Abstract
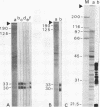
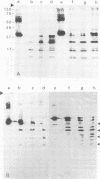
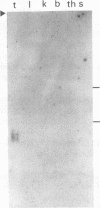
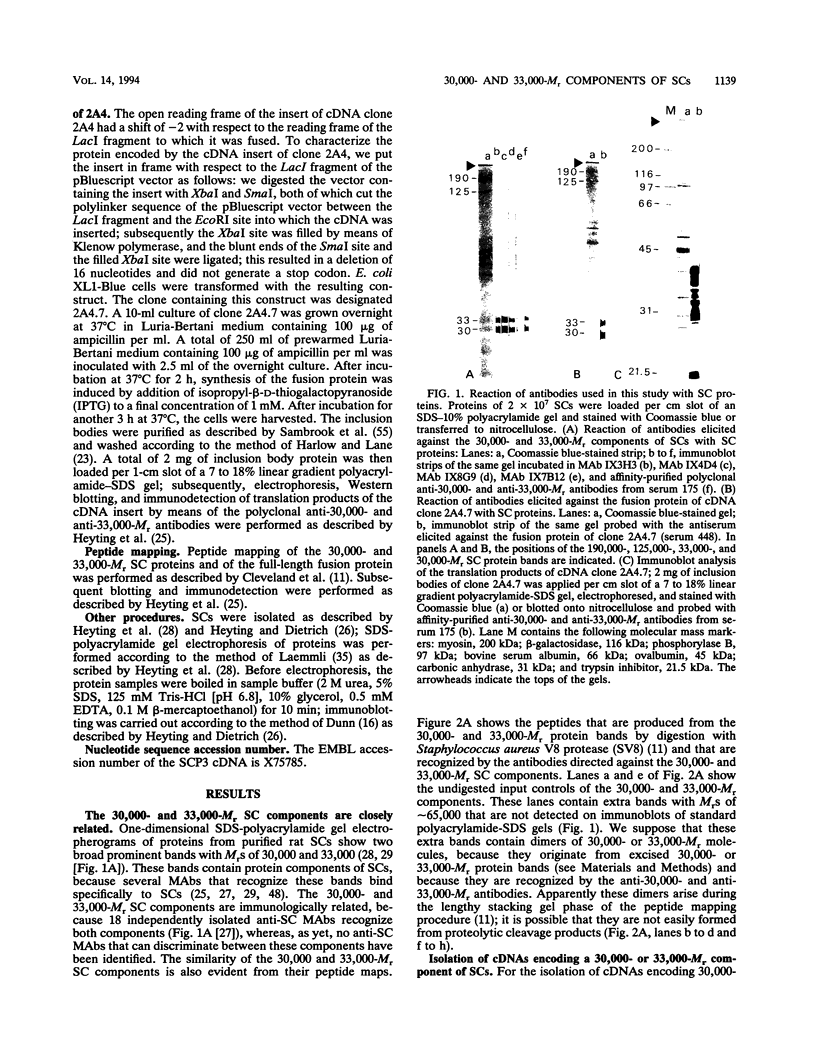
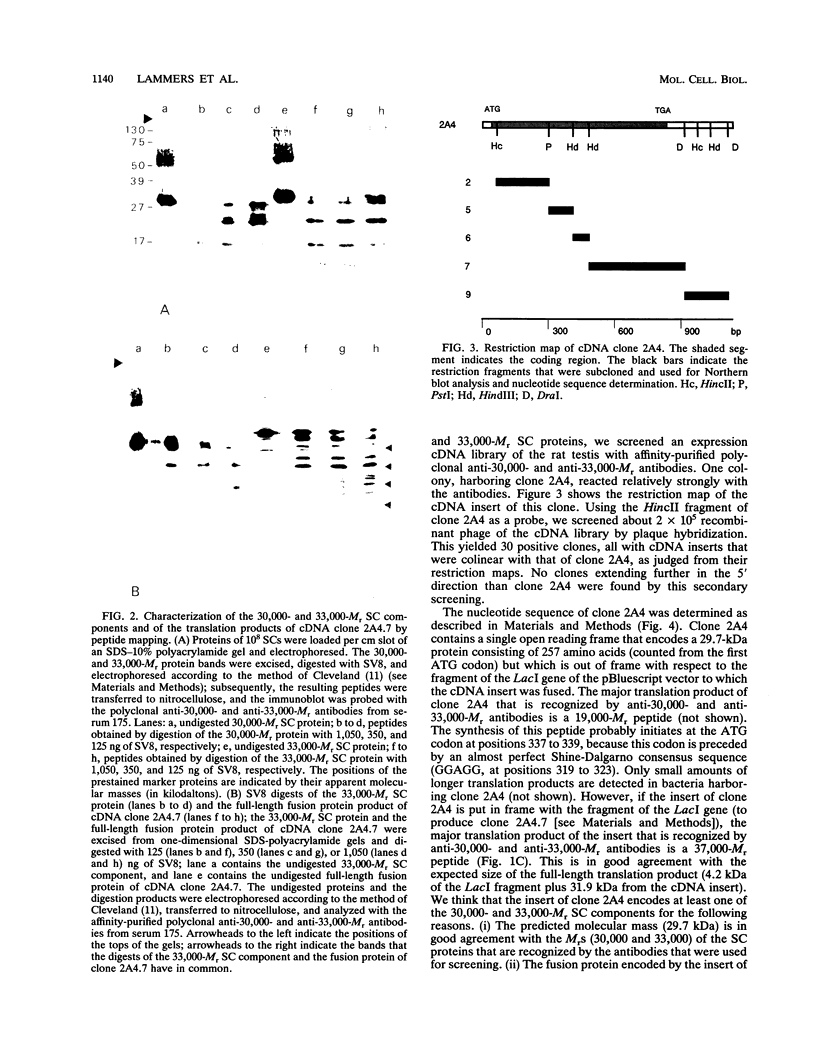
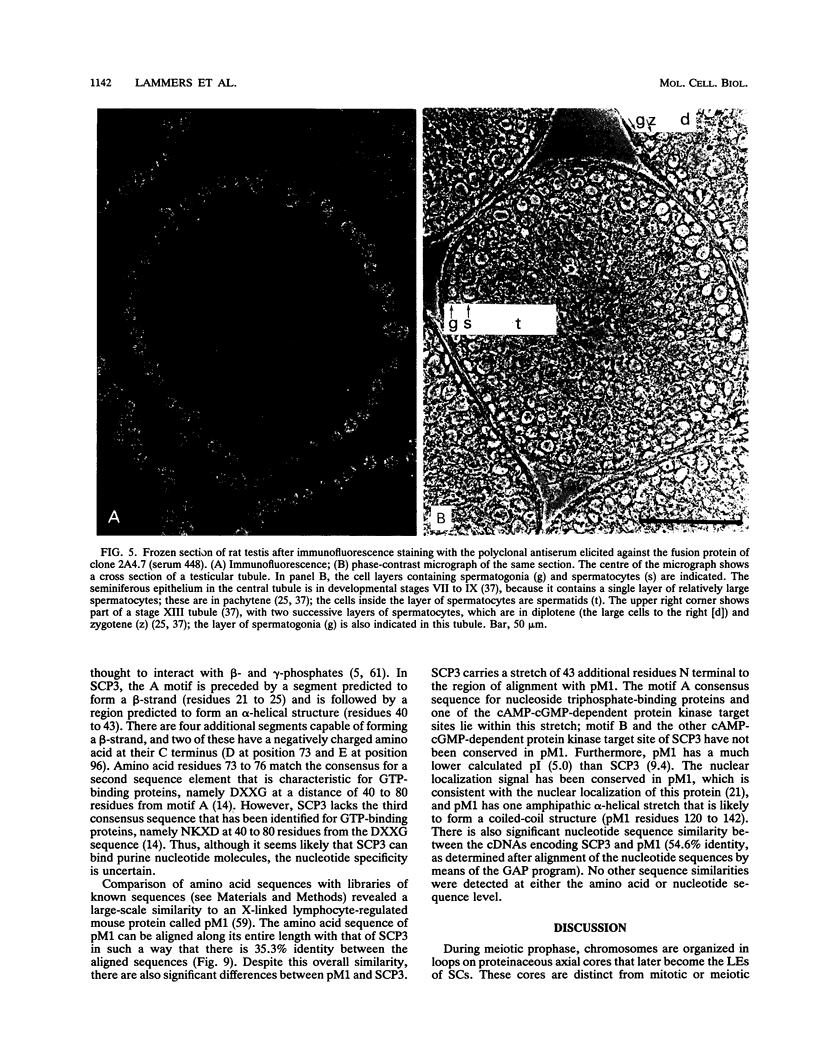
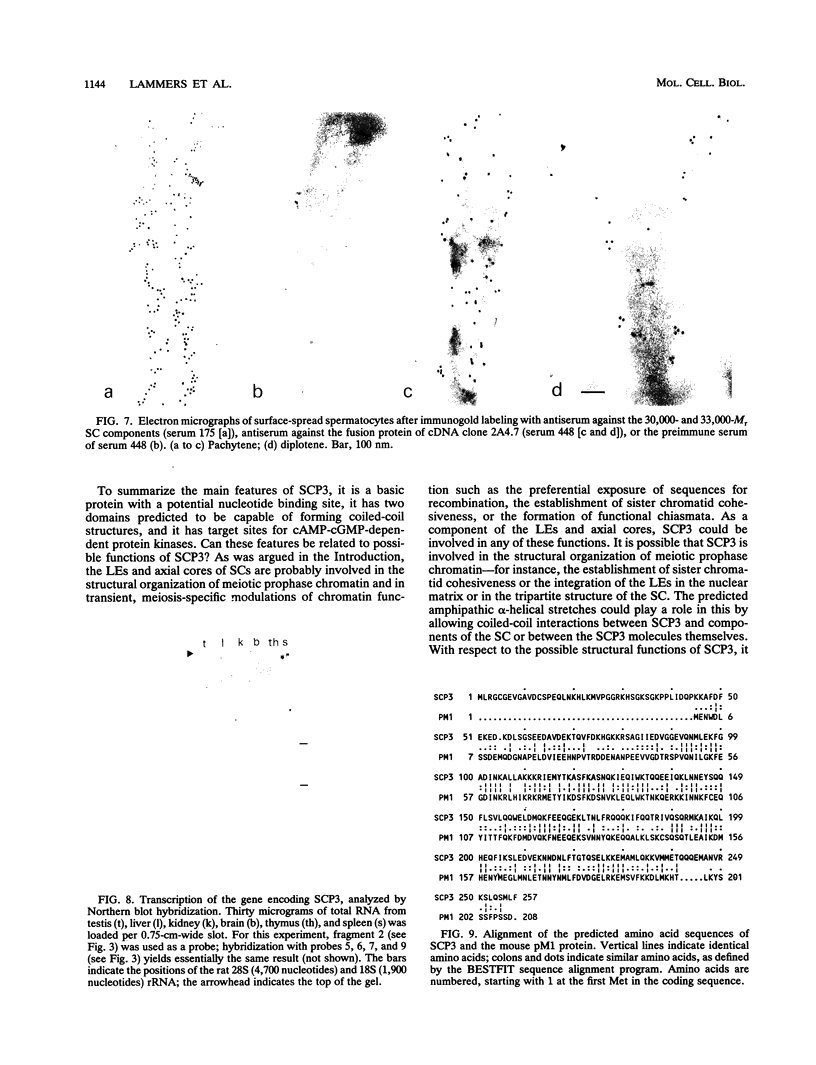
The lateral elements of synaptonemal complexes (SCs) of the rat contain major components with relative electrophoretic mobilities (M(r)S) of 30,000 and 33,000. After one-dimensional separation of SC proteins on polyacrylamide-sodium dodecyl sulfate gels, these components show up as two broad bands. These bands contain closely related proteins, as judged from their peptide maps and immunological reactivity. Using affinity-purified polyclonal anti-30,000- and anti-33,000-M(r) component antibodies, we isolated a cDNA encoding at least one of the 30,000- or 33,000-M(r) SC components. The protein predicted from the nucleotide sequence of the cDNA, called SCP3 (for synaptonemal complex protein 3), has a molecular mass of 29.7 kDa and a pI value of 9.4. It has a potential nucleotide binding site and contains stretches that are predicted to be capable of forming coiled-coil structures. In the male rat, the gene encoding SCP3 is transcribed exclusively in the testis. SCP3 has significant amino acid similarity to the pM1 protein, which is one of the predicted products of an X-linked lymphocyte-regulated gene family of the mouse: there are 63% amino acid sequence similarity and 35% amino acid identity between the SCP3 and pM1 proteins. However, SCP3 differs from pM1 in several respects, and whether the proteins fulfill related functions is still an open question.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alani E., Subbiah S., Kleckner N. The yeast RAD50 gene encodes a predicted 153-kD protein containing a purine nucleotide-binding domain and two large heptad-repeat regions. Genetics. 1989 May;122(1):47–57. doi: 10.1093/genetics/122.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Oltz E. M., Young F., Gorman J., Taccioli G., Chen J. VDJ recombination. Immunol Today. 1992 Aug;13(8):306–314. doi: 10.1016/0167-5699(92)90043-7. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bishop D. K., Park D., Xu L., Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992 May 1;69(3):439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- Bradley M. K., Smith T. F., Lathrop R. H., Livingston D. M., Webster T. A. Consensus topography in the ATP binding site of the simian virus 40 and polyomavirus large tumor antigens. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4026–4030. doi: 10.1073/pnas.84.12.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Alani E., Kleckner N. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell. 1990 Jun 15;61(6):1089–1101. doi: 10.1016/0092-8674(90)90072-m. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chelsky D., Ralph R., Jonak G. Sequence requirements for synthetic peptide-mediated translocation to the nucleus. Mol Cell Biol. 1989 Jun;9(6):2487–2492. doi: 10.1128/mcb.9.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. D., Chan C. S., Pirrotta V. Conserved DNA binding and self-association domains of the Drosophila zeste protein. Mol Cell Biol. 1992 Feb;12(2):598–608. doi: 10.1128/mcb.12.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Compton D. A., Szilak I., Cleveland D. W. Primary structure of NuMA, an intranuclear protein that defines a novel pathway for segregation of proteins at mitosis. J Cell Biol. 1992 Mar;116(6):1395–1408. doi: 10.1083/jcb.116.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever T. E., Glynias M. J., Merrick W. C. GTP-binding domain: three consensus sequence elements with distinct spacing. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1814–1818. doi: 10.1073/pnas.84.7.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
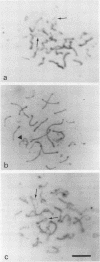
- Dietrich A. J., Kok E., Offenberg H. H., Heyting C., de Boer P., Vink A. C. The sequential appearance of components of the synaptonemal complex during meiosis of the female rat. Genome. 1992 Jun;35(3):492–497. doi: 10.1139/g92-072. [DOI] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Glass D. B., Krebs E. G. Optimal spatial requirements for the location of basic residues in peptide substrates for the cyclic AMP-dependent protein kinase. J Biol Chem. 1980 May 10;255(9):4240–4245. [PubMed] [Google Scholar]
