Abstract
We evaluated the effectiveness of rhamnogalacturonan II (RG-II)-stimulated bone marrow-derived dendritic cells (BMDCs) vaccination on the induction of antitumor immunity in a mouse lymphoma model using EG7-lymphoma cells expressing ovalbumin (OVA). BMDCs treated with RG-II had an activated phenotype. RG-II induced interleukin (IL)-12, IL-1β, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) production during dendritic cell (DC) maturation. BMDCs stimulated with RG-II facilitate the proliferation of CD8+ T cells. Using BMDCs from the mice deficient in Toll-like receptors (TLRs), we revealed that RG-II activity is dependent on TLR4. RG-II showed a preventive effect of immunization with OVA-pulsed BMDCs against EG7 lymphoma. These results suggested that RG-II expedites the DC-based immune response through the TLR4 signaling pathway.
Keywords: adjuvant, dendritic cells, rhamnogalacturonan II, Toll-like receptor 4, tumor
Introduction
Previous research has shown that various compounds extracted from ginseng or grapes, including ginsenosides and resveratrol, have prominent effects on suppressing tumor progression and enhancing immune response.1, 2 Resveratrol has been defined as an outstanding compound for tumor therapy via various mechanisms.3 Rhamnogalacturonan II (RG-II), another component of ginseng and grapes, has unusual activity on antitumor responses via immune enhancement in a different manner than resveratrol. RG-II, a low-molecular-mass polysaccharide (5–10 KDa), was first identified in 1978 as a polysaccharide complex that is solubilized by endopolygalacturonase treatment of suspension-cultured sycamore cell walls.4 RG-II is a pectic polysaccharide, referred to as a substituted galacturonan, and has a backbone composed of linear 1,4-linked α-𝒟-GalpA residues.5 The generation of a strong cellular immune response is important for successful immunotherapeutic cancer treatment. Immune systems can recognize tumor antigens and elicit CD8+ T-cell responses against tumor antigens through the process of cross-presentation, and optimal cross-presentation of tumor antigens by antigen-presenting cells is required for sufficient priming of CD8+ T-cell responses capable of resolving tumors.6 Dendritic cells (DCs) play a critical role in antigen presentation for the initiation and maintenance of immune responses.7 Thus, recent research trends have concentrated on the development of a DC-based vaccination protocol for cancer immunotherapy because of the ability of DCs to process and present antigens to elicit a strong T-cell response.8 Animal experiments as well as clinical experience indicate that DC-based cancer vaccination mediates potent antitumor immune responses.7, 9, 10
For improved efficacy of DC-based vaccines in cancer immunotherapy, upregulation of adhesive and co-stimulatory molecules on DCs is required. The B7 superfamily (CD80 and CD86) plays a key role in activating T cells, producing cytokines and generating cytotoxic T lymphocytes (CTLs).11 At the same time, CD40 signaling leads to the upregulation of co-stimulatory molecules and, in particular, promotes the generation of long-lasting memory CTLs.12, 13 Several studies have reported that CD40 stimulation of DCs is essential in the priming phase, because the ability of DCs to migrate to secondary lymphoid organs is important in the induction of tumor-specific T cells, including CTLs.12, 13 Experimental mouse studies have shown that mice lacking the CD40 ligand have defective priming ability accompanied by disrupted DC migration toward regional lymph nodes.14
Toll-like receptor (TLR) agonists are a class of vaccine adjuvants that have been the topic of intense study in recent years. Various bacterial- or viral-derived molecular structures are agonists for 1 of 10 known human TLRs and are thus potent activators of innate immunity.15 Stimulation of DCs through TLRs is important to elicit a switch in the activation state from an immature phenotype to a mature state to produce an effective systemic antitumor response. Several studies have reported that TLR agonist stimulation serves an adjuvant function in DC–tumor fusion vaccination.16, 17 Among the aforementioned TLR agonists, TLR4 agonists are more suitable for immunostimulatory adjuvants because of DC-based immune boosting potency via the TLR4 signaling cascade.18 In a previous report, we also revealed that Mycobacterium avium subspecies paratuberculosis fibronectin attachment protein, a TLR4 agonist, enhances DC-based cancer vaccine potency.19 Attenuated vaccines typically generate some degree of cellular immunity, but clinical use often provokes numerous public health problems, including adverse reactions or reversion to virulence in some portion of the population.20 Therefore, a primary goal of vaccine development is to create a noninfectious vaccine that mimics the ability of a natural infection to stimulate a strong cellular immune response. As such, significant effort has concentrated on the development of a novel and potent vaccine adjuvant. The majority of vaccine adjuvants developed thus far have not generated clinically significant cell-mediated immunity.
Previously, we reported that RG-II is an ameliorator in asthmatic inflammation.21 Here, we determined the anticancer effect of RG-II as a potential cancer preventive compound and the mechanism of action of RG-II. We showed that RG-II is a potent adjuvant that can enhance the stimulatory capacity of DCs in vitro. By using an ovalbumin (OVA)-expressing EL-4 (EG7) tumor model, we also determined DC-based vaccines (a combination of OVA and RG-II) elicit strong induction of antigen-specific CTL responses and tumor regression in vivo.
Materials and methods
Mice
C57BL6 mice (Orient Bio, Seoul, Korea) were used at 6 to 8 weeks of age. C57BL/6 OT-1 T-cell receptor (TCR) transgenic mice, C57BL/6J TLR2 knockout mice (TLR2−/−; B6.129-Tlr2tm1Kir/J) and C57BL/10 TLR4 knockout mice (TLR4−/−; C57BL/10ScNJ) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). MyD88 −/− mice were generously provided by Dr Byungsuk Kwon (University of Ulsan, Ulsan, South Korea), and TRIF −/− mice were generously provided by Dr Sung Jae Shin (Yonsei University, Seoul, South Korea). The animals were housed in a specific pathogen-free environment within our animal facility and used in accordance with the institutional guidelines for animal care.
Reagents and antibodies
RG-II isolated from the leaves of Panax ginseng was provided by Mazence (Gjyeonggido, Korea). Recombinant mouse (rm) granulocyte macrophage colony-stimulating factor, rm interleukin-4 (IL-4) and rm interferon-γ (rmIFN-γ) were purchased from R&D Systems (Minneapolis, MN, USA). Anti-mouse CD11c FITC (clone N418), CD40 PE (clone 1C10), CD80 (B7-1) PE (clone 16-10A1), CD86 (B7-2) PE (clone GL1), major histocompatibility complex (MHC) class I (H-2Db) PE (clone 28-14-8), MHC class II (I-A/I-E) PE (clone M5/114.15.2), and isotype-matched control mAbs were purchased from eBioscience (San Diego, CA, USA).
