Abstract
Please cite this paper as: Moseley et al. (2010) Peroxisome proliferator‐activated receptor and AMP‐activated protein kinase agonists protect against lethal influenza virus challenge in mice. Influenza and Other Respiratory Viruses 4(5), 307–311.
Background A novel influenza A (H1N1) virus was isolated from humans in North America and has developed into the first pandemic of the 21st century. Reports of a global shortage of antiviral drugs, the evolution of drug‐resistant influenza virus variants, and a 6‐month delay in vaccine availability underline the need to develop new therapeutics that may be widely distributed during future pandemics.
Methods In an effort to discover alternatives to the conventional therapeutic strategies available, we screened several classes of immunomodulatory agents possessing the potential to mitigate the effects of influenza virus‐induced immunopathology.
Results Here, we provide preliminary evidence that two classes of drugs, peroxisome proliferator‐activated receptor‐γ agonists and AMP‐activated protein kinase agonists, provide protection in mice infected with highly pathogenic and pandemic strains of influenza virus.
Conclusions The extensive production in the developed world, combined with the significant degree of protection described here, establishes these drugs as a potential therapeutic option that may be broadly implemented to combat serious disease caused by future influenza epidemics or pandemics.
Keywords: inflammation, Influenza, PPAR
Introduction
Historically, an epidemiological comparison of influenza A pandemics to the seasonal influenza A epidemics has resulted in a paradox. While seasonal influenza epidemics often result in a heavy burden of disease among infants and the elderly, influenza pandemics often cause severe disease in young (adolescents and young adults), immuno‐competent individuals. 1 Maintaining this trend, the majority of clinical infections with manifesting complications following infection with the novel 2009 H1N1 pandemic virus (pH1N1), thus far, have occurred in patients aged 18 and younger, 2 and the median age of infected individuals has been found to be 25.
Until recently, most influenza virologists have concentrated on developing therapeutic strategies that target virus replication. 3 However, the pathologic effects of an exuberant immune response to infection with highly pathogenic (HP) influenza viruses, often referred to as a cytokine storm, are well documented. 4 , 5 , 6 , 7 This, along with the report demonstrating that the level of virus replication and host survival are not always correlated, 8 suggest that new strategies focused on modulating the influenza‐induced inflammation are needed. Several recent reviews discuss therapeutic agents that dampen inflammation in acute lung injury associated with microbial pathogens and with conditions such as multiple trauma not associated with any known infectious agents. 8 , 9 , 10 , 11
Recently, our group and others have demonstrated that the immune‐induced pathology following infection with HP influenza viruses is associated with the enhanced recruitment of a specific subset of chemokine (C‐C motif) receptor 2 (CCR2) responsive, monocyte‐derived cells capable of damaging the lung when recruited in large numbers. 12 , 13 , 14 While searching for a pharmacological means to dampen the CCR2‐dependent recruitment of inflammatory monocytes (iM), we found that the peroxisome proliferator‐activated receptor (PPAR)‐γ agonist, pioglitazone, had been shown to reduce innate inflammation and in particular MCP‐1 expression, which is the primary ligand for CCR2. 15 , 16 Thus we attempted to therapeutically decrease the recruitment of iM via prophylactic treatment with pioglitazone. This treatment demonstrated that prophylactic treatment blunted the recruitment of iM to the airways and provided a significant degree of protection against HP influenza virus challenge. 12 The protection appeared to be mediated by abating the excessive inflammation, as there was no measurable change in virus titers.
