Abstract
It is now well established that inhibitory interneurons of the cerebral cortex display large diversity, but where each subclass originates and how they acquire final position and physiological characteristics is only begin to be elucidated. Recent studies indicate that the phenotypes of many forebrain interneurons are specified in the ganglionic eminence (GE) at the time of their origin. However, developmental history of cannabinoid type 1 receptor (CB1) positive (+) interneurons is not known. Here, we focus on the origin and migratory routs of prospective CB1/cholecystokinin (CCK)+ and CB1/reelin/calretinin+ γ-aminobutyric acid (GABA)-ergic hippocampal interneurons. We have used variety of markers and a combination of methods, including immunocytochemistry at light and electron microscopic level, and in utero electroporation, to identify a subpopulation of CB1+ cells at the time of their origin in the caudal GE and pallial–subpallial boundary at the 11th–12th embryonic days. We have followed their migration, first radially to the marginal zone, then tangentially in the lateral-to-medial direction within the dorsal telencephalon, before they reach their final destination in the hippocampus proper and the dentate gyrus where they differentiate into CB1/CCK+ or CB1/reelin/calretinin+ GABAergic interneurons. Thus, the specific subclasses of CB1+ inhibitory interneurons, similar to the projection neurons, are determined at the time and place of last cell division and follow their own complex migratory pattern to the final positions.
Keywords: brain development, cholecystokinin, ganglionic eminence, hippocampus, neuronal migration
Introduction
It has recently become evident that basic phenotypes as well as the prospective laminar and area positions of major interneuronal types, similar to the projection neurons, may be determined at the time of their last cell division at distinct sites in the proliferative zones. However the details for the specific subclasses are still missing. It is generally agreed that the interneurons, including those of the hippocampal formation, are generated before projection neurons (Lubbers et al. 1985; Soriano et al. 1986) and are imported from the ganglionic eminence (GE) of the ventral telencephalon (Anderson et al. 1997; Lavdas et al. 1999; Wichterle et al. 1999; Nery et al. 2002; Ang et al. 2003; Lopez-Bendito et al. 2004). However, it is unknown whether different subclasses of hippocampal interneurons have a distinct time and place of their origin. The present study is focused on the origin and fate of a small but distinct and functionally significant (Freund et al. 2003; Piomelli 2003) subclass of γ-aminobutyric acid (GABA)-ergic cannabinoid type 1 receptor (CB1)+ hippocampal interneurons.
Although, CB1 has been identified in glutamatergic neurons (Berrendero et al. 1998; Marsicano and Lutz 1999; Fernandez-Ruiz et al. 2000; Kawamura et al. 2006; Katona et al. 2006; Monory et al. 2006; Yoshida et al. 2006), accumulation of CB1 in the cell bodies and proximal dendrites was found only in a subclass of GABAergic interneurons, which also contain neuropeptide CCK (Katona et al. 1999; Morozov and Freund 2003b). Some CB1/cholecystokinin (CCK)+ interneurons are transiently stationed, and even synaptically interconnected, in the stratum moleculare of the developing hippocampus before they translocate their somas across the full width of the dentate gyrus and settle in the hilus (Morozov et al. 2006). In this final position, they acquire connectivity into the synaptic network and the morphologic characteristics of typical basket interneurons (Freund and Buzsaki 1996). In the present study, we used immunocytochemical labeling at the light and electron microscopic level as well as in vivo electroporation of fluorescent DNA plasmid to trace the origin and developmental history of hippocampal CB1+ cells in the embryonic and early postnatal rodent brain. Our findings of early commitment of CB1+ hippocampal interneurons, identification of the site of their origin, description of their complex migratory routes and morphogenetic transformations have relevance for understanding their function and the potential effects of cannabinoids on prenatal brain development.
Materials and Methods
All animal protocols were approved by the Institutional Animal Care and Use Committee and comply with the National Institutes of Health guidelines for animal care and use. For terminal surgery, the animals were deeply anaesthetized with pentobarbital (0.03 mL/10 g of body weight).
Immunocytochemistry for Light and Electron Microscopy
Timed-pregnant C57BL mice at embryonic day 11.0 (E11.0) (n = 7 embryos from 3 litters), E11.5 (n = 11 embryos from 5 litters), E12.5 (n = 17 embryos from 6 litters), E13.5 (n = 16 embryos from 6 litters), E15.5 (n = 18 embryos from 5 litters), and E18.5 (n = 15 embryos from 6 litters) were decapitated and the embryo brains were removed and immersed overnight in a fixative containing 4% paraformaldehyde, 0.2% picric acid, and 0.2% glutaraldehyde. Postnatal Sprague–Dawley rats (postnatal day 2 [P2], n = 3; P4, n = 3; P8, n = 3; P12, n = 3; and P20, n = 3) and postnatal P5 (n = 2) and young adult C57BL mice (P30, n = 2) were perfused transcardially by the same fixative. Vibratome 100-μm-thick or 60-μm-thick (for embryos or postnatal animals, respectively) coronal sections were used for pre-embedding immunocytochemistry and evaluated in light photomicroscope Axioplan 2 (Zeiss, Jena, Germany) or further prepared for transmission electron microscopy as previously described (Morozov and Freund 2003a, 2003b; Morozov et al. 2006). Specificity of the method was tested by omitting the primary antibody from the staining protocol. No staining was observed in negative control sections.
The polyclonal anti-CB1 antibody was raised against the last 31 amino acids (C-terminus) of mouse CB1 (made in guinea pig; Frontier Science, Japan), the last 15 amino acids (C-terminus) or first 77 amino acids (NH-terminus) of rat CB1 (both made in rabbit; gifts from K. Mackie, University of Washington, WA) and was used at 1:1000, 1:2000, and 1:4000, respectively. The monoclonal mouse anti-reelin antiserum (CR-50; gift from M. Ogawa, Kochi Medical School, Japan) was used at a dilution of 1:2000. The following primary antibodies were also used: monoclonal mouse anti-CCK (CURE Gastroenteric Biology Center, Los Angeles, CA; 1:5000) or anti-Tuj1 (Covance, Berkeley, CA; 1:1000), rabbit anti-calretinin (Swant, Switzerland; 1:5000), rabbit anti-GABA (Sigma, St Louis, MO; 1:5000), rabbit anti-Tbr1 (Chemicon International, Inc., Temecula, CA; 1:1000) and rabbit anti-Dlx (Distal-less homeodomain) (GenWay Biotech, Inc., San Diego, CA; Panganiban et al. 1995; 1:1000). For fluorescent microscopy, corresponding combination of Alexa A-594- and Alexa A-488-anti-rabbit or anti-mouse IgGs (all from Molecular Probes, Eugene, OR; 1:300) or Cy3-anti-guinea pig (Jackson ImmunoResearch, Inc., West Grove, PA; 1:300) was used as secondary antibodies. For conventional light and electron microscopy, biotinylated anti-rabbit, anti-guinea pig or anti-mouse IgGs (1:300) and Elite ABC kit (all from Vector Laboratories, Burlingame, CA) with 3,3′-diaminobenzidine-4HCl (DAB) or Ni-intensified DAB as a chromogen were used. We performed immunogold labeling of CB1 using goat anti-rabbit IgGs conjugated with 1-nm gold particles (1:80) and subsequent silver intensification with R-Gent SE-LM kit (all from Aurion, Wageningen, The Netherlands). Specificity of the anti-CB1 antisera was confirmed in the embryos of CB1 knockout mice (Ledent et al. 1999); the results are published in our previous study (Berghuis et al. 2007). In cases of the double immunolabeling with the antibodies made in the same host species (rabbit for CB1/GABA, CB1/Tbr1, and CB1/calretinin, or mouse for reelin/CCK), a complete cycle of immunolabeling for CB1 or reelin was fulfilled first, then immunolabeling for another antigen was done using a contrast immunofluorescent or immunoperoxidase marker. Although cross reactivity was seen in several specimens, the antigens could be detected by their morphology (dotted staining for CB1 and reelin vs. diffuse for GABA, calretinin and CCK or nuclear for Tbr1).