Purification of RG-II
A crude polysaccharide fraction (GL-2) was prepared from the leaves of P. ginseng by hot water extraction, ethanol precipitation and dialysis.22 GL-2 was fractionated by Cetavlon (cetyltrimethylammonium bromide) precipitation, and a weakly acidic polysaccharide fraction (GL-4) was obtained. The Fc receptor expression-enhancing polysaccharide (RG-II) was purified from GL-4 by anion-exchange chromatography on diethylaminoethyl Sepharose CL-6B (Sigma, St Louis, MO, USA), as described previously.23 In order to remove the colored materials in the polysaccharide, RG-II was further purified on a QSepharose column (C1 form) (Sigma). The column was washed with water and eluted sequentially with 0.1, 0.2, 0.3, 0.4, 0.5 and 1.0 ℳ NaC1. The major fraction, which was eluted with 0.3 ℳ NaC1, was further fractionated by gel filtration on a Bio-Gel P-30 column to obtain the purified RG-II (yield: 5.8 mg kg−1 dry leaves).
Confocal laser scanning microscopy
Bone marrow-derived dendritic cells (BMDCs) were treated with fluorescein isothiocyanate (FITC)-conjugated RG-II (0.5 mg ml−1) for 30 min, fixed and stained with anti-TLR4-PE-conjugated antibody overnight at 4 °C and then stained with Alexa568-conjugated anti-rat and Alexa488-conjugated anti-rabbit antibodies (Invitrogen, Grand Island, NY, USA) for 1 h at room temperature. Cell morphology and fluorescence intensity were analyzed using the Zeiss LSM510 Meta confocal laser scanning microscope (Zeiss, Jena, Germany). Images were acquired using the LSM510 Meta software and processed using the LSM image examiner.
Generation and culture of BMDCs
BMDCs were isolated and cultured as previously described.24 Briefly, bone marrow was flushed from the tibiae and femurs of C57BL/6 mice, and red blood cells were depleted with ammonium chloride. The cells were plated in six-well culture plates (106 cells per ml, 3 ml per well) and cultured at 37 °C in the presence of 5% CO2 using OptiMEM (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum, 2 mℳℒ-glutamine, 100 U ml−1 penicillin, 100 μg/ml−1 streptomycin, 5 × 10−5 ℳ β-mercaptoethanol, 10 mℳ HEPES (pH 7.4), 20 ng ml−1 recombinant mouse granulocyte macrophage colony-stimulating factor and 20 ng ml−1 rmIL-4. On day 3 of culture, floating cells were gently removed, and fresh medium was added. On day 6 of culture, nonadherent cells and loosely adherent proliferating DC aggregates were harvested and re-plated in 60-mm dishes (1 × 106 cells per ml; 5 ml per dish) for stimulation and analysis. On day 7, ⩾80% of the nonadherent cells expressed CD11c. To obtain highly purified populations for subsequent analyses, the DCs were labeled with bead-conjugated anti-CD11c monoclonal antibody (Miltenyi Biotec, Bergisch Gladbach, Germany), followed by positive selection on paramagnetic columns (LS columns; Miltenyi Biotec) according to the manufacturer's instructions. The purity of the cell fraction selected was >95%.
Cytokine measurements
Cell culture supernatants were analyzed for IL-1β, tumor necrosis factor-α (TNF-α), IL-12p70, IL-10 and IFN-γ content in triplicate using an enzyme-linked immunosorbent assay (ELISA) kit, as described by the manufacturer (R&D Systems).
Quantitative real-time PCR
Total RNA from 5 × 106 cells was rapidly isolated using TRIzol (Invitrogen, Foster City, CA, USA) following the manufacturer's instructions. Total RNA (5 μg) was used for the synthesis of the first strand of complementary DNA. Quantitative real-time PCR was performed as previously described.25 The oligonucleotides used for amplification of CCR7 (C-C motif chemokine receptor 7) and CCR1 (C-C motif chemokine receptor 1) were as follows: CCR7 primer, forward 5′-GTGTGCTTCTGCCAAGATGA-3′ and reverse 5′-CCACGAAGCAGATGACAGAA-3′. The CCR1 PCR primers were forward 5′-AGGGCCCGAACTGTTACTTT-3′ and reverse 5′-TTCCACTGCTTCAGGCTCTT-3′. Quantitative amounts of each gene were standardized against the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping gene. Real-time PCR was performed using a Bio-Rad MiniOpticon System (Bio-Rad Laboratories, Hercules, CA, USA) with SYBR green fluorophore. Reactions were performed in a total volume of 20 μl, which included 10 μl 2 × SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 1 μl of each primer at a 10-μℳ concentration and 1 μl of the previously reverse-transcribed complementary DNA template. The reaction protocols used were as follows: denaturation (95 °C for 10 min), amplification repeated 40 times (95 °C for 30 s, 52 °C for 30 s, 72 °C for 30 s and acquisition temperature for 15 s). For each sample, ΔΔ threshold cycle (ΔΔCT) (crossing point) values were calculated as the Ct of the target gene minus the Ct of the GAPDH gene. Gene expression was derived according to the equation 2–ΔΔCt; changes in gene expression were expressed as relative to basal levels.
In vitro T-cell proliferation
Transgenic OVA-specific CD8+ T-cells were purified from bulk splenocytes via negative selection by using a mouse CD8+ T-cell kit (Miltenyi Biotec). The purity of the obtained cell population was assessed to be >93% by flow cytometry after staining with a Cy5-conjugated anti-CD8 antibody. Briefly, the cells were resuspended in 5 μℳ carboxyfluorscein diacetate succinimidyl ester (CFSE) in phosphate-buffered saline and shaken for 10 min at room temperature. Next, the cells were washed once in pure fetal bovine serum and twice in phosphate-buffered saline with 10% fetal bovine serum. Unpulsed, OVA-pulsed or OVA (1 μg ml−1)-pulsed RG-II (0.5–1 mg ml−1)-treated BMDCs (1 × 105 cells) were cultured with CFSE-labeled splenocytes of OT-1 TCR transgenic mice (1 × 106 cells per well) for 96 h. After 4 days, the cells were harvested and stained with Cy5-labeled anti-CD8 monoclonal antibody (to gate OT-1 T-cells) and analyzed by flow cytometry.
CTL assay
Cytolytic T-cell assays were performed as described by Chan et al6, 26 Briefly, lymph nodes and spleen cells were collected and pooled from mice that had been immunized 21 days earlier by intraperitoneal injection (three times at 1-week intervals) with unpulsed, OVA (1 μg ml−1)-pulsed or OVA-pulsed RG-II (0.5 mg ml−1)-treated BMDCs (1 × 106 cells). The cells were restimulated in culture with peptide and rmIL-2 (2 ng ml−1) for 5 days and tested for the ability to lyse EG7 (OVA-expressing EL4) or EL4. Lysis was detected using the CytoTox 96 assay kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. After background subtraction, lysis was calculated as follows: 100 × ((test release−spontaneous release)/ (maximum−spontaneous release)).
Preventive tumor challenge experiments
Mice were injected intraperitoneally (three times at 1-week intervals) with unpulsed, OVA (1 μg ml−1)-pulsed or OVA-pulsed RG-II (0.5 mg ml−1)-treated BMDCs (5 × 105 cells), followed by subcutaneous injection of EG7 lymphoma cells (4 × 105 cells) into the right lower back. The tumor growth was monitored at regular intervals, and the tumor mass was calculated as follows: V=(2A × B)/2, where A is the length of the short axis, and B is the length of the long axis.
