Abstract
Elucidation of abacavir hypersensitivity syndrome in HIV-1 patients establishes a new pharmacogenetic paradigm. A drug can alter the bodies’ surface display of peptides bound to a given HLA molecule, thereby initiating life threatening T-cell reactions against self-peptides not previously exposed to the immune system.
The nucleoside reverse-transcriptase inhibitor abacavir has been shown to be an effective drug against HIV-1 but is associated with hypersensitivity reactions appearing in the first 6 weeks of administration in 5–10% of patients 1. These mandate immediate drug cessation to avoid life threatening sequelae. Two groups previously reported an association between the abacavir hypersensitivity syndrome (AHS) and the major histocompatibility complex (MHC) class I allele HLA-B*57:012,3. HLA-B*57:01 genetic screening is currently an effective way to prevent AHS4. But what is the molecular basis of AHS? An elegant study on page ____ of this issue by Illing et al now provides the answer.5
Thymus-derived T-lymphocytes detect perturbations among the body’s own cellular surface constituents, distinguishing abnormal from normal cells(Reviewed in 6). Self-vs. non-self-discrimination is at the core of T-lymphocyte recognition, endowed by clonal surface-bound αβ T-cell receptors (TCRs). Within any given mammal, there are billions of T-cells with their own unique TCR. Each T-lymphocyte identifies a peptide bound to the groove of an MHC molecule (HLA or H-2 in human and mouse, respectively) displayed as a complex (pMHC) on a nucleated cell. T-lymphocytes search for peptides arrayed on other cell surfaces during immune surveillance. Aberrant processes within a human cell are reflected by alterations of surface HLA-bound peptides. Once a T-lymphocyte recognizes a variant peptide, for example, a foreign peptide derived from a viral protein bound to a self-MHC molecule on an epithelial surface, signaling is initiated for CD8 cytotoxic T-lymphocytes (CTL) to proliferate, differentiate and eliminate the “flagged” cell7 (Figure 1A). Several copies of one peptide displayed among a sea of unrelated self-peptides (100,000) on a cell surface can be detected.8
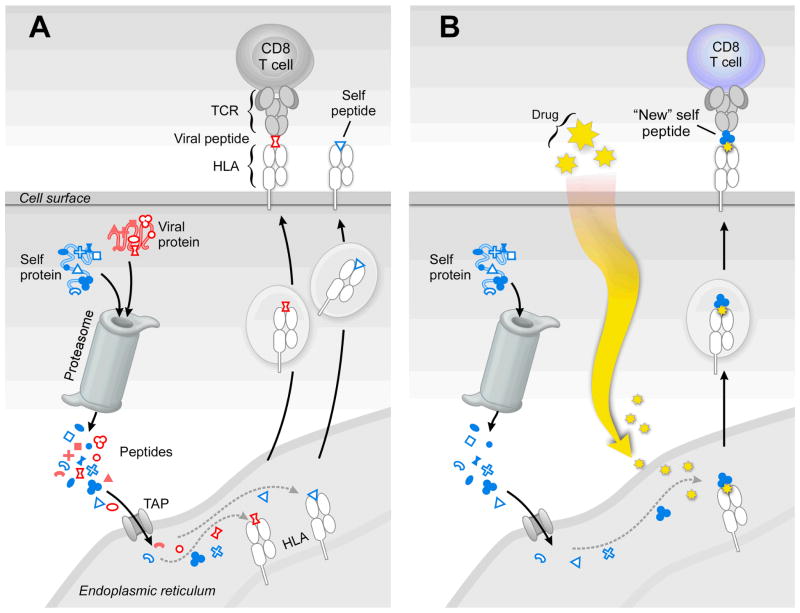
Figure 1. A case of mistaken identity.