In Utero Electroporation
In utero electroporation was performed in CD1 timed-pregnant mice at E11.5 and E12.5 following previously described procedures (Saito and Nakatsuji 2001; Sarkisian et al. 2006). The dams were anesthetized by intraperitoneal injection of ketamine (37 mg/kg)/xylazine (1.9 mg/kg). A DNA solution of pCAGGS-EGFP (enhanced green fluorescent protein) plasmid (0.5 mg/mL) (Hashimoto-Torii et al. 2003) was injected into the cerebral lateral ventricle using a pulled glass capillary and electroporation was applied across the brain using a BTX ECM830 Square Wave Electroporator with 5 electric pulses at 25 V for 5 ms each with 950-ms intervals. Dames were sutured and placed on heating pads until they awoke. After 3–6 days, the dames were sacrificed and the surviving embryos were removed and checked for EGFP expression under a Stemi SV11 Apo microscope (Zeiss) equipped with epifluorescent light source. EGFP+ brains were dissected, fixed, sectioned and evaluated in the fluorescent microscope or immunolabeled with goat anti-GFP (Abcam, Cambridge, MA; 1:300) and guinea pig anti-CB1 antibodies (Frontier Science, Japan; 1:1000) as above.
Transgenic Mice
We used the Emx1IRESCre transgenic mouse in which Cre recombinase was under the transcriptional control of the Emx1 locus (Gorski et al. 2002) crossed to LacZ Rosa 26 reporter mouse in which the expression of β-galactosidase could be activated by Cre (Soriano 1999). 15-day-old mice (n = 2) were perfused transcardially by 4% paraformaldehyde. Immunolabeling for fluorescent microscopy was performed using mouse anti-β-galactosidase (Promega, Madison, WI; 1:100) and guinea pig anti-CB1 antibodies (Frontier Science, Japan; 1:1000) as above.
Cell Birth Dating with Bromodeoxyuridine Administration
Intraperitoneal bromodeoxyuridine (BrdU) injection (50 μg/g body weight, twice with 2-h interval) was performed in timed-pregnant CD1 mice at E10.5 (n = 2), E11.5 (n = 5), E12.5 (n = 2), E13.5 (n = 3), and E14.5 (n = 4), which were allowed to survive until E15.5 or E18.5. The embryo brains were fixed and sectioned as above. For singular BrdU detection, the sections were first kept in 2N HCl at +40 °C during 40 min then rinsed in buffer, permeabilized in 0.5% Triton X-100 and immersed in mouse anti-BrdU antiserum (Becton Dickinson, San Jose, CA; dilution 1:100) overnight at room temperature. The sections were further prepared for fluorescent microscopy and photographed as above. For double BrdU and CB1 immunolabeling, CB1 was first detected as above (DAB as the chromogen) then HCl treatment and anti-BrdU immunoperoxidase labeling, omitting Triton X-100 and using DAB-Ni as the chromogen were performed. The specimens were then postfixed, embedded and analyzed in light and electron microscopes as above.
Results
Emergence of CB1 in Postmitotic Neurons in the Developing Mouse Forebrain
We took advantage of the unexpectedly early expression of CB1 in the embryonic mouse telencephalon. Postmitotic CB1+ neurons could be identified by clusters of immunolabeled vesicles in the cytoplasm of the cell bodies and proximal dendrites (Fig. 1) what was further confirmed by colocalization of CB1 and neuron specific class III β-tubulin (Tuj1) at early developmental stages (Supplementary Fig. 1). We used antisera against distinct epitopes of rodent CB1 that are reliable markers for these cells in the postnatal rodent brain (Katona et al. 1999; Morozov and Freund 2003b). Selectivity of the antisera for mouse embryo brain was confirmed in our previous study (Berghuis et al. 2007) as well as our pilot experiments using preabsorption of IgGs with fusion proteins and embryos of CB1 knockout mice. In our hands, the anti-C-terminus and the anti-NH-terminus antisera produced the reproducible patterns of immunolabeling which were complimentary at the electron microscopic level (Fig. 1).
Figure 1.
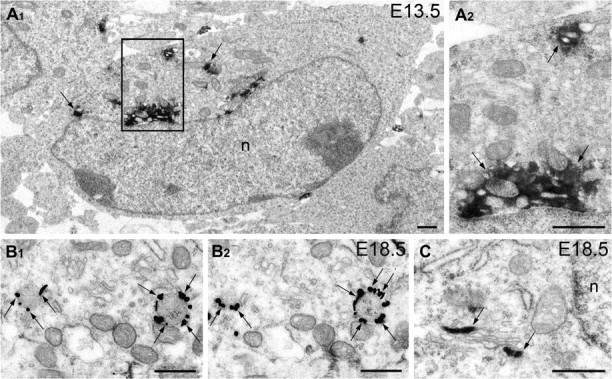
Electron microscopy detection of intracellular CB1+ vesicles in mouse embryo brain. Anti-CB1 immunolabeling of neuron cell bodies in the mouse cerebral MZ at E13.5 (A) and in the hippocampus at E18.5 (B, C). Immunoperoxidase DAB-Ni reaction end-product (arrows in A) and immunogold silver amplified granules in serial electron micrographs (arrows in B) are situated on the outer surface of the membrane of intracellular vesicles, consistent with the location of the CB1-C-terminus. Anti-CB1-NH-terminus, in contrast, situates inside the vesicles and cisterns of the smooth endoplasmic reticulum (arrows in C). Framed area in A1 is enlarged in A2. Abbreviation: n, cell nucleus. Scale bars: 0.5 μm.
CB1+ cells were not detected in the embryonic mouse telencephalon before or at E11. The occasional CB1+ cells could be observed in the wall of the cerebral vesicle at E11.5; but, 1 day later, at E12.5, we identified numerous intensely labeled CB1+ cells in the subpial area of the GE as well as in the marginal zone (MZ) of the dorsal telencephalon (Fig. 2A). At subsequent stages of development, CB1+ cells could also be seen in other parts of the GE (not shown), particularly in the vicinity of the prospective amygdala, where CB1/CCK+ interneurons were probably destined for the basolateral amygdalar nucleus as has been previously reported (Katona et al. 2001; Medina et al. 2004). In the present study, we focus on the CB1+ cells stationed in the caudal side of the GE, which enter the dorsal telencephalon and then undergo a characteristic change in their distribution. More specifically, in E12.5 embryos, numerous CB1+ cells could be seen in the wall of the cerebral vesicle, but they were not present in the hippocampal primordium or cortical hem (Fig. 2A). However, 1 day later, at E13.5, CB1+ cells were easily detectable also in the hippocampal primordium (Fig. 2B). Significantly, 2 days later, at E15.5, CB1+ cells became rare in the neocortex and cingular cortex, whereas a large number was accumulating in the prospective subiculum and hippocampal primordium, particularly in the segment of the incipient fissure. Finally, at E18.5, CB1+ cells became very numerous in the hippocampal primordium, whereas they are seen very rarely in the entorhinal cortex and subiculum (Fig. 2C). The dramatic and systematic changes in the distribution of the CB1+ cells over time are suggestive of neuronal migration from the GE to the hippocampus and call for additional exploration of the spatio-temporal course of their origin and translocation to the place of final residence. Although detailed birth dating analysis is not completed, our preliminary, short time survival, experiments show that after BrdU injections at E11.5-E13.5, BrdU+ cells can be located in the MZ of the prospective neocortex and hippocampus in the E15.5 mouse embryo. Three days later, at E18.5, there are fewer BrdU+ cells in the neocortical MZ, whereas some of them now can be detected as double labeled (BrdU/CB1+ cells in the hippocampal formation, indicating that they may have been generated around E11.5–E13.5 in the GE (Supplementary Fig. 2).
Figure 2.
