Abstract
Although numerous risk-prediction models exist in patients presenting with acute coronary syndromes (ACS), they are subject to important short-comings, including lack of contemporary information. Short-term models are frequently biased by in-hospital events. Accordingly, we sought to create contemporary risk-prediction models for clinical outcomes following ACS up to 1 year following discharge. Models were constructed for death at 30 days and 1 year, death/myocardial infarction (MI)/revascularization at 30 days and death/MI at 1 year in consecutive patients presenting with ACS at our institution between 2006 and 2008, and discharged alive. Logistic regression was used to model the 30 day outcomes and Cox proportional hazards were used to model the 1 year outcomes. No linearity assumptions were made for continuous variables. The final model coefficients were used to create a prediction nomogram, which was incorporated into an online risk calculator. A total of 2,681 patients were included, of which about 9.5% presented with ST-elevation MI. All-cause mortality was 2.6% at 30 days and 13% at 1 year. Demographic, past medical history, laboratory, pharmacological and angiographic parameters were identified as being predictive of adverse ischemic outcomes at 30 days and 1 year. The c-indices for these models ranged from 0.73 to 0.82. Our study thus identified risk factors that are predictive of short- and long-term ischemic and revascularization outcomes in contemporary patients with ACS, and incorporated them into an easy-to-use online calculator, with equal or better discriminatory power than currently available models.
Keywords: Mortality, myocardial infarction, predictors, registry, revascularization
Introduction
The management of acute coronary syndromes (ACS), defined as unstable angina, non ST-elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI), has made considerable progress in the past few years. This has been possible due to a number of factors, including procedural advances in coronary reperfusion techniques, a greater proportion of patients receiving timely mechanical reperfusion, newer additions to the pharmaco-invasive armamentarium, and increased adherence to evidence-based therapies, both acutely and at discharge [1-4].
Numerous models have been developed to identify patients at highest risk for short- and intermediate-term adverse outcomes after ACS [5-8]. However, these models have substantial disadvantages, including the lack of information on the use of current pharmacological agents and angiographic severity of disease, which have been shown to be significantly associated with outcomes in these patients [9-12]. Moreover, two of the commonly used models: the Thrombolysis in Myocardial Infarction (TIMI) models for STEMI and NSTE-ACS, and the Platelet glycoprotein IIb/IIIa in Unstable angina: Receptor Suppression Using Integrilin (eptifibatide) Therapy (PURSUIT) model predict short-term mortality (up to 30 days) only; the Global Registry of Acute Coronary Events (GRACE) model predicts 6 month mortality following hospital discharge [5]. Clinical decision models predicting longer-term mortality in these patients are scant. Moreover, short-term models can be heavily influenced by in-hospital mortality [13]. Accordingly, we sought to create prediction models for death, death/ recurrent myocardial infarction, and death/recurrent MI/ repeat revascularization in a contemporary cohort of patients presenting with acute coronary syndromes, who were discharged alive from the hospital. Since predictors of short- and long-term mortality are likely to be different, we constructed models for these 3 outcomes at 2 separate time-points: 30 days and 1 year.
Methods
Data source
Successive patients presenting to the Cleveland Clinic, Cleveland, OH between January 1, 2006 and December 31, 2008 were included. A detailed review of their baseline demographics, past medical history, clinical presentation, laboratory values, medications given, procedures performed, complications and outcomes was conducted using our electronic health record (EHR) system, which includes the eResearch Data Repository, the diagnostic catheterization registry, the percutaneous coronary intervention (PCI) registry, the coronary artery bypass graft (CABG) registry, the nuclear perfusion imaging stress test registry, procedure billing data, and inpatient pharmacy data.
Study population
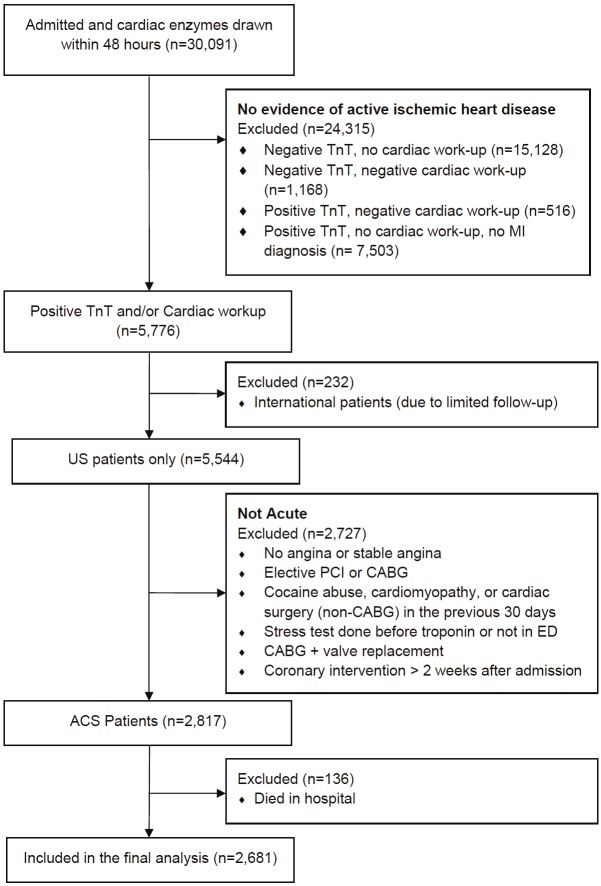
Patients with acute coronary syndromes were identified from the PCI registry and from encounters in the Cleveland Clinic main campus hospital or emergency department (ED). PCI patients undergoing elective procedures were excluded. ED and hospital patients were included only if they underwent serial serum troponin measurements and these values were either elevated, further cardiac testing (e.g. cardiac catheterization or stress nuclear imaging) revealed ischemic heart disease, or they underwent a coronary revascularization procedure during the index admission. We excluded patients undergoing coronary artery bypass graft (CABG) surgery if they also underwent concomitant coronary valve replacement surgery, since the vast majority of these patients appeared to be having elective procedures. Figure 1 demonstrates the CONSORT flow diagram used to identify patients for this study.
