendorf tubes. Protein concentration was measured using the bichinchoninic acid (BCA) protein assay (Thermo Fischer, Rockford, IL). 20 µg of protein was mixed with reducing agent (NuPAGE Sample Reducing Agent)) (Invitrogen; Carlsbad, CA) and sample buffer (NuPAGE LDS Sample Buffer) (Invitrogen, Carlsbad, CA) then heated for 10 minutes at 80° C. Proteins were separated by electrophoresis using sodium dodecyl sulfate (SDS) on a 7.5% Tris-HCL minigel (Bio-Rad, Hercules, CA). Gels were then transferred onto polyvinylidene fluoride (PVDF) membranes (Sigma Aldrich, St. Louis, MO). Membranes were blocked with 5% bovine serum albumin (BSA, Sigma Aldrich) for one hour, then incubated with primary antibody against EGFR (1:250, Capralogics, Hardwick, MA), ErbB2 (1:250, Santa Cruz Biotechnology, Santa Cruz, CA) or ErbB3 (1:250, Santa Cruz Biotechnology) overnight. Membranes were then rinsed in PBS and incubated with appropriate horseradish peroxidase (HRP)-labeled secondary antibody (1:10,000) for one hour, then rinsed in PBS, and incubated with enhanced chemiluminescence (Positioning Pierce; Rockford, IL) reagent for 5 min. Blots were then exposed to film (Kodak) and developed using an SRX-101A film processor (Konica Minolta Medical and Graphic Inc, NJ).
Immunofluorescence Staining
VS cells were plated on glass cover slides until they reached desired confluence. Cells were fixed with 4% paraformaldehyde, and after washing with PBS, paraformaldehyde fixed cells were permeabilized with 0.2% Triton X-100. Cells were then blocked with 10% BSA and incubated in primary antibodies 1/100 in 4% BSA (sheep anti-human EGFR (Capralogics; Hardwick, MA); Mouse anti-human ERbB2 (Abcam; Cambridge, MA); Rabbit anti-human ErbB3 (Santa Cruz Bio; CA) for 2 hours at room temperature in a humidified chamber. Then, primary antibodies were removed and cells were washed three times with PBS and incubated with the appropriate secondary antibodies (Anti-sheep conjugated to Texas Red (TR), Anti-mouse conjugated to FITC, anti-Rabbit conjugated to Alexa Fluor 350) (Jackson ImmunoResearch Laboratories, Bar Harbor, Maine) for 1 hour at room temperature in the dark in a humidified chamber. Slides were mounted with Vectashield® mounting medium (Vector Laboratories, Inc, Burlingame, CA) and coverslipped. Images were acquired with an Olympus FV1000 point scanning confocal microscope (UCSD School of Medicine Light Microscopy Facility supported by grant P30 NS047101).
Cell Cycle Distribution
To determine the cell cycle distribution after treating HEI193 cells with Lapatinib and AG825, as described above, cells were harvested and fixed in 50% cold ethanol by vortexing the PBS solution and adding ethanol drop-wise. Cells were then put on ice and treated with a solution of PBS plus 0.1% Triton- X100, 2-mg/mL DNAase-free RNaseA, and 0.02-mg/mL propidium iodide. Cells were analyzed by flow cytometry on a FACScan flowcytometer (Becton, Dickinson, and Company, San Jose, California). Multicycle AV Cell Cycle software (Phoenix Flow Systems) was used to calculate the fraction of cells in each phase of the cell cycle.
Annexin V Assay for Cell Death
To assess the extent to which Lapatinib and AG825induced VS cell apoptosis, we used flow cytometry. HEI193 cells were treated with the desired concentrations of the inhibitors and Annexin-V Apoptosis Detection kit (Calbiochem, San Diego, CA) was used per manufacturer’s instructions. The position of quadrants on Annexin V/PI dot plots was performed and live cells (Annexin V−/PI−, F3), early/primary apoptotic cells (Annexin V+/PI−, F4), late/secondary apoptotic cells (Annexin V+/PI+, F2) and necrotic cells (Annexin V−/PI+, F1) were distinguished based on FITC-labeled Annexin-V binding and propidium iodide (PI) uptake.
Cellular Proliferation
VS primary cultures were maintained in 24-well plates and treated with Lapatinib, AG825, or control as described above for seventy-two hours. Zymed® labeling medium (Invitrogen) was diluted in DMEM to a concentration of 100 µmol/L and added to cells 48 hours prior to 5-bromo-2-deoxyuridine labeling. Cultures were treated with Zymed® BrdU Staining Kit according to manufacturer’s instructions (Invitrogen). After staining, cells were kept in PBS. The number of BrdU positive nuclei was determined by counting 10 randomly selected fields for each condition. The percent of BrdU positive VS cells was expressed as a percent of the total number of VS cells.
Statistical Analyses
For cell cycle, apoptosis, and proliferation assay, statistical significance was calculated using Chi-square analyses with a two-tailed p-value.