Peroxisome proliferator‐activated receptors are a family of lipid‐activated transcription factors that are key regulators of lipid and glucose metabolism as well as inflammation. 17 , 18 There are three distinct members of the PPAR family: PPAR‐α, PPAR‐δ (sometimes referred to as PPAR‐β), and PPAR‐γ. In addition to our work revealing that PPAR‐γ stimulation decreases influenza‐induced inflammation, a recent study found that treatment with gemfibrozil (a PPAR‐α agonist) 4 days after infection resulted in an increase in survival from 26% to 52%. 19
In light of the protective effects realized by treatment with these PPAR agonists, we sought to expand our study to other compounds or combinations that target the PPAR family. In addition to pioglitazone, we tested the efficacy of rosiglitazone (another PPAR‐γ agonist), aminoimidazole carboxamide ribonucleotide (AICAR), or the combination of pioglitazone and AICAR to reduce the excessive inflammation induced by HP influenza virus infection. AICAR is an activator of AMP‐activated protein kinase (AMPK), which is well recognized as a stimulator of PPARs and is known to complement the action of immunosuppression by pharmacological PPAR agonists. 20 The results of our study provide preliminary evidence that stimulating the PPAR axis may prove effective in dampening the immune‐induced pathology often associated with HP influenza virus infection.
Methods
Viruses
The mouse‐adapted H1N1 A/Puerto Rico/8/34 (PR8) influenza A virus was obtained from the St. Jude Children’s Research Hospital (Memphis, TN, USA) repository. The pH1N1 A/California/04/09 (CA04) influenza A virus was obtained from the Centers for Disease Control and Prevention (Atlanta, GA, USA) and was mouse adapted by nine serial passages in mice every 2 days.
Mice
Female C57BL/6 (B6) mice (8–10 weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in specific pathogen‐free conditions. All mice in this study were used according to protocols approved by the Institutional Animal Care and Use Committee at St. Jude Children’s Research Hospital.
Infection and treatment
Naive mice were anesthetized by intraperitoneal (i.p.) injection with avertin (2,2,2‐tribromoethanol) before intranasal (i.n.) challenge with the appropriate dose of virus diluted in 30 μl of sterile, endotoxin‐free phosphate buffered saline (PBS). Animals were weighed prior to infection and then monitored daily for weight loss as a measure of morbidity. Mice found to have lost 25% of their body weight were euthanized in accordance with protocols approved by the Institutional Animal Care and Use Committee at St. Jude Children’s Research Hospital. Pioglitazone and rosiglitazone were purchased from Molcan Corporation (ON, Canada). AICAR was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). The mice were treated with pioglitazone (60 mg/kg) or rosiglitazone (60 mg/kg) by oral gavage or AICAR (125 mg/kg) by i.p. injection. All drugs were suspended in 100 μl of sterile, endotoxin‐free PBS, and treatment was initiated beginning day 3 prior to infection and continued daily thereafter.
Statistics
Data are presented as mean ± SEM. Survival data were analyzed with the Mantel–Cox test using prism (GraphPad Software, Inc.; La Jolla, California, USA). Weight loss data were compared with a multiple regression analysis, including mixed effects to account for repeated measures, using R2.9.1 (http://www.R‐project.org).
Results
PPAR‐γ agonists and an AMPK activator protect against a lethal influenza virus challenge in mice
For this study, we used mice infected with the mouse‐adapted H1N1 A/Puerto Rico/8/31 (PR8) virus. PR8 infection of mice results in the expeditious accumulation of cytokines/chemokines, and the leukocyte trafficking kinetics are similar to that seen in murine and primate infections with the 1918 “Spanish” or H5N1 influenza viruses, 6 , 12 making this a realistic model to rapidly search for new pharmacological agents to reduce the influenza‐induced immunopathology.
Mice were treated with PBS, pioglitazone (60 mg/kg), rosiglitazone (60 mg/kg), AICAR (125 mg/kg), or a combination of pioglitazone (60 mg/kg), and AICAR (125 mg/kg) beginning 3 days prior to infection. Animals were challenged with 500 pfu PR8 (LD50 = 102·4 pfu) and monitored daily for weight loss and mortality. The results demonstrated that all treatment regimens were associated with a significant reduction in weight loss when compared to PBS‐treated animals (Figure 1A). To determine whether one treatment was more effective than the others in reducing morbidity, a multiple regression analysis was performed on the treatment groups without the inclusion of the PBS‐treated group. This analysis demonstrated that the animals treated with rosiglitazone experienced significantly less weight loss than animals receiving the other treatments (P < 0·0012). There were no measurable differences in weight loss between animals receiving pioglitazone, AICAR, or the pioglitazone/AICAR combination (P > 0·3695).