Figure 1.
CONSORT flow diagram of study participants. ACS represents acute coronary syndromes; CABG, coronary artery bypass graft surgery; ED, emergency department; MI, myocardial infarction; PCI, percutaneous coronary intervention; TnT, troponin T.
Assessment of outcomes
All outcomes were assessed only in patients discharged alive from the hospital after their index ACS hospitalization. Mortality was assessed from the EHR and from the Social Security Death Index. Reinfarction (or new infarction, if the index event was not acute MI) was defined as a serum troponin T ≥ 0.08mcg/L on any occasion after the index hospitalization. A patient was considered to undergo repeat revascularization (or new revascularization, if the index event did not result in revascularization) if he/she was identified in either the CABG or PCI database or if one of the relevant Current Procedural Terminology (CPT) codes were identified in the EHR. All events except mortality were censored on the date of the patient’s last encounter.
Predictor variables
Table 1 shows the predictor variables that were considered for inclusion in the final models due to their known association with the outcomes of interest. Admission, inpatient and discharge medications were separately considered. The same high sensitivity troponin assay was utilized throughout the duration of the study.
Table 1.
Baseline characteristics of the study population*
| Characteristic | NSTEMI | STEMI | Unstable Angina |
|---|---|---|---|
| (n =1187) | (n = 253) | (n =1,222) | |
| Demographic characteristics | |||
| Age (years) | 69.6 (59.5,78.2) | 62.3 (53.3,71.9) | 65.5 (57.1,75.2) |
| Female | 419 (35.3) | 86 (34) | 402 (32.9) |
| Race | |||
| White | 847 (72.4) | 160 (64) | 969 (80.1) |
| Black | 296 (25.3) | 83 (33.2) | 211 (17.4) |
| Other | 27 (2.3) | 7 (2.8) | 30 (2.5) |
| Body mass index (kg/m2) | 27.2 (23.7,31.4) | 27.3 (24.7,31.1) | 28.1 (24.9,32.7) |
| Past medical history | |||
| Diabetes | 561 (47.3) | 84 (33.2) | 603 (49.3) |
| Peripheral vascular disease | 301 (25.4) | 31 (12.3) | 270 (22.1) |
| History of prior stroke | 241 (20.3) | 20 (7.9) | 258 (21.1) |
| History of prior CHF | 333 (28.1) | 27 (10.7) | 290 (23.7) |
| On lipid medication prior to hospitalization | 875 (73.7) | 214 (84.6) | 1101 (90.1) |
| Chronic kidney disease | 466 (39.3) | 40 (15.8) | 336 (27.5) |
| Chronic obstructive lung disease | 260 (21.9) | 36 (14.2) | 217 (17.8) |
| Admission characteristics | |||
| Pulse at presentation (bpm) | 97 (95,98) | 96 (95,98) | 97 (95,98) |
| Serum ALT (IU/L) | 25 (16,42.2) | 30 (20.5,50) | 20 (14,31) |
| HDL cholesterol (mg/dl) | 43 (35,53) | 44 (37,54) | 42 (35,52) |
| Ejection fraction (%) | 50 (35,55) | 45 (35,55) | 55 (45,55) |
| Maximum troponin T (mcg/L) | 0.4 (0.2,1.1) | 3.5 (1.8,7.5) | 0 (0,0) |
| Serum creatinine (mg/dl) | 1.1 (0.9,1.7) | 0.9 (0.8,1.2) | 1 (0.8,1.3) |
| Serum albumin (g/dl) | 3.5 (3,3.9) | 3.6 (3.3,3.9) | 3.9 (3.6,4.2) |
| Serum hematocrit (%) | 33.1 (30,37.4) | 36.2 (33,40.4) | 37.4 (33.1,40.8) |
| Events during hospitalization | |||
| Number of vessels with ≥50% stenoses | |||
| 0 | 76 (10.5) | 21 (8.3) | 155 (12.7) |
| 1 | 341 (47) | 232 (91.7) | 735 (60.3) |
| 2 | 73 (10.1) | 0 (0) | 128 (10.5) |
| 3 | 213 (29.4) | 0 (0) | 174 (14.3) |
| 4 | 22 (3) | 0 (0) | 26 (2.1) |
| Treatment | |||
| CABG | 197 (16.6) | 0 (0) | 92 (7.5) |
| Medical Management | 638 (53.7) | 0 (0) | 386 (31.6) |
| PCI-BMS | 149 (12.6) | 155 (61.3) | 216 (17.7) |
| PCI-DES | 175 (14.7) | 62 (24.5) | 456 (37.3) |
| PCI-Other | 28 (2.4) | 36 (14.2) | 72 (5.9) |
| Number of Vessels Treated | |||
| 0 | 641 (56.9) | 0 (0) | 386 (32.6) |
| 1 | 285 (25.3) | 230 (93.1) | 586 (49.5) |
| 2 | 84 (7.5) | 16 (6.5) | 144 (12.2) |
| 3 | 116 (10.3) | 1 (0.4) | 68 (5.7) |
| 4 | 0 (0) | 0 (0) | 1 (0.1) |
| Stroke during hospitalization | 33 (2.8) | 2 (0.8) | 23 (1.9) |
| New CHF in hospital | 137 (11.5) | 59 (23.3) | 60 (4.9) |
| Discharged to home | 967 (81.5) | 241 (95.3) | 1187 (97.1) |
| Medication utilization | |||
| Beta blocker in hospital | 1002 (84.4) | 213 (84.2) | 1091 (89.3) |
| Gp IIb/IIIa inhibitor for PCI | 255 (23.7) | 158 (86.8) | 135 (12.8) |
| Clopidogrel pre-procedure | 481 (44.2) | 248 (99.2) | 965 (81) |
| Clopidogrel at discharge | 753 (63.4) | 250 (98.8) | 1037 (84.9) |
There were 19 patients that had missing ACS type prior to imputation.