Results
ErbB Receptor Identity and Activation State
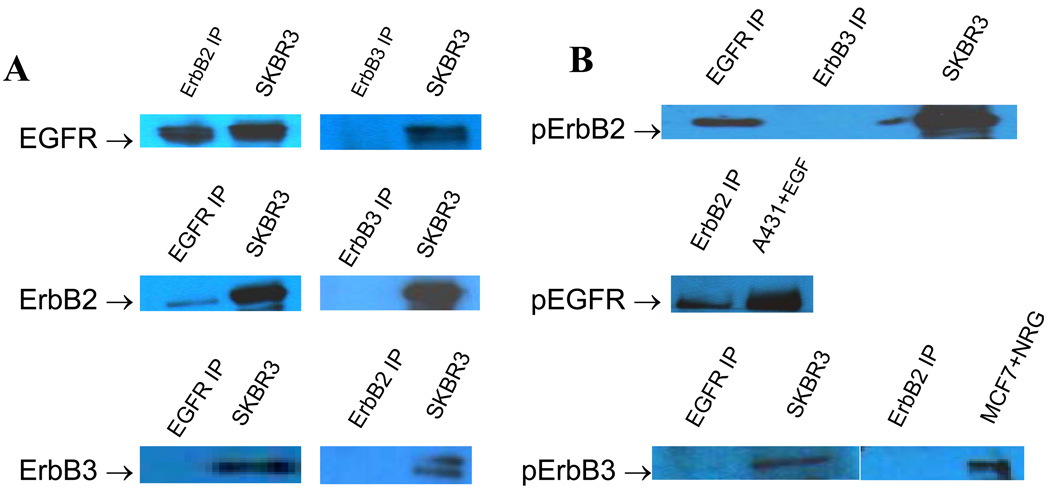
Co-immunopreciptation (co-IP) and immunoblotting demonstrated primarily EGFR-ErbB2 hetero-dimerization in VS tumor samples (Figure 1). A breast cancer cell line, SKBR3, served as positive control (Figure 1A). Phosphorylated ErbB2 was demonstrated in co-immunoprecipitated ErbB receptors with EGFR, indicating activation of ErbB2 in EGFR/ErbB2 heterodimer pairs. Western blots confirmed activation (phosphorylation) of ErbB2 with EGFR co-IP and activation of EGFR with ErbB2 co-IP (not shown), but not with ErbB3 co-IP (Figure 1B),.
Figure1.
Co-Immunoprecipitation (Co-IP) studies in VS tumor samples. A: representative Co-IP Western blot showing identity of ErbB dimer partners (SKBR3 = positive control). B: Phosphorylated p-ErbB Co-IP Western bands, indicating activation levels of ErbB dimer partners in human VS.
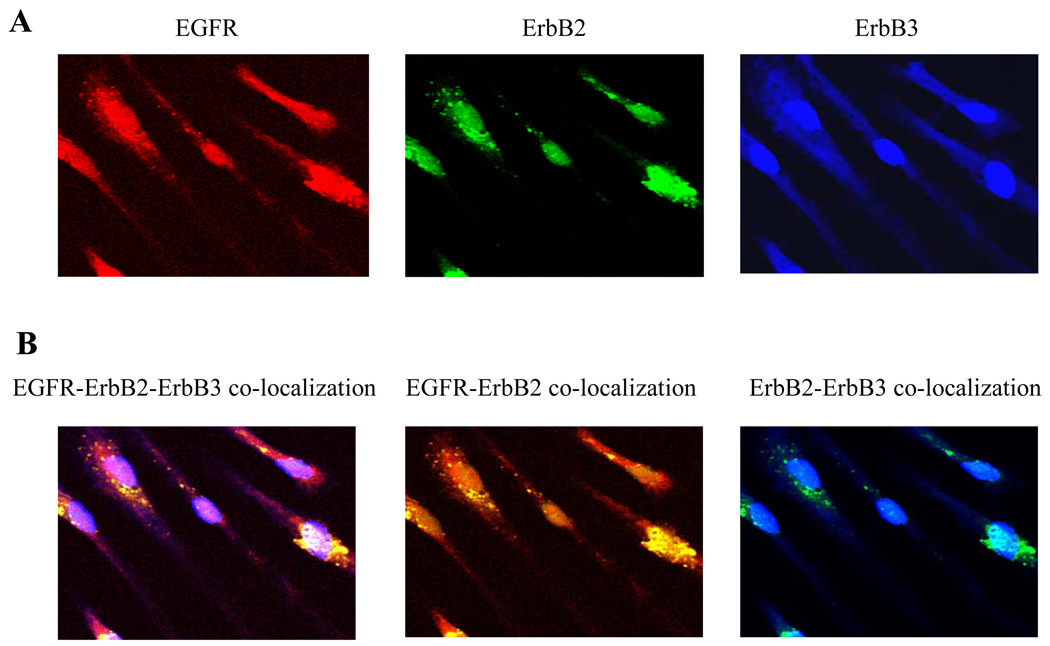
Immunofluoresence staining of human VS cell primary cultures demonstrated EGFR, ErbB2 and ErbB3 expression (Figure 2A). Co-localization of the three ErbB receptors demonstrated EGFR-ErbB2 dimerization, confirming co-IP data. (Figure 2B).
Figure2.
Immunofluorescence staining of VS cells depicting the expression of EGFR, ErbB2, and ErbB3 receptors: (A) Cofocal images of individual ErbB receptors (B) Confocal images of co-localized ErbB receptors depicting predominently EGFR-ErbB2 dimerization.
