Abstract
BACKGROUND
Engagement in cognitively stimulating activities (CA) and leisure time physical activity (PA) have been associated with maintaining cognitive performance and reducing the likelihood of cognitive decline in older adults. However, neural mechanisms underlying protective effects of these lifestyle behaviors are largely unknown. In the current study, we investigated the effect of self-reported PA and CA on hippocampal volume and semantic processing activation during a fame discrimination task, as measured by functional magnetic resonance imaging (fMRI). We also examined whether possession of the apolipoprotein E (APOE) ε4 allele could moderate the effect of PA or CA on hippocampal structure or function.
METHODS
Seventy-eight healthy, cognitively intact older adults underwent baseline neuropsychological assessment, hippocampal volume measurement via manually-traced structural MRI, and task-activated fMRI.
RESULTS
After 18 months, 27 participants declined by one standard deviation or more on follow-up neuropsychological testing. Logistic regression analyses revealed that CA alone or in combination with baseline hippocampal structure or functional activity did not predict the probability of cognitive decline. In contrast, PA interacted with APOE ε4 status such that engagement in PA reduced the risk of cognitive decline in APOE ε4 carriers only. Furthermore, the benefits of PA appeared to diminish with reduced functional activity or volume in the hippocampus.
CONCLUSIONS
Our findings suggest that increased leisure time PA is associated with reduced probability of cognitive decline in persons who are at high risk for AD. The beneficial effects of PA in this group may be related to enhancement of the functional and structural integrity of the hippocampus.
Keywords: Apolipoprotein E, Cognitive Activity, Cognitive Decline, Functional Magnetic Resonance Imaging, Hippocampus, Physical Activity
Introduction
The current lack of effective treatments for Alzheimer's disease (AD) has stimulated considerable interest in developing strategies for prevention of cognitive decline [1, 2]. A multidisciplinary expert panel recently reviewed the existing evidence for such interventions or modifiable risk factors that may delay AD onset or mitigate cognitive decline [3]. The panel concluded that there is currently insufficient evidence supporting the use of pharmaceutical or nutritional supplements for preventing AD or cognitive decline. However, the panel also noted positive preliminary data supporting cognitive engagement and physical activity as possible interventions for maintaining or enhancing cognitive function. Indeed, if effective in preventing cognitive decline, such lifestyle behaviors have considerable promise because they are cost-effective and have few adverse side effects.
Participation in cognitively stimulating activities (CA; e.g., reading, creative writing) has been proposed as a way to enhance cognitive reserve [4] by promoting resistance to cognitive decline despite the presence of brain damage or disease pathology [5]. Longitudinal epidemiological studies have demonstrated that participation in CA is associated with a reduced incidence of cognitive decline in late life [6-8], mild cognitive impairment (MCI) [9], and AD [4, 10-14]. CA has also been associated with superior cognitive functioning in healthy older adults [15, 16]. Despite numerous epidemiological studies showing benefits of CA for maintaining cognitive function, a biological mechanism for these effects remains elusive. Increased blood flow and metabolism to the brain during CA or the effects of repetitive behavioral rehearsal are possible explanations. However, effects of CA on brain structure or function have not been systematically examined. While the broader construct of cognitive reserve involves enhanced neural network efficiency associated with increasing task difficulty [5], no imaging studies have looked specifically at the impact of CA on neural functioning.
Engagement in leisure-time physical activity (PA) is another common lifestyle modification for managing and preventing a variety of chronic health conditions (e.g., hypertension, obesity, diabetes) [17-19]. However, it may also play a role in reducing likelihood of cognitive decline [20]. Longitudinal epidemiological studies suggest that late-life participation in PA (e.g., walking, calisthenics) is associated with a reduced incidence of cognitive decline [21-28], MCI [29], and AD [13, 21, 30-33], and intervention studies have demonstrated that engagement in PA can improve cognitive and attentional task performance [34-36]. One recent report [37] suggests that engagement in PA may also be associated with prolonged survival in AD patients. Animal models have suggested possible biological mechanisms underlying the positive effects of PA on cognitive function. For example, PA may enhance neural functioning by increasing levels of brain-derived neurotrophic factor (BDNF), which in turn mediates synaptogenesis, neurogenesis, and plasticity [38, 39]. Exercise interventions can stimulate hippocampal neurogenesis in rodents [40-47]. These enhancements may lead to more efficient brain functioning and may confer resistance to future cognitive decline. Importantly, several studies have suggested that the beneficial effects of PA may be moderated by the presence of one or both apolipoprotein E (APOE) ε4 alleles, a well-established risk factor for AD. In several studies, PA appears to exert a greater protective effect for ε4 carriers than for non-carriers [22, 30, 48]. In contrast another study found PA preferentially benefits APOE ε4 non-carriers [31]. Thus, the nature of the, association between PA and cognitive decline, and how the APOE ε4 allele influences this relationship, are unclear.
Functional neuroimaging studies evaluating lifestyle modifications, such as PA and CA, may elucidate biological mechanisms by which these interventions confer resistance to late-life cognitive decline. Several of the previously discussed animal studies suggest that biological changes in the hippocampus may underlie some of the benefits of interventions such as PA. The purpose of the current study was to evaluate whether PA and CA may reduce the risk of cognitive decline independent of hippocampal volume and function. We used a famous name discrimination task during functional magnetic resonance imaging (fMRI) that has consistently revealed greater semantic processing activity in persons at risk for AD (cognitively intact older APOE ε4 carriers [49] and persons with MCI [50]) relative to persons not at risk. A longitudinal follow-up study using this task demonstrated that healthy older adults with greater baseline semantic processing activity in cortical and hippocampal regions and larger baseline hippocampal volumes (particularly in APOE ε4 carriers) showed greater stability in cognitive functioning over 18 months [51]. In a subsequent cross-sectional study, persons who reported engaging in regular PA exhibited greater cortical semantic processing activity on this task, particularly among APOE ε4 positive participants [52]. Thus, the current study was designed to extend the results of these previous studies by comparing the effects of PA, CA, and hippocampal activity and volume on cognitive decline. Because we observed the protective effect of increased semantic processing activation to be strongest in APOE ε4 allele carriers [51] and because PA was associated with the greatest increase in semantic processing activation for APOE ε4 allele carriers [52], we predicted that the presence of the APOE ε4 allele and increased hippocampal activation would potentiate the protective influences of PA or CA on resistance to cognitive decline.