Values represent numbers (%) for dichotomous/categorical variables, and median (interquartile range) for continuous variables. ALT represents alanine aminotransferase; BMS, bare-metal stent; bpm, beats per minute; CABG, coronary artery bypass graft surgery; CHF, congestive heart failure; DES, drug-eluting stent; Gp, glycoprotein; HDL, high density lipoprotein; PCI, percutaneous coronary intervention.
Statistical analysis
Prediction models were constructed for death at 30 days and 1 year, death/recurrent MI/revascularization at 30 days, and death/recurrent MI at 1 year. As no censoring was done prior to 30 days, logistic regression was used to model the 30 day outcomes. Multivariate Cox proportional hazards were used to model the 1 year outcomes. For each of the Cox models besides mortality, patients were followed until the time of event or until the date of the last contact in our EHR. Time zero was the time of discharge from the hospital. Variable selection was performed using the “stepdown” method suggested by Harrell [14], and the models were reduced until achieving the best model discrimination according to the bias-corrected c-statistic using tenfold cross-validation. A calibration curve was created by plotting the quintiles of the predicted probabilities on the observed estimates for the entire cohort. The final model coefficients were used to create a Web-based risk calculator that calculated the predictions automatically (prediction nomogram), and is available free of charge at http://rcalc.ccf.org.
Missing values were imputed using Multiple Imputation by Chained Equations package, version 2.3, for R. (Van Burren, 2010) Imputation was performed to maximize the available information and to reduce the potential bias introduced by deleting incomplete records. Imputation was performed using regression techniques that include all baseline patients and all baseline variables as predictors, including the outcome [15]. All statistical analyses were performed using SAS software (version 9.1, SAS Institute, Cary, North Carolina) and R version 2.10. All p- values were two-tailed, with statistical significance set at 0.05. All confidence intervals (CI) were calculated at the 95 percent level.
Results
A total of 2,681 patients met our study criteria (44.6% with unstable angina, 45.9% with NSTEMI and 9.5% with STEMI on presentation). Baseline characteristics of the study population are listed in Table 1. About 51% of the patients received PCI, while 38.2% were medically managed, and 10.8% underwent CABG. Overall in-hospital mortality was 4.8%. Of all the patients discharged alive, 90% were discharged to home.
30-day mortality
Of the patients discharged alive from the hospital, a total of 69 deaths (2.6%) occurred within 30 days. The nomogram for 30-day mortality is demonstrated in Figure 2. Significant risk factors of 30-day mortality included medical management, male gender, history of CKD, lack of use of a glycoprotein IIb/IIIa inhibitor in the hospital in patients undergoing PCI, lack of discharge on clopidogrel, older age, lower serum alanine transaminase (ALT) at admission, lower high density lipoprotein (HDL) cholesterol at admission, higher maximal troponin T, lower serum albumin at admission, and discharge to facility other than home; BMI and hematocrit demonstrated a U-shaped relationship. The c-index was 0.82.
Figure 2.
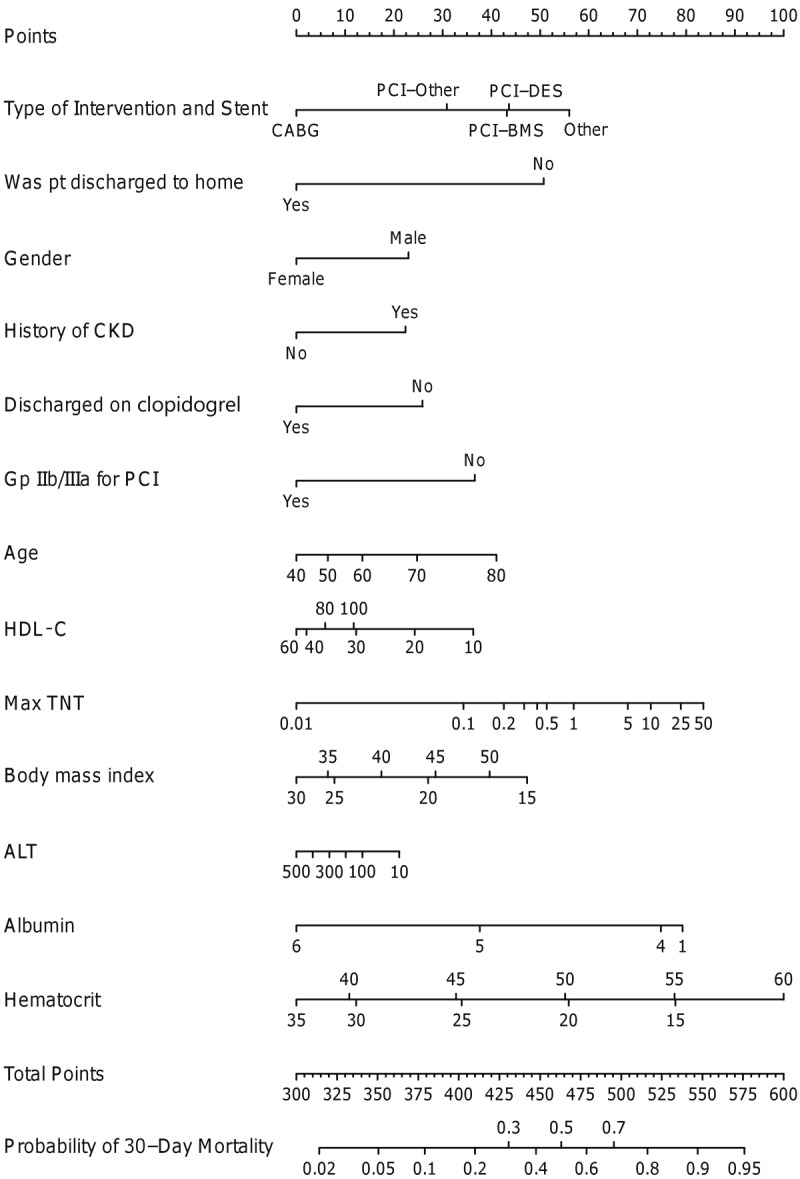
Nomogram for 30-day mortality. ALT represents alanine aminotransferase; BMS, bare-metal stents; CABG, coronary artery bypass graft surgery; CKD, chronic kidney disease; DES, drug-eluting stent; Gp, glycoprotein; HDL, high density lipoprotein; PCI, percutaneous coronary intervention; TNT, troponin T.