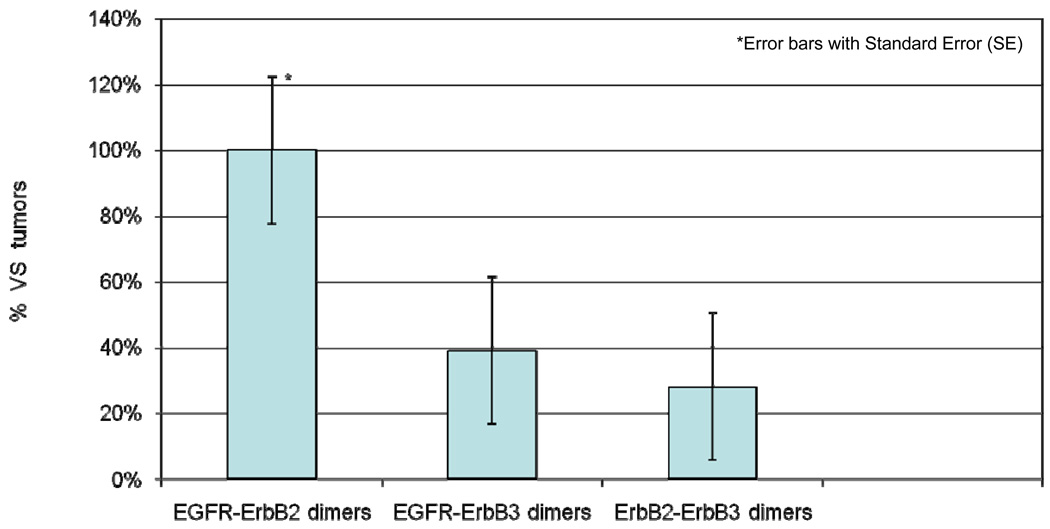
A bar graph demonstrating the percentage of ErbB receptor dimers in 18 VS tumors is shown in Figure 3. EGFR-ErbB2 heterodimers were detected in all 18 VS specimens (100%). Seven VS specimens (39%) expressed EGFR-ErbB3 heterodimers, and 5 VS specimens (28%) expressed ErbB2-ErbB3 heterodimers.
Figure 3.
The prevalence (expressed as %) of specific ErbB dimer pairs in VS tumors (n = 18), determined by comprehensive co-immunoprecipitation and immunoblotting studies.
Cell Cycle effects using ErbB Inhibitors
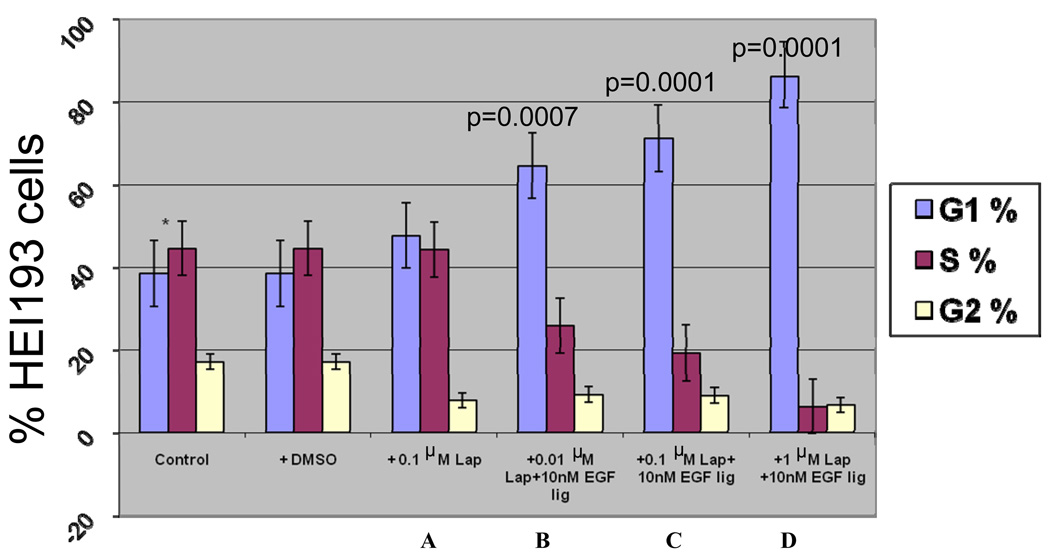
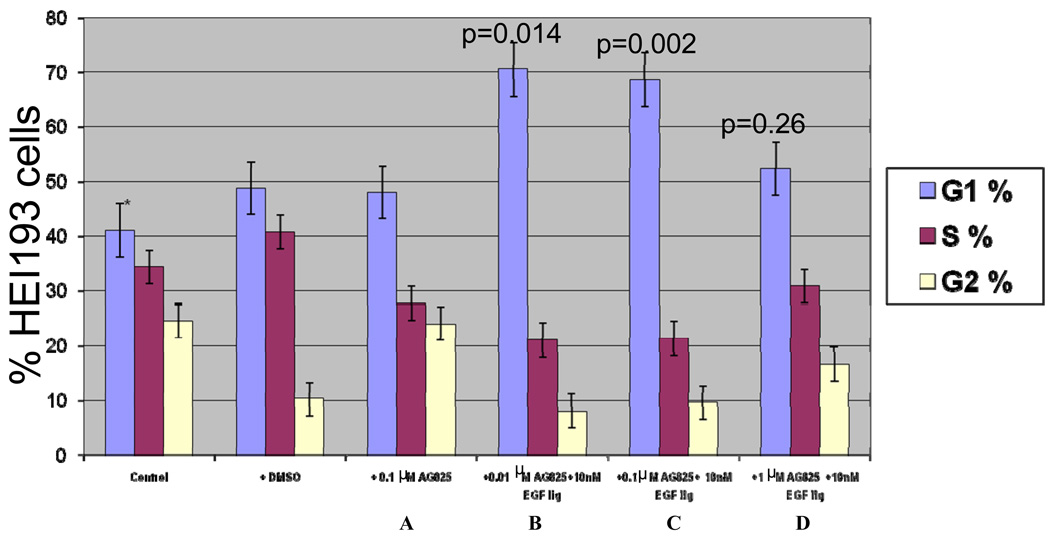
Cell Cycle analysis in HEI193 cells demonstrated increased G1 accumulation and decreased S1 phase entry with increasing doses of Lapatinib (0.01,0.1, and 1 µM); EGF ligand was added to cells to stimulate the ErbB receptors and downstream signaling, and enhanced the inhibitory effect of Lapatinib (Figure 4). Increased G1 accumulation and decreased S1 phase at 0.01 µM and 0.1 µM