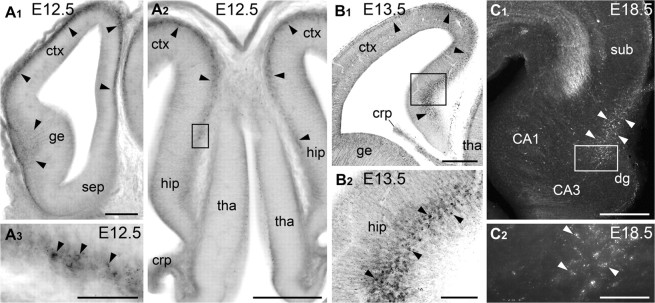
Sequential change in the distribution of CB1+ cells in the mouse cerebrum during mid-late corticogenesis. (A) At E12.5, a high density of CB1+ cell bodies (arrowheads) are present in the subpial area of the GE (ge) and in the MZ of the neocortex (ctx); but not in the septum (sep), thalamus (tha), or hippocampal primordium (hip). In A3 (rotated clock wise), numerous heavily labeled CB1+ cells are detectable in the right side (cingular cortex); but, no immunolabeling is seen in the left side (the hippocampal primordium). (B) At E13.5, plenty of CB1+ cell bodies are detectable in the hippocampal primordium as well (arrowheads). (C) At E18.5, CB1+ cell bodies are numerous in the hippocampus (white arrowheads) and are virtually absent in the subiculum (sub) and entorhinal cortex. CB1+ cells are revealed with DAB-Ni immunoprecipitation and conventional microscopy in (A), (B), and with immunofluorescent microscopy (Z-stack merged) in (C). Framed areas in (A2), (B1), and (C1) are enlarged in (A3), (B2), and (C2), respectively. Abbreviations: crp, choroid plexus; dg, dentate gyrus primordium. Scale bars: (A1, A2, B1, C1) 200 μm (A3, B2, C2) 50 μm.
Hippocampal CB1+ Cells Originate in the Pallial–Subpallial Boundary and/or the Caudal GE
To prove that CB1+ cells originate at the specific sites of the GE and then migrate to the hippocampus via the neocortical plate, we had to exclude the possibilities that the timing of their appearance and disappearance is not due to differential cell death and/or sequence of CB1 expression across these areas. First, our electron microscopy analysis of the distribution of necrotic, apoptotic and autophagy cells in the telencephalon did not display any particular spatio-temporal pattern in these 3 areas. Furthermore, the possibility that CB1+ neocortical cells are losing their immunochemical marker in the MZ of the dorsal cerebral wall, whereas other cells upregulate the very same peptide in the hippocampal primordium was eliminated by our detailed analysis of immunochemical properties of these cells (see next sections below). Finally, to exclude the possibility that all CB1+ cells are generated locally in each area, with a systematic delay of the onset of generation in the hippocampus compared with the neocortex, we used DNA plasmid to transfect neural precursors by in utero focal electroporation in each area and traced VZ cells and their progeny by the expression of EGFP. We found that electroporation in the hippocampal primordium at E12.5, with sacrificing after 6 days, at E18.5, yields numerous cells in the hippocampus that are expressing either EGFP or CB1 but not a single cell that expresses both markers (Fig. 3A,B). Thus, we concluded that delay of development of the hippocampus cannot explain changes in the distribution of CB1+ neurons in the cerebrum during the course of prenatal development, but rather that they are generated in another place, then migrate and arrive to their final destination as postmitotic cells.
Figure 3.
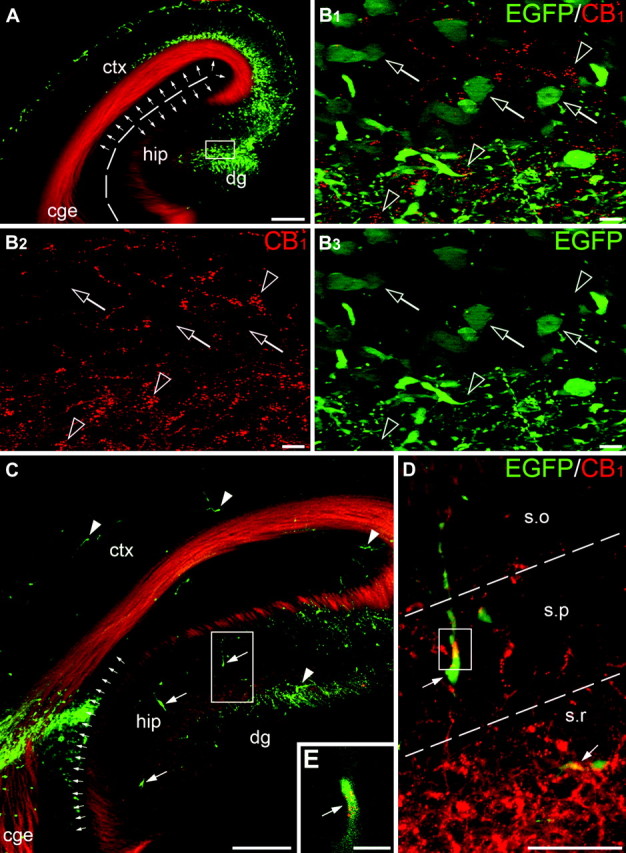
Origin of hippocampal CB1+ neurons. Telencephalic progenitors were labeled by in utero electroporation at E12.5 and the brains were fixed at E18.5. EGFP is depicted in green; CB1+ cell bodies are detected as clusters of red spots. (A, B) Electroporation into the dorsal neocortex and hippocampus (notice robust labeling of vertical cells; set of small arrows) does not reveal colocalization of EGFP and CB1 in the same cells although EGFP+ (empty arrows) and CB1+ (empty arrowheads) cells are abundant in the zone of the hippocampal fissure. This is clearly seen at high magnification in (B) depicting the framed area in (A). (C–E) Tracing the migration pathway of EGFP+ cells from their origin at the proliferative zone of the caudal GE and the pallial–subpallial boundary to the hippocampus. Robust labeling of the radial glial cells (set of small arrows) marks the peak site of electroporation between the dorsal neocortex (ctx) and the caudal GE (cge). Numerous horizontally oriented EGFP+ cells can be detected in the neocortex and hippocampus (hip; arrowheads in C). Most of the EGFP+ cells demonstrate detectable CB1 (arrows in D and E). The framed area in (C) is enlarged in (D). Insert (E) depicts a single optical section taken at high magnification from the framed area in (D). Several EGFP/CB1+ cells (arrows in C) have vertical morphology indicating their distribution through the hippocampus during perinatal period. The ventricular surface in (A) and the borders between the hippocampal layers in (D) are demarked with dashed lines. Abbreviations: dg, dentate gyrus primordium; s.o, stratum oriens; s.p, stratum pyramidale; s.r, stratum radiatum. Scale bars: (A, C) 200 μm, (D) 50 μm, (B, E) 10 μm.
To determine the exact site of origin of CB1+ cells and the possible routes of their migration, we performed multiple electroporations into various locations of the telencephalon. The electroporation was performed at E11.5–E12.5 and the surviving embryos (n = 22) were fixed at E14.5–E18.5 and analyzed for the presence of EGFP using fluorescent microscopy. The systematic analysis revealed that the site of electroporation was critical for the subsequent distribution of EGFP+ cells in the telencephalon. Thus, electroporation into the dorsal neocortex (n = 10) did not lead to the horizontal translocation of EGFP+ cells (not shown), which is in harmony with previous reports (Takiguchi-Hayashi et al. 2004). However, injections into the lateral GE (n = 6) revealed numerous EGFP+ cells in the developing neocortex (latero-caudal migratory stream), which also confirm findings reported in numerous publications (for review see Marin and Rubenstein 2001). But in contrast to the large number of CB1+ cells, only few if any EGFP+ cells have reached the hippocampus by E14.5–E16.5 (not shown). Conversely, electroporation into the caudal portion of the GE and its pallial–subpallial boundary (n = 6) resulted in numerous EGFP+ cells in the MZ of the cingular cortex and hippocampus (Fig. 3C; Supplementary Fig. 3). Importantly, many EGFP+ cells in the hippocampus clearly express CB1, indicating that the hippocampal CB1+ interneurons originate from the caudal GE or pallial–subpallial boundary (Fig. 3D,E), although we cannot exclude the possibility that some of the CB1+ cells might be generated in other sites, such as the medial GE, anterior entopeduncular area, ventral thalamus and so on. Migration of CB1+ cells is further confirmed with our findings that in the E16.5 embryos, EGFP+ cells assumed a horizontal or quasirandom orientation of their processes, which is characteristic of migrating or more mature nonpyramidal neurons, respectively (Supplementary Fig. 3); whereas, 2 days later (E18.5), many EGFP+ cells in the hippocampus became vertically oriented, suggesting their migratory mode and redistribution within the hippocampal formation (Fig. 3C,D).