Statistical analysis
All experiments were repeated at least three times with consistent results. Unless otherwise stated, data are expressed as s.e.m. Analysis of variance was used to compare experimental groups with control values, whereas comparisons between multiple groups were made using Tukey's multiple comparison tests. A P-value of <0.05 was considered to indicate statistical significance.
Results
BMDCs treated with RG-II have a highly activated phenotype
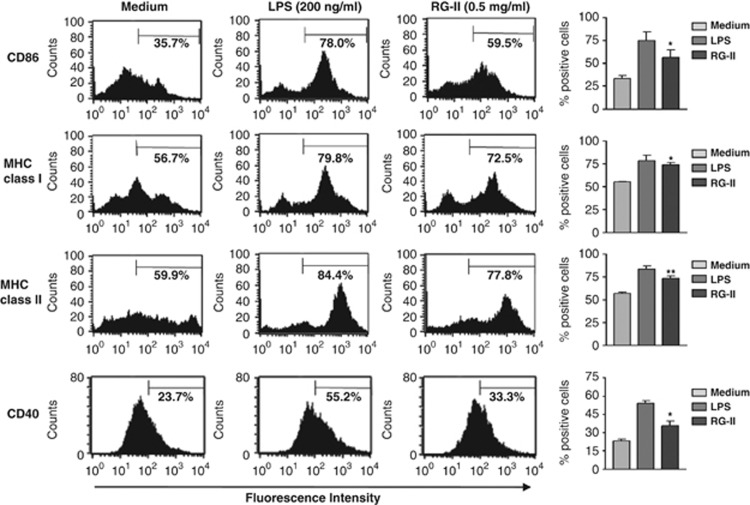
The immunostimulatory capacity of DCs depends on the extent to which MHC class and co-stimulatory molecules, including MHC class I, MHC class II, CD80, CD86, and CD40, are expressed. Highly increased expression of these surface molecules during maturation may enhance the ability of DCs to potently induce T-cell responses. Because of the fact that induction of protective tumor immunity by a DC vaccine is required to generate large numbers of fully mature and stable DCs, we evaluated whether or not RG-II promotes enhanced maturation of DCs. To assess the cytotoxicity of RG-II, we performed a cell death assay. Flow cytometric analysis using Annexin V and propidium iodide staining showed no marked change in the percentage of dead cells until a concentration of 1 mg ml−1 RG-II was reached (Supplementary Figure S1). Thus, we demonstrated that cell death is not influenced by the above-mentioned concentration of RG-II. BMDCs were cultured in the presence of lipopolysaccharide (LPS) as a positive control or RG-II (0.5 mg ml−1) for 24 h. Figure 1 depicts the effect of RG-II on DC maturation. Flow cytometric analysis of MHC class and co-stimulatory molecule expression showed that a strong increase in the expression of CD86, MHC class I and MHC class II was induced by RG-II. RG-II also significantly enhanced CD40 expression. However, co-stimulation of BMDCs with LPS (200 ng ml−1) and RG-II did not induce further increase in the expression of CD86, CD80, MHC class I and MHC class II (data not shown), indicating that stimulation of RG-II (0.5 mg ml−1) was sufficient to induce the expression of these surface molecules in BMDCs to an equivalent level as LPS stimulation.
Figure 1.
Rhamnogalacturonan II (RG-II) activates dendritic cell (DC) phenotypes. Bone marrow-derived dendritic cells (BMDCs) were incubated with 200 ng ml−1 lipopolysaccharide (LPS; positive control) or 0.5 mg ml−1 RG-II for 24 h. Co-stimulatory molecules were then analyzed by two-color flow cytometry. Cells were gated on a CD11c-positive population. DCs were stained with phycoerythrin (PE)-conjugated anti-CD86, anti-CD40 anti-MHC class I, or anti-MHC class II antibodies. Bar charts represent the mean (s.e.m.) of the percentages of positive DCs expressing CD86, major histocompatibility complex (MHC) class I, MHC class II or CD40. * And ** indicate significant differences at P<0.05 and P<0.01, respectively, compared with unstimulated cells. Results are representative of three independent experiments.
RG-II activates MAPKs and nuclear translocation of NF-κB and induces cytokine production (IL-12, IL-1β, TNF-α and IFN-γ) during DC maturation
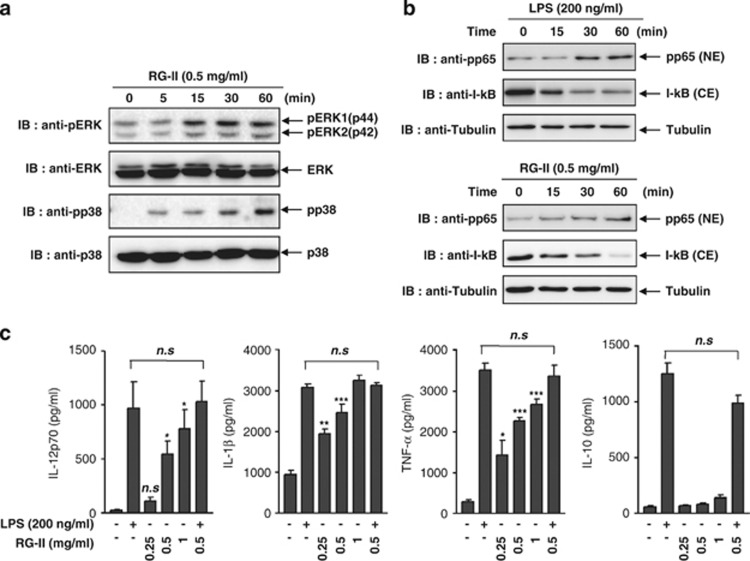
Activation of mitogen-induced protein kinases (MAPKs), including extracellular signal-regulated kinases (ERKs), p38 MAPK and nuclear factor (NF)-κB, is important in DC maturation. Recent studies suggest that these MAPK signaling pathways differentially regulate all aspects of phenotypic maturation, cytokine production and functional maturation of DCs.27 A crucial pathway for DC maturation by inflammatory stimuli involves the transcription factor NF-κB.28 Particularly, NF-κB activation is crucial for the expression of CD40, CD86 and MHC class II during DC maturation. To characterize the effects of RG-II on the MAPK and NF-κB signaling pathways, BMDCs were treated with 0.5 mg ml−1 RG-II and harvested at the indicated times. The results showed that the phosphorylation of ERK1/2 and p38 MAPK was significantly induced by RG-II stimulation (Figure 2a). In addition, nuclear translocation of the p65 subunit was observed in the RG-II-treated BMDCs (Figure 2b).
Figure 2.
Rhamnogalacturonan II (RG-II) activates both mitogen-induced protein kinases and nuclear translocation of nuclear factor-κB and induces cytokine production (interleukin (IL)-12, IL-1β, tumor necrosis factor-α (TNF-α) and interferon-γ IFN-γ)) during DC maturation. Bone marrow-derived dendritic cells (BMDCs) were treated with 0.5 mg ml−1 RG-II for 5, 15, 30 or 60 min. (a) Cell lysates were prepared and blotted with anti-phospho-ERK1/2, anti-ERK1/2, anti-phosopho-p38 and anti-p38 antibodies. (b) Nuclear extracts (NEs) were blotted with anti-phospho-p65 (Thr 254) antibody and cytosolic extracts (CEs) were also blotted with anti-I-κB antibody. BMDCs incubated with lipopolysaccharide (LPS; 200 ng ml−1) were used as a positive control. (c) BMDCs were stimulated with LPS (200 ng ml−1), RG-II (0.25–1 mg ml−1) or RG-II (0.5 mg ml−1) in the presence of LPS for 24 h. Cytokine concentrations in culture supernatants were measured in triplicate by enzyme-linked immunosorbent assay (ELISA). The mean (s.e.m.) values shown represent three independent experiments. *, ** And *** indicate significant differences at P<0.05, P<0.01 and P<0.001, respectively, compared with unstimulated cultures (NS, not significant).