- Game J. C., Sitney K. C., Cook V. E., Mortimer R. K. Use of a ring chromosome and pulsed-field gels to study interhomolog recombination, double-strand DNA breaks and sister-chromatid exchange in yeast. Genetics. 1989 Dec;123(4):695–713. doi: 10.1093/genetics/123.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garchon H. J., Davis M. M. The XLR gene product defines a novel set of proteins stabilized in the nucleus by zinc ions. J Cell Biol. 1989 Mar;108(3):779–787. doi: 10.1083/jcb.108.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garchon H. J. The Xlr (X-linked lymphocyte regulated) gene family (a candidate locus for an X-linked primary immune deficiency). Immunodefic Rev. 1991;2(4):283–302. [PubMed] [Google Scholar]
- Gutz H. Site Specific Induction of Gene Conversion in SCHIZOSACCHAROMYCES POMBE. Genetics. 1971 Nov;69(3):317–337. doi: 10.1093/genetics/69.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyting C., Dettmers R. J., Dietrich A. J., Redeker E. J., Vink A. C. Two major components of synaptonemal complexes are specific for meiotic prophase nuclei. Chromosoma. 1988;96(4):325–332. doi: 10.1007/BF00286921. [DOI] [PubMed] [Google Scholar]
- Heyting C., Dietrich A. J. Meiotic chromosome preparation and protein labeling. Methods Cell Biol. 1991;35:177–202. doi: 10.1016/s0091-679x(08)60573-7. [DOI] [PubMed] [Google Scholar]
- Heyting C., Dietrich A. J., Moens P. B., Dettmers R. J., Offenberg H. H., Redeker E. J., Vink A. C. Synaptonemal complex proteins. Genome. 1989;31(1):81–87. doi: 10.1139/g89-016. [DOI] [PubMed] [Google Scholar]
- Heyting C., Moens P. B., van Raamsdonk W., Dietrich A. J., Vink A. C., Redeker E. J. Identification of two major components of the lateral elements of synaptonemal complexes of the rat. Eur J Cell Biol. 1987 Feb;43(1):148–154. [PubMed] [Google Scholar]
- KAFER E. The processes of spontaneous recombination in vegetative nuclei of Aspergillus nidulans. Genetics. 1961 Dec;46:1581–1609. doi: 10.1093/genetics/46.12.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyk L. C., Hartwell L. H. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics. 1992 Oct;132(2):387–402. doi: 10.1093/genetics/132.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A., Nishiyama K., Nakanishi H., Uratsuji Y., Nomura H., Takeyama Y., Nishizuka Y. Studies on the phosphorylation of myelin basic protein by protein kinase C and adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1985 Oct 15;260(23):12492–12499. [PubMed] [Google Scholar]