concentrations of AG825 were observed (Figure 5). However, the cell cycle arrest with AG825 is less than that seen with Lapatinib treatment, especially, at 1 µM concentration (compare Figures 4 and 5).
Figure 4.
Cell cycle study of HEI193 cells after in culture treatment with Lapatinib at: A) 0.1 µM, (B, C,D) 0.01, 0.1, and 1 µM Lapatinib with 10 nM EGF ligand, respectively.
Figure 5.
Cell cycle analyses of HEI193 cells after in culture treatment with AG825 at: (A) 0.1 µM, (B, C,D) 0.01, 0.1, and 1 µM AG825 with 10 nM EGF ligand, respectively.
Apoptosis
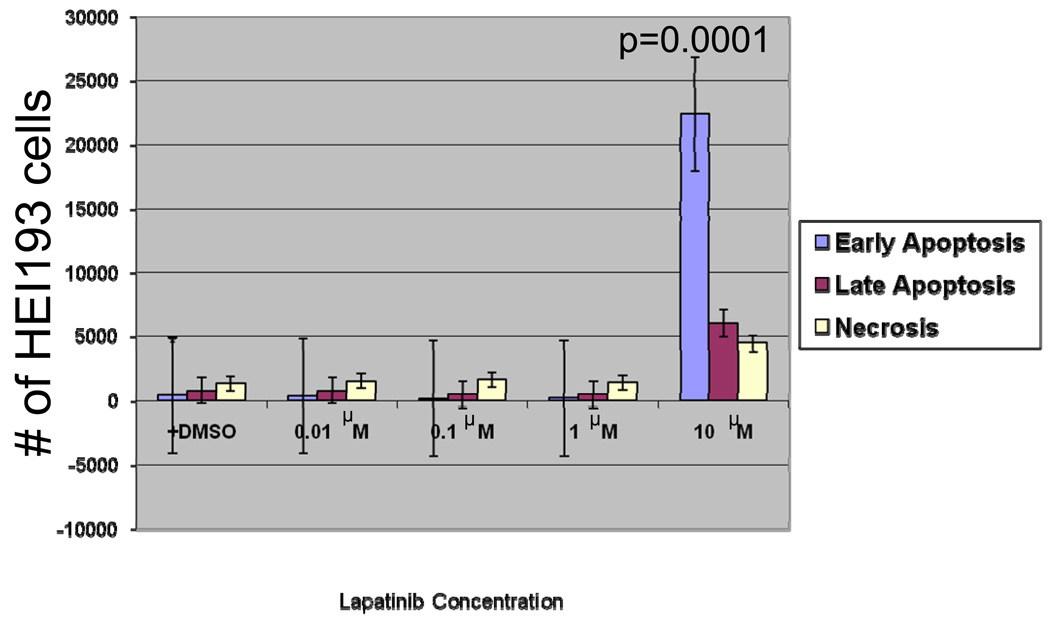
Following treatment with 10 µM Lapatinib, HEI193 cells demonstrated a dramatic increase in early apoptosis as well as significant increase in late apoptosis and necrosis (Figure 6). This finding suggests that cell cycle arrest could be the main mechanism controlling cellular growth at lower concentrations of the inhibitor.
Figure 6.
Annexin-V assay of HEI193 cells after treatment with 0.01, 0.1, 1, and 10 µM Lapatinib.