Migrating CB1+ Cells Acquire Dlx and GABA upon Arrival to the Hippocampus
Our observations are suggestive that CB1 is a novel marker of a specific subclass of prospective interneurons which is identifiable before expression of other interneuronal markers such as proteins encoded by Dlx and GABA. Addressing this point, we performed another set of immunocytochemical examinations using antibodies recognizing all known Dlx proteins considered reliable markers of prospective interneurons (Panganiban et al. 1995). In accordance with our data described above (Fig. 2), as well as extensive literature (e.g., Anderson et al. 1997), double immunolabeling of CB1 and Dlx revealed that these cell populations have different locations in the mouse embryonic telencephalon during early gestation (Fig. 4A). Nevertheless, at E18.5 (Fig. 4B) as well as in young postnatal mice (Supplementary Fig. 4), Dlx/CB1+ cells are detectable in the hippocampus. Accordingly, at E12.5, GABA is well-detectable in the GE but not in the neocortex (Hevner et al. 2003; our not shown data), where CB1+ cells transiently locate; but, at E16.5 onwards, when GABA is upregulated in the neocortex and hippocampus, nearly all CB1+ cells coexpress GABA (Fig. 5A–D). Thus, CB1+ cells do not express other interneuronal markers while migrating through the neocortex, but acquire Dlx, GABA (present research), and CCK (Morozov and Freund 2003a, 2003b) upon arrival to the hippocampus.
Figure 4.
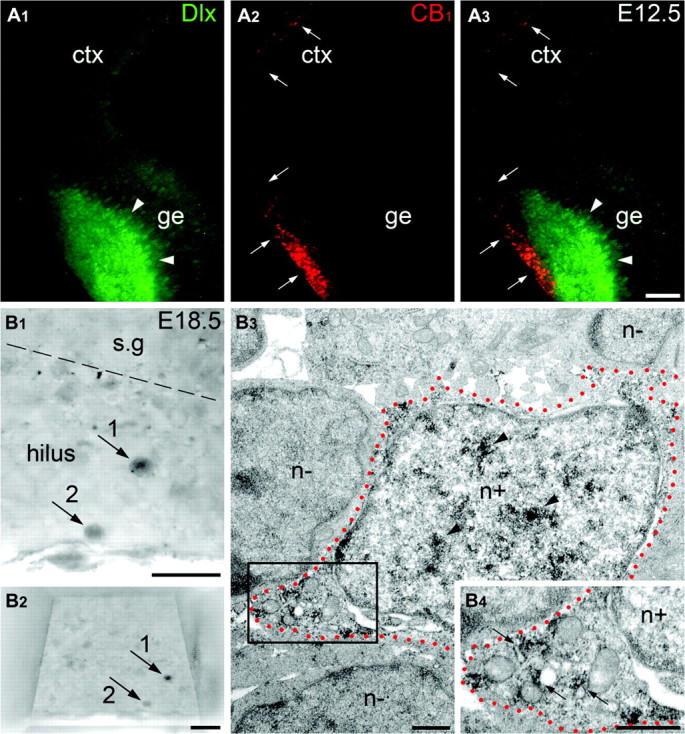
CB1+ and Dlx+ cells have different locations in the mouse embryo telencephalon during early gestation; but later, they are coexpressed in the hippocampal interneurons. (A) At E12.5, Dlx+ and CB1+ cells have complimentary patterns of location. As shown by immunofluorescent microscopy, Dlx+ nuclei (green; arrowheads in A1 and A3) are located in the GE of the telencephalon, whereas CB1+ vesicles (red; arrows in A2 and A3) are distributed through the GE and neocortical MZ (see main text for further explanation). (B) Two arbitrarily chosen Dlx+ cells (numbered arrows) are depicted in the hilus of the dentate gyrus at E18.5. Notice these cells in the specimen trimmed for ultrathin sectioning before electron microscopy examination in (B2). The cell #1 is shown in electron micrographs in (B3) and (B4) where its profile is demarked with red dotted lines. Dlx+ nucleus (n+; notice anti-Dlx DAB-Ni immunoprecipitation; arrowheads) of the cell is surrounded by several Dlx-negative (n−) nuclei. A fragment of cytoplasm of the cell framed in (B3) is enlarged in (B4) demonstrating plural CB1+ vesicles (small arrows). The border between the stratum granulosum (s.g) and the hilus is demarked with dashed line. Abbreviations: ctx, neocortex; ge, ganglionic eminence. Scale bars: (A) 100 μm, (B1, B2) 20 μm, (B3, B4) 0.5 μm.
Figure 5.
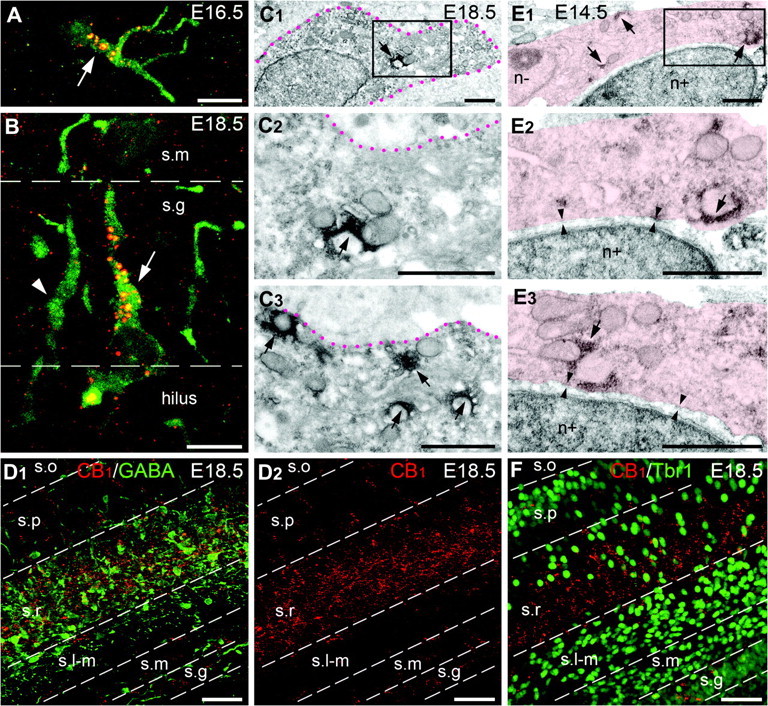
CB1 is coexpressed with GABA, but not with Tbr1, in the mouse embryo hippocampus and neocortical MZ. (A–D) Although, CB1+ and GABA+ cell populations demonstrate different locations in the mouse telencephalon at E12.5–E14.5 (not shown; see main text for further explanation), most of CB1+ cells (red and yellow spots) reveals detectable amount of GABA (green) in the hippocampus at E16.5–E18.5. CB1/GABA+ cells are numerous in the zone of the hippocampal fissure at E16.5 (white arrow in A), in the strata granulosum (s.g; white arrow in B) and radiatum (demarked with red dotted lines in C) at E18.5. GABA+ CB1-negative cells (white arrowhead in B) are also numerous at E18.5. Both GABA+ and CB1+ cells have similar patterns of location and are robust in the stratum radiatum (s.r) at E18.5 as shown in (D) (D1—merged image, D2—anti-CB1 labeling). Electron microscopy analysis of an arbitrarily chosen GABA+ cell in the developing hippocampus demonstrates plural CB1+ vesicles (arrows in C) in the cell, as shown in enlarged serial not adjacent sections (C2, C3) from the framed area in (C1). CB1 is observed as red and yellow spots (in A, B, and D) and with black DAB-Ni immunoprecipitation (in C). GABA is revealed with green immunofluorescence (in A, B, and D1) and with diffuse DAB immunoprecipitation (in C). (E) A CB1+ cell (demarked in pink) and a Tbr1+ cell (revealed with DAB-immunolabeled nucleus; n+) are adjacent in the cerebral MZ at E14.5 (see main text for further explanation). Nevertheless, CB1+ vesicles visualized with black DAB-Ni immunoprecipitation (arrows) belong to the Tbr1-negative cell (notice Tbr1-negative nucleus; n−); whereas, the Tbr1+ cell does not contain CB1+ vesicles. Two cell membranes are normally seen between the cells in a number of serial sections (arrowheads in enlarged serial not adjacent sections from the framed area). (F) Merged image of double immunolabeling for CB1 and Tbr1 in the mouse hippocampus at E18.5 demonstrates different patterns of localization (compared with CB1/GABA in D). CB1+ cells (red) concentrate in the stratum radiatum (s.r). In contrast, Tbr1+ nuclei (green) are numerous in the strata moleculare (s.m) and lacunosum-moleculare (s.l-m). The borders between the hippocampal layers are designated with dashed lines. Abbreviations: s.o, stratum oriens; s.p, stratum pyramidale. Scale bars: (A, B), 10 μm, (C, E) 1 μm, (D, F) 50 μm.