- Kleckner N., Padmore R., Bishop D. K. Meiotic chromosome metabolism: one view. Cold Spring Harb Symp Quant Biol. 1991;56:729–743. doi: 10.1101/sqb.1991.056.01.082. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P., CLERMONT Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952 Nov 20;55(4):548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb N. J., Cavadore J. C., Labbe J. C., Maurer R. A., Fernandez A. Inhibition of cAMP-dependent protein kinase plays a key role in the induction of mitosis and nuclear envelope breakdown in mammalian cells. EMBO J. 1991 Jun;10(6):1523–1533. doi: 10.1002/j.1460-2075.1991.tb07672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl K. F. His and hers recombinational hotspots. Trends Genet. 1991 Sep;7(9):273–276. doi: 10.1016/0168-9525(91)90306-B. [DOI] [PubMed] [Google Scholar]
- Lindsley J. E., Wang J. C. Proteolysis patterns of epitopically labeled yeast DNA topoisomerase II suggest an allosteric transition in the enzyme induced by ATP binding. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10485–10489. doi: 10.1073/pnas.88.23.10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl J. The initiation of meiotic chromosome pairing: the cytological view. Genome. 1990 Dec;33(6):759–778. doi: 10.1139/g90-115. [DOI] [PubMed] [Google Scholar]
- Maguire M. P. Sister chromatid cohesiveness: vital function, obscure mechanism. Biochem Cell Biol. 1990 Nov;68(11):1231–1242. doi: 10.1139/o90-183. [DOI] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. Binding of the recA protein of Escherichia coli to single- and double-stranded DNA. J Biol Chem. 1981 Aug 25;256(16):8835–8844. [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic features in the amino acid sequence of nematode myosin rod. J Mol Biol. 1983 Mar 15;164(4):605–626. doi: 10.1016/0022-2836(83)90053-0. [DOI] [PubMed] [Google Scholar]