Material and Methods
Participants
Participants were recruited via newspaper advertisements. In order to enrich our sample with participants who were at-risk for AD, half of the participants were selected on the basis of having a family history of dementia, defined as having a first-degree relative with a formal diagnosis of AD prior to death or a reported history of dementia-like symptoms without a diagnosis. Because of the elevated proportion of individuals with a family history of dementia, the representation of APOE ε4 carriers was also greater in our sample than in the general population.
Participants were required to be cognitively intact and non-demented upon study entry. They were excluded if they scored less than 25 on the Mini-Mental State Exam (MMSE) [53] or more than 1.5 standard deviations below age-appropriate means on the Rey Auditory Verbal Learning Test (RAVLT) [54]; Mattis Dementia Rating Scale-2 (MDRS-2) [55-57]. Participants were also excluded if they obtained a score greater than 10 on the Geriatric Depression Scale (GDS) [58, 59] or if they demonstrated any impairment of activities of daily living on the Lawton and Brody Self-Maintaining and Instrumental Activities of Daily Living Scale [60]. Finally, participants were excluded if they had a history or evidence of: 1) significant neurological illnesses/conditions; 2) medical illnesses/conditions that may affect brain function; 3) current psychiatric disturbance or substance abuse or dependence meeting DSM-IV Axis I criteria; 4) contraindications specific to MR scanning: pregnancy, weight inappropriate for height, ferrous objects within the body, or a history of claustrophobia; 5) left-handedness or ambidexterity (laterality quotient [LQ] < 50) as assessed with the Edinburgh Handedness Inventory [61]; or 6) use of medications that may affect the hemodynamic response in the scanner.
Seventy-eight community-dwelling older adults meeting study inclusion and exclusion requirements served as study participants (Table 1). Family history of dementia was present in 51.3% of participants; 33.3% of the sample carried the APOE ε4 allele. One participant was a homozygous carrier of the ε4 allele; the remaining 25 carriers were heterozygous (ε3, ε4). Among the non-carriers, there were 44 persons who were homozygous ε3 carriers and 8 persons who were heterozygous (ε2, ε3). At baseline, all participants completed the PA survey, but three participants did not complete the CA survey. Baseline neuropsychological evaluation revealed that all participants were initially cognitively intact according to the measures described above. All participants were Caucasian. Written informed consent was obtained and all participants received financial compensation for time and travel.
Table 1.
Mean demographic and neuropsychological test results for stable and declining participants. SDs are provided in parentheses
| Variable | Stable (n = 51) | Declining (n = 27) |
|---|---|---|
| Age (years) | 72.6 (5) | 73.6 (4.7) |
| Education (years) | 15.1 (2.4) | 14.5 (3.2) |
| Test-Retest Interval (days) | 551.7 (43.5) | 560.6 (47.0) |
| Ethnicity | 100% Caucasian | 100% Caucasian |
| Family History Status | 27 Dementia -, 24 Dementia + | 11 Dementia -, 16 Dementia + |
| APOE ε4+* | 39 ε4- (5 ε2ε3, 34 ε3ε3), 12 ε4+ (11 ε3ε4, 1 ε4ε4) | 13 ε4- (3 ε2ε3, 10 ε3ε3), 14 ε4+ (all ε3ε4) |
| Gender | 13 Males, 38 Females | 8 Males, 19 Females |
| Cognitive Activity (Sum) | 25.9 (2.8) | 26.7 (2.8) |
| Physical Activity | 25 Low PA, 26 High PA | 16 Low PA, 11 High PA |
| Baseline Cognitive Results | ||
| DRS-2 Total | 140.7 (3.2) | 139.7 (3.8) |
| RAVLT Trials 1-5 | 50.6 (8.8) | 46.8 (8.1) |
| RAVLT DR | 10.1 (2.6) | 9.0 (2.8) |
| Lawton IADL | 5 (0) | 5 (0) |
| GDS* | 2.7 (2.5) | 1.4 (1.8) |
| MMSE | 29.4 (0.8) | 28.9 (1.2) |
| Follow-up Cognitive Results | ||
| DRS-2 Total** | 139.5 (2.1) | 135.6 (5.0) |
| RAVLT Trials 1-5** | 49.5 (7.5) | 40.1 (7.1) |
| RAVLT DR** | 10.2 (2.4) | 6 (2.3) |
| Lawton IADL | 4.98 (0.14) | 4.96 (0.19) |
| GDS* | 2.6 (2.6) | 1.3 (1.8) |
| MMSE | 29.5 (1.1) | 29.0 (1.2) |
Note: IADL=Instrumental Activities of Daily Living; GDS=Geriatric Depression Scale; MMSE=Mini-Mental State Examination; DRS-2=Mattis Dementia Rating Scale-2; RAVLT=Rey Auditory Verbal Learning Test; RT=Reaction Time.
p<.05 between-groups.
p<.001
Neuropsychological Assessment and APOE Genotyping
Participants underwent baseline neuropsychological testing, fMRI scanning, and APOE genotyping. The neuropsychological battery included the MMSE, GDS, MDRS-2, RAVLT, and Lawton Instrumental Activities of Daily Living Scale. Each of these measures is commonly used in dementia assessment. The MMSE is a brief, 30-point measure of cognitive status. The GDS is a 30-item measure of the presence or absence of depressive symptoms that has been well validated with older adults. The MDRS-2 provides an index of global cognitive functioning as well as domain-specific performance measures in five cognitive domains (Attention, Initiation/Perseveration, Construction, Conceptualization, and Memory). The RAVLT consists of a list of 15 unrelated words that is presented to the participant over five study-test trials. In order to test susceptibility to proactive and retroactive interferences, a distractor list of words is then presented in a study-test format, followed by a trial requiring the participant to recall the original list of words. Finally, 30-minute delayed recall and recognition of the original list are assessed. Although the RAVLT provides a number of memory performance indices, the current study examined the Sum of Words Recalled across Trials 1-5 (a measure of immediate learning capability) and 30-minute Delayed Recall (as a measure of delayed retention). Finally, the Lawton Instrumental Activities of Daily Living Scale evaluates the extent of functional independence by surveying capacity to carry out both instrumental and personal self-maintenance activities of daily living. APOE genotype was determined from whole blood using a PCR method [62]. An 18-month follow-up neuropsychological evaluation was completed using alternate forms of the DRS-2 [63, 64] and RAVLT [65].
