Abstract
Objective
To investigate the effect of coping strategies, depression, physical health, and cognition on National Eye Institute Visual Function Questionnaire scores obtained at baseline in a sample of older patients with age-related macular degeneration (AMD) enrolled in the Improving Function in AMD Trial, a randomized controlled clinical trial that compares the efficacy of problem-solving therapy with that of supportive therapy to improve vision function in patients with AMD.
Methods
Baseline evaluation of 241 older outpatients with advanced AMD who were enrolled in a clinical trial testing the efficacy of a behavioral intervention to improve vision function. Vision function was characterized as an interval-scaled, latent variable of visual ability based on the near-vision subscale of the National Eye Institute Vision Function Questionnaire-25 plus Supplement.
Results
Visual ability was highly correlated with visual acuity. However, a multivariate model revealed that patient coping strategies and cognitive function contributed to their ability to perform near-vision activities independent of visual acuity.
Conclusions
Patients with AMD vary in their coping strategies and cognitive function and in their visual acuity, and that variability determines patients’ self-report of vision function. Understanding patient coping mechanisms and cognition may help increase the precision of vision rating scales and suggest new interventions to improve vision function and quality of life in patients with AMD.
Trial Registration
clinicaltrials.gov Identifier: NCT00572039
Age-related macular de-generation (AMD) is the leading cause of severe vision loss in older adults, with nearly 2 million having advanced disease (ie, neovascular AMD or geographic atrophy) and more than 7 million having early signs of AMD.1 These numbers will double by 2020, dramatically increasing the number of visually impaired adults who cannot read, drive, or live independently.2 The anti–vascular endothelial growth factor (anti-VEGF) antibodies ranibizumab and bevacizumab have greatly improved the prognosis of neovascular AMD.3 The MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration) and ANCHOR (Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration) trials3–5 found that ranibizumab treatment prevented vision loss in 95.5% of patients. Approximately 30% of patients gained 15 or more letters, and 50% had improved mental health.6 Although these are unprecedented outcomes, the converse is informative: the vision function of 70% of patients and the mental health of 50% did not improve to this extent. Patients with visual acuity worse than 20/70 in the better eye after treatment, for example, would still have disabling impairment and rehabilitative needs. Thus, despite the success of anti-VEGF treatment, AMD-related disability remains a major public health problem.7
To measure AMD-related disability, many investigators have used the National Eye Institute Visual Function Questionnaire (NEI-VFQ).8,9 Massof and Fletcher10 demonstrated that the NEI-VFQ items that assess difficulty with everyday tasks yield a latent visual ability variable that strongly relates to visual acuity. The correlation of 0.523, however, indicates that visual acuity accounts for only approximately 27% of the variance and suggests that other factors affect NEI-VFQ scores. Depression and general health are 2 such factors.11,12 Other clinical variables may also contribute and, unless accounted for, may introduce un-measured sources of variability or “noise” into disability measurements in patients with AMD.
In this study, we investigated the effect of coping strategies, depression, physical health, and cognition on NEI-VFQ scores obtained at baseline in a sample of older patients with AMD enrolled in the Improving Function in AMD Trial, a randomized controlled clinical trial that compares the efficacy of problem-solving therapy with that of supportive therapy to improve vision function in patients with AMD.
METHODS
This study reports baseline data obtained before randomization into the Improving Function in AMD Trial. We recruited 241 patients with AMD from the retina clinics associated with the Wills Eye Institute, Philadelphia, Pennsylvania, between January 1, 2006, and December 31, 2009, and randomized patients to receive problem-solving therapy or supportive therapy in a 1:1 allocation ratio.13,14 The primary aims of the Improving Function in AMD Trial are to test the immediate (3-month) and longer-term (6-month) efficacy of problem-solving therapy to improve the primary outcome of vision function.