- Meuwissen R. L., Offenberg H. H., Dietrich A. J., Riesewijk A., van Iersel M., Heyting C. A coiled-coil related protein specific for synapsed regions of meiotic prophase chromosomes. EMBO J. 1992 Dec;11(13):5091–5100. doi: 10.1002/j.1460-2075.1992.tb05616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzayan C., Copeland C. S., Snyder M. The NUF1 gene encodes an essential coiled-coil related protein that is a potential component of the yeast nucleoskeleton. J Cell Biol. 1992 Mar;116(6):1319–1332. doi: 10.1083/jcb.116.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki W. Y., Orr-Weaver T. L. Sister-chromatid misbehavior in Drosophila ord mutants. Genetics. 1992 Dec;132(4):1047–1061. doi: 10.1093/genetics/132.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Heyting C., Dietrich A. J., van Raamsdonk W., Chen Q. Synaptonemal complex antigen location and conservation. J Cell Biol. 1987 Jul;105(1):93–103. doi: 10.1083/jcb.105.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenberg H. H., Dietrich A. J., Heyting C. Tissue distribution of two major components of synaptonemal complexes of the rat. Chromosoma. 1991 Nov;101(2):83–91. doi: 10.1007/BF00357057. [DOI] [PubMed] [Google Scholar]
- Pearson W. R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Pinna L. A. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990 Sep 24;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Roca J., Wang J. C. The capture of a DNA double helix by an ATP-dependent protein clamp: a key step in DNA transport by type II DNA topoisomerases. Cell. 1992 Nov 27;71(5):833–840. doi: 10.1016/0092-8674(92)90558-t. [DOI] [PubMed] [Google Scholar]
- Rockmill B., Roeder G. S. Meiosis in asynaptic yeast. Genetics. 1990 Nov;126(3):563–574. doi: 10.1093/genetics/126.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S. Chromosome synapsis and genetic recombination: their roles in meiotic chromosome segregation. Trends Genet. 1990 Dec;6(12):385–389. doi: 10.1016/0168-9525(90)90297-j. [DOI] [PubMed] [Google Scholar]