1-year mortality
Of the patients discharged alive from the hospital, a total of 348 deaths (13.0%) were noted within 1 year. The nomogram for 1-year mortality is demonstrated in Figure 3. Significant risk factors for 1-year mortality included presentation with NSTEMI/unstable angina (as compared with STEMI), medical management, multi-vessel revascularization, male gender, history of stroke, stroke during hospitalization, history of CHF, new onset CHF during hospitalization, history of CKD, history of COPD, absence of the use of beta-blocker in the hospital and lipid lowering medications and clopidogrel at discharge, older age, lower ejection fraction, higher troponin T, lower albumin at admission, lower hematocrit on admission and discharge to a facility other than home. BMI and pulse on presentation demonstrated a U-shaped relationship, with very low and high values being associated with a higher risk of mortality. The c-index was 0.80.
Figure 3.
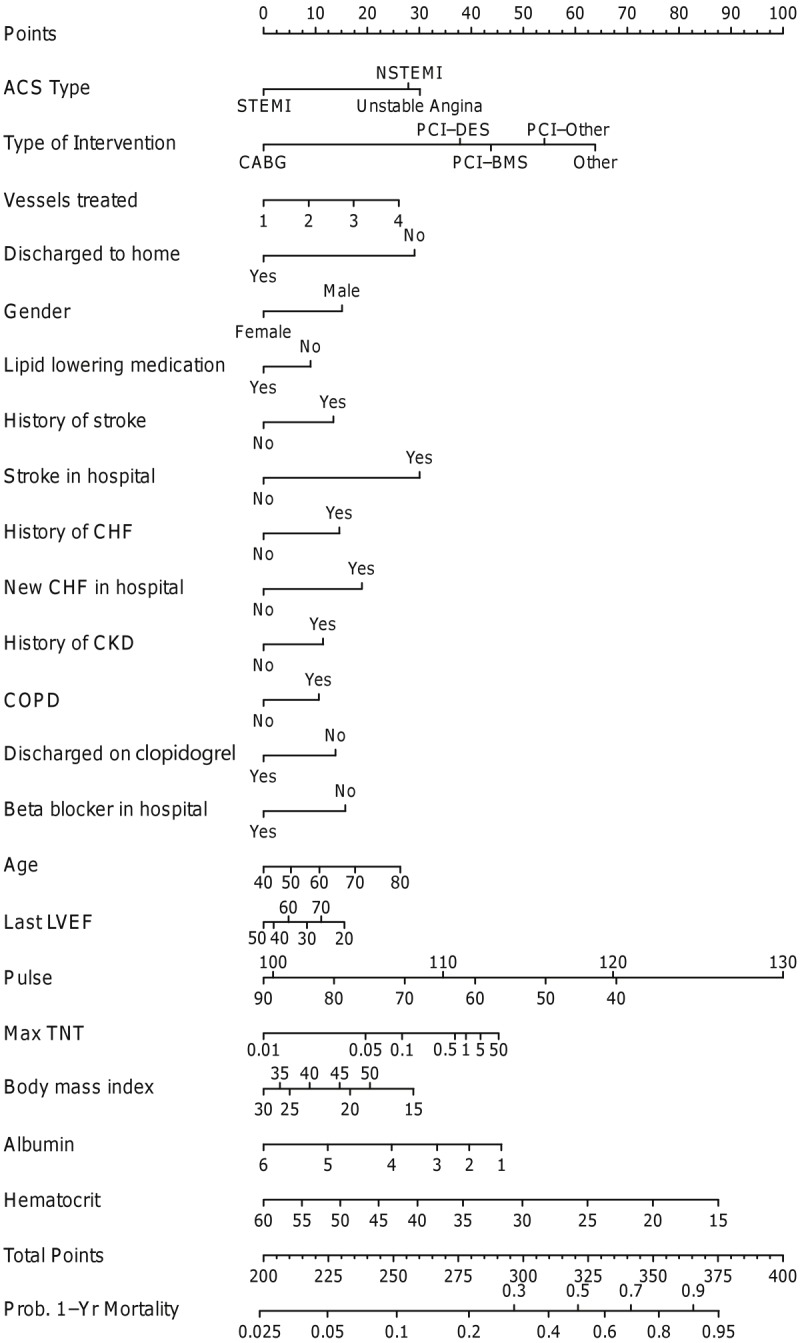
Nomogram for 1-year mortality. ACS represents acute coronary syndrome; BMS, bare-metal stents; CABG, coronary artery bypass graft surgery; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DES, drug-eluting stent; LVEF, left ventricular ejection fraction; NSTEMI, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; TNT, troponin T.
30-day composite of mortality/recurrent MI/revascularization
Of the patients discharged alive from the hospital, a total of 326 composite events (13.8%) were noted within 30 days. The nomogram for 30-day events is demonstrated in Figure 4. Significant risk factors included medical management, smoking, diabetes mellitus, presence of peripheral vascular disease, stroke during hospitalization, lower ejection fraction, higher serum low density lipoprotein (LDL) cholesterol, higher maximum troponin T, higher serum creatinine, lower ALT, higher pulse rate on admission, no clopidogrel loading in patients undergoing PCI, absence of use of angiotensin converting enzyme inhibiting agents or angiotensin receptor blocking agents in-hospital/at discharge, and discharge to a facility other than home. The c-index was 0.73.
Figure 4.