Proliferation
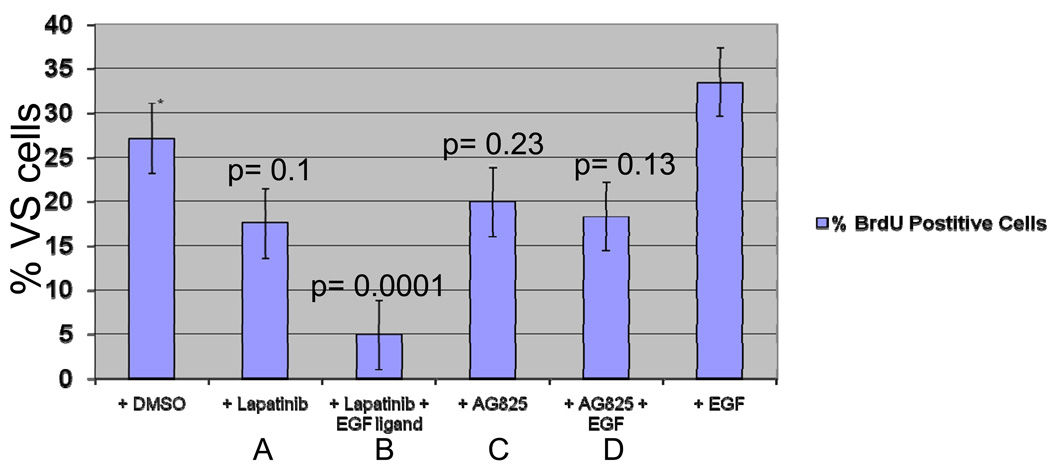
Seventy-two hours following administration of 0.2 µM Lapatinib, primary VS cultures demonstrated decreased proliferation (figure 7A). Similar to cell-cycle effects, addition of EGF ligand enhanced the inhibitory effect of Lapatinib and led to further decrease in proliferation (figure 7B). Treatment with AG825 demonstrated a similar pattern, but with less effect (figure 7C, D).
Figure 7.
BrdU staining of cultured VS cells after treatment with (A) 0.2 µM Lapatinib (B) 0.2 µM Lapatinib with 10 nM EGF ligand (C) 0.2 µM AG825 (D) 0.2 µM AG825 with 10 nM EGF ligand.
Discussion
The ErbB family of receptor tyrosine kinases, notably EGFR and ErbB2, exhibit increased expression and activation in many malignancies, particularly breast, lung, and colon cancers.9,10 Targeted inhibition of specific kinases with small molecule inhibitors has been the focus of research to eradicate many human tumors. Recently, a study showed that Erlotinib, a small molecule tyrosine kinase inhibitor of EGFR, retarded VS xenograft growth in nude mice.11 However, treatment of 11 NF2-related VS with erlotinib showed no radiographic or hearing responses.12 Lapatinib (Tykerb), a dual small molecule inhibitor of EGFR and ErbB2,13 has been approved for the treatment of advanced metastatic ErbB2-positive breast and lung cancers. Lapatinib binds the ATP-binding site located in the kinase domain of EGFR and ErbB2. This effectively prevents auto-phosphorylation, and inhibits subsequent activation of downstream signaling cascades including the Ras/Raf/MAPK and PI3-K/AKT pathways.14
It has been shown that EGFR-ErbB2 heterodimerization with ErbB2 over-expression leads to strong mitogenic and proliferative signaling, while ErbB homodimers lead to weaker signaling .15
Similarly, it has been demonstrated that human tumors over-expressing both EGFR and ErbB2 are more aggressive than tumors over-expressing either receptor alone, or other receptor combinations.16,17 It has also been shown that EGFR and ErbB2 over-expression in many tumors correlates with poor prognosis.18,19,20 EGFR is expressed in 36–100% of head and neck cancers while ErbB2 is expressed in 17–53%.21 Recent studies have demonstrated that simultaneous inhibition of EGFR and ErbB2 prevent cancer cell growth compared with the inhibition of either EGFR or ErbB2 alone.22 Different ErbB-initiated cell signaling pathways persist when using single inhibitors to treat solid tumors.23 Similarly, tumor cells expressing a variety of ErbB receptors can shift expression from one predominant heterodimer pair to another if one ErbB receptor is inhibited. This problem may explain the inefficacy of erlotinib in NF2-related VS.12 In other cancers, this ‘resistance’ is being managed clinically using dual inhibitors such as Lapatinib, alone or in combination with other inhibitors. Lapatinib is a potent and reversible inhibitor of the tyrosine domains of both EGFR and ErbB2 receptors.24 Lapatinib effectively inhibited cellular proliferation in a cell line resistant to the anti-ErbB2 antibody, Trastuzumab (Herceptin®; Genentech).25,26 In addition, Lapatinib was shown to increase radiation sensitivity of carcinogenic cells exhibiting upregulation of Ras, which is another downstream effector of EGFR.27
In this study, we examined ErbB family member expression and dimerization in vestibular schwannoma tumor samples and primary cultures. We predominantly found activated EGFR-ErbB2 heterodimers (figures 1 and 3). While Figure 2 shows expression of the EGFR, ErbB2, and ErbB3 receptors (top row) and co-localization of all three receptors (bottom row); it does not show activation of these receptors, demonstrated by phosphorylation, which is the hallmark of receptor signaling. Figure 1 shows that EGFR is activated with ErbB2 but not with ErbB3. Previous authors have demonstrated expression of all 3 ErbB receptors in VS, but neither their expression nor co-localization indicates activation state. Rather, activation state depends on ligand activity and receptor dimerization.