PA Survey
Participation in PA was assessed at baseline with the Stanford Brief Activities Survey [SBAS 66], which has been validated for assessment of habitual PA in older adults [66, 67] and has shown significant, dose-dependent relationships with cardiovascular risk factors and estimated energy expenditure in a large sample [66]. Survey items pertained to PA performed over the previous year. Participants who reported two or fewer instances of low intensity PA (e.g., going for walks, doing chores, or playing golf) per week were assigned to the low PA group. Participants who reported moderate (e.g., brisk walking for 15 minutes; performing moderately difficult chores for 45 minutes) to heavy (e.g., jogging for 30 minutes; moderately difficult chores for 60 minutes) PA at least three times a week were assigned to the high PA group. The group designation criteria for the high PA group are roughly consistent with the recommendations for minimum physical activity participation in healthy older adults that are expected to confer health benefits, while the low PA group did not meet this recommendation [19].
CA Survey
Participation in CA was assessed at baseline using the Cognitive Activities Scale [CAS 16], a previously established composite index of CA frequency [6, 10, 11, 16]. Participants were asked about their frequency of participation in seven cognitively stimulating activities: viewing television, listening to radio, reading newspapers, reading magazines, reading books, playing games (e.g., cards, checkers, crosswords, puzzles), and going to museums. Participants used a five-point Likert-type rating scale to indicate their current frequency of participation for each activity, where 5 = every day or about every day, 4 = several times a week, 3 = several times a month, 2 = several times a year, and 1 = once a year or less. Scores from all seven activities were summed to form a composite index of participation in CA, ranging from 7 to 35.
Definition of Cognitive Decline
Significant cognitive decline was defined as exhibiting a one SD reduction or greater on at least one of the three principal outcome indices (DRS-2, RAVLT Sum of Trials 1-5, RAVLT Delayed Recall). Standardized residual change scores were computed [51] to adjust for baseline performance, practice effects, and regression to the mean [68-70]. Participants with standardized residual scores of -1.0 or lower for at least one neuropsychological measure constituted the cognitively declining group; the remaining participants were classified as cognitively stable. Because age may influence the likelihood of cognitive decline, age-corrected scores on each of these measures were also used to compute standardized residual change scores. The results using age-corrected residual change scores did not differ from non-corrected change scores, which may have been a function of the relatively brief test-retest interval of 18 months. Therefore, our definition of cognitive decline in the analyses that follow used residual change scores that are not corrected for age.
fMRI Task
For the famous name discrimination task [71], participants viewed individually presented names of well-known public figures (famous) or names drawn at random from a local telephone book (unfamiliar). Stimuli consisted of 30 famous and 30 unfamiliar names that were randomly interspersed with 20 presentations of a centrally placed crosshair to introduce “jitter” into the fMRI time course. Stimuli were presented using a fixed interstimulus interval of 4 sec. The imaging run started and ended with 12 secs of fixation. Total time for the single imaging run is 5 min and 24 sec. By using an event-related fMRI design, we are able to exclude the occasional incorrect item from the derived activation maps and avoid other methodological concerns associated with a blocked design format (e.g., strategy deployment). Participants made a right index or right middle finger key press for famous or unfamiliar names, respectively. Accuracy and reaction time were recorded.
Image Acquisition
Whole-brain, event-related fMRI was conducted on a General Electric (Waukesha, WI) Signa Excite 3.0 Tesla short bore scanner equipped with a quad split quadrature transmit/receive head coil. Echoplanar images were collected using an echoplanar pulse sequence (TE=25 ms; flip angle=77 degrees; field of view (FOV)=24 cm; matrix size=64 × 64; TR=2 secs). Thirty-six contiguous axial 4-mm-thick slices provided whole brain coverage (voxel size = 3.75 × 3.75 × 4 mm). High-resolution, three-dimensional spoiled gradient-recalled at steady-state (SPGR) anatomic images were acquired (TE = 3.9 ms; TR = 9.5 ms; inversion recovery (IR) preparation time = 450 ms; flip angle = 12 degrees; number of excitations (NEX) = 2; slice thickness = 1.0 mm; FOV = 24 cm; resolution = 256 × 224). Foam padding was used to reduce head movement within the coil.
Hippocampal Activation
Functional images were generated with the Analysis of Functional NeuroImages (AFNI) software package [72]. Each image series was time-shifted to the beginning of the TR and spatially registered to reduce head motion effects using a rigid body iterative linear least squares method. A deconvolution analysis was used to extract separate hemodynamic response functions (HRFs) for correctly recognized famous and unfamiliar names. HRFs were modeled for the 0-16 second period post-stimulus onset. Motion parameters and incorrect trials were incorporated into the model as nuisance regressors. Area under the curve (AUC) was calculated by summing the hemodynamic responses at time points 4, 6, and 8 seconds post-trial onset. Anatomical and functional scans were transformed into standard stereotaxic space [73], and functional images were blurred using a 6-mm Gaussian filter.
A voxelwise t-test contrasting semantic activation during correct discrimination of famous names versus unfamiliar names (AUCs) was performed on all participants. The statistical threshold was based on an individual voxel probability (p = 0.005) coupled with a minimum cluster volume (0.73 ml). These values were derived from 3,000 Monte Carlo simulations [74] and correspond to a whole brain family-wise error threshold of p < 0.05. This approach identified significant clusters representing functional regions of interest (fROIs; [51]). Across all voxels within each fROI, an “average AUC” was computed for each participant. Using these data, a principal components analysis (PCA) further reduced the number of regions that would serve as predictors in the logistic regression analysis. Two components were identified, representing cortical (bilateral posterior cingulate/precuneus, left angular gyrus, left superior frontal gyrus, right angular gyrus, right superior middle frontal gyrus) and hippocampal (right and left parahippocampal gyrus/hippocampus) regions of increased semantic activation during correct identification of famous names relative to unfamiliar names. Because this study focused on hippocampal volume and function, only the latter component was used in logistic regression models.
Hippocampal Volume
Left and right hippocampal volumes were obtained using Freesurfer [75, 76] and manually edited on T1-weighted SPGR images by two raters blinded to participant group membership as described previously [51]. Hippocampal volumes were normalized by dividing by the total intracranial volume. Intraclass correlation for the two raters was 0.87. The left and right hippocampal volumes were then summed to create a single value.