Prospective CB1+ Interneurons are Distinct from the Pallium-Derived Cajal–Retzius Cells
To exclude the possibility that CB1 may be transiently expressed in the GABA-negative cells in the embryonic cerebral MZ, and that this marker later appears in the GABA+ hippocampal interneurons, we performed immunocytochemical analysis of CB1 and T-box transcription factor (Tbr1) which is specific for pallium-derived glutamatergic Cajal–Retzius neurons, that are numerous in the cerebral MZ after E11.5 (Hevner et al. 2003). Although, the patterns of anti-CB1 and anti-Tbr1 labeling appeared similar at E12.5–E16.5, repeated analysis did not reveal colocalization of these 2 markers at the electron microscopic level. In contrast, we have observed that CB1+ vesicles and Tbr1+ nuclei are labeled in adjacent cells (Fig. 5E). Furthermore, distribution patterns of the CB1+ and Tbr1+ cells become different at E18.5, when CB1+ cells disappear from the MZ and emerge in the dentate gyrus and the hippocampus proper (Fig. 5F). In rare cases in the stratum radiatum, CB1+ vesicles and Tbr1+ nuclei appear adjacent to each other at the light microscopic level; nevertheless, electron microscopic examination shows unequivocally that they are localized in separate cells (n = 4; not shown). This led us to the conclusion that CB1+ cells are Tbr1-negative in both the embryonic neocortex and in the hippocampus. Finally, the subpallial origin of CB1+ cells is further confirmed by the absence of coexpression of CB1 and β-galactosidase in the Emx1IRESCre-LacZ Rosa 26 transgenic mouse which constantly trace Emx1+ pallium-derived neurons (Gorski et al. 2002) in the hippocampus of early postnatal mice (n = 2; Supplementary Fig. 5). Thus, in contrast to a recent report (Mulder et al. 2008), our detailed analysis at the light and electron microscopic levels indicates that CB1+ neurons do not express pallial markers while they are migrating across the embryonic cerebrum.
Because CB1+ cells originate and enter the MZ simultaneously with Cajal–Retzius neurons, we examined whether these 2 cell classes can be distinguished from each other by cell class-specific immunochemical markers, such as calretinin and reelin (Ogawa et al. 1995). Double immunolabeling of CB1 and reelin or CB1 and calretinin revealed that the majority of CB1+ cells were negative to other markers. However, coexpression of these markers was detected in some cells during the prenatal and postnatal periods (Fig. 6); and in some cases, both CB1 and reelin could be colocalized in the very same vesicle (Fig. 6C). Unfortunately, the exact fraction of CB1+ cells that also express reelin or calretinin was difficult to estimate in densely packed tissues such as the cortical and hippocampal primordia. CB1/reelin+ cells could be transiently located in the molecular and granule cell layers of the dentate gyrus during the first postnatal week (Fig. 6E) and later in the hilus (Fig. 6F), confirming their secondary migration in this brain tissue (Morozov et al. 2006). Remarkably, in spite of an extensive search, we did not find colocalization of CCK and reelin in the hippocampus of young adult mice, being in harmony with the absence of colocalization of CCK and calretinin in rat cerebral cortex (Somogyi et al. 2004). Thus, we conclude that a small number of CB1+ cells coexpress reelin and calretinin, what is also in harmony with the reported migration of reelin/calretinin+ cells which originate in the pallial–subpallial boundary from the developing brain homeobox 1 (Dbx1) expressing cells (Bielle et al. 2005). Thus, the main body of the CB1+ cell population is negative for reelin and calretinin. These cells likely originate in the caudal part of the GE and migrate to the hippocampal formation where they acquire CCK during the perinatal period (Morozov and Freund 2003a, 2003b).
Figure 6.
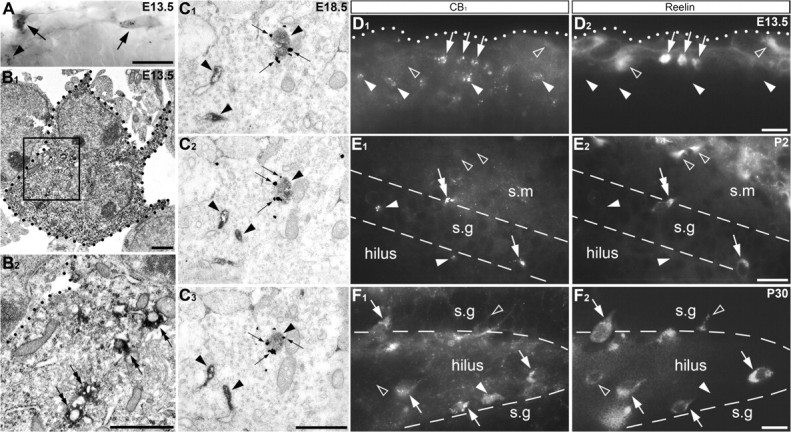
CB1 is present within a subset of Cajal–Retzius cells. (A, B) CB1/calretinin+ cells (arrows) in the mouse cerebral MZ at E13.5. A CB1+ calretinin-negative cell is marked with arrowhead in (A). Calretinin is visualized with diffuse DAB immunoprecipitation, whereas CB1+ vesicles—with black DAB-Ni staining. The profiles of a calretinin+ cell are depicted with black dotted lines in electron micrographs in (B). The framed area is also shown at high power micrograph in (B2). Notice number of CB1+ vesicles (double arrows) in the calretinin+ cell. (C–F) Coexpression of CB1 and reelin was detected in a subset of neocortical and hippocampal neurons at all time-points analyzed in prenatal and postnatal rodent. (C) Double immunolabeling of CB1 (silver intensified immunogold particles; small arrows) and reelin (DAB immunoprecipitation in the cisterns of the endoplasmic reticulum and vesicles, characteristic for intracellular reelin location; arrowheads) shown in serial electron micrographs in mouse embryo hippocampus at E18.5. Notice that one of the vesicles contains both markers (arrows and arrowheads). (D–F) Arbitrarily chosen sections of mouse embryo neocortex (E13.5; D), early postnatal rat (P2; E) and young adult mouse hippocampal dentate gyrus (P30; F) immunolabeled for CB1 (left column) and reelin (right column). The cells expressing both markers are indicated with white arrows. Number of CB1+ cells is reelin-negative (white arrowheads). Reelin+ but CB1-negative cells are marked with empty arrowheads. Notice a CB1/reelin+ vertical bipolar cell (white double arrow in E) near the border of the strata moleculare (s.m) and granulosum (s.g) presumed to be migrating toward the hilus in accordance with previously demonstrated migration of CB1+ cells (Morozov and Freund 2003a, 2003b). (F) Adult location pattern of CB1+ and reelin+ cells in the hilus with number of cells bordering the granule cell layer (s.g), again in accordance with the pattern of the cells terminated their relocation (Morozov et al. 2006). The pial surface in (D) is demarked with a white dotted line. The borders between the hippocampal layers in (E) and (F) are demarked with dashed lines. Scale bars: (A, D–F) 20 μm, (B, C) 1 μm.