The inclusion criteria were (1) age 65 years or older, (2) bilateral AMD (neovascular or dry), (3) visual acuity between 20/70 and 20/400 (inclusive; best corrected) in the better-seeing eye and no worse than 20/400 in the fellow eye, and (4) moderate difficulty in at least 1 valued vision function goal. The exclusion criteria were (1) the presence of uncontrolled glaucoma, diabetic retinopathy, or planned cataract surgery within 6 months; (2) dementia, using a version of the Mini-Mental State Examination that omits vision-dependent items15; (3) the presence of life-threatening illness; and (4) residence in a skilled nursing facility. All the participants signed an informed consent form approved by the Thomas Jefferson University institutional review board. At baseline, a research nurse conducted clinical assessments in patients’ homes and gathered demographic information and assessed the following clinical variables: vision, physical health, depression, cognition, vision function, and coping strategies.
VISION
Best-corrected vision was assessed using the Lighthouse Ferris-Bailey Early Treatment Diabetic Retinopathy Study chart to measure distance visual acuity and the Pelli-Robson Contrast Sensitivity chart to measure contrast sensitivity. Near and distance visual acuities were assessed at 16 in and 5 ft (41 cm and 1.5 m), respectively. A gooseneck lamp was used to standardize luminance levels. For statistical analyses, log transformations (ie, logMAR and log contrast) were used for visual acuity and contrast sensitivity, respectively.
PHYSICAL HEALTH
We used the Chronic Disease Score, which provides an index of medical comorbidity based on a weighted sum of medications taken for chronic illness, and the Multilevel Assessment Inventory Health Conditions Check List, which lists specific acute and chronic conditions.16,17
DEPRESSION
We used the Patient Health Questionnaire-9 to assess depression. This questionnaire includes the 9 criteria that comprise Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) diagnoses of major or minor depressive disorders.18 It is a dual-function instrument in that it generates categorical diagnoses of depression and grades depressive symptom severity as a continuous measure. Symptoms are scored on an ordinal scale from 0 (not at all) to 3 (every day). The raw score for each patient is the sum of symptom scores across the 9 items. The raw scores range from 0 to 27, with higher scores indicating worse depression. Symptoms are scored from 0 (not at all) to 3 (every day).
COGNITION
We administered the Animal Fluency Test to obtain a brief assessment of cognitive function that is relevant to the completion of daily activities.19 This verbal fluency test requires patients to name as many different animals as possible in 60 seconds and is scored as the number of animals named. The test requires semantic knowledge of categories, vocabulary storage, speeded mental processing, and intact executive function. A reduction in the number of retrieved items, repetition of the same word, and listing of disqualified words indicate difficulty with sustained output, concentration, and retrieval. The mean (SD) score for white women aged 70 to 89 years with 12 years of education is 17.2 (4.2).
VISION FUNCTION
We used the NEI-VFQ-25 plus Supplement, which consists of 25 items and a supplement of 14 additional items, derived from the original 52-item NEI-VFQ.8,9 It is used to assess self-reported vision function and generates 11 subscale scores and an overall score. In this investigation, we focused on items included in part 2 of the NEI-VFQ because they all require difficulty ratings of vision-dependent activities that many patients highly value. In particular, the near-vision subscale consists of 6 items rating difficulties with reading newsprint, doing housework or hobbies (eg, sewing and using tools), finding something on a crowded shelf, reading small print on a medication bottle or legal form, determining whether bills are accurate, and performing personal hygiene tasks (eg, shaving and putting on makeup). Patients rate these items on an ordinal scale from 1 to 5, with higher numbers indicating increasing levels of difficulty (ie, no difficulty, a little difficulty, moderate difficulty, extreme difficulty, or stopped doing this because of your eyesight), or they can respond that they stopped doing the activity described by the item for other reasons/not interested (scored as missing data). Previous studies6,10,20–23 have demonstrated that the items of the near-vision subscale are responsive to low-vision rehabilitation and anti-VEGF treatment and can be used to estimate an interval scale suitable for the analyses we conducted.
COPING STRATEGIES
We used the Optimization in Primary and Secondary Control Scale (OPS) to assess the characteristic approaches, or control strategies, that patients enact to achieve valued goals.24 This instrument draws from the life-span theory of control, which posits that people use different health-related control strategies to greater and lesser extents when faced with adverse health conditions. We selected the OPS because of its applicability to patients with chronic disabling diseases, such as AMD, who must find ways to adjust to vision loss. The reliability and validity and psychometric properties of the OPS have been demonstrated in studies of older persons and patients with AMD.25 Brennan et al26 adapted items specifically for patients with vision loss. The OPS is divided into 4 control strategies, each composed of 8 items rated from 0, “never true,” to 4, “almost always true,” yielding a raw score range of 0 to 32 for each control strategy; higher scores indicate greater use of the particular strategy.