Data Analysis
We tested two initial logistic regression models that predicted cognitive decline at 18-month follow-up using APOE ε4 allele status, either baseline PA or CA, and the APOE ε4 allele status by activity interaction. Next, we tested several logistic regression models to determine whether addition of the baseline neuroimaging variables as covariates would enhance prediction of cognitive decline beyond APOE ε4 allele status and participation in CA or PA alone.
Results
Demographics and Baseline and Follow-up Neuropsychological Scores
Twenty-seven of the 78 participants demonstrated a one SD decline on one or more of the three principal neuropsychological measures (DRS-2, RAVLT Sum of Trials 1-5, RAVLT Delayed Recall). Participant demographics, neuropsychological performance and PA and CA data for the stable (n=51) and declining (n=27) groups are presented in Table 1. Importantly, there were no significant differences between the stable and declining groups on age, education, or gender representation. There was a non-significant trend for the declining group to have slightly lower baseline scores on the MMSE, RAVLT Sum of Trials 1-5, and RAVLT Delayed Recall relative to the stable group. The stable group demonstrated significantly greater baseline mean scores on the GDS, although neither the stable nor declining groups scored in the clinical range on this measure. Although there was a significantly higher proportion of APOE ε4 carriers in the declining group, there were no significant differences between groups with respect to other demographic variables or extent of engagement in PA or CA. At follow-up, the neuropsychological scores in Table 1 reveal that the declining group clearly demonstrated significant reductions on all three of the measures used to define the groups (DRS-2, RAVLT Sum of Trials 1-5, and RAVLT Delayed Recall). There was no significant change in the level of depressive symptoms reported on the GDS over the two assessments.
Influence of CA and PA on Cognitive Decline
Table 2 shows the results of several logistic regression models investigating how well CA, APOE ε4 allele status, hippocampal volume, and fMRI-measured semantic processing activity help predict probability of cognitive decline after 18 months. Each model's ability to discriminate between stable and declining participants was evaluated with Nagelkerke R2 and the concordance or C index (related to the area under the receiver operating characteristic curve; [77]). The Nagelkerke R2 reflects the collective importance of the predictors in each model as compared to a “perfectly fitting” null model [78]. The C index reflects the proportion of all possible pairs of declining and stable subjects in which the declining participant in the pair had a higher predicted probability of decline than the stable participant [77].
Table 2.
Logistic regression results testing effects of neuroimaging results, APOE ε4 allele status, and physical or cognitive activity on cognitive decline
| Baseline Model 1A | Baseline Model 1B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C=.719 | R2=.203 | C=.716 | R2=.178 | ||||||
| Effect | Coefficient | SE | Wald Z | p | Effect | Coefficient | SE | Wald Z | p |
| Intercept | -1.23 | 0.35 | -3.54 | 4E-4 | Intercept | 3.30 | 2.16 | 1.52 | .127 |
| Hippocampal fMRI | -0.70 | 0.31 | -2.23 | .025 | Hippocampal volume | -0.99 | 0.49 | -2.02 | .043 |
| APOE ε4 genotype | 1.40 | 0.54 | 2.60 | .009 | APOE ε4 genotype | 1.06 | 0.53 | -2.00 | .045 |
| Cognitive Activity Models | Physical Activity Models | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 2A | C=.640 | R2=.104 | Model 3A | C=.680 | R2=.208 | ||||
| Effect | Coefficient | SE | Wald Z | p | Effect | Coefficient | SE | Wald Z | p |
| Intercept | -1.01 | 0.41 | -2.45 | .014 | Intercept | -1.19 | 0.432 | -2.76 | .006 |
| APOE ε4 genotype | 0.61 | 0.77 | 0.79 | .429 | APOE ε4 genotype | 2.69 | 0.893 | 3.02 | .003 |
| CA level | -0.09 | 0.66 | -0.13 | .895 | PA status | 0.21 | 0.645 | 0.32 | .746 |
| APOE ε4 genotype * CA level | 0.90 | 1.06 | 0.84 | .399 | APOE ε4 genotype * PA level | -2.41 | 1.152 | -2.09 | .037 |
| Model 2B | C=.724 | R2=.209 | Model 3B | C=.741 | R2=.304 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | Coefficient | SE | Wald Z | p | Effect | Coefficient | SE | Wald Z | p |
| Intercept | -1.09 | 0.43 | -2.51 | .012 | Intercept | -1.42 | 0.47 | -3.01 | .003 |
| Hippocampal fMRI | -0.72 | 0.32 | -2.28 | .023 | Hippocampal fMRI | -0.81 | 0.35 | -2.29 | .022 |
| APOE ε4 genotype | 0.63 | 0.79 | 0.79 | .428 | APOE ε4 genotype | 3.04 | 0.96 | 3.17 | .002 |
| CA level | -0.20 | 0.69 | -0.29 | .773 | PA level | 0.36 | 0.68 | 0.53 | .597 |
| APOE ε4 genotype * CA level | 1.17 | 1.12 | 1.04 | .296 | APOE ε4 genotype * PA level | -2.75 | 1.22 | -2.24 | .025 |
| Model 2C | C=.703 | R2=.163 | Model 3c | C=.742 | R2=.258 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | Coefficient | SE | Wald Z | p | Effect | Coefficient | SE | Wald Z | p |
| Intercept | 2.92 | 2.18 | 1.34 | .181 | Intercept | 3.02 | 2.37 | 1.27 | .202 |
| Hippocampal volume | -0.90 | 0.50 | -1.81 | .070 | Hippocampal volume | -0.93 | 0.52 | -1.78 | .075 |
| APOE ε4 genotype | 0.56 | 0.78 | 0.72 | .474 | APOE ε4 genotype | 2.36 | 0.92 | 2.56 | .011 |
| CA status | 0.09 | 0.68 | 0.13 | .897 | PA level | -0.01 | 0.67 | -0.02 | .985 |
| APOE ε4 genotype * CA level | 0.67 | 1.09 | 0.62 | .538 | APOE ε4 genotype * PA level | -2.08 | 1.18 | -1.76 | .079 |
Baseline Models
Two baseline models were established in order to assess the combined influence of factors other than CA and PA that might also be predictive of cognitive decline at 18-month follow-up. These models included APOE ε4 allele status and either baseline hippocampal fMRI semantic processing activity (Model 1A) or baseline hippocampal volume (Model 1B). Although both models exhibited comparable C indices (.72), the model that included APOE ε4 allele status and hippocampal fMRI activity yielded a slightly stronger R2 (.203). Models assessing the impact of CA and PA on cognitive decline at follow-up were contrasted against these baseline models.