Together, our findings suggest that CB1+ hippocampal interneurons originate in the caudal GE and/or the pallial–subpallial boundary (ventral and lateral pallium) during early gestation (∼E11.5–E12.5); then, they migrate in the lateral-to-medial direction and can be seen transiently in the neocortical MZ (∼E12.5–E14.5) before relocating to the hippocampal primordium (∼E13.5–E15.5). Their final distribution within the developing hippocampal formation occurs during the perinatal and early postnatal period (Fig. 7).
Figure 7.
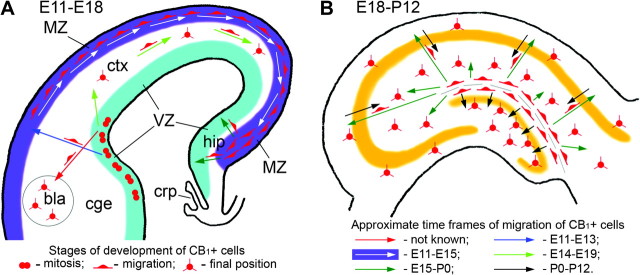
Schemas of migration pathways of CB1+ cells in the prenatal telencephalon and the early postnatal hippocampus in rodent. (A) First CB1+ cells are generated in the VZ of the caudal GE and pallial–subpallial boundary (lateral and ventral pallium) at about E11.5–E12.5. The lateral pallium-derived CB1+ cells probably migrate to the basolateral amygdalar nucleus (bla; red arrow; Medina et al. 2004) and develop into CB1/CCK+ interneurons (Katona et al. 2001). The ventral pallium- and caudal GE-derived cells migrate vertically (blue arrow) to the MZ and subsequently horizontally in the lateral-to-medial direction (white arrows) through the cerebral MZ and finally concentrate in the hippocampal primordium (hip) by E14–E15. CB1/CCK+ cells also migrate in the lateral-to-medial direction later during the prenatal period (E14–E19; light green arrows) to settle in the neocortex (Bodor et al. 2005). CB1+ cells do not move farther toward the septum or thalamus during the prenatal period, presumably due to the presence of chemorepulsive proteins in the choroid plexus (crp; Hu 1999); instead, they distribute through the hippocampus during perinatal period (dark green arrows). (B) The cells arrived in the hippocampus nevertheless remain transitional and perform another step of migration (dark green arrows), populating the strata oriens, pyramidale and radiatum of the hippocampus proper or traversing the developing granule cell layer and settling in the hilus of the dentate gyrus during the last prenatal days and first postnatal weeks (black arrows; Morozov and Freund 2003a, 2003b; Morozov et al. 2006).
Discussion
The cannabinoid receptor, CB1, is present in a subclass of GABAergic hippocampal interneurons which also express the neuropeptide CCK (e.g., Katona et al. 1999; Monory et al. 2006). This cell subclass can be distinguished by the presence of CB1 in the membrane of intracellular vesicles, within both the somatic cytoplasm and proximal dendrites, allowing their positive identification at the ultastructural level (Katona et al. 1999; Morozov and Freund 2003b). Our detailed analysis of the developmental history of the CB1+ hippocampal interneurons in the embryonic mouse telencephalon reveals several conceptually and biomedically important findings, including 1) the early commitment to CB1+ phenotype following the last cell division and relocation from the VZ to MZ; 2) the selective and relatively sharply defined place of their origin at the specific area of the caudal GE and its border to the pallium in the dorsal telencephalon; 3) their complex, 3-phasic migratory pathway crosses the borders of 3 major telencephalic subdivisions—GE, neocortex, and hippocampus; and, 4) delay of detectable expression of Dlx and GABA in CB1+ cells while they are migrating through the neocortex and acquiring these interneuronal markers upon arrival to the hippocampus. These results provide new insight into evolution and the possible role of this relatively rare, but important, cellular subclass of the hippocampal formation.
Early Commitment in the Proliferative Zone
Our finding that CB1/CCK+ and CB1/reelin/calretinin+ hippocampal interneurons originate already at E11.5–E12.5 was unexpected for the GABAergic interneurons, because such early generation precedes not only most inhibitory neurons, but also generation of the majority of projection cortical neurons in the embryonic mouse telecephalon. Their early commitment suggests that these cells may be related to other classes of pioneering neurons in the developing cerebrum. This link is suggestive by the coexpression of CB1 with reelin and calretinin, indicating some similarity to the Cajal–Retzius cells. This heterogeneous neuron subgroup is known to play a critical role in the lamination of both the cerebral neocortex and hippocampus (reviewed in Soriano and Del Rio 2005). Some Cajal–Retzius cells that express Tbr1 (Hevner et al. 2003) are generated in the cortical hem (hippocampal primordium) and distribute through the cerebral MZ by medial-to-lateral migration (Takiguchi-Hayashi et al. 2004; Borrell and Marin 2006). Another subgroup of Cajal–Retzius cells which transiently express Dbx1 is generated at the pallial–subpallial boundary and migrates in the opposite direction (Bielle et al. 2005). Our data indicate that CB1+ cells are Tbr1-negative and display the pattern of migration as well as immunochemical properties like Dbx1-derived cells (Bielle et al. 2005). This indicates that CB1/reelin/calretinin+ neurons most likely are generated in the pallial–subpallial boundary from the Dbx1+ precursors. In contrast, CB1/CCK+ cells probably are generated in the caudal GE and their genetic lineage is unknown.
Our present study uncovered not only the early genesis of CB1+ subtype of GABAergic interneurons, but also show that they are produced at a very restricted segment of the proliferative VZ and subsequently follow a very specific migratory route to a very distant part of the telencephalon. These findings support the concept of early regionalization of neuronal production in the proliferative zones (Rakic 1988). This concept, known as the protomap hypothesis, was initially based on the ablation experiments and heterologous transplantations and indicated that cortical projection neurons are committed to their phenotypes as well as to areal and laminar positions at the time of their last cell division in the proliferative VZ of the telencephalon (McConnell 1988; Rakic 1988). Similar regionalization of neuronal production has been observed in the spinal cord (Jessellm 2000). The protomap hypothesis has been amply confirmed by the application of modern methods of molecular genetics, which shows that cortical pyramidal cells are initially prespecified independently of their synaptic input (Fukuchi-Shimogori and Grove 2003; Chen, Schaevitz, et al. 2005; Chen, Rasin, et al. 2005; O'Leary and Borngasser 2006; Shen et al. 2006; Storm et al. 2006; Cholfin and Rubenstein 2007; Molyneaux et al. 2007). In spite of this, most developmental neurobiologists have assumed that developing interneurons are not committed from the moment of generation to the specific morphogenetic phenotypes, but rather, that they are initially equipotent and disperse randomly throughout the cortical plate to be only subsequently modeled by the specific afferents in order to serve their inhibitory function appropriate for a given cortical area and/or layer. Our present findings, together with an increasing number of studies (Lavdas et al. 1999; Nery et al. 2002; Lopez-Bendito et al. 2004; Xu et al. 2004; Fogarty et al. 2007; Merkle et al. 2007; Batista-Brito et al. 2008), show that cerebral interneurons are committed early in the specific segments of the proliferative zones as has been predicted by the protomap hypothesis (Rakic 1988).