- STRICKLAND W. N. An analysis of interference in Aspergillus nidulans. Proc R Soc Lond B Biol Sci. 1958 Jul 1;149(934):82–101. doi: 10.1098/rspb.1958.0053. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultes N. P., Szostak J. W. Decreasing gradients of gene conversion on both sides of the initiation site for meiotic recombination at the ARG4 locus in yeast. Genetics. 1990 Dec;126(4):813–822. doi: 10.1093/genetics/126.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara A., Ogawa H., Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992 May 1;69(3):457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- Siegel J. N., Turner C. A., Klinman D. M., Wilkinson M., Steinberg A. D., MacLeod C. L., Paul W. E., Davis M. M., Cohen D. I. Sequence analysis and expression of an X-linked, lymphocyte-regulated gene family (XLR). J Exp Med. 1987 Dec 1;166(6):1702–1715. doi: 10.1084/jem.166.6.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Benavente R. Identification of a structural protein component of rat synaptonemal complexes. Exp Cell Res. 1992 Feb;198(2):291–297. doi: 10.1016/0014-4827(92)90382-i. [DOI] [PubMed] [Google Scholar]
- Story R. M., Steitz T. A. Structure of the recA protein-ADP complex. Nature. 1992 Jan 23;355(6358):374–376. doi: 10.1038/355374a0. [DOI] [PubMed] [Google Scholar]
- Sym M., Engebrecht J. A., Roeder G. S. ZIP1 is a synaptonemal complex protein required for meiotic chromosome synapsis. Cell. 1993 Feb 12;72(3):365–378. doi: 10.1016/0092-8674(93)90114-6. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigley D. B., Davies G. J., Dodson E. J., Maxwell A., Dodson G. Crystal structure of an N-terminal fragment of the DNA gyrase B protein. Nature. 1991 Jun 20;351(6328):624–629. doi: 10.1038/351624a0. [DOI] [PubMed] [Google Scholar]
- Wu L. C., Fisher P. A., Broach J. R. A yeast plasmid partitioning protein is a karyoskeletal component. J Biol Chem. 1987 Jan 15;262(2):883–891. [PubMed] [Google Scholar]
- Yang C. H., Lambie E. J., Snyder M. NuMA: an unusually long coiled-coil related protein in the mammalian nucleus. J Cell Biol. 1992 Mar;116(6):1303–1317. doi: 10.1083/jcb.116.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wettstein D., Rasmussen S. W., Holm P. B. The synaptonemal complex in genetic segregation. Annu Rev Genet. 1984;18:331–413. doi: 10.1146/annurev.ge.18.120184.001555. [DOI] [PubMed] [Google Scholar]