Effect of CA on Cognitive Decline
A logistic regression model that included only APOE ε4 allele status, participation in CA and the interaction between these two variables (Model 2A) was not statistically significant. For Model 2B, which added baseline hippocampal fMRI semantic processing activity as a covariate, only the hippocampal fMRI index emerged as a significant predictor. For Model 2C, there were no significant effects, although baseline hippocampal volume demonstrated a non-significant trend (p=.07) toward significance. For Models 2B and 2C, addition of the neuroimaging variables enhanced the C index and R2 values relative to Model 2A. Because these increases were principally driven by the neuroimaging variables, it does not appear that participation in CA affects the predicted probability of cognitive decline. In addition, relative to the two baseline models (Models 1A and 1B), the models that added CA and its interaction with APOE ε4 allele status did not result in appreciably different C indices or R2 values.
Effect of PA on Cognitive Decline
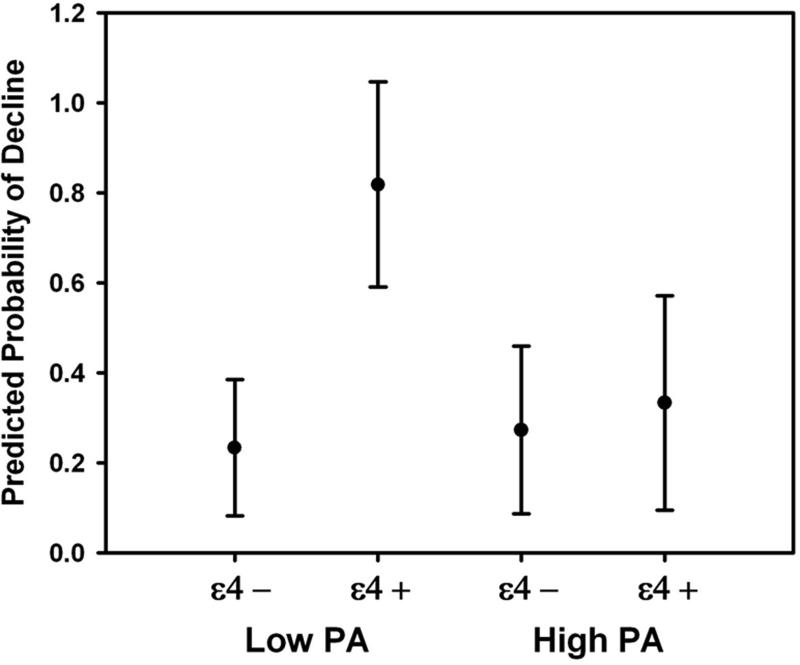
The logistic regression model that included PA (high vs. low), APOE ε4 allele status, and their interaction (Model 3A) was statistically significant. APOE ε4 allele status and the interaction between PA and APOE ε4 status were significant predictors. Inspection of the simple effects of this interaction revealed that the predicted probability of decline for the APOE ε4 positive participants who reported low PA was significantly higher compared to APOE ε4 carriers who reported high PA. However, the predicted probability of decline was not affected by PA level for APOE ε4 negative participants (Figure 1).
Figure 1.
Differences between predicted probabilities of decline for high and low PA groups, as a function of APOE ε4 allele status. Error bars reflect the 95% confidence intervals. Low PA ε4 carriers (n=11) demonstrated a higher probability of decline than low PA ε4 non-carriers (n=30), high PA ε4 carriers (n=15), and high PA ε4 non-carriers (n=22).
For models 3B and 3C, we added indices of baseline hippocampal function and structure as covariates to determine their relationship with prediction of cognitive decline at 18-month follow-up when combined with APOE ε4 allele status and PA level. Relative to Model 3A, addition of the neuroimaging covariates increased the Nagelkerke R2 and C index values by 5-10%, depending on the model. For Model 3B, APOE ε4 allele status and baseline hippocampal fMRI semantic activation were significant predictors of decline. For Model 3C, although APOE ε4 allele status was a significant predictor, total hippocampal volume at baseline demonstrated a non-significant trend (p=.075) as a covariate. For both Models 3B and 3C, the main effect of PA status was not significant. However, the interaction between PA and APOE ε4 status was significant in Model 3B (p=.032) but demonstrated a non-significant trend in Model 3C, where baseline total hippocampal volume was included as a covariate (p=.079).
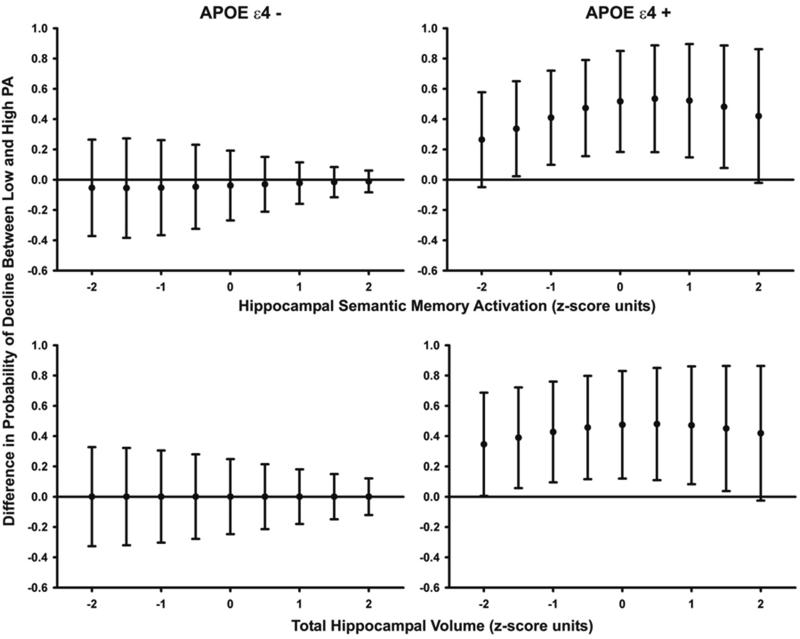
To better elucidate the interaction between PA and APOE ε4 allele status in Model 3B, we held the covariate (baseline hippocampal fMRI semantic processing activity) constant at the mean and at several 0.5 SD increments above and below the mean. Then, for APOE ε4 positive and negative individuals separately, we plotted the differences between the predicted probabilities of decline for high and low PA participants. The upper two graphs in Figure 2 display the difference in predicted probabilities of decline for the low PA group minus the high PA group at each incremental level of baseline hippocampal activity.