Next, to demonstrate the effect of RG-II on cytokine production by DCs, secretion of IL-12p70, IL-1β, TNF-α and IL-10 by DCs was assessed by measuring the concentrations of cytokines in supernatants conditioned by BMDCs in the presence or absence of RG-II. RG-II enhanced the secretion of IL-12p70, IL-1β and TNF-α in BMDCs in a dose-dependent fashion, whereas RG-II had little effect on IL-10 production (Figure 2c). The data indicate that RG-II functions as a stimulus of MAPK and NF-κB signaling cascades and is crucial for the maturation of DCs and the production of pro-inflammatory cytokines, including IL-12, IL-1β and TNF-α, rather than the anti-inflammatory cytokine, IL-10.
RG-II leads to TLR4-mediated DC maturation
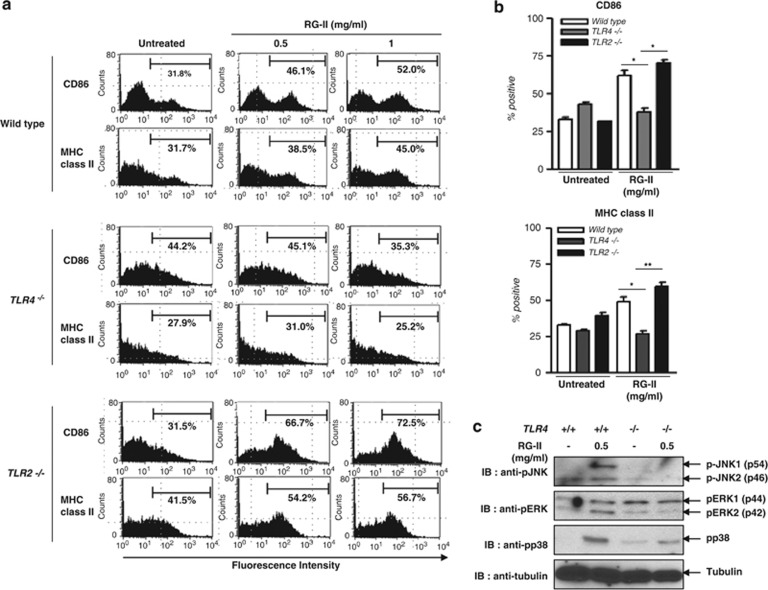
Stimulation of DCs via TLR4 can lead to the activation of MAPKs and NF-κB and expression of various inflammatory mediators, including IL-6, TNF-α and IL-12 (p40/p70). We next determined whether or not a specific TLR was involved in DC maturation and cytokine production induced by RG-II. We observed that RG-II was able to induce upregulation of the maturation markers, CD86 and MHC class II, in BMDCs from wild-type (WT) and TLR2 knockout (KO) mice, but not in TLR4 KO mice (Figures 3a and b). To prove that RG-II is an agonist of TLR4, BMDCs from WT and TLR4 KO mice were stimulated with RG-II (0.5 mg ml−1) for 30 min, and whole-cell lysates were probed for MAPKs. RG-II triggered the phosphorylation of JNK, ERK and p38 MAPK in a TLR4-dependent manner (Figure 3c). Because of the similarity of RG-II to LPS, to examine the possibility of LPS contamination, we performed IL-12 ELISA using polymyxin B (a LPS contamination inhibitor) and showed that RG-II still stimulates IL-12 expression in the presence of polymyxin B (Supplementary Figure S2). Therefore, we confirmed that LPS contamination does not occur in a RG-II sample. Taken together, these results suggest that DC maturation is mediated by RG-II, a novel agonist of TLR4, rather than TLR2.
Figure 3.
Rhamnogalacturonan II (RG-II) signals through Toll-like receptor 4 (TLR4). Bone marrow-derived dendritic cells (BMDCs), derived from wild-type (WT, TLR2 and TLR4 knockout (KO mice), were unstimulated or stimulated with lipopolysaccharide (LPS; 200 ng ml−1) or RG-II (0.5 or 1 mg ml−1) for 24 h. (a) Flow cytometric analysis of CD86 and major histocompatibility complex (MHC) class II surface expression. (b) Bar charts represent the mean±s.e.m. of the percentages of positive DCs expressing CD86 or MHC class II. *P<0.05; **P<0.01. (c) BMDCs (3 × 106 cells) derived from WT and TLR4 KO mice were stimulated with RG-II (0.5 mg ml−1) for 30 min. Whole-cell lysates were probed for the phosphorylated forms of c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38 mitogen-induced protein kinase (MAPK) by western blotting. α-Tubulin served as a protein-loading control. Results are representative of two independent experiments.
RG-II colocalizes with TLR4, and CD14 and LBP are crucial for the interaction between RG-II and TLR4
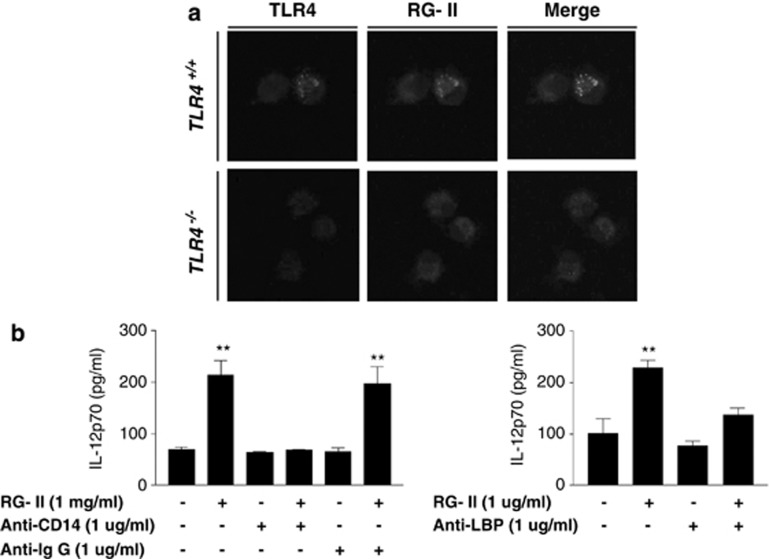
Because of TLR4 dependency on RG-II, we investigated the localization of RG-II on TLR4 using confocal microscopy to confirm the influence of RG-II on TLR4, and showed that RG-II colocalizes with TLR4 (Figure 4a). In addition, to define the interaction between RG-II on TLR4, we examined the relevance of accessory proteins, CD14 and LPS-binding protein (LBP), which are crucial for the interaction between TLR4 and LPS (a representative TLR4 agonist). We performed IL-12 ELISA in neutralization conditions using the above-mentioned antibodies. In CD14- or LBP-depleted conditions, RG-II-induced secretion of IL-12 was significantly attenuated compared with control antibody-depleted conditions (Figure 4b). The data indicated that RG-II colocalizes with TLR4, and accessory proteins (CD14 and LBP) are essential for the interaction between RG-II and TLR4.