Figure 1.

Reduced morbidity and mortality in drug‐treated mice following challenge with a lethal dose of PR8 virus. (A) Weight loss was measured to assess morbidity after infection with 500 pfu PR8 in mice (n ≥ 10/group) treated with pioglitazone (60 mg/kg), rosiglitazone (60 mg/kg), aminoimidazole carboxamide ribonucleotide (AICAR) (125 mg/kg), a combination of pioglitazone (60 mg/kg) and AICAR (125 mg/kg), or PBS. Treatments began 3 days prior to infection and continued daily thereafter. Groups of animals receiving drug treatments displayed a significant reduction in weight loss when compared to PBS‐treated animals (Multiple regression with mixed effects: same letter, P > 0·0012; different letter, P < 0·0012). There was no difference in weight loss between groups receiving any of the drug treatments (Multiple regression with mixed effects: P > 0·3695). (B) All drug treatment regimens resulted in a significant increase in the rate of survival when compared to PBS‐treated animals (Mantel–Cox test: P < 0·01).
In addition to decreasing host morbidity, all of the treatment regimens significantly reduced both mortality and time to death when compared to the control group (P < 0·01; Figure 1B). Surprisingly, rosiglitazone treatment provided absolute protection from death, whereas the pioglitazone‐treated animals displayed a 20% increase in survival over control animals. AICAR alone provided a 40% increase in protection, whereas the AICAR/pioglitazone combination increased survival by 60%, thus indicating that there is an additive benefit of using the two drugs in combination.
Rosiglitazone protects from a highly lethal pH1N1 challenge
Because of the high degree of protection achieved with rosiglitazone, we speculated that it may also protect against a lethal pH1N1 challenge. To test this hypothesis, we adapted the A/California/04/09 (CA04) influenza virus to mice by serial passage. The mouse‐adapted CA04 (mCA04) variant had a lethal dose50 (LD50) in mice that was about 2 logs lower than the original virus. Typically, humans infected with the pH1N1 virus manifest relatively mild symptoms; however, in some cases serious disease develops in the infected airways and may ultimately progress to death. We sought to mimic these severe conditions in our experiments to determine whether rosiglitazone, which provided 100% protection against a lethal challenge with PR8 virus, would protect mice infected with the pH1N1 virus. Groups of mice were treated with either PBS or rosiglitazone as described for the previous experiments and then infected with 5000 egg infectious dose50 (EID50) of mCA04, a dose that results in the rapid onset of symptoms and uniform mortality within a week. Animals were monitored for weight loss and mortality as earlier.
The results show that rosiglitazone‐treated animals experienced a significant reduction in morbidity when compared to PBS‐treated animals (P < 0·0001; Figure 2A). Furthermore, control mice receiving only PBS demonstrated a rapid onset of disease and reached 100% mortality by day 8 after infection, whereas there was a 20% increase in survival in the rosiglitazone‐treated group (Figure 2B). Rosiglitazone treatment significantly decreased both mortality and the time to death when compared to controls (Mantel–Cox test, P < 0·001). Although the protective effects were modest, the mice were challenged with a dose of virus that was uniformly lethal. Thus, our finding that rosiglitazone pre‐treatment induced a statistically significant degree of protection is promising.
Figure 2.