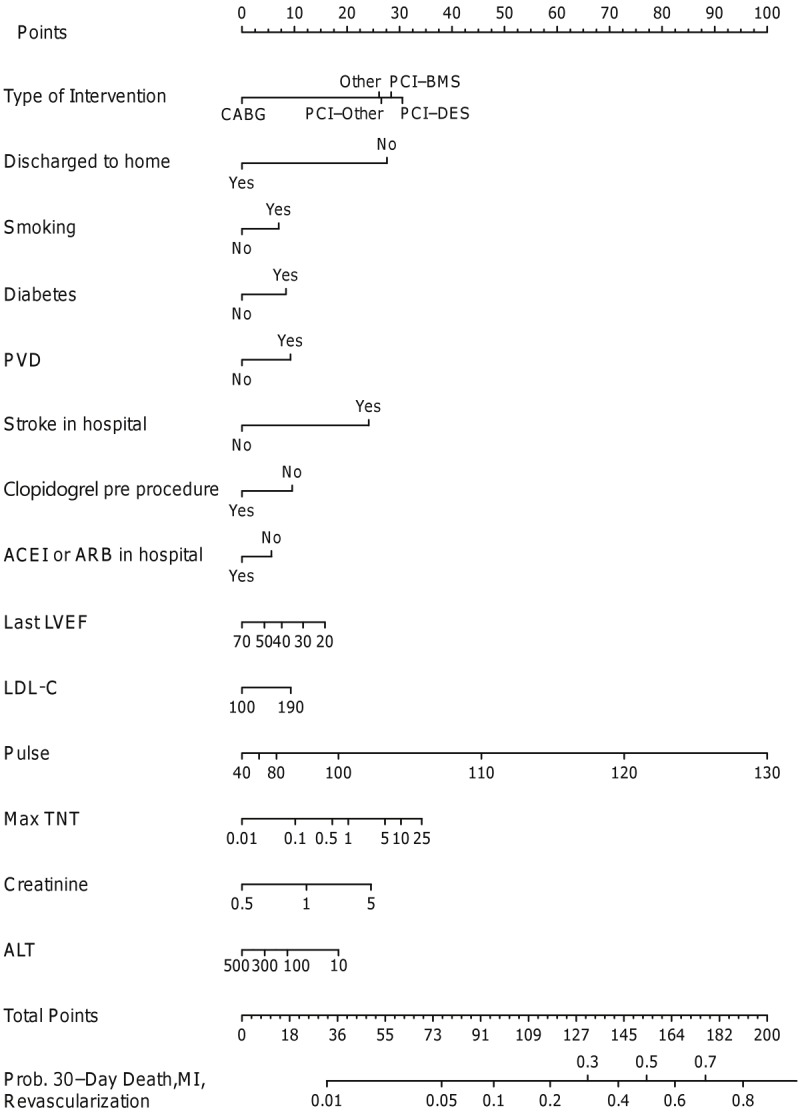
Nomogram for 30-day death/repeat myocardial infarction/revascularization. ACEI represents angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker, ALT, alanine aminotransferase; BMS, bare-metal stents; CABG, coronary artery bypass graft surgery; DES, drug-eluting stent; LDL-C, low density lipoprotein cholesterol, LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; TNT, troponin T.
1-year composite of mortality/recurrent MI
Of the patients discharged alive from the hospital, a total of 723 composite events (30.1%) were noted within 1 year. The nomogram for 1-year events is demonstrated in Figure 5. Significant risk factors included presentation with NSTEMI/unstable angina (as compared with STEMI), medical management, multivessel disease, African American race, smoking, diabetes mellitus, history of stroke, history of CHF, history of CKD, history of COPD, absence of the use of beta-blocker in the hospital and lipid lowering medications, lower ejection fraction, higher maximum troponin T, higher serum creatinine at admission, higher serum LDL cholesterol at admission, lower serum albumin at admission, lower serum hematocrit at admission, and discharge to a facility other than home. BMI on presentation seemed to have a U-shaped relationship, with very low and high values being associated with a higher risk of mortality/re-infarction. The c-index was 0.74.
Figure 5.
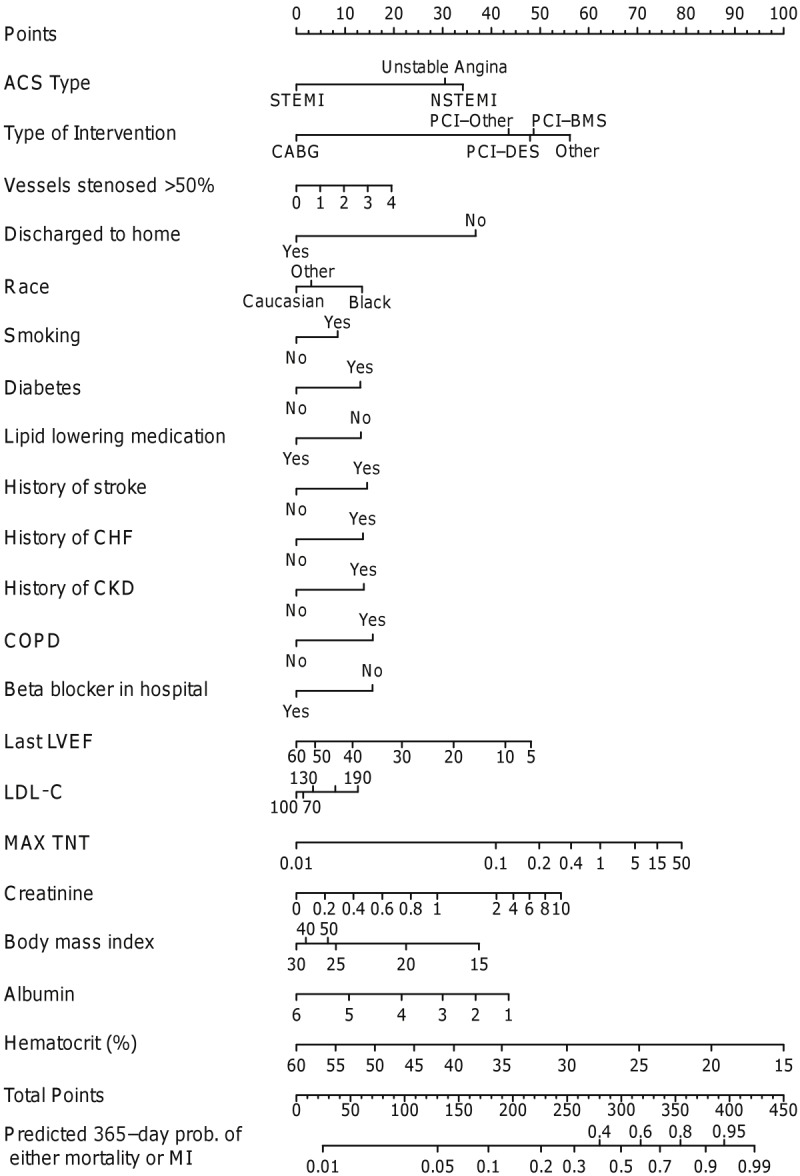
Nomogram for 1-year death/repeat myocardial infarction. ACS represents acute coronary syndrome; ALT, alanine aminotransferase; BMS, bare-metal stents; CABG, coronary artery bypass graft surgery; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DES, drug-eluting stent; LDL-C, low density lipoprotein cholesterol, LVEF, left ventricular ejection fraction; NSTEMI, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; TNT, troponin T.