Figure 4.
Rhamnogalacturonan II (RG-II) interacts with Toll-like receptor 4 (TLR4). (a) Colocalization of RG-II and TLR4 on bone marrow-derived dendritic cells (BMDCs). BMDCs were treated with fluorescein isothiocyanate (FITC)-conjugated RG-II (0.5 mg ml−1) for 30 min, and fixed and stained with anti-TLR4-PE-conjugated antibody overnight at 4 °C. Cell morphology and fluorescence intensity were analyzed using the Zeiss LSM510 Meta confocal laser scanning microscope. (b) CD14 and lipopolysaccharide-binding protein (LBP) are crucial for RG-II-mediated interleukin-12 (IL-12) production. BMDCs were pretreated with anti-CD14, anti-LBP and anti-IgG (1 μg ml−1) for 30 min and then stimulated with RG-II (1 mg ml−1) for 24 h. Cytokine concentrations in culture supernatants were measured in triplicate by enzyme-linked immunosorbent assay (ELISA). **Significant difference at P<0.01 compared with unstimulated cells. The results are representative of the three experiments performed.
RG-II-mediated activation of TLR4 stimulates the MyD88 and TRIF signaling pathways
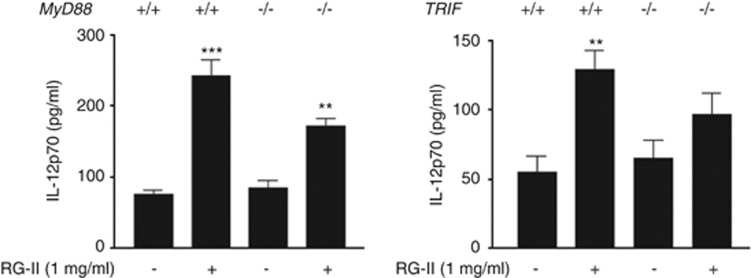
To define the detailed downstream signaling of TLR4 by RG-II, we performed IL-12 ELISAs using myeloid differentiation primary response gene 88 (MyD88) or TIR-domain-containing adapter-inducing interferon-β (TRIF) knockout BMDCs. In these experiments, we found that RG-II-mediated IL-12 secretion is partially impaired in MyD88 or TRIF knockout BMDCs compared with normal BMDCs (Figure 5). Thus, we concluded that RG-II is via MyD88- and TRIF-dependent pathways, similar to existing LPS.
Figure 5.
Rhamnogalacturonan II (RG-II)-mediated activation of Toll-like receptor 4 (TLR4) stimulates the MyD88 (myeloid differentiation primary response gene 88) and TRIF (TIR-domain-containing adapter-inducing interferon-β) signaling pathways. Bone marrow-derived dendritic cells (BMDCs; 3 × 106 cells), derived from wild-type (WT), MyD88 and TRIF knockout mice (KO mice), were stimulated with RG-II (1 mg ml−1) for 24 h. Cytokine concentrations in culture supernatants were measured in triplicate by enzyme-linked immunosorbent assay (ELISA). ** And *** indicate significant differences at P<0.01 and P<0.001, respectively, compared with unstimulated cultures.
RG-II regulates chemokine receptor expression
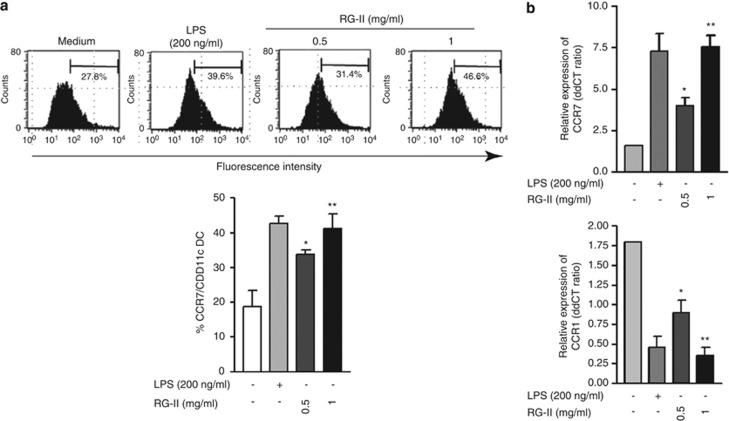
CCL19 (C-C motif chemokine ligand 19), which induces LPS-treated DC migration, is a specific ligand for CCR7 .29 During DC maturation, the differential regulation of chemokine receptors is crucial for trafficking of DCs. Whereas mature DCs induce migration to the draining lymph node by upregulating CCR7, CCR1 expressed with a high level in immature DCs is downregulated in the maturation process.30 We hypothesized that RG-II treatment alters chemokine receptor (CKR) expression, which in turn enhances DC migration to the draining lymph node. To test this hypothesis, we measured the level of protein expression of CCR7 after stimulation with LPS or RG-II using flow cytometry and quantitative PCR. Similar to the increased CCR7 expression observed in LPS-treated BMDCs, RG-II induced the upregulation of CCR7 expression in BMDCs (Figures 6a and b). Conversely, the expression of CCR1, which is reduced during DC maturation, was downregulated by RG-II treatment or LPS stimulation (Figure 6b). Consistent with this finding, based on the mouse-injected CFSE-labeled DC migration assay, we found an increment in the number of CFSE-positive RG-II-treated BMDCs in the draining lymph node compared with CFSE-positive RG-II-untreated BMDCs in vivo (data not shown). These results suggest that RG-II-mediated differential regulation of CCR7 and CCR1 is crucial for DC migration.
Figure 6.
Chemokine receptor expression is regulated by rhamnogalacturonan II (RG-II) in bone marrow-derived dendritic cells (BMDCs). (a) Effect of RG-II on CCR7 (C-C motif chemokine receptor 7) expression. On day 6, BMDCs were incubated with RG-II (0.5 mg ml−1) or lipopolysaccharide (LPS; 200 ng ml−1) for 24 h. On day 7, phycoerythrin (PE)-conjugated CCR7 cells were analyzed by flow cytometry. Cells were gated on CD11c+. (b) Effect of RG-II on CCR1 and CCR7 mRNA expression. On day 6, BMDCs were incubated with RG-II (0.5 or 1 mg ml−1) or were incubated with or without LPS (200 ng ml−1) for 24 h. Quantitative mRNA expression was measured as described in the Materials and Methods section. * And ** indicate significant differences at P<0.05 and P<0.01, respectively, compared with unstimulated cells. The results shown are representative of three independent experiments.