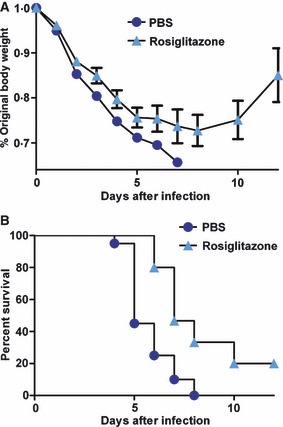
Reduced morbidity and mortality in rosiglitazone‐treated mice following infection with a uniformly lethal dose of mouse‐adapted H1N1 pandemic virus (pH1N1). (A) Weight loss was measured to assess morbidity after infection with 5000 egg infectious dose50 (EID50) mouse‐adapted A/California/04/09 in mice (n = 20/group) treated with rosiglitazone (60 mg/kg) or PBS. Treatment began 3 days prior to infection and continued daily thereafter. Rosiglitazone‐treated animals demonstrated a significant reduction in weight loss when compared to PBS‐treated animals (Multiple regression with mixed effects: P < 0·0001). (B) Rosiglitazone treatment resulted in a significant increase in the rate of survival when compared to PBS‐treated animals (Mantel–Cox test: P < 0·001).
Conclusions
The emergence of the pH1N1 virus underscores the importance of developing new pharmacological agents to treat influenza virus infection. Vaccination is currently the best option available to combat the spread of influenza. Although this is effective in reducing the burden of disease associated with seasonal influenza viruses, the 6 months required for vaccine production after identification of a new pandemic virus precludes the use of vaccines to combat its early stages. This was clearly shown this past year; the pH1N1 emerged in April 2009, yet vaccines did not become widely available until November 2009. Further complicating matters, 10 months after the emergence of the pandemic virus, pH1N1 vaccine had been supplied to only two developing countries. In the absence of vaccine, therapeutic treatment is limited to the currently licensed antiviral drugs. However, their continued utility is not assured. During the 2008–2009 influenza season, more than 98% of all seasonal H1N1 viruses tested were resistant to oseltamivir, 21 and oseltamivir‐resistant pH1N1 viruses have emerged, although the transmission of these viruses appears to be limited for now. Thus, it is becoming increasingly clear that new pharmacological interventions are needed that target not only the virus but the host response as well.
It is well recognized that the clinical complications because of influenza virus infection are often related to a hyperactive immune response. 6 , 7 , 12 , 22 Therapeutic interventions that target the host response, rather than virus replication alone, are therefore becoming more appealing. Although this type of intervention would not preclude the spread of disease, it might prevent the development of severe disease in those who are infected before vaccines become available or be used as prophylaxis by healthcare workers. Additionally, the odds of drug resistance developing are almost non‐existent, because treatment would target the host response rather than the virus.
Our preliminary data demonstrate that targeting the PPAR axis may be a viable option for reducing the immune‐induced pathology caused by HP influenza virus infection. We previously found that prophylactic treatment with pioglitazone was effective in reducing both morbidity and mortality in mice following a lethal influenza challenge. 12 In this study, we showed that pre‐treatment with another PPAR agonist, rosiglitazone, produced a significantly greater degree of protection than that observed with pioglitazone. Further, we observed an additive effect when pioglitazone was used in combination with AICAR, an AMPK agonist. This mimics the combination of pioglitazone and metformin (also an AMPK agonist), a safe and effective treatment regimen currently used for patients with diabetes mellitus. Another benefit of using PPAR‐γ agonists to treat influenza‐induced immunopathology is that they are currently licensed in the United States and produced as an inexpensive generic agent by companies located in developing countries. Therefore, the cost, with respect to both money and time, involved in the development of novel therapies is negated. Further studies are needed to test the efficacy of a combination of rosiglitazone and metformin, another AMPK agonist currently licensed in the United States, and their use not only in prophylaxis but also in the treatment of established infection.
Thus far, experiments testing the efficacy of PPAR and AMPK agonists have been limited to pre‐treatment in laboratory models of HP influenza. These agents are safe, well tolerated and widely used to treat cardiovascular diseases and diabetes mellitus in humans. Our results suggest that they should be tested in the prophylaxis and treatment of human influenza infection, either alone or in combination with currently approved antiviral agents.