Discussion
In our analysis of a contemporary population of 2,681 presenting with entire spectrum of ACS to a large quarternary care institution, we confirm the prognostic importance of several baseline characteristics reported from previous models developed from other databases [5-8,16]. In addition, we report on the prognostic significance of several pharmacological and angiographic characteristics, which although routinely encountered in clinical practice, are not incorporated into most of the currently available risk stratification models.
The prognosis of patients presenting with ACS is heterogeneous, with high-risk patients having a significant risk of progression to MI and death. Although risk stratification models such as the TIMI and PURSUIT models are well validated, they are often underutilized and have a number of shortcomings [17]. For one, although some factors such as age, gender, past medical history, and hemodynamic measurements are relatively easy to characterize, others factors such as those based on clinical examination findings (for example, the presence of a summation gallop) or symptoms (for example, angina class) can be quite subjective, and are often difficult to characterize. Continuous variables such as age and pulse rate are also included in most models and are either included as linear (age in 10-year increments, for example) [5], or dichotomous (age ≥ 65 years, for example) [6], although these relationships are not linear [18,19]. Further, the hazard of an adverse ischemic outcome within 30 days after an ACS event is non-linear, with the majority of the risk concentrated in the initial hours and days following presentation [13]. Thus, by including in-hospital events, the majority of the currently available 30-day risk prediction models are likely unduly influenced by factors predictive of in-hospital events rather than those after discharge. A number of these limitations were addressed by the GRACE 6 months post-discharge model, and it remains one of the most widely cited ACS risk scores in clinical practice today [5]. Despite this, most of ACS models including GRACE cannot be considered to be totally relevant in today’s ACS population, since they were constructed at least a decade ago. Information on adjunctive pharmacotherapy (example, dual antiplatelet therapy) and angiographic severity were not incorporated into these models. When included, these models only studied a subset of the ACS population (for example, patients undergoing PCI only in the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) sub-analysis [12]). Our current models attempts to address all the above issues. We only included variables that can be reliably measured and reproduced. We also made no assumptions regarding the linearity of the associations between continuous variables and outcomes. We think that this significantly improves the prognostic utility of our models over other existing models. As is evident from the nomograms, none of the continuous predictors tested including age, BMI, pulse, hematocrit, LDL/HDL cholesterol, creatinine, troponin or LVEF had a true linear relationship with the endpoints; BMI, pulse and hematocrit in fact demonstrated a U-shaped relationship. We also did not attempt to reduce the model using variable selection techniques (example, stepwise regression) because the omission of insignificant predictors tends to harm predictive accuracy [14]. This is evidenced by the fact that the c-indices in our models are at least as high as or higher than those reported for currently available models, especially for composite endpoints [19]. Further, by excluding peri-procedural and in-hospital events, our 30-day and 1 year models are likely predictive of only those factors that influence adverse outcomes in patients being discharged alive. This is thus more likely to be meaningful to physicians taking care of these patients after an ACS hospitalization. Finally, we included data on the angiographic severity of coronary artery disease, and also on pharmacological agents utilized during the index admission and at discharge in all patients, including in patients who were medically managed only. We believe that our model is thus very contemporary, and highly representative of “all-comers” ACS patients.
Several of the identified predictors deserve special comment. Firstly, we note that 1-year death and death/ MI are both higher in patients presenting with NSTEMI or unstable angina versus those presenting with STEMI. Earlier analyses have suggested similar 1-year ischemic outcomes in patients presenting with STEMI or NSTEMI [20]. Since we included patients discharged alive only, this is likely a reflection that STEMI patients have higher “up-front” mortality (not captured in this analysis), while NSTE-ACS patients may be at higher risk for recurrent ischemic events due to “non-completion” of the event. They are also more likely to be older with more advanced and complex CAD, and less likely to be completely revascularized and receive evidence-based secondary prevention therapies at discharge [21-23]. Secondly, our models suggest that in patients undergoing PCI, lack of pretreatment with clopidogrel and lack of use of glycoprotein IIb/IIIa inhibitors are detrimental, which corroborates with current literature [10,24]. Similarly, we confirm the prognostic significance of the use of evidence-based therapies such as beta-blockers and clopidogrel at discharge on ischemic outcomes in these patients [25]. Improving adherence to these measures is critically important. Quality improvement national initiatives such as the American Heart Association’s Get With the Guidelines program and the American College of Cardiology’s Guidelines Applied in Practice (GAP) program have been launched to improve adherence in patients with CAD, and have resulted in significant improvements [26-28]. However certain subgroups remain vulnerable [17,28]. Thirdly, the type of management was associated with each of the 4 models, with medically managed patients having the highest risk of adverse outcomes and patients undergoing CABG the lowest. Although the distribution of patients undergoing various management strategies is similar to that reported from large national registries and clinical trials [29,30], the choice of management strategy was not randomized, and could be influenced by patient characteristics [31]. A propensity matched analysis might thus be more instructive. Nonetheless, our findings support data from randomized controlled trials that suggest that patients with NSTE-ACS who are medically managed have worse outcomes when compared with those who undergo an invasive strategy [4]. As far as the comparison between CABG and PCI is concerned, the completeness of revascularization with CABG over PCI may play a role, especially in reducing the need for repeat revascularization [32]; factors influencing perceived suitability for CABG may also independently influence outcome.