RG-II-stimulated BMDCs enhance CD8+ T-cell proliferation
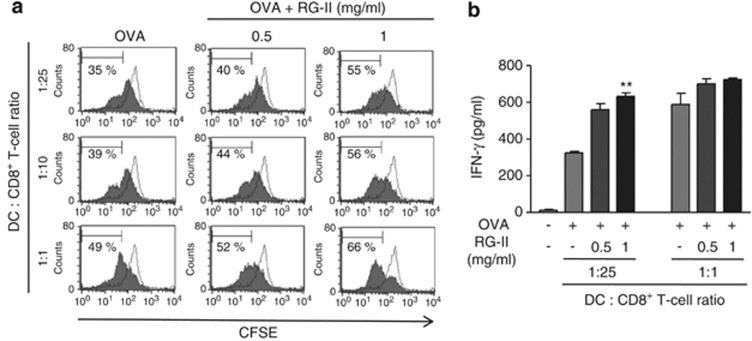
An important feature of vaccines is the ability to induce T-cell proliferation. To precisely characterize the effect of RG-II on CD8+ T-cell proliferation, we established a mixed lymphocyte reaction system using the OT-1 TCR in transgenic CD8+ T cells, which expresses a TCR specific for the MHC I-restricted OVA peptide antigen in DCs. Proliferation of transgenic OVA-specific CD8+ T cells co-cultured with OVA/RG-II-stimulated BMDCs was significantly increased compared with OVA-specific CD8+ T cells treated with OVA alone, as revealed by CFSE dilution (Figure 7a). These results were supported by ELISA experiments showing that the combination OVA/RG-II-stimulated CTLs produced twofold higher IFN-γ than OVA-stimulated OT-1 T cells (Figure 7b).
Figure 7.
Bone marrow-derived dendritic cell (BMDC) stimulation with rhamnogalacturonan II (RG-II) increases the expansion of CD8+ T cells in vitro. (a) CD8+ OT-1 T cells were labeled with carboxyfluorscein diacetate succinimidyl ester (CFSE) and activated using ovalbumin (OVA) peptide-pulsed BMDCs in the presence or absence of RG-II. As a control, unpulsed BMDC was co-cultured with CD8+ OT-1 T cells. Proliferation rates after 3 days were measured by flow cytometry. The percentage of proliferating cells of each genotype is indicated. (b) In the same set of experiments, interferon-γ (IFN-γ) production levels by CD8+ T cells were measured by enzyme-linked immunosorbent assay (ELISA) in each culture supernatant 72 h after culture initiation. **Significant difference at P<0.01 compared with OVA alone-treated cultures. Results are expressed as the mean (s.e.m.) of triplicate cultures from one of three representative experiments.
RG-II provokes the CTL response against EG7 cells, and immunization with OVA and RG-II-pulsed BMDCs significantly inhibits tumor growth and enhances the rate of survival
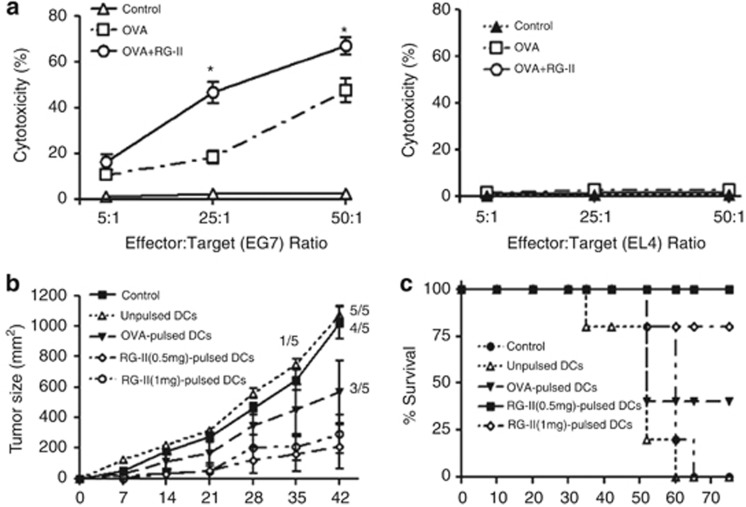
To evaluate RG-II as a potent immune adjuvant on antigen-specific CTL induction, we established the ex vivo CTL assay. When a CTL assay was conducted using purified CD8+ T cells from the OVA or RG-II-loaded BMDC-injected mouse groups, CD8+ T cells elicited different CTL activity levels against EG7, an OVA-expressing EL4 variant target cell (Figure 8a). Immunization of mice with OVA and RG-II-loaded BMDCs induced a strong CTL response against EG7 cells compared with mice immunized with OVA-pulsed BMDCs but not against the parental EL4 cells, as expected.
Figure 8.
Effect of rhamnogalacturonan II (RG-II) on the induction of ovalbumin (OVA)-specific cytotoxic T lymphocyte (CTL) activity, and dendritic cell (DC) vaccination using RG-II as an adjuvant against EG7 cells slows tumor progression. (a) Mice were intraperitoneally (i.p.) injected 3 times (1 week apart) with bone marrow-derived dendritic cells (BMDCs; 1 × 106 cells) pulsed with phosphate-buffered saline (PBS), OVA alone or OVA plus RG-II. CD8+ cells were isolated with antibody-coated magnetic beads from the pooled splenocytes and lymphocytes of each group. Purified CD8+ cells were restimulated in vitro with OVA peptide, and its cytotoxicity against EG7 or EL4 cells was measured using a CTL assay kit after 5 days of culture. Data are representative of two separate experiments. (b) Mice were vaccinated i.p. with unpulsed, OVA (1 μg ml−1)-pulsed or OVA-pulsed RG-II (0.5 or 1 mg ml−1)-treated BMDCs (5 × 105 cells), followed by subcutaneous (s.c.) injection of EG7 lymphoma cells (4 × 105 cells) into the right lower back. Growth curve data shown are the mean (s.e.m.; n=5 mice) of two separate experiments. (c) Survival response after transfer of BMDCs pulsed with PBS, OVA alone or OVA plus RG-II (0.5 or 1 mg ml−1). Data shown are pooled from two separate experiments. *P<0.05; OVA+RG-II-pulsed BMDC group was compared with OVA-pulsed BMDC group.
We next assessed the antitumor activity of RG-II using an EG7 lymphoma model. We showed that immunization with BMDCs pulsed with OVA peptide in combination with RG-II cures or delays tumor occurrence in the majority of mice subcutaneously challenged with EG7-OVA lymphoma cells. Average tumor sizes varied between the groups of immunized mice. Nonimmunized mice had the largest, fastest growing tumors. Figure 8b shows that immunized group with OVA/RG-II-pulsed BMDCs had significantly slowed tumor growth at day 42 (P<0.01) compared with the tumor growth pattern of others. All control mice and mice injected with nonpulsed BMDCs were culled by day 62. Three of the five mice injected with BMDCs pulsed with OVA alone without RG-II stimulation were culled by day 52. All mice vaccinated with BMDCs pulsed with OVA+RG-II (0.5 mg ml−1) and four of the five mice vaccinated with BMDCs pulsed with OVA+RG-II (1 mg ml−1) survived until day 65 (Figure 8c). These results suggested that RG-II is not only a strong elicitor of CTL responses to kill target cells in an OVA-specific manner, but is also an effective agent for DC-based tumor vaccination.