Selective primary control refers to the investment of behavioral resources (ie, time, effort, and skills) into pursuing a goal. Representative items are “I do whatever I can to continue my everyday activities despite my vision problem” and “If I invest enough time, I can continue my everyday activities despite my vision problem.” Selective secondary control serves to enhance and maintain motivation and commitment to a goal, particularly when obstacles (ie, vision loss) make achieving the goal difficult. Items include “I think how important it is to me to keep up my daily activities despite my vision problem” and “I tell myself that it is up to me to make sure my vision problem does not interfere with what I want to do.” Compensatory primary control refers to the recruitment of help from others or the use of assistive devices (eg, magnifiers) when an individual has difficulty attaining a goal. Items include “If there is something that I can no longer do because of my vision problem, I actively seek out help from others” and “If I’m having trouble doing something because of my vision problem, I look for a device or aid that will help get it done.” Compensatory secondary control refers to goal disengagement when the goal becomes unattainable, thereby freeing up the person to pursue other goals that are attainable. It also includes self-protective strategies, such as focusing on successes in other domains. Typical items include “I can accept that there are things I can no longer do since I started having problems with my vision” and “I spend my time doing what I can do rather than struggling with the things that have become difficult because of my vision problem.”
STATISTICAL METHODS
Descriptive statistics for baseline demographic and clinical variables are presented as mean (SD) for continuous data and as frequency (percentage) for categorical data. We used a latent variable model to investigate the relationship between the 6 items of the NEI-VFQ near-activities subscale and various clinical and psychological characteristics.27,28 Because we expect patient responses to depend on multiple variables, we used a structural equation model that assumes that each patient has an ability to perform near activities (ie, the composite latent variable) manifested by the 6 NEI-VFQ items. The ability to perform a specific near-vision NEI-VFQ activity item is obtained by multiplying the factor loading for that item by the underlying latent ability. A patient should perform at a given level for a specific item when the product of the item factor loading and the underlying ability crosses a given threshold for that item. We assumed that the ability to perform near activities is a linear function of 1 or more clinical or psychological characteristics (eg, age, visual acuity, cognition, and coping strategies). That is, we modeled the ability to perform near activities using a linear regression model that assumes that the component variables have independent effects on the estimated functional ability variable. We considered each of the potential predictors individually and selected all with P<.20 for inclusion in a multivariable model. We used the latter liberal statistical criterion to maximize our ability to detect any significant associations. Latent variable models were fit using Mplus version 6.29
RESULTS
Patient clinical and psychological characteristics are summarized in Table 1. The mean (SD) age of the patients was 82.8 (6.9) years, and 63.1% were women. One hundred two patients (42.3%) had received anti-VEGF injections. Depressive symptoms, as reflected by mean Patient Health Questionnaire-9 scores, were low in the sample as a whole; however, 31 patients (12.9%) met the criteria for a depressive disorder. This rate is consistent with a recent study30 of depression prevalence rates in patients with AMD.
Table 1.
Clinical and Vision Characteristics of the Samplea
| Characteristic | |
|---|---|
| Age, mean (SD), y | 82.8 (6.9) |
| Female sex, No. (%) | 153 (63.5) |
| Education, mean (SD), y | 13.2 (3.1) |
| Chronic Disease Score, mean (SD)b | 5.6 (2.9) |
| Vision characteristics | |
| Best eye logMAR, mean (SD)c | 0.57 (0.29) |
| Best eye log contrast, mean (SD)d | 0.69 (0.41) |
| Anti-VEGF treatment in past 3 mo, No. (%) | 102 (42.3) |
| NEI-VFQ near activities, mean (SD)e | 53.3 (20.7) |
| Coping strategies, mean (SD)f | |
| Selective primary control (range, 6–24) | 22.3 (2.4) |
| Compensatory primary control (range, 9–36) | 26.7 (6.0) |
| Selective secondary control (range, 9–36) | 30.1 (4.9) |
| Compensatory secondary control (range, 7–28) | 21.8 (4.0) |
| Depression | |
| PHQ-9 scores, mean (SD) (range, 0–27)g | 1.3 (2.5) |
| Cognition | |
| Animal Fluency Test, mean (SD)h | 14.8 (4.7) |
Abbreviations: NEI-VFQ, National Eye Institute Visual Function Questionnaire; PHQ-9, Patient Health Questionnaire-9; VEGF, vascular endothelial growth factor.