Acknowledgements
We thank John Franks for his excellent technical support, Nicholas Negovetich for assistance with statistical analysis, and Heather Forrest, Patrick Seiler, and Natasha Ilyushina for assistance in creating the mouse‐adapted CA04 virus. This work was supported by Contract HHSN266200700005C awarded to all authors and grant 1F32AI078667 awarded to JRA, both from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, and by the American Lebanese Syrian Associated Charities. RGW also receives research support from Hoffmann‐La Roche.
References
- 1. Ahmed R, Oldstone MB, Palese P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nat Immunol 2007; 8:1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dawood FS, Jain S, Finelli L et al. Emergence of a novel swine‐origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605–2615. [DOI] [PubMed] [Google Scholar]
- 3. Basler CF. Influenza viruses: basic biology and potential drug targets. Infect Disord Drug Targets 2007; 7:282–293. [DOI] [PubMed] [Google Scholar]
- 4. Chan MC, Cheung CY, Chui WH et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res 2005; 6:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheung CY, Poon LL, Lau AS et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 2002; 360:1831–1837. [DOI] [PubMed] [Google Scholar]
- 6. Kobasa D, Jones SM, Shinya K et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 2007; 445:319–323. [DOI] [PubMed] [Google Scholar]
- 7. Loo YM, Gale M Jr. Influenza: fatal immunity and the 1918 virus. Nature 2007; 445:267–268. [DOI] [PubMed] [Google Scholar]
- 8. Fedson DS. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis 2008; 8:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fedson DS. Meeting the challenge of influenza pandemic preparedness in developing countries. Emerg Infect Dis 2009; 15:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fedson DS. Confronting the next influenza pandemic with anti‐inflammatory and immunomodulatory agents: why they are needed and how they might work. Influenza Other Respi Viruses 2009; 3:129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fedson DS, Dunnill P. Commentary: from scarcity to abundance: pandemic vaccines and other agents for “have not” countries. J Public Health Policy 2007; 28:322–340. [DOI] [PubMed] [Google Scholar]
- 12. Aldridge JR Jr, Moseley CE, Boltz DA et al. TNF/iNOS‐producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A 2009; 106:5306–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herold S, Von Wulffen W, Steinmueller M et al. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol 2006; 177:1817–1824. [DOI] [PubMed] [Google Scholar]
- 14. Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte‐derived dendritic cells and exudate macrophages produce influenza‐induced pulmonary immune pathology and mortality. J Immunol 2008; 180:2562–2572. [DOI] [PubMed] [Google Scholar]
- 15. Di Gregorio GB, Yao‐Borengasser A, Rasouli N et al. Expression of CD68 and macrophage chemoattractant protein‐1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes 2005; 54:2305–2313. [DOI] [PubMed] [Google Scholar]
- 16. Haraguchi G, Kosuge H, Maejima Y et al. Pioglitazone reduces systematic inflammation and improves mortality in apolipoprotein E knockout mice with sepsis. Intensive Care Med 2008; 34:1304–1312. [DOI] [PubMed] [Google Scholar]
- 17. Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid‐activated nuclear receptors. Nature 2008; 454:470–477. [DOI] [PubMed] [Google Scholar]
- 18. Keller H, Dreyer C, Medin J et al. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator‐activated receptor‐retinoid X receptor heterodimers. Proc Natl Acad Sci USA 1993; 90:2160–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Budd A, Alleva L, Alsharifi M et al. Increased survival after gemfibrozil treatment of severe mouse influenza. Antimicrob Agents Chemother 2007; 51:2965–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pitocco D, Giubilato S, Zaccardi F et al. Pioglitazone reduces monocyte activation in type 2 diabetes. Acta Diabetol 2009; 46:75–77. [DOI] [PubMed] [Google Scholar]
- 21. Dharan NJ, Gubareva LV, Meyer JJ et al. Infections with oseltamivir‐resistant influenza A (H1N1) virus in the United States. JAMA 2009; 301:1034–1041. [DOI] [PubMed] [Google Scholar]
- 22. De Jong MD, Simmons CP, Thanh TT et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]