Discussion
In cancer immunotherapy, various DC-based vaccines against cancer have been designed and extensively studied. The potential ability of DCs for use as immune therapeutic strategies are now being investigated.9 Recent studies indicate that DC vaccination can induce immunologic and clinical responses in cancer patients.31 The mechanism of antigen presentation by DCs and the ability to stimulate antitumor T-cell responses are probably influenced by stimulation with strong adjuvants. In DC-based cancer vaccination strategies, various adjuvants for antigen priming of DCs have been reported.32 However, the development of tumor vaccines using these adjuvants has met with only limited success; adjuvants have often proved ineffective and highly toxic for therapeutic tests. Moreover, it remains unclear as to which of the adjuvants can polarize T cells for the most efficacious antitumor immunity. In the current study, we showed that RG-II has the ability to act as an effective adjuvant to promote tumor-specific cell-mediated immune responses in mice. DCs engulf and process OVA, and then convey antigen information to CD8+ T cells. Therefore, OVA-pulsed DCs are cytotoxic. Moreover, DCs pulsed with the H-2Kb binding OVA-peptide, SIINFEKL, in combination with RG-II treatment showed more effective cytotoxicity against EG7-OVA lymphoma cells and cures or delays in tumor occurrence in nearly all mice challenged subcutaneously with EG7-OVA lymphoma cells.
As nearly all T-cell responses are dependent on initial CD28 stimulation, expression of CD80/86 is necessary for mediating the potent expansion of antigen-specific CD8+ T cells.11 Crosslinking of CD40 onto DCs is also critical for long-term cellular immunity induction through the activation of many DC functions.33, 34 Thus, CD40/CD154 interactions resulted in the induction of IFN-γ-producing T helper type 1 (Th1) CD4+ T-cell clones. Moreover, CD40-activated DCs are capable of expanding CD8+ CTL clones.35, 36
Our data showed that DC stimulation with RG-II alone increased the expression of classical DC activation markers, such as CD80, CD86 and CD40 (Figure 1). We also found that RG-II is highly effective in inducing CD8+ T-cell proliferation, suggesting that RG-II can be used as a potent adjuvant. In correlation with the enhancement of surface expression of CD80, CD86 and MHC class II, we observed that BMDCs stimulated with RG-II produced the highest amount of IL-12p70, IL-1β and TNF-α (Figure 2). IL-12 is the key factor that shifts the immune balance toward a Th1 response, and it induces IFN-γ production and facilitates the switch from an established Th2 to a Th1 response.37 The Th1-based cytokine balance is desirable for sensitization of CTLs specific for tumor-associated antigens by antigen-presenting cells. Recently, synergy between DC-based vaccination and IL-12 was shown in an animal model.38, 39 In fact, potent antitumor effects of DCs genetically engineered with IL-12 have been shown in several murine models by vaccination using DCs pulsed with a tumor-associated antigen-derived peptide.40 Moreover, TNF-α and IL-1β are known to mediate innate immune responses. These cytokines are required for efficient DC maturation and mobilization to tumor-draining lymph nodes. These inflammatory mediators are critical for activating the adaptive immune response by helping IL-12 secretion.41, 42 On the basis of the important roles of these cytokines in eliciting immune responses, the increase in the production of IL-12p70, IL-1β, TNF-α and IFN-γ by BMDCs stimulated with RG-II suggests that RG-II is an effective adjuvant of cell-mediated vaccines. Whereas RG-II increases the production of pro-inflammatory cytokines, RG-II had little effect on IL-10 production (Figure 2c). With respect to IL-10 induction, p38 activity has been shown to be crucial for IL-10 expression in previous reports.43, 44 Therefore, we examined LPS- or RG-II-mediated p38 activity in WT or MyD88 knockout BMDCs and found that RG-II-induced p38 activation is weaker compared with LPS-induced p38 activation, and LPS- or RG-II-mediated p38 activity in MyD88 knockout BMDCs is partially blocked compared with WT cells (Supplementary Figure S3). Thus, the lack of IL-10 induction by RG-II, in contrast to the robust induction by LPS, is due to p38 activity.
We further characterized the mechanisms underlying the recognition of RG-II and immune responses induced by RG-II. Using KO mice, we provided evidence that RG-II activates DCs through TLR4, leading to phenotypic DC maturation and MAPK signal pathway activation (Figure 3). These are characteristics that are important for the potential use of RG-II as a vaccine adjuvant, because such characteristics constitute some of the prerequisites for the development of tumor antigen-specific T cells. However, we did not observe a synergistic effect of LPS and RG-II under co-treated conditions (Figure 2a and Supplementary Figure S4). Thus, we thought that this phenomenon is probably because RG-II and LPS share the same receptor (TLR4). Namely, RG-II and LPS have competitive actions against TLR4. In addition, our findings show that strong activation of murine DC by RG-II renders these cells competent to strongly stimulate T-cell proliferation and differentiation.
Herein, we used RG-II as an adjuvant to develop a DC-based cancer vaccine. As RG-II is a natural compound derived from the Korean traditional herbal medicine ‘ginseng' and red grape skin, this agent is safe and does not have or induce systemic toxic effects. RG-II elicits strong tumor antigen-specific CTL activity and inhibits tumor growth. In addition, when DCs were stimulated with RG-II in the current study, there was a significant increase in the production of IL-12 and IFN-γ by the DCs. These are important properties for an adjuvant that can be used in a tumor model, because these properties may be important in promoting a Th1 immune response. Overall, our results suggest that RG-II has significant potential for cancer treatment because of enhanced anticancer activity. The potency of RG-II to stimulate protective immunity in relevant animal models suggests that it could be a very attractive candidate.