N=241.
A higher score indicates worse medical morbidity.
A higher score indicates worse vision.
A higher score indicates better contrast.
Scored from 0 to 100, with higher scores indicating better function.
A higher score indicates more frequent use of the coping strategy.
A higher score indicates worse depression.
A higher score indicates better cognitive function.
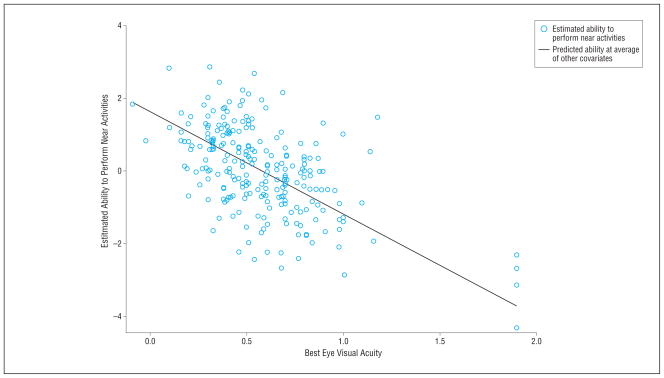
The value of the latent visual ability variable was estimated for each patient from the multivariable model. Estimated values ranged from −4.3 to 2.85 (mean [SD], 0 [1.2]). The Figure depicts the relationship of the latent visual ability with visual acuity and shows the strong relationship between the 2 variables but also the considerable variability that remains. Table 2 provides the results of the univariable models wherein we evaluated the relationship between the predictor variables and the latent visual ability variable. The values represent the increase in visual ability associated with a 1-U increase in that predictor. For example, a 1-U increase in contrast sensitivity was associated with a 0.32 increase in visual ability. Of the 12 possible predictors, visual acuity, compensatory primary control, cognitive verbal fluency score (Animal Fluency Test), age, selective primary control, selective secondary control, and contrast sensitivity were significantly associated with the latent visual ability variable (at the P<.20 level) and were included in the multivariate model. Table 3 lists the results of the multivariable model wherein we considered the unique effect of each significant variable of the univariable model after controlling for the effects of the other variables. This model reveals that visual acuity, compensatory primary control, selective secondary control, and verbal fluency were independently associated with self-reported difficulty with near activities.
Figure.
Scatterplot of visual ability vs visual acuity.
Table 2.
Bivariable Relationships of Predictor Variables to Vision Function
| Variable | Estimate (95% CI)a | P Value |
|---|---|---|
| Visual acuity | −2.02 (−2.63 to −1.41) | <.001 |
| Compensatory primary control | −0.046 (−0.071 to −0.020) | <.001 |
| Animal Fluency Test | 0.056 (0.024 to 0.088) | .001 |
| Age | −0.030 (−0.052 to −0.008) | .006 |
| Selective primary control | 0.08 (0.018 to 0.14) | .01 |
| PHQ-9 severity | −0.055 (−0.120 to 0.005) | .07 |
| Selective secondary control | 0.027 (−0.004 to 0.057) | .08 |
| Contrast sensitivity | 0.32 (−0.05 to 0.68) | .09 |
| Chronic Disease Score | −0.025 (−0.075 to 0.025) | .33 |
| Education | 0.020 (−0.028 to 0.068) | .41 |
| Male sex (vs female) | 0.068 (−0.240 to 1.310) | .66 |
| Compensatory secondary control | 0.005 (−0.032 to 0.041) | .81 |
Abbreviations: CI, confidence interval; PHQ-9, Patient Health Questionnaire-9.
Estimates are the increase in visual ability associated with a 1-U increase in each predictor variable.
Table 3.