We investigated changes in the cell cycle after treating HEI193, an immortalized human schwannoma cell line derived from an NF2-associated VS, with three concentrations of Lapatinib and AG825. We also examined initiation of apoptosis after treating cells with four concentrations of Lapatinib and AG825. Our results suggest that Lapatinib suppresses cellular growth more than AG825 at 1 µM (figures 4,5). Additionally, we found that Lapatinib causes increased apoptosis at 10 µM concentration (figure 6) while AG825 does not have any effect. While HEI193 cells were derived from a human NF2-related VS, they are an immortalized cell line, and do not behave as primary VS cultures in significant ways. They can be repeatedly passaged and do not senesce; therefore, they can be grown to large numbers to be used in studies of cell cycle analysis and apoptosis, which require large numbers of cells for quantitative results. Therefore, our results on HEI193 cells may not be applicable to primary VS cells or tumors. However, our studies of cellular proliferation performed in primary cultured VS cells suggest that 0.2 µM Lapatinib suppresses the proliferation of VS cells more than AG825 (figure 7).
Evidence suggests that patients with tumors over-expressing ErbB2 benefit from Lapatinib treatment and experience few side effects.6 While we observed that AG825 efficacy is similar to Lapatinib in suppressing cellular growth at lower concentrations (0.01 and 0.1 µM), Lapatinib demonstrated both growth inhibition and induction of early and late apoptosis at 10 µM (figure 6). As expected, changes in cell cycle, apoptosis and proliferation were more pronounced upon the addition of EGF, the preferred ligand of EGFR-ErbB2 heterodimer (figures 4, 5, 6 and 7). Since proliferation signaling increases upon the addition of the EGF ligand and the inhibitor effect depends on receptor activation state, as it inhibits the tyrosine kinase activity resulting from activation, the suppressive activity of Lapatinib is better demonstrated in this state (figure 6). The addition of EGF alone to cultured VS cells lead, as expected, to increased proliferation compared to VS cells treated with DMSO alone (figure 7). The addition of EGF was two-fold: (1) to simulate the in vivo activation state, and (2) to observe the maximal inhibitor effect on VS cells. It is difficult to determine, however, whether lapatinib would be more or less effective in vivo than in our in vitro model, since it would depend on receptor activation, but our results on primary tumor tissue specimens (Co-IP studies, Figure 1) indicate that EGFR and ErbB2 receptors are present in an activated state in vivo. In primary cultures, the activation state is short-lived (on the order of minutes) upon the addition of ligand, and this may not represent the in vivo milieu, in which EGF is present in the blood stream and extracellular fluid. Certainly, the in vivo effect would be variable and depend on receptor activation state.
Importantly, these results have implications that suggest Lapatinib could have greater efficacy in VS control in NF2 patients. NF2-related VS have a greater average growth rate and incur a greater management dilemma due to their size and bilaterality, and would be ideally treated with targeted molecular therapy. NF2 patients tend to present with VS at a younger age, and therefore, would be expected to have greater levels of circulating EGF because of their young age and growth phase. However, further studies are necessary to investigate the relationship between age, circulating EGF, and the affects of Lapatinib on VS both in vitro (i.e., repeating cell cycle and apoptosis assays on primary cultured VS cells) and in vivo, alone and in combination with other targeted molecular therapies. Recently, lapatanib was shown to inhibit both ErbB2 phosphorylation and upregulation of survivin, an inhibitor of apoptosis. Additionally, downstream ERK1/2 and AKT activation was inhibited, resulting in decreased proliferation in a human vestibular schwannoma model in vitro. 28
Here we have demonstrated that activated ErbB family receptor heterdimers in VS contain predominantly EGFR and ErbB2, and that inhibition of EGFR-ErbB2 signaling with lapatinib suppressed VS cell growth and proliferation. Lapatinib could potentially provide an alternative treatment for VS. Lapatanib is FDA approved for the treatment of metastatic breast cancer, is orally bioavailable, crosses the blood-brain barrier28, and its side effects are predictable and manageable during long-term therapy, similar to those observed with other tyrosine kinase inhibitors.29,30 The effective half-life of Lapatinib is twenty-four hours, thus once daily oral administration would be sufficient to produce enough systemic exposure.31 Further elucidation of the effectiveness of Lapatinib in vitro and in vivo is warranted.
Conclusion
The predominant ErbB receptor dimerization pattern we observed in VS was EGFR and ErbB2 heterodimers. Furthermore, these heterodimers demonstrated an activated state as evidenced by their phosphorylation. Lapatinib demonstrated suppression of VS proliferation at low concentrations, and inhibited EGF-induced VS proliferation. This suggests that lapatinib may be effective at abrogating the growth effects of EGF in VS. Further studies are necessary to determine its efficacy both in vitro and in vivo and clinically in VS, particularly NF2, patients.