Acknowledgments
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health Welfare & Family Affairs, Republic of Korea (A091047).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Experimental & Molecular Medicine website (http://www.nature.com/emm)
Supplementary Material
References
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Li T, Fan GX, Wang W, Li T, Yuan YK. Resveratrol induces apoptosis, influences IL-6 and exerts immunomodulatory effect on mouse lymphocytic leukemia both in vitro and in vivo. Int Immunopharmacol. 2007;7:1221–1231. doi: 10.1016/j.intimp.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Subramanian L, Youssef S, Bhattacharya S, Kenealey J, Polans AS, van Ginkel PR. Resveratrol: challenges in translation to the clinic-a critical discussion. Clin Cancer Res. 2010;16:5942–5948. doi: 10.1158/1078-0432.CCR-10-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill AG, McNeil M, Albersheim P. Structure of plant cell walls: VIII. A new pectic polysaccharide. Plant Physiol. 1978;62:418–422. doi: 10.1104/pp.62.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MA, Ishii T, Albersheim P, Darvill AG. Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu Rev Plant Biol. 2004;55:109–139. doi: 10.1146/annurev.arplant.55.031903.141750. [DOI] [PubMed] [Google Scholar]
- Brode S, Macary PA. Cross-presentation: dendritic cells and macrophages bite off more than they can chew! Immunology. 2004;112:345–351. doi: 10.1111/j.1365-2567.2004.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94:459–473. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- Clarke SR. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J Leukoc Biol. 2000;67:607–614. doi: 10.1002/jlb.67.5.607. [DOI] [PubMed] [Google Scholar]
- French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- Moodycliffe AM, Shreedhar V, Ullrich SE, Walterscheid J, Bucana C, Kripke ML, et al. CD40-CD40 ligand interactions in vivo regulate migration of antigen-bearing dendritic cells from the skin to draining lymph nodes. J Exp Med. 2000;191:2011–2020. doi: 10.1084/jem.191.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- Broomfield SA, van der Most RG, Prosser AC, Mahendran S, Tovey MG, Smyth MJ, et al. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J Immunol. 2009;182:5217–5224. doi: 10.4049/jimmunol.0803826. [DOI] [PubMed] [Google Scholar]
- Datta SK, Redecke V, Prilliman KR, Takabayashi K, Corr M, Tallant T, et al. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J Immunol. 2003;170:4102–4110. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh KT, Shin SJ, Son KH, Jung ID, Kang HK, Lee SJ, et al. The Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein, a toll-like receptor 4 agonist, enhances dendritic cell-based cancer vaccine potency. Exp Mol Med. 2012;44:340–349. doi: 10.3858/emm.2012.44.5.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audibert F. Adjuvants for vaccines, a quest. Int Immunopharmacol. 2003;3:1187–1193. doi: 10.1016/S1567-5769(03)00011-0. [DOI] [PubMed] [Google Scholar]
- Jung ID, Kim HY, Park JW, Lee CM, Noh KT, Kang HK, et al. RG-II from Panax ginseng C.A. Meyer suppresses asthmatic reaction. BMB Rep. 2012;45:79–84. doi: 10.5483/BMBRep.2012.45.2.79. [DOI] [PubMed] [Google Scholar]
- Gao QP, Kiyohara H, Cyong JC, Yamada H. Chemical properties and anti-complementary activities of polysaccharide fractions from roots and leaves of Panax ginseng. Planta Med. 1989;55:9–12. doi: 10.1055/s-2006-961765. [DOI] [PubMed] [Google Scholar]
- Shin KS, Kiyohara H, Matsumoto T, Yamada H. Rhamnogalacturonan II from the leaves of Panax ginseng C.A. Meyer as a macrophage Fc receptor expression-enhancing polysaccharide. Carbohydr Res. 1997;300:239–249. doi: 10.1016/s0008-6215(97)00055-4. [DOI] [PubMed] [Google Scholar]
- Noh KT, Son KH, Jung ID, Kang HK, Hwang SA, Lee WS, et al. Protein kinase C delta (PKCdelta)-extracellular signal-regulated kinase 1/2 (ERK1/2) signaling cascade regulates glycogen synthase kinase-3 (GSK-3) inhibition-mediated interleukin-10 (IL-10) expression in lipopolysaccharide (LPS)-induced endotoxemia. J Biol Chem. 2012;287:14226–14233. doi: 10.1074/jbc.M111.308841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong YI, Kim SW, Jung ID, Lee JS, Chang JH, Lee CM, et al. Curcumin suppresses the induction of indoleamine 2,3-dioxygenase by blocking the Janus-activated kinase-protein kinase Cdelta-STAT1 signaling pathway in interferon-gamma-stimulated murine dendritic cells. J Biol Chem. 2009;284:3700–3708. doi: 10.1074/jbc.M807328200. [DOI] [PubMed] [Google Scholar]
- Chan T, Chen Z, Hao S, Xu S, Yuan J, Saxena A, et al. Enhanced T-cell immunity induced by dendritic cells with phagocytosis of heat shock protein 70 gene-transfected tumor cells in early phase of apoptosis. Cancer Gene Ther. 2007;14:409–420. doi: 10.1038/sj.cgt.7701025. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Moroi Y, Uchi H, Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J Dermatol Sci. 2006;42:1–11. doi: 10.1016/j.jdermsci.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Chen G, Zheng Y, Lu L, Wu C, Zhang Y, et al. TLR4 signaling induces functional nerve growth factor receptor p75NTR on mouse dendritic cells via p38MAPK and NF-kappa B pathways. Mol Immunol. 2008;45:1557–1566. doi: 10.1016/j.molimm.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Marsland BJ, Battig P, Bauer M, Ruedl C, Lassing U, Beerli RR, et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity. 2005;22:493–505. doi: 10.1016/j.immuni.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Yanagawa Y, Onoe K. CCR7 ligands induce rapid endocytosis in mature dendritic cells with concomitant up-regulation of Cdc42 and Rac activities. Blood. 2003;101:4923–4929. doi: 10.1182/blood-2002-11-3474. [DOI] [PubMed] [Google Scholar]
- Rodeberg DA, Erskine C, Celis E. In vitro induction of immune responses to shared tumor-associated antigens in rhabdomyosarcoma. J Pediatr Surg. 2007;42:1396–1402. doi: 10.1016/j.jpedsurg.2007.03.041. [DOI] [PubMed] [Google Scholar]
- Adams S, O'Neill D, Bhardwaj N. Maturation matters: importance of maturation for antitumor immunity of dendritic cell vaccines. J Clin Oncol. 2004;22:3834–3835. doi: 10.1200/JCO.2004.99.019. [DOI] [PubMed] [Google Scholar]
- Mauri C, Mars LT, Londei M. Therapeutic activity of agonistic monoclonal antibodies against CD40 in a chronic autoimmune inflammatory process. Nat Med. 2000;6:673–679. doi: 10.1038/76251. [DOI] [PubMed] [Google Scholar]
- Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J Immunol. 2007;178:1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Terheyden P, Straten P, Brocker EB, Kampgen E, Becker JC. CD40-ligated dendritic cells effectively expand melanoma-specific CD8+ CTLs and CD4+ IFN-gamma-producing T cells from tumor-infiltrating lymphocytes. J Immunol. 2000;164:6633–6639. doi: 10.4049/jimmunol.164.12.6633. [DOI] [PubMed] [Google Scholar]
- Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- Portielje JE, Gratama JW, van Ojik HH, Stoter G, Kruit WH. IL-12: a promising adjuvant for cancer vaccination. Cancer Immunol Immunother. 2003;52:133–144. doi: 10.1007/s00262-002-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi T, Takehara T, Kanto T, Miyagi T, Kuzushita N, Sugimoto Y, et al. Administration of interleukin-12 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines in mouse hepatocellular carcinoma. Cancer Res. 2001;61:7563–7567. [PubMed] [Google Scholar]
- Yano S, Nishioka Y, Nokihara H, Sone S. Macrophage colony-stimulating factor gene transduction into human lung cancer cells differentially regulates metastasis formations in various organ microenvironments of natural killer cell-depleted SCID mice. Cancer Res. 1997;57:784–790. [PubMed] [Google Scholar]
- Geissmann F, Dieu-Nosjean MC, Dezutter C, Valladeau J, Kayal S, Leborgne M, et al. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J Exp Med. 2002;196:417–430. doi: 10.1084/jem.20020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevejo JM, Marino MW, Philpott N, Josien R, Richards EC, Elkon KB, et al. TNF-alpha -dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc Natl Acad Sci USA. 2001;98:12162–12167. doi: 10.1073/pnas.211423598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodeller F, Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The p38 mitogen-activated protein kinase regulates effector functions of primary human CD4 T cells. Eur J Immunol. 2005;35:3631–3642. doi: 10.1002/eji.200535029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.