Multivariable Relationships of Predictor Variables to Vision Function: Regression Variables
| Variable | Estimate (95% CI) | P Value |
|---|---|---|
| Visual acuity | −1.93 (−2.59 to −1.27) | <.001 |
| Compensatory primary control | −0.076 (−0.110 to −0.044) | <.001 |
| Animal Fluency Test | 0.054 (0.019 to 0.090) | .003 |
| Selective primary control | 0.08 (0.010 to 0.018) | .14 |
| Selective secondary control | 0.047 (0.003 to 0.090) | .04 |
| Contrast sensitivity | 0.20 (−0.210 to 0.610) | .33 |
| PHQ-9 severity | −0.032 (−0.100 to 0.035) | .35 |
| Age, y | −0.008 (−0.033 to 0.016) | .51 |
Abbreviations: CI, confidence interval; PHQ-9, Patient Health Questionnaire-9.
COMMENT
We found that patients with AMD vary in their coping strategies and cognitive function and in their visual acuity and that variability in these factors determines patients’ self-report of vision function independent of the effect of visual acuity. The patients we studied were drawn from outpatients of an academic retina vitreous practice, had specific vision characteristics, and had enrolled in a clinical trial to improve vision function. These unique characteristics limit the generalizability of these findings. Nevertheless, the sample represents a large group of patients commonly seen in ophthalmologic practices whose severity of vision loss and disability present a challenge to patients and their ophthalmologists.
The strengths of this study include the large sample size; systematic ascertainment and assessment of patients whose visual, affective, medical, and functional characteristics were evaluated using instruments of known reliability and validity; and the use of latent variable modeling to estimate an interval scale of visual ability based on the NEI-VFQ near-vision subscale. Although previous studies31–33 have demonstrated the validity of the NEI-VFQ in a conventional sense, they used ordinal rather than interval-scaled item responses (ie, categorical responses, where the difference between responses may not be the same, vs numerical values, where the difference between values is the same). Because ordinal responses have uncertain quantitative relationships with each another, there is an increased risk of measurement error.31–33 Our use of an interval-scaled, latent visual variable yields a more precise measure that has enabled us to identify new clinical variables that illuminate patient perceptions of disability. This study’s limitations, however, include lack of measures of central scotomas, glare sensitivity, binocular vision, reading, and other performance-based tests that might better discriminate patients in terms of the direct effects of AMD on ability.
All vision-dependent tasks require a specific level of vision to perform them successfully and independently. A patient’s rating of “difficulty” reflects the difference between the level of required vision and the patient’s visual ability, which depends, these data show, on his or her visual acuity and coping strategies and cognitive function.34 We found that higher use of the coping strategy of compensatory primary control, such as relying on others for help and using optical devices, was associated with greater difficulty with near-vision activities. This intuitively correct association indicates that this coping strategy, which aims to increase a patient’s control over his or her life circumstances, represents a healthy psychological adaptation to vision loss and contributes to what drives his or her perceptions of disability. This finding provides support for ophthalmologists’ recommendations to patients with AMD to pursue low-vision rehabilitative interventions.
A second control strategy that was associated with visual ability was selective secondary control. A higher use of this strategy, which represents the willingness to persevere in the face of potential failure, predicted lower ratings of vision disability. Patients with AMD who use this strategy tend to look forward to the positive consequences of achieving a goal even as they work hard to achieve it. Understandably, they would tend to perceive less difficulty than others who lack the same level of motivation but who are otherwise similar in their vision characteristics. The treatment implication of this finding is that interventions that strengthen the ability to tolerate frustration and keep on trying, similar to cognitive behavior therapies, might reduce disability levels in vulnerable people. Although depression is often related to vision function in patients with vision loss, in this sample, it was not.35–37 The unique characteristics of the sample (ie, patients who enrolled in a clinical trial who had, on average, low levels of depressive symptoms) constrained the scores and limited the ability to detect any significant associations.