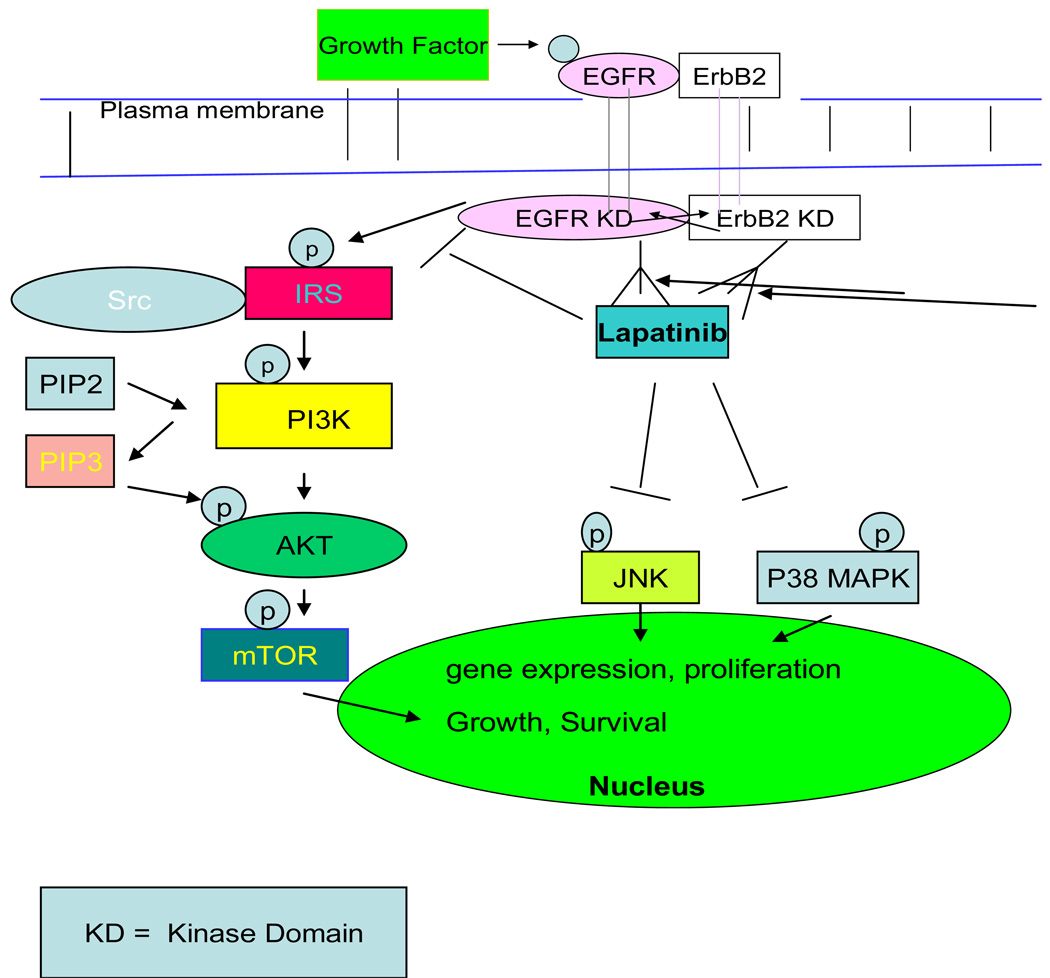
Figure 8.
Schematic depicting signaling pathways initiated by ligand binding to EGFR and subsequent dimerization of EGFR and ErbB2 are inhibited as Lapatinib, a dual tyrosine kinase inhibitor of EGFR and ErbB2 receptors, binds to the kinase domain on the intracellular portions of both receptors, mimicking ATP activity. The inhibition of phosphorylation at tyrosine residues effectively prevents a platform for the initiation of intracellular signaling cascades and activation of downstream effectors. This results in the inhibition of activation of transcription factors responsible for altered DNA transcription, cellular proliferation and survival. The PI3K, the JNK, and the P38 MAPK,32,33 among other pathways, are inhibited as a consequence of Lapatinib binding.
Acknowledgments
This research was supported by Grant 5 K08 DC008523-01A1 from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders Career Development Award (to J.K.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans DG, Huson SM, Donnai D, et al. A clinical Study of type 2 neurofibromatosis. Quart J Med. 1992;84:603–618. [PubMed] [Google Scholar]
- 2.Evans DG. Neurobibromatosis type 2: genetic and clinic features. ENT J. 199;78:97–100. [PubMed] [Google Scholar]
- 3.Taschudi DC, Liinder TE, Fish U. Concervative Management of Unilateral Accoustic Neuromas. Am J Otol. 2000;21:722–728. [PubMed] [Google Scholar]
- 4.Chang LS, Welling DB. Molecular Biology of Vestibular Schwannomas. Methods Mol Biol. 2009;493:163–177. doi: 10.1007/978-1-59745-523-7_10. [DOI] [PubMed] [Google Scholar]
- 5.Neff BA, Welling DB, Akhmametyeva E, Chang LS. The Molecular Biology of Vestibular Schwannomas: Dissecting the Pathogenic Process at the Molecular Level. Otol Neurotol. 2006 Feb;27(2):197–208. doi: 10.1097/01.mao.0000180484.24242.54. [DOI] [PubMed] [Google Scholar]
- 6.Nelson MH, Dolder CR. Lapatinib: A Novel Dual Tyrosine Kinase Inhibitor with Activity in Solid Tumors. Ann Pharmacotherapy. 2006;40:261–269. doi: 10.1345/aph.1G387. [DOI] [PubMed] [Google Scholar]
- 7.Sridhar SS, Seymour L, Shepherd FA. Inhibitors of epidermal-growth factor receptors: a review of clinical research with a focus on non–small celllung cancer. Lancet Oncol. 2003;4:397–406. doi: 10.1016/s1470-2045(03)01137-9. [DOI] [PubMed] [Google Scholar]
- 8.Doherty JK, Ongkeko O, Crawley B, Andalibi A, Ryan AF. ErbB and Nrg: Potential Molecular Targets for Vestibular Schwannoma Pharmacotherapy. Otol & Neurotol. 2008;29:50–57. doi: 10.1097/mao.0b013e31815d4429. [DOI] [PubMed] [Google Scholar]
- 9.Gross ME, Shazer RL, Agus DB. Targeting the HER-kinase axis in cancer. Sen, in Onco. 2004;31(Suppl 3):9–20. doi: 10.1053/j.seminoncol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Harari PM, Allen GW, Bonner JA. Biology of interactions: Antiepidermal growth factor receptor agents. J C [in Onco] 2007;25:4057–4065. doi: 10.1200/JCO.2007.11.8984. [DOI] [PubMed] [Google Scholar]
- 11.Clark JJ, Provenzano M, Diggelmann HR, Xu N, Hansen SS, Hansen MR. The ErbB Inhibitors Trastuzumab and Erlotinib Inhibit Growth of Vestibular Schwannoma Xenografts in Nude Mice: A Preliminary Study. Otol & Neurotol. 2008;29:846–853. doi: 10.1097/MAO.0b013e31817f7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plotkin SR, Halpin C, McKenna MJ, et al. Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol. 2010;31:1135–1143. doi: 10.1097/MAO.0b013e3181eb328a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: adual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 anddownstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 14.Spector NL, Xia W, Bums H, III, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Onco[ 2005;23:2502–2512. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 15.Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mo[Cell Bio] 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 16.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 17.Cohen BD, Kiener PA, Green JM, Foy L, Fell HP, Zhang K. The relationship between human epidermal growth-like factor receptor expression and cellular transformation in NIH3T3 cells. J Biol Chem. 1996;271:30897–30903. doi: 10.1074/jbc.271.48.30897. [DOI] [PubMed] [Google Scholar]
- 18.Pavelic K, Banjac Z, Pavelic J, Spaventi S. Evidence for a role of EGF receptor in the progression of human lung carcinoma. Anticancer Res. 1993;13:1133–1137. [PubMed] [Google Scholar]
- 19.Menard S, Casalini P, Campiglio M, Pupa S, Tagliabue E. Role of HER2/neu in tumor progression and therapy. Cell Mol Life Sci. 2004;61:2965–2978. doi: 10.1007/s00018-004-4277-7. [DOI] [PubMed] [Google Scholar]
- 20.Slamon DJ, Godolphin W. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 21.Montemurro F, Valabrega G, Aglietta M. Lapatinib: a dual inhibitor of EGFR and HER2 tyrosine kinase activity. Expert Opin. Biol. Ther. 2007;7(2):257–268. doi: 10.1517/14712598.7.2.257. [DOI] [PubMed] [Google Scholar]
- 22.Burris HA., III Dual kinase inhibition in the treatment of breast cancer: initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist. 2004;9(suppl 3):10–15. doi: 10.1634/theoncologist.9-suppl_3-10. [DOI] [PubMed] [Google Scholar]
- 23.Lin NU, Winer EP. Small molecule tyrosine kinase inhibitors. Breast Cancer Res. 2004;6:204–210. doi: 10.1186/bcr919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H, Kim Y-S, Peletier A, McCall W, Earp HS, Sartor CI. Effects of the EGFR/HER2 kinase inhibitor GW572016 on EGFR and HER2– overexpressing breast cancer cell line proliferation, radiosensitization, and resistance. Int J Radiat Oncol Biol Phys. 2004;58:344–352. doi: 10.1016/j.ijrobp.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 26.Xia W, Liu L-H, Ho P, Spector NL. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene. 2004;23:646–653. doi: 10.1038/sj.onc.1207166. [DOI] [PubMed] [Google Scholar]
- 27.Grana TM, Sartor CI, Cox AD. Epidermal growth factor receptor autocrine signaling in RIE-1 cells transformed by the ras oncogene enhances radiation resistance. Cancer Res. 2003;63:7807–7814. [PubMed] [Google Scholar]
- 28.Ammoun S, Cunliffe CH, Allen JC, et al. ErbB/HER receptor activation and preclinical efficacy of lapatanib in vestibular schwannoma. Neuro Oncol. 2010;12(8):834–843. doi: 10.1093/neuonc/noq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burris HA, III, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 30.Richter M, Zhang H. Receptor-targeted cancer therapy. DNA Cell Biol. 2005;24:271–282. doi: 10.1089/dna.2005.24.271. [DOI] [PubMed] [Google Scholar]
- 31.Medina PJ, Goodin S. Lapatinib: A Dual Inhibitor of Human Epidermal Growth Factor Receptor Tyrosine Kinases. Clinical Therapeutics. 2008;30:1426–1447. doi: 10.1016/j.clinthera.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting The PI3K/AKT Pathways for Cancer Drug Discovery. Nature. 2005 Dec.vol 4 doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 33.Johnson GL, Lapadat R. Mitogen-Activated Protein Kinase Pathways -Mediated by ERK, JNK, and p38 Protein Kinases. Science. 2002 Dec 6;vol 298 doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]