Limitations of our study include the relatively modest sample size, and that this represents a single quaternary referral center experience. Data on pharmacological agents used was abstracted from inpatient and outpatient pharmacy data. Data on outcomes such as reinfarction and revascularization were determined from the electronic medical records using standard definitions. However, the definition of reinfarction could suffer from some misclassification bias due to the sole reliance on troponin T to define recurrence, without the inclusion of electrocardiographic changes. Re-infarction and revascularization procedures occurring outside our institution were not captured in the current analysis. However, the event rates noted in our study are comparable to those reported by other investigators [33]. Finally, we are unable to directly compare our results with the GRACE score due to non-capture of EKG variables. Validation of the currently identified predictors in other ACS registries is necessary to determine the generalizability of our findings.
Conclusions
In our analysis of a contemporary cohort of patients presenting with ACS, we identified a number of demographic, past medical history, laboratory, pharmacological and angiographic parameters that are associated with adverse ischemic outcomes at 30 days and 1 year in patients discharged alive from the hospital. These models have comparative or superior discriminatory power as currently available models. Our easy-to-use online nomogram allows for accurate risk characterization of these patients at discharge. Future studies are necessary to validate our findings in other ACS registries.
Disclosures
D.J. Kumbhani: Honoraria: American College of Cardiology, SomahLutions. B.J. Wells: None A.M. Lincoff: research grants (to the institution) from AstraZeneca, Atherogenics, Bristol-Myers Squibb, Edwards Lifesciences, Esperion, Johnson & Johnson, Kai Pharmaceuticals, Lilly, Medicines Company, Medtronic, Novartis, Novo Nordisk, Pfizer, Roche, Sankyo, Sanofi-Aventis, Schering-Plough, Scios, Takeda, and VasoGenix. A. Jain: None. S. Arrigain: None. C. Yu: None. M. Goormastic: None. S.G. Ellis: None. E. Blackstone: None. M.W. Kattan: None.
Funding sources
This study was funded by AstraZeneca. The sponsor was not involved in the analysis or interpretation of data or the preparation of the manuscript.
Supporting Information
References
- 1.Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA Jr, Granger CB, Flather MD, Budaj A, Quill A, Gore JM. Decline in rates of death and heart failure in acute coronary syndromes, 1999-2006. JAMA. 2007;297:1892–1900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 2.Venkitachalam L, Kip KE, Selzer F, Wilensky RL, Slater J, Mulukutla SR, Marroquin OC, Block PC, Williams DO, Kelsey SF. Twenty-year evolution of percutaneous coronary intervention and its impact on clinical outcomes: a report from the National Heart, Lung, and Blood Institute-sponsored, multicenter 1985-1986 PTCA and 1997-2006 Dynamic Registries. Circ Cardiovasc Interv. 2009;2:6–13. doi: 10.1161/CIRCINTERVENTIONS.108.825323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 4.Bavry AA, Kumbhani DJ, Quiroz R, Ramchandani SR, Kenchaiah S, Antman EM. Invasive therapy along with glycoprotein IIb/IIIa inhibitors and intracoronary stents improves survival in non-ST-segment elevation acute coronary syndromes: a meta-analysis and review of the literature. Am J Cardiol. 2004;93:830–835. doi: 10.1016/j.amjcard.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month post-discharge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 6.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 7.Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, Akkerhuis KM, Harrington RA, Deckers JW, Armstrong PW, Lincoff AM, Califf RM, Topol EJ, Simoons ML. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation. 2000;101:2557–2567. doi: 10.1161/01.cir.101.22.2557. [DOI] [PubMed] [Google Scholar]
- 8.Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, Giugliano RP, McCabe CH, Braunwald E. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031–2037. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 9.Helton TJ, Bavry AA, Kumbhani DJ, Duggal S, Roukoz H, Bhatt DL. Incremental effect of clopidogrel on important outcomes in patients with cardiovascular disease: a meta-analysis of randomized trials. Am J Cardiovasc Drugs. 2007;7:289–297. doi: 10.2165/00129784-200707040-00006. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Topol EJ. Current role of platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes. JAMA. 2000;284:1549–1558. doi: 10.1001/jama.284.12.1549. [DOI] [PubMed] [Google Scholar]
- 11.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW. Percutaneous coronary intervention versus coronaryartery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 12.Lansky AJ, Goto K, Cristea E, Fahy M, Parise H, Feit F, Ohman EM, White HD, Alexander KP, Bertrand ME, Desmet W, Hamon M, Mehran R, Moses J, Leon M, Stone GW. Clinical and angiographic predictors of short- and long-term ischemic events in acute coronary syndromes: results from the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial. Circ Cardiovasc Interv. 2010;3:308–316. doi: 10.1161/CIRCINTERVENTIONS.109.887604. [DOI] [PubMed] [Google Scholar]