Better scores on a cognitive task that assesses verbal fluency were associated with lower perceived vision function difficulties. Greater ability in this cognitive domain indicates better sustained output, concentration, and executive function.38 The latter refers to a group of complex cognitive abilities that include organizing, understanding, and appreciating information and planning, initiating, and monitoring behavior, which, in turn, enables rational problem solving.39 Thus, we might expect that patients with AMD who possess these cognitive skills would find ways to compensate for their vision disabilities and devise strategies to reduce task difficulty. This interpretation agrees with other studies that find that coexisting visual and cognitive impairments are highly disabling and that patients with AMD who relinquish valued activities are at risk for incident dementia.40–43 These studies emphasize the importance of assessing cognition in AMD studies, even in patients without dementia, and encouraging patients to remain active despite vision loss to promote optimal cognitive and physical health.
The introduction of anti-VEGF treatments for AMD has spared many patients from progressive vision loss and severe disability. Although these treatments have expanded rapidly in the community in recent years, we know little of their impact outside of clinical trials.44 The present data suggest that recognizing the role of patient coping strategies and cognition may inform outcome studies of anti-VEGF treatment and may have direct implications for the clinical care of patients. For researchers who use the NEI-VFQ in clinical trials, characterizing patient coping strategies and cognitive function may improve the precision of vision rating scales, reduce measurement error, and suggest new interventions to improve vision function and quality of life. For ophthalmologists in clinical practice, encountering patients whose vision function is worse than expected given their visual acuity should prompt brief assessments of how patients are coping or of their cognition. These assessments might then lead to referrals for neurologic or psychiatric evaluation to identify modifiable factors that may optimize functional vision. For highly motivated patients who use active control strategies, positive reinforcement and referral to low-vision rehabilitation may help them achieve their goals. For patients who more passively accept their disability, sympathetic understanding of their functional limitations and expressions of support may be valuable interpersonal interventions. From the clinical standpoint, these findings highlight the need for evidence-based models to improve care at the interface of ophthalmology and psychiatry and to develop a comprehensive national health care policy to assist older persons with their visual needs.
Acknowledgments
Funding/Support: This work was supported by grant U01 EY 015839 from the NEI and by the Farber Institute for Neurosciences of Thomas Jefferson University.
Footnotes
Financial Disclosure: None reported.
Additional Contributions: The Wills Eye AMD Study Group provided assistance with recruitment of the sample and data collection. Members of this group include William E. Benson, MD; Gary C. Brown, MD; Jay L. Federman, MD; Mitchell S. Fineman, MD; David H. Fischer, MD; Sunir J. Garg, MD; Allen C. Ho, MD; Jason Hsu, MD; Richard S. Kaiser, MD; Alfred C. Lucier, MD; Joseph I. Maguire, MD; J. Arch McNamara, MD (deceased); Carl H. Park, MD; Carl D. Regillo, MD; Lov K. Sarin, MD; Arunan Sivalingam, MD; Marc J. Spirn, MD; and James F. Vander, MD.
References
- 1.Congdon N, O’Colmain B, Klaver CC, et al. Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J Vision Health Cost-Effectiveness Study Group. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127(4):533–540. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld PJ, Rich RM, Lalwani GA. Ranibizumab: phase III clinical trial results. Ophthalmol Clin North Am. 2006;19(3):361–372. doi: 10.1016/j.ohc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355 (14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 6.Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J, Klesert TR MARINA Study Group. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 2007;125(11):1460–1469. doi: 10.1001/archopht.125.11.1460. [DOI] [PubMed] [Google Scholar]