Figure 2.
(upper two graphs). Simple effects of the APOE ε4 * PA interaction, holding hippocampal fMRI semantic processing activity constant at selected values 2 SD above and below the mean. Closed circles represent the predicted probability of decline for the low PA group minus the predicted probability of decline for the high PA group, and error bars reflect the 95% confidence intervals. Note that there is no significant difference between predicted probabilities of decline for low and high PA participants for hippocampal fMRI activity values at +2 and -2 SD from the mean. The difference between predicted probabilities of decline for high and low PA participants appears to be greatest at 0.5 SD above the mean and becomes smaller as hippocampal activity values decrease (possibly reflecting the loss of hippocampal function) or increase (possibly reflecting the effects of cognitive reserve).
Figure 2 (lower two graphs). Simple effects of the APOE ε4 * PA interaction, holding hippocampal volume constant at selected values 2 SD above and below the mean. Closed circles represent the predicted probability of decline for the low PA group minus the predicted probability of decline for the high PA group, and error bars reflect the 95% confidence intervals. Note that there is no significant difference between predicted probabilities of decline for high and low PA participants for hippocampal volumes that are +2 SD from the mean, and the difference approaches 0 for hippocampal volumes that are -2 SD from the mean. As in Figure 1, the difference between predicted probabilities of decline for low and high PA participants appears to be greatest at 0.5 SD above the mean and becomes smaller as hippocampal volumes decrease (possibly reflecting the loss of hippocampal function) or increase (possibly reflecting the effects of cognitive reserve)
For APOE ε4 negative participants (Figure 2, upper left panel), there was no significant difference between the predicted probabilities of decline for high and low PA participants. However, for APOE ε4 positive participants (Figure 2, upper right panel), the predicted probability of decline associated with low PA is significantly higher than that for high PA. Further inspection suggests that the relative advantage conferred by PA in reducing the probability of cognitive decline decreases as hippocampal fMRI semantic processing activity decreases from values that are 0.5 SD above the mean. Smaller PA-related differences in predicted probability of decline were also seen as fMRI semantic processing activity increased above 0.5 SD beyond the mean. Importantly, there were no significant differences in predicted probability of decline for high and low PA participants when hippocampal semantic processing activity was 2 SD above or below the mean.
Although the overall interaction was not significant, Figure 2 (lower two panels) demonstrates similar trends for baseline hippocampal volume. The protective effect of PA over physical inactivity is not significantly different when baseline hippocampal volume is held constant at 2.0 SD above or below the mean.
Discussion
Our major finding is that high PA was associated with a reduced likelihood of cognitive decline in APOE ε4 carriers. In contrast, PA level did not influence the probability of decline in APOE ε4-negative participants. Moreover, greater engagement in PA tended to confer progressively smaller advantages over low PA as hippocampal fMRI semantic processing activity decreased. That is, as hippocampal function declines, the effectiveness of PA as an intervention against cognitive decline may become attenuated. A similar trend was seen with smaller baseline hippocampal volumes. These findings suggest the importance of beginning a PA regimen as early as possible, prior to the occurrence of substantial hippocampal volume or functional activity loss that might be attributable to disease-related factors. In addition, higher levels of hippocampal fMRI semantic processing activity were also associated with smaller differences in predicted probability of decline between the high and low PA groups. This finding could be attributable to the effects of cognitive reserve, suggesting that greater levels of hippocampal integrity may buffer against cognitive decline [5]. In addition, PA may play a neuroprotective role by reducing age-related declines in gray and white matter density [79] and hippocampal atrophy [34]. Individuals with intact and highly functional hippocampi may be at lower risk of cognitive decline regardless of their PA level [80-85].
High and low PA APOE ε4 non-carriers did not show any difference in predicted probability of decline, regardless of fMRI semantic processing activity or baseline hippocampal volume. While generally beneficial associations between PA and cognitive functioning have been reported in humans, other studies have reported null findings [4, 10-12, 86]. Our findings are consistent with results from a study showing that a lack of PA posed a greater risk of cognitive decline in older male APOE ε4 carriers than non-carriers [22]. In addition, our results support findings that midlife physical activity was associated with greater reductions in the risk of incident dementia and AD in APOE ε4 carriers relative to non-carriers after an average follow-up of 21 years [30]. In contrast, in a large, population-based study, lack of PA was associated with an increased number of incident dementia cases only in APOE ε4 non-carriers [31]. Important differences between this study and ours that could account for the apparent discrepant effects of PA in APOE ε4 non-carriers include outcome measure (e.g., cognitive decline vs. incident dementia), timing and duration of PA, and the approach used to assess PA. These methodological differences will be important to examine in future research.
Finally, participation in CA was not predictive of subsequent cognitive decline in our logistic regression models. This finding contrasts with results from a prior study utilizing the CAS [6]. However, in that study, the sample size was very large (n = 4392), follow-up occurred over a longer time frame than in the current study (average follow-up: 5.3 years vs. 18 months), and cognitive decline was coded as a continuous rather than a dichotomous variable, utilizing a composite z-score from four tests of cognitive function [6].
Our results complement the findings of a recent study [34] demonstrating that a one-year PA intervention can increase hippocampal volume in older adults. Specific PA-associated improvement in spatial memory was associated with increased volume of the anterior hippocampus. Our study extends these results by directly examining the longitudinal effects of both CA and PA on risk of cognitive decline using both sMRI and fMRI and by focusing on APOE ε4 allele as a moderator of the relationship between cognitive decline and hippocampal structure and function. Future interventional research should examine whether different intensities of PA exert varying effects on hippocampal volume and function and whether they have differential impacts on the trajectory of cognitive decline. Additionally, future studies might investigate whether the benefits of PA interventions are reduced in asymptomatic elders with below average hippocampal volume and/or functional integrity, as suggested by our results.