Three-Phase Migratory Routes
Our data indicate that CB1+ cells first migrate radially, from the VZ of the ventral telencephalon to the MZ of the dorsal neocortex where they acquire robust CB1 accumulation. Then, we could follow their tangential, lateral-to-medial, migration confined to the MZ all the way to the hippocampal primordium, where they arrive between E13.5 and E15.5. Significantly, CB1+ cells do not move farther, across the fimbria toward the septum, presumably due to chemorepulsive proteins detected in the septum and the choroid plexus (Hu 1999). Instead, during the last prenatal days and the early postnatal period, about one half of them assume a vertical orientation (Fig. 7B) and migrate across the full width of the developing granule cell layer to settle in the hilus of the dentate gyrus (Morozov et al. 2006). The remaining half stays in the hippocampus proper, where they settle in the strata oriens, pyramidale, and radiatum (present research; Morozov and Freund 2003a). Thus, we conclude that prospective CB1+ hippocampal interneurons are a unique subclass of the early generated neurons, which vanguards (E12–E15) lateral-to-medial migration of the main cohort of the GABAergic interneurons peaking between E14 and E19 (Anderson et al. 1997; Pleasure et al. 2000; Polleux et al. 2002; Ang et al. 2003).
Perhaps the most intriguing finding in the present study is the complex migratory pathways of CB1+ cells which change the direction of movement several times and cross the borders of 3 major telencephalic subdivisions, whereas the majority of postmitotic neurons in all tissues of the vertebrate central nervous system migrate in one direction, usually remaining within the radial domain superjacent to the place of their origin (for review see Rakic 1988; Jessellm 2000). There are, however, several notable exceptions where the postmitotic migrating cells cross the borders of brain subdivisions in their route to the final destination. The oldest and well established example is migration of neurons from the rhombic lip of the romhencephalon to the gray nucleus of the pons that is preserved in all mammals, including human, where it is most prominent (Essick 1912; Rakic 1990, 2000; Rodriguez and Dymecki 2000). Other examples of a transdivisional migratory routes are the migration stream from the telencephalic GE to the dorsal thalamus of the diencephalon that so far can only be observed in human (Rakic and Sidman 1969; Letinic and Rakic 2001) and Gonadotropin-releasing hormone neurons migration from the nasal placode across the nasal compartment and the cribriform plate through the basal forebrain to the hypothalamus (Cariboni et al. 2007). Cerebellar granule cells also undergo several distinct phases of migration (Komuro and Rakic 1998); but, they stay within the same segment of the neuraxis. In the present study, we not only have identified the site of origin of a subclass of interneurons, but also found that they have an unusually complex itinerary (Fig. 7). The evolutional significance of migration of CB1+ neurons across 3 brain subdivisions and their plural reorientation is enigmatic. Why should the cells generated in the ventral telencephalon undergo so complex journey to reach the hippocampal formation? Why cannot they be generated locally? The fact that such unique routes are repeated from individual to individual, in spite of the complex molecular machinery that is orchestrating in their translocation, suggests a biological necessity (Rakic 1990). However, why it is needed is a puzzle still waiting for an answer. One possibility is that it is a remnant of a phylogenetically simpler route, that became complex during evolutionary expansion of the neocortex between the GE and the hippocampus.
Functional Considerations
The role of CB1 located in the cell membrane of axons in depolarization-induced suppression of neurotransmitter release from the CB1+ synaptic boutons is well-documented (Llano et al. 1991; Pitler and Alger 1992; Kreitzer and Regehr 2001; Wilson and Nicoll 2002; Freund et al. 2003; Piomelli 2003). Simultaneously, CB1 located in the membrane of intracellular vesicles in the cell bodies and proximal dendrites transmits a unique form of slow self-inhibition detected in CB1/CCK+ interneurons in postnatal rats. Such self-inhibition was shown independent on neurotransmission, but rather on Ca2+-dependent autocrine action of cannabinoids, synthesized by and targeted to the same neuron (Bacci et al. 2004). Tangentially migrating neurons in the fetal neocortical MZ, which accumulate CB1 in the cell bodies but have not yet extended CB1+ axons, likely follow the cannabinoid-dependant maintenance of [Ca2+] oscillations (Rakic et al. 1996; Komuro and Rakic 1996; Garaschuk et al. 2000) and may be particularly vulnerable to cannabis self administration or medical use of CB1 agonists and antagonists.
The colocalization of CB1 and reelin in the same intracellular vesicles suggests a possibility of interaction between these 2 molecular pathways. Indeed, externalization and internalization of CB1+ vesicles are known to be cannabinoid-dependant (Rinaldi-Carmona et al. 1998; Leterrier et al. 2006). Incorporation of CB1/reelin+ vesicles into the outer cell membrane may result in secretion of reelin that is considered essential for proper laminar positioning of migrating projection neurons as well as for the appropriate laminar termination of the developing hippocampal afferents (Del Rio et al. 1997; Super et al. 1998; Borrell et al. 1999; Deller et al. 1999). Whereas most reelin+ Cajal–Retzius cells disappear from the brain due of programmed cell death during the early postnatal period (Super et al. 1998), CB1/reelin+ interneurons undergo a secondary migration (Morozov and Freund 2003b; Morozov et al. 2006) and appear to continue secreting reelin in a cannabinoid-dependent manner during late postnatal development and adulthood. This apparent interaction warrants further investigation because several CB1 agonists and antagonists are used in clinical practice (Murray et al. 2007).
Finally, it should be emphasized that CB1/CCK+ GABAergic interneurons are also unique in the sense that they establish synapses while stationed transiently in the stratum moleculare, before translocation across the full width of the dentate gyrus and settling in the hilus (Morozov et al. 2006). Their dual function in early and late hippocampal development may be related to the fact that GABA synapses in this brain tissue become active before glutamatergic ones (Hennou et al. 2002) and that they may initially serve an excitatory rather than inhibitory function (e.g., Ben-Ari 2002). This early functional activity of CB1+ cells should be taken into consideration in assessment of the possible effect of canabinoid exposure during pregnancy.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
USA Public Health Service grant, the March of Dimes Birth Defects Foundation, and the Kavli Institute for Neuroscience at Yale.
Acknowledgments
We are grateful to Matthew Sarkisian, Tibor Harkany, Tamas Freund, and Istvan Katona for their helpful discussion, as well as Viktor Tarabykin for Emx1IRESCre-LacZ Rosa 26 transgenic mouse and Ken Mackie for anti-CB1 antibodies. Conflict of Interest: None declared.