- 7.Bressler SB. Anti-VEGF therapy impact on daily life: what we have learned from quality-of-life research. Ophthalmol Times. 2006;31:10–12. [Google Scholar]
- 8.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 9.Mangione CM, Berry S, Spritzer K, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol. 1998;116(2):227–233. doi: 10.1001/archopht.116.2.227. [DOI] [PubMed] [Google Scholar]
- 10.Massof RW, Fletcher DC. Evaluation of the NEI visual functioning questionnaire as an interval measure of visual ability in low vision. Vision Res. 2001;41(3):397–413. doi: 10.1016/s0042-6989(00)00249-2. [DOI] [PubMed] [Google Scholar]
- 11.Owsley C, McGwin G., Jr Depression and the 25-item National Eye Institute Visual Function Questionnaire in older adults. Ophthalmology. 2004;111(12):2259–2264. doi: 10.1016/j.ophtha.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Miskala PH, Bressler NM, Meinert CL. Relative contributions of reduced vision and general health to NEI-VFQ scores in patients with neovascular age-related macular degeneration. Arch Ophthalmol. 2004;122(5):758–766. doi: 10.1001/archopht.122.5.758. [DOI] [PubMed] [Google Scholar]
- 13.Cuijpers P, van Straten A, Warmerdam L. Problem solving therapies for depression: a meta-analysis. Eur Psychiatry. 2007;22(1):9–15. doi: 10.1016/j.eurpsy.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Borkovec TD, Newman MG, Pincus AL, Lytle R. A component analysis of cognitive-behavioral therapy for generalized anxiety disorder and the role of interpersonal problems. J Consult Clin Psychol. 2002;70(2):288–298. [PubMed] [Google Scholar]
- 15.Reischies FM, Geiselmann B. Age-related cognitive decline and vision impairment affecting the detection of dementia syndrome in old age. Br J Psychiatry. 1997;171:449–451. doi: 10.1192/bjp.171.5.449. [DOI] [PubMed] [Google Scholar]
- 16.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45(2):197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 17.Lawton MP, Moss M, Fulcomer M, Kleban MH. A research and service oriented multilevel assessment instrument. J Gerontol. 1982;37(1):91–99. doi: 10.1093/geronj/37.1.91. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas JA, Ivnik RJ, Smith GE, et al. Mayo’s Older African Americans Normative Studies: norms for Boston Naming Test, Controlled Oral Word Association, Category Fluency, Animal Naming, Token Test, Wrat-3 Reading, Trail Making Test, Stroop Test, and Judgment of Line Orientation. Clin Neuropsychol. 2005;19(2):243–269. doi: 10.1080/13854040590945337. [DOI] [PubMed] [Google Scholar]
- 20.Stelmack JA, Stelmack TR, Massof RW. Measuring low-vision rehabilitation outcomes with the NEI VFQ-25. Invest Ophthalmol Vis Sci. 2002;43(9):2859–2868. [PubMed] [Google Scholar]
- 21.Ryan B, Court H, Margrain TH. Measuring low vision service outcomes: Rasch analysis of the seven-item National Eye Institute Visual Function Questionnaire. Optom Vis Sci. 2008;85(2):112–121. doi: 10.1097/OPX.0b013e31816225dc. [DOI] [PubMed] [Google Scholar]
- 22.Marella M, Pesudovs K, Keeffe JE, O’Connor PM, Rees G, Lamoureux EL. The psychometric validity of the NEI VFQ-25 for use in a low-vision population. Invest Ophthalmol Vis Sci. 2010;51(6):2878–2884. doi: 10.1167/iovs.09-4494. [DOI] [PubMed] [Google Scholar]
- 23.Pesudovs K, Gothwal VK, Wright T, Lamoureux EL. Remediating serious flaws in the National Eye Institute Visual Function Questionnaire. J Cataract Refract Surg. 2010;36(5):718–732. doi: 10.1016/j.jcrs.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Heckhausen J, Wrosch C, Schulz R. A motivational theory of life-span development. Psychol Rev. 2010;117(1):32–60. doi: 10.1037/a0017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahl HW, Schilling O, Becker S. Age-related macular degeneration and change in psychological control: role of time since diagnosis and functional ability. J Gerontol B Psychol Sci Soc Sci. 2007;62(2):90–97. doi: 10.1093/geronb/62.2.p90. [DOI] [PubMed] [Google Scholar]
- 26.Brennan M, Boerner K, Reinhardt JP, Horowitz A. Applying the life-span theory of control in adjustment to chronic illness: the development of the Vision-Specific OPS Scale. Poster presented at: Annual Meeting of the Gerontological Society of America; November 2004; Washington, DC.. [Google Scholar]