Although this study represents the first to incorporate both functional and anatomical brain imaging to assess the effects of lifestyle and genetic risk on cognitive functioning, limitations of our study should be considered. Our sample size and relatively brief follow-up period may have limited our ability to detect a relationship between cognitively stimulating activities and cognitive decline. A more expanded survey of CA, including activities such as playing musical instruments, going to classes, studying, painting, cooking food, and going to theatres or concerts, may provide greater sensitivity to predicting cognitive decline [9, 12-14, 87-90]. Additionally, in our study we dichotomized participants into high and low PA groups; other PA questionnaires estimate energy expenditure during PA as a continuous variable [91-94]. An even better approach would involve the use of accelerometry to objectively measure PA or the prospective engagement in a controlled physical exercise training program.
Conclusion
Results of our study revealed that engagement in leisure-time PA, but not CA, is associated with a reduced probability of cognitive decline over 18 months in healthy older APOE ε4 carriers. Furthermore, addition of baseline fMRI semantic processing activity or baseline hippocampal volume as covariates enhanced prediction of future decline. We did not find an effect of participation in PA or CA on risk of cognitive decline in APOE ε4 non-carriers. When the simple effects of the APOE ε4 allele status by PA interaction were examined, the difference between the predicted probabilities of decline for high and low PA APOE ε4 carriers decreased with lower levels of hippocampal fMRI semantic processing activity and hippocampal volume. This trend may suggest decreasing benefits of PA in persons with lower levels of hippocampal function or reduced hippocampal volume. Smaller differences in predicted probability of decline for the two PA groups were also observed with greater hippocampal volume or higher levels of hippocampal fMRI activity. This finding suggests that greater hippocampal volume and function reflect greater cognitive reserve buffering against cognitive decline [5].
Acknowledgements
1. The authors thank Qi Zhang, Amelia Gander, and Nicole Klos for their help with subject recruitment and data collection.
2. This project was supported by NIH grant, R01 AG022304, awarded to SMR, the Medical College of Wisconsin General Clinical Research Center (M01 RR00058), and the Medical College of Wisconsin Advancing a Healthier Wisconsin Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Clark CM, Davatzikos C, Borthakur A, et al. Biomarkers for early detection of Alzheimer pathology. Neurosignals. 2008;16:11–8. doi: 10.1159/000109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiman EM, Langbaum JB, Tariot PN. Alzheimer's prevention initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomark Med. 2010;4:3–14. doi: 10.2217/bmm.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daviglus ML, Bell CC, Berrettini W, et al. National Institutes of Health State-of-the-Science Conference Statement: Preventing Alzheimer's disease and cognitive decline. NIH Consens State Sci Statements. 2010;27:1–30. [PubMed] [Google Scholar]
- 4.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69:1911–20. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- 5.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61:812–6. doi: 10.1212/01.wnl.0000083989.44027.05. [DOI] [PubMed] [Google Scholar]
- 7.Bosma H, van Boxtel MP, Ponds RW, et al. Engaged lifestyle and cognitive function in middle and old-aged, non-demented persons: a reciprocal association? Z Gerontol Geriatr. 2002;35:575–81. doi: 10.1007/s00391-002-0080-y. [DOI] [PubMed] [Google Scholar]
- 8.Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356–61. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–7. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–4. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–8. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 12.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–16. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 13.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001;57:2236–42. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155:1081–7. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- 15.Schooler C, Mulatu MS. The reciprocal effects of leisure time activities and intellectual functioning in older people: a longitudinal analysis. Psychol Aging. 2001;16:466–82. doi: 10.1037//0882-7974.16.3.466. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54:P155–60. doi: 10.1093/geronb/54b.3.p155. [DOI] [PubMed] [Google Scholar]
- 17.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 18.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 19.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–45. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 20.Rolland Y, Abellan van Kan G, Vellas B. Physical activity and Alzheimer's disease: from prevention to therapeutic perspectives. J Am Med Dir Assoc. 2008;9:390–405. doi: 10.1016/j.jamda.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 22.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–7. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Etgen T, Sander D, Huntgeburth U, Poppert H, Forstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med. 2010;170:186–93. doi: 10.1001/archinternmed.2009.498. [DOI] [PubMed] [Google Scholar]
- 24.Jedrziewski MK, Ewbank DC, Wang H, Trojanowski JQ. Exercise and cognition: results from the National Long Term Care Survey. Alzheimers Dement. 2010;6:448–55. doi: 10.1016/j.jalz.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–61. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 26.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–8. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 27.van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology. 2004;63:2316–21. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- 28.Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a metaanalysis of prospective studies. Journal of internal medicine. 2011;269:107–17. doi: 10.1111/j.1365-2796.2010.02281.x. [Meta-Analysis Review] [DOI] [PubMed] [Google Scholar]
- 29.Geda YE, Roberts RO, Knopman DS, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80–6. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–11. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 31.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–51. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 32.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–53. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 33.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA : the journal of the American Medical Association. 2009;302:627–37. doi: 10.1001/jama.2009.1144. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3017–22. doi: 10.1073/pnas.1015950108. [Randomized Controlled Trial Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3316–21. doi: 10.1073/pnas.0400266101. [Clinical Trial Comparative Study Randomized Controlled Trial Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voelcker-Rehage C, Godde B, Staudinger UM. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front Hum Neurosci. 2011;5:26. doi: 10.3389/fnhum.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scarmeas N, Luchsinger JA, Brickman AM, et al. Physical activity and Alzheimer disease course. Am J Geriatr Psychiatry. 2011;19:471–81. doi: 10.1097/JGP.0b013e3181eb00a9. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 39.Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004;76:356–62. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- 40.Brown J, Cooper-Kuhn CM, Kempermann G, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–6. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 41.Fabel K, Tam B, Kaufer D, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–12. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 42.Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci. 2009;3:50. doi: 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 44.Kronenberg G, Reuter K, Steiner B, et al. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–63. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- 45.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 47.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Etnier JL, Caselli RJ, Reiman EM, et al. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39:199–207. doi: 10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- 49.Seidenberg M, Guidotti L, Nielson KA, et al. Semantic memory activation in individuals at risk for developing Alzheimer disease. Neurology. 2009;73:612–20. doi: 10.1212/WNL.0b013e3181b389ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodard JL, Seidenberg M, Nielson KA, et al. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132:2068–78. doi: 10.1093/brain/awp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodard JL, Seidenberg M, Nielson KA, et al. Prediction of cognitive decline in healthy older adults using fMRI. J Alzheimers Dis. 2010;21:871–85. doi: 10.3233/JAD-2010-091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith JC, Nielson KA, Woodard JL, et al. Interactive effects of physical activity and APOE-epsilon4 on BOLD semantic memory activation in healthy elders. Neuroimage. 2011;54:635–44. doi: 10.1016/j.neuroimage.2010.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 54.Rey A. L'examen clinique en psychologie. Presses Universitaires de France; Paris: 1958. [Google Scholar]
- 55.Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2 professional manual. Psychological Assessment Resources; Lutz, FL: 2001. [Google Scholar]
- 56.Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellak L, Karasu T, editors. Geriatric psychiatry: A handbook for psychiatrists and primary care physicians. Grune and Stratton; New York: 1976. pp. 77–121. [Google Scholar]
- 57.Mattis S. Dementia Rating Scale professional manual. Psychological Assessment Resources; Odessa, Florida: 1988. [Google Scholar]
- 58.Yesavage J, Brink T, Rose T, et al. Development and validation of a geriatric depression scale: A preliminary report. JPsychiatrRes. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 59.Yesavage JA, Brink TL, Rose TL, Adey M. The Geriatric Depression Rating Scale: Comparison with other self-report and psychiatric rating scales. In: Poon L, editor. Handbook for clinical memory assessment of older adults. American Psychological Association; Washington, DC: 1986. pp. 153–67. [Google Scholar]
- 60.Lawton M, Brody E. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 61.Oldfield RC. The assessment of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–111. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 62.Saunders AM, Hulette O, Welsh-Bohmer KA, et al. Specificity, sensitivity, and predictive value of apolipoprotein-E genotyping for sporadic Alzheimer's disease. Lancet. 1996;348:90–3. doi: 10.1016/s0140-6736(96)01251-2. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt KS. DRS-2: Alternate form professional manual. Psychological Assessment Resources; Lutz, FL: 2004. [Google Scholar]
- 64.Schmidt KS, Mattis PJ, Adams J, Nestor P. Alternate-form reliability of the Dementia Rating Scale-2. Arch Clin Neuropsychol. 2005;20:435–41. doi: 10.1016/j.acn.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt M. Rey Auditory and Verbal Learning Test: A handbook. Western Psychological Services; Los Angeles: 1996. [Google Scholar]
- 66.Taylor-Piliae RE, Norton LC, Haskell WL, et al. Validation of a new brief physical activity survey among men and women aged 60-69 years. Am J Epidemiol. 2006;164:598–606. doi: 10.1093/aje/kwj248. [DOI] [PubMed] [Google Scholar]
- 67.Taylor-Piliae RE, Haskell WL, Iribarren C, et al. Clinical utility of the Stanford brief activity survey in men and women with early-onset coronary artery disease. J Cardiopulm Rehabil Prev. 2007;27:227–32. doi: 10.1097/01.HCR.0000281768.97899.bb. [DOI] [PubMed] [Google Scholar]
- 68.Frerichs RJ, Tuokko HA. A comparison of methods for measuring cognitive change in older adults. Arch Clin Neuropsychol. 2005;20:321–33. doi: 10.1016/j.acn.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 69.McSweeny AJ, Naugle RI, Chelune GJ, Luders H. “T scores for change”: An illustration of a regression approach to depicting change in clinical neuropsychology. The Clinical Neuropsychologist. 1993;7:300–12. [Google Scholar]
- 70.Temkin NR, Heaton RK, Grant I, Dikmen SS. Detecting significant change in neuropsychological test performance: a comparison of four models. J Int Neuropsychol Soc. 1999;5:357–69. doi: 10.1017/s1355617799544068. [DOI] [PubMed] [Google Scholar]
- 71.Douville K, Woodard JL, Seidenberg M, et al. Medial temporal lobe activity for recognition of recent and remote famous names: an event-related fMRI study. Neuropsychologia. 2005;43:693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 73.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- 74.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 75.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 76.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 77.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer; New York: 2001. [Google Scholar]
- 78.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–2. [Google Scholar]
- 79.Colcombe SJ, Erickson KI, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58:176–80. doi: 10.1093/gerona/58.2.m176. [Comparative Study Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 80.de Leon MJ, George AE, Stylopoulos LA, Smith G, Miller DC. Early marker for Alzheimer's disease: the atrophic hippocampus. Lancet. 1989;2:672–3. doi: 10.1016/s0140-6736(89)90911-2. [DOI] [PubMed] [Google Scholar]
- 81.Henneman WJ, Sluimer JD, Barnes J, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72:999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jack CR, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morra JH, Tu Z, Apostolova LG, et al. Automated 3D mapping of hippocampal atrophy and its clinical correlates in 400 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, Detoledo-Morrell L. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: Relation to memory function. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolf H, Jelic V, Gertz HJ, Nordberg A, Julin P, Wahlund LO. A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurol Scand Suppl. 2003;179:52–76. doi: 10.1034/j.1600-0404.107.s179.10.x. [DOI] [PubMed] [Google Scholar]
- 86.Sturman MT, Morris MC, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Physical activity, cognitive activity, and cognitive decline in a biracial community population. Arch Neurol. 2005;62:1750–4. doi: 10.1001/archneur.62.11.1750. [DOI] [PubMed] [Google Scholar]
- 87.Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 2006;21:65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- 88.Fabrigoule C, Letenneur L, Dartigues JF, Zarrouk M, Commenges D, Barberger-Gateau P. Social and leisure activities and risk of dementia: a prospective longitudinal study. J Am Geriatr Soc. 1995;43:485–90. doi: 10.1111/j.1532-5415.1995.tb06093.x. [DOI] [PubMed] [Google Scholar]
- 89.Valenzuela MJ, Sachdev P. Assessment of complex mental activity across the lifespan: development of the Lifetime of Experiences Questionnaire (LEQ). Psychol Med. 2007;37:1015–25. doi: 10.1017/S003329170600938X. [DOI] [PubMed] [Google Scholar]
- 90.Paillard-Borg S, Fratiglioni L, Winblad B, Wang HX. Leisure activities in late life in relation to dementia risk: principal component analysis. Dement Geriatr Cogn Disord. 2009;28:136–44. doi: 10.1159/000235576. [DOI] [PubMed] [Google Scholar]
- 91.McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med. 1989;5:65–72. [PubMed] [Google Scholar]
- 92.Taylor HL, Jacobs DR, Jr., Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 93.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6. [PubMed] [Google Scholar]
- 94.Bowles HR, FitzGerald SJ, Morrow JR, Jr., Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. Am J Epidemiol. 2004;160:279–86. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]