References
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Ang E, Jr, Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci. 2003;23:5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R, Machold R, Klein C, Fishell G. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb Cortex. 2008;18:2306–2317. doi: 10.1093/cercor/bhm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Garcia-Gil L, Hernandez ML, Romero J, Cebeira M, de Miguel R, Ramos JA, Fernandez-Ruiz JJ. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–3188. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell V, Del Rio JA, Alcantara S, Derer M, Martinez A, D'Arcangelo G, Nakajima K, Mikoshiba K, Derer P, Curran T, et al. Reelin regulates the development and synaptogenesis of the layer-specific entorhino-hippocampal connections. J Neurosci. 1999;19:1345–1358. doi: 10.1523/JNEUROSCI.19-04-01345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell V, Marin O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–1293. doi: 10.1038/nn1764. [DOI] [PubMed] [Google Scholar]
- Cariboni A, Maggi R, Parnavelas JG. From nose to fertility: the long migratory journey of gonadotropin-releasing hormone neurons. Trends Neurosci. 2007;30:638–644. doi: 10.1016/j.tins.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Chen JG, Rasin MR, Kwan KY, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17792–17797. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL. Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci USA. 2007;104:7652–7657. doi: 10.1073/pnas.0702225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio JA, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, et al. A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature. 1997;385:70–74. doi: 10.1038/385070a0. [DOI] [PubMed] [Google Scholar]
- Deller T, Drakew A, Heimrich B, Forster E, Tielsch A, Frotscher M. The hippocampus of the reeler mutant mouse: fiber segregation in area CA1 depends on the position of the postsynaptic target cells. Exp Neurol. 1999;156:254–267. doi: 10.1006/exnr.1999.7021. [DOI] [PubMed] [Google Scholar]
- Essick CR. The development of the nuclei pontis and the nucleus arcuatus in man. Am J Anat. 1912;13:25–54. [Google Scholar]
- Fernandez-Ruiz JJ, Berrendero F, Hernandez ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends Neurosci. 2000;23:14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marín O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:345–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Emx2 patterns the neocortex by regulating FGF positional signaling. Nat Neurosci. 2003;6:825–831. doi: 10.1038/nn1093. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Motoyama J, Hui CC, Kuroiwa A, Nakafuku M, Shimamura K. Differential activities of Sonic hedgehog mediated by Gli transcription factors define distinct neuronal subtypes in the dorsal thalamus. Mech Dev. 2003;120:1097–1111. doi: 10.1016/j.mod.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Hennou S, Khalilov I, Diabira D, Ben-Ari Y, Gozlan H. Early sequential formation of functional GABA(A) and glutamatergic synapses on CA1 interneurons of the rat foetal hippocampus. Eur J Neurosci. 2002;16:197–208. doi: 10.1046/j.1460-9568.2002.02073.x. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Neogi T, Englund C, Daza RA, Fink A. Cajal-Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin. Brain Res Dev Brain Res. 2003;141:39–53. doi: 10.1016/s0165-3806(02)00641-7. [DOI] [PubMed] [Google Scholar]
- Hu H. Chemorepulsion of neuronal migration by Slit2 in the developing mammalian forebrain. Neuron. 1999;23:703–711. doi: 10.1016/s0896-6273(01)80029-5. [DOI] [PubMed] [Google Scholar]
- Jessellm TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz E, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17:275–285. doi: 10.1016/s0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Distinct modes of neuronal migration in different domains of developing cerebellar cortex. J Neurosci. 1998;15:1478–1490. doi: 10.1523/JNEUROSCI.18-04-01478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert J, Beslot F, Bohme G, Imperato A, Pedrazzini T, Roques B, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Leterrier C, Lainé J, Darmon M, Boudin H, Rossier J, Lenkei Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J Neurosci. 2006;26:3141–3153. doi: 10.1523/JNEUROSCI.5437-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Rakic P. Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci. 2001;4:931–936. doi: 10.1038/nn0901-931. [DOI] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Sturgess K, Erdelyi F, Szabo G, Molnar Z, Paulsen O. Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb Cortex. 2004;14:1122–1133. doi: 10.1093/cercor/bhh072. [DOI] [PubMed] [Google Scholar]
- Lubbers K, Wolff JR, Frotscher M. Neurogenesis of GABAergic neurons in the rat dentate gyrus: a combined autoradiographic and immunocytochemical study. Neurosci Lett. 1985;62:317–322. doi: 10.1016/0304-3940(85)90568-3. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JLR. A long remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- McConnell SK. Fates of visual cortical-neurons in the ferret after isochronic and heterochronic transplantation. J Neurosci. 1988;8:945–974. doi: 10.1523/JNEUROSCI.08-03-00945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina L, Legaz I, Gonzalez G, De Castro F, Rubenstein JL, Puelles L. Expression of Dbx1, Neurogenin 2, Semaphorin 5A, Cadherin 8, and Emx1 distinguish ventral and lateral pallial histogenetic divisions in the developing mouse claustroamygdaloid complex. J Comp Neurol. 2004;474:504–523. doi: 10.1002/cne.20141. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov YM, Ayoub AE, Rakic P. Translocation of synaptically connected interneurons across the dentate gyrus of the early postnatal rat hippocampus. J Neurosci. 2006;26:5017–5027. doi: 10.1523/JNEUROSCI.0272-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov YM, Freund TF. Postnatal development and migration of cholecystokinin-immunoreactive interneurons in rat hippocampus. Neuroscience. 2003a;120:923–939. doi: 10.1016/s0306-4522(03)00409-3. [DOI] [PubMed] [Google Scholar]
- Morozov YM, Freund TF. Post-natal development of type 1 cannabinoid receptor immunoreactivity in the rat hippocampus. Eur J Neurosci. 2003b;18:1213–1222. doi: 10.1046/j.1460-9568.2003.02852.x. [DOI] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabás K, Ballester Rosado CJ, Nguyenm L, Monory K, Marsicano G, Di Marzo V, Hurd YL, et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci USA. 2008;105:8760–8765. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- O'Leary DDM, Borngasser D. Cortical ventricular zone progenitors and their progeny maintain spatial relationships and radial patterning during preplate development indicating an early protomap. Cereb Cortex. 2006;16:i46–i56. doi: 10.1093/cercor/bhk019. [DOI] [PubMed] [Google Scholar]
- Panganiban G, Sebring A, Nagy L, Carroll S. The development of crustacean limbs and the evolution of arthropods. Science. 1995;270:1363–1366. doi: 10.1126/science.270.5240.1363. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABA(a) responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure S, Anderson S, Hevner R, Bagri A, Marin O, Lowenstein D, Rubenstein J. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. Principles of neuronal cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- Rakic P. Illegal immigrations. Neuron. 2000;27:409–410. doi: 10.1016/s0896-6273(00)00047-7. [DOI] [PubMed] [Google Scholar]
- Rakic P, Knyihar-Csillik E, Csillik B. Polarity of microtubule assemblies during neuronal cell migration. Proc Natl Acad Sci USA. 1996;93:9218–9222. doi: 10.1073/pnas.93.17.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Sidman RL. Telencephalic origin of pulvinar neurons in the fetal human brain. Z Anat Entwickl-Gesch. 1969;l29:53–82. doi: 10.1007/BF00521955. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Le Duigou A, Oustric D, Barth F, Bouaboula M, Carayon P, Casellas P, Le Fur G. Modulation of CB1 cannabinoid receptor functions after a long-term exposure to agonist or inverse agonist in the Chinese hamster ovary cell expression system. J Pharmacol Exp Ther. 1998;287:1038–1047. [PubMed] [Google Scholar]
- Rodriguez CI, Dymecki SM. Origin of the precerebellar system. Neuron. 2000;27:475–466. doi: 10.1016/s0896-6273(00)00059-3. [DOI] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001;240:237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- Sarkisian MR, Bartley CM, Chi H, Nakamura F, Hashimoto-Torii K, Torii M, Flavell RA, Rakic P. MEKK4 signaling regulates filamin expression and neuronal migration. Neuron. 2006;52:789–801. doi: 10.1016/j.neuron.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, Fukaya M, Shigemoto R, Watanabe M, Somogyi P. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19:552–569. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- Soriano E, Cobas A, Fairen A. Asynchronism in the neurogenesis of GABAergic and non-GABAergic neurons in the mouse hippocampus. Brain Res. 1986;395:88–92. doi: 10.1016/s0006-8993(86)80012-9. [DOI] [PubMed] [Google Scholar]
- Soriano E, Del Rio JA. The cells of Cajal-Retzius: still a mystery one century after. Neuron. 2005;46:389–394. doi: 10.1016/j.neuron.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Super H, Martinez A, Del Rio JA, Soriano E. Involvement of distinct pioneer neurons in the formation of layer-specific connections in the hippocampus. J Neurosci. 1998;18:4616–4626. doi: 10.1523/JNEUROSCI.18-12-04616.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi-Hayashi K, Sekiguchi M, Ashigaki S, Takamatsu M, Hasegawa H, Suzuki-Migishima R, Yokoyama M, Nakanishi S, Tanabe Y. Generation of reelin-positive marginal zone cells from the caudomedial wall of telencephalic vesicles. J Neurosci. 2004;24:2286–2295. doi: 10.1523/JNEUROSCI.4671-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll R. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