- 27.Moustaki I. A general class of latent variable models for ordinal manifest variables with covariate effects on the manifest and latent variables. Br J Math Stat Psychol. 2003;56(pt 2):337–357. doi: 10.1348/000711003770480075. [DOI] [PubMed] [Google Scholar]
- 28.Skrondal A, Rabe-Hesketh S. Multilevel, Longitudinal and Structural Equation Models. Boca Raton, FL: Chapman Hall/CRC; 2004. Generalized Latent Variable Modeling. [Google Scholar]
- 29.Muthén LK, Muthén BO. Mplus User’s Guide. 6. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- 30.Rees G, Tee HW, Marella M, Fenwick E, Dirani M, Lamoureux EL. Vision-specific distress and depressive symptoms in people with vision impairment. Invest Ophthalmol Vis Sci. 2010;51(6):2891–2896. doi: 10.1167/iovs.09-5080. [DOI] [PubMed] [Google Scholar]
- 31.Submacular Surgery Trials Research Group. Evaluation of minimum clinically meaningful changes in scores on the National Eye Institute Visual Function Questionnaire (NEI-VFQ): SST report number 19. Ophthalmic Epidemiol. 2007;14 (4):205–215. doi: 10.1080/09286580701502970. [DOI] [PubMed] [Google Scholar]
- 32.Lindblad AS, Clemons TE. Responsiveness of the National Eye Institute Visual Function Questionnaire to progression to advanced age-related macular degeneration, vision loss, and lens opacity: AREDS report No. 14. Arch Ophthalmol. 2005;123(9):1207–1214. doi: 10.1001/archopht.123.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miskala PH, Hawkins BS, Mangione CM, et al. Submacular Surgery Trials Research Group. Responsiveness of the National Eye Institute Visual Function Questionnaire to changes in visual acuity: findings in patients with subfoveal choroidal neovascularization—SST report No. 1. Arch Ophthalmol. 2003;121(4):531–539. doi: 10.1001/archopht.121.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massof RW. A systems model for low vision rehabilitation, II: measurement of vision disabilities. Optom Vis Sci. 1998;75(5):349–373. doi: 10.1097/00006324-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 35.Rovner BW, Casten RJ, Tasman WS. Effect of depression on vision function in age-related macular degeneration. Arch Ophthalmol. 2002;120(8):1041–1044. doi: 10.1001/archopht.120.8.1041. [DOI] [PubMed] [Google Scholar]
- 36.Williams RA, Brody BL, Thomas RG, Kaplan RM, Brown SI. The psychosocial impact of macular degeneration. Arch Ophthalmol. 1998;116(4):514–520. doi: 10.1001/archopht.116.4.514. [DOI] [PubMed] [Google Scholar]
- 37.Horowitz A, Reinhardt JP, Kennedy GJ. Major and subthreshold depression among older adults seeking vision rehabilitation services. Am J Geriatr Psychiatry. 2005;13(3):180–187. doi: 10.1176/appi.ajgp.13.3.180. [DOI] [PubMed] [Google Scholar]
- 38.Weintraub S. Neuropsychological assessment of mental state. In: Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. 2. Oxford, England: Oxford University Press; 2000. pp. 121–173. [Google Scholar]
- 39.Ardila A. On the evolutionary origins of executive functions. Brain Cogn. 2008;68 (1):92–99. doi: 10.1016/j.bandc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Whitson HE, Cousins SW, Burchett BM, Hybels CF, Pieper CF, Cohen HJ. The combined effect of visual impairment and cognitive impairment on disability in older people. J Am Geriatr Soc. 2007;55(6):885–891. doi: 10.1111/j.1532-5415.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 41.Baker ML, Wang JJ, Rogers S, et al. Early age-related macular degeneration, cognitive function, and dementia: the Cardiovascular Health Study. Arch Ophthalmol. 2009;127(5):667–673. doi: 10.1001/archophthalmol.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rovner BW, Casten RJ, Leiby BE, Tasman WS. Activity loss is associated with cognitive decline in age-related macular degeneration. Alzheimers Dement. 2009;5(1):12–17. doi: 10.1016/j.jalz.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clemons TE, Rankin MW, McBee WL Age-Related Eye Disease Study Research Group. Cognitive impairment in the Age-Related Eye Disease Study: AREDS report No. 16. Arch Ophthalmol. 2006;124(4):537–543. doi: 10.1001/archopht.124.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell RJ, Bronskill SE, Bell CM, Paterson JM, Whitehead M, Gill SS. Rapid expansion of intravitreal drug injection procedures, 2000 to 2008: a population-based analysis. Arch Ophthalmol. 2010;128(3):359–362. doi: 10.1001/archophthalmol.2010.19. [DOI] [PubMed] [Google Scholar]