- 13.Krumholz HM, Wang Y, Chen J, Drye EE, Spertus JA, Ross JS, Curtis JP, Nallamothu BK, Lichtman JH, Havranek EP, Masoudi FA, Radford MJ, Han LF, Rapp MT, Straube BM, Normand SL. Reduction in acute myocardial infarction mortality in the United States: risk-standardized mortality rates from 1995-2006. JAMA. 2009;302:767–773. doi: 10.1001/jama.2009.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Moons KG, Donders RA, Stijnen T, Harrell FE Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59:1092–1101. doi: 10.1016/j.jclinepi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Kumbhani DJ, Shishehbor MH, Willis JM, Karim S, Singh D, Bavry AA, Zishiri E, Ellis SG, Menon V. Influence of Gender on Long-Term Mortality in Patients Presenting With Non-ST-Elevation Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention. Am J Cardiol. 2012;109:1087–1091. doi: 10.1016/j.amjcard.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 17.Motivala AA, Cannon CP, Srinivas VS, Dai D, Hernandez AF, Peterson ED, Bhatt DL, Fonarow GC. Changes in myocardial infarction guideline adherence as a function of patient risk: an end to paradoxical care? J Am Coll Cardiol. 2011;58:1760–1765. doi: 10.1016/j.jacc.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 18.Lee KL, Woodlief LH, Topol EJ, Weaver WD, Betriu A, Col J, Simoons M, Aylward P, Van de Werf F, Califf RM. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation. 1995;91:1659–1668. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 19.Ohman EM, Granger CB, Harrington RA, Lee KL. Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284:876–878. doi: 10.1001/jama.284.7.876. [DOI] [PubMed] [Google Scholar]
- 20.Montalescot G, Dallongeville J, Van Belle E, Rouanet S, Baulac C, Degrandsart A, Vicaut E. STEMI and NSTEMI: are they so different? 1 year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry) Eur Heart J. 2007;28:1409–1417. doi: 10.1093/eurheartj/ehm031. [DOI] [PubMed] [Google Scholar]
- 21.Polonski L, Gasior M, Gierlotka M, Osadnik T, Kalarus Z, Trusz-Gluza M, Zembala M, Wilczek K, Lekston A, Zdrojewski T, Tendera M. A comparison of ST elevation versus non-ST elevation myocardial infarction outcomes in a large registry database: are non-ST myocardial infarctions associated with worse long-term prognoses? Int J Cardiol. 2011;152:70–77. doi: 10.1016/j.ijcard.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Carruthers KF, Dabbous OH, Flather MD, Starkey I, Jacob A, Macleod D, Fox KA. Contemporary management of acute coronary syndromes: does the practice match the evidence? The global registry of acute coronary events (GRACE) Heart. 2005;91:290–298. doi: 10.1136/hrt.2003.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124:40–47. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabatine MS, Cannon CP, Gibson CM, Lopez-Sendon JL, Montalescot G, Theroux P, Lewis BS, Murphy SA, McCabe CH, Braunwald E. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA. 2005;294:1224–1232. doi: 10.1001/jama.294.10.1224. [DOI] [PubMed] [Google Scholar]
- 25.Lahoud R, Howe M, Krishnan SM, Zacharias S, Jackson EA. Effect of use of combination evidence-based medical therapy after acute coronary syndromes on long-term outcomes. Am J Cardiol. 2012;109:159–164. doi: 10.1016/j.amjcard.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 26.LaBresh KA, Gliklich R, Liljestrand J, Peto R, Ellrodt AG. Using “get with the guidelines” to improve cardiovascular secondary prevention. Jt Comm J Qual Saf. 2003;29:539–550. doi: 10.1016/s1549-3741(03)29064-x. [DOI] [PubMed] [Google Scholar]
- 27.Mehta RH, Montoye CK, Gallogly M, Baker P, Blount A, Faul J, Roychoudhury C, Borzak S, Fox S, Franklin M, Freundl M, Kline-Rogers E, LaLonde T, Orza M, Parrish R, Satwicz M, Smith MJ, Sobotka P, Winston S, Riba AA, Eagle KA. Improving quality of care for acute myocardial infarction: The Guidelines Applied in Practice (GAP) Initiative. JAMA. 2002;287:1269–1276. doi: 10.1001/jama.287.10.1269. [DOI] [PubMed] [Google Scholar]
- 28.Kumbhani DJ, Fonarow GC, Cannon CP, Hernandez AF, Peterson ED, Peacock WF, Laskey WK, Pan W, Schwamm LH, Bhatt DL Get With the Guidelines Steering Committee Investigators. Predictors of Adherence to Performance Measures in Patients with Acute Myocardial Infarction. Am J Med. 2013;126:74e1–9. doi: 10.1016/j.amjmed.2012.02.025. Epub 2012 Aug 24. [DOI] [PubMed] [Google Scholar]
- 29.Levine GN, Lincoff AM, Ferguson JJ 3rd, Mahaffey KW, Goodman SG, Cannon CP, Theroux P, Fox KA. Utilization of catheterization and revascularization procedures in patients with non-ST segment elevation acute coronary syndrome over the last decade. Catheter Cardiovasc Interv. 2005;66:149–157. doi: 10.1002/ccd.20469. [DOI] [PubMed] [Google Scholar]
- 30.Gogo PB Jr, Dauerman HL, Mulgund J, Ohman EM, Patel MR, Cohen DJ, Saucedo JF, Harrington RA, Gibler WB, Smith SC Jr, Peterson ED, Roe MT. Changes in patterns of coronary revascularization strategies for patients with acute coronary syndromes (from the CRUSADE Quality Improvement Initiative) Am J Cardiol. 2007;99:1222–1226. doi: 10.1016/j.amjcard.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 31.Hiratzka LF, Eagle KA, Liang L, Fonarow GC, LaBresh KA, Peterson ED. Atherosclerosis secondary prevention performance measures after coronary bypass graft surgery compared with percutaneous catheter intervention and nonintervention patients in the Get With the Guidelines database. Circulation. 2007;116:I207–212. doi: 10.1161/CIRCULATIONAHA.106.681247. [DOI] [PubMed] [Google Scholar]
- 32.Weintraub WS, Grau-Sepulveda MV, Weiss JM, O’Brien SM, Peterson ED, Kolm P, Zhang Z, Klein LW, Shaw RE, McKay C, Ritzenthaler LL, Popma JJ, Messenger JC, Shahian DM, Grover FL, Mayer JE, Shewan CM, Garratt KN, Moussa ID, Dangas GD, Edwards FH. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–1476. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen M, Stinnett SS, Weatherley BD, Gurfinkel EP, Fromell GJ, Goodman SG, Fox KA, Califf RM. Predictors of recurrent ischemic events and death in unstable coronary artery disease after treatment with combination antithrombotic therapy. Am Heart J. 2000;139:962–970. doi: 10.1067/mhj.2000.106915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.