a) CD8 CTL kill virally infected cells whose surface HLA molecules display peptides derived from viral proteins. In the cytoplasm, both foreign viral proteins (red) and endogenous self-proteins (blue) are cleaved by the proteosome into multiple peptides (represented by different shapes). Then a subset is transported via TAP into the endoplasmic reticulum. Those peptides matching a specific HLA molecule’s binding motif are loaded for export and cell surface display. Viral peptides associated with self-HLA are recognized as foreign, engendering a brisk CTL response to destroy the infected cell. In contrast, CTL do not kill uninfected cells, since self-peptide reactive T cells were deleted by thymic tolerance mechanisms early in life. b) Administration of a specific HLA-binding drug, like abacavir, alters the HLA peptide binding characteristics of the allele with which it interacts (HLA-B*57:01), thereby loading and displaying “new” self-peptides on the antigen-presenting cell surface. Since this “new” self-peptide (3-lobe clover symbol) was not displayed during thymic tolerance induction, “new” self-peptide specific CTL (blue) were not deleted and, hence, unleash an immune attack causing AHS. In panels A and B, only an individual viral or self-peptide and one corresponding CD8 CTL is shown for simplicity.
Illing et al now show that the abacavir prodrug binds directly to the HLA-B*57:01 molecule. In general, peptides associate with HLA molecules through inserting several of their amino acid residue side chains and amino and carboxyl termini9 into a set of highly allele-specific HLA binding pockets (termed A–F). Abacavir binds to the F pocket of the HLA-B*57:01 molecule, shifting the preference for the carboxy-terminal peptide anchor residues from tryptophan or phenylalanine to include isoleucine or leucine. This drug-bound alteration in HLA-B*57:01-presented peptides drives different CD8+ T-cells to become activated and destructive (Figure 1B).
Several interesting mass spectrometry observations were made. First, the unmodified abacavir compound binds directly to HLA-B*57:01, but not the closely related HLA-B*57:03 allele. Second, sequence determination of up to 2,500 HLA-B*57:01 bound peptides indicated that there was a drug-induced shift in the peptides. By contrast, following drug treatment of HLA-B*57:03, an allele not linked to AHS, no such shift was observed. Third, the magnitude of the peptide differences was enormous, representing 20–25% of the recovered peptides. As expected, there was no change in peptides bound to HLA-B*57:03 upon drug treatment. These data clearly imply that HLA-B*57:01 enters the endoplasmic reticulum and complexes with abacavir prodrug prior to peptide loading, thereby altering the pool of self-peptides bound to this allele and displayed on the cell surface for CTL recognition (Fig. 1).
T-cells responding to the HLA-B*57:01 peptide display in the presence of abacavir were examined by assessing Vβ usage as a proxy for TCR diversity since the Vβ and Vα domains form the TCR recognition surface for pMHC binding. There was no obvious preferred TCR specificity, in keeping with an earlier study revealing large scale activation of CD8 T-cells as the cellular basis of the systemic drug hypersensitivity.10 To examine the structural cause of the change in bound peptides, the authors refolded HLA-B*57:01 in the presence of abacavir plus LTTKLTNTNI, a self-peptide derived from cytochrome C oxidase subunit 2 exclusively isolated from HLA-B*57:01 in the presence of drug. This x-ray crystallographic structure was compared to that of an HLA-B*57:01 complex with the self-peptide LFSPVTKSF in the absence of drug. The cyclopropyl moiety of abacavir protruded into the F pocket accounting for the smaller amino acid side chain preference following drug binding. The structure revealed little drug-peptide interaction while the HLA-B*57:01 molecule buried more than 75% of the Abacavir surface area. The purinyl moiety hydrogen bonded to Asp 114 and Ser 116, residues unique to this HLA molecule, explaining abacavir’s allele specificity.
Within the thymus, central tolerance is the key mechanism by which T lineage cells are rendered non-reactive against self. Autoreactive T-cells stimulated by self-peptides bound to self-MHC molecules are deleted via an apoptotic process termed negative selection.11 As a consequence, a repertoire of T-cells is selected whose activation is dependent upon detecting “foreign” peptides derived from viruses, other infectious pathogens or tumor-related peptides bound to self-MHC molecules on the altered cell.
The αβ TCR mediates recognition with extraordinary sensitivity and specificity due to its structural features.6 Even slight perturbation from self can trigger severe consequences. For example, the urushiol contained in various plants including poison ivy, causes an allergic contact dermatitis mediated primary by CD8 T-cells. That T-cell response is directed against self-peptides derivatized by the oxidized urushiol and hence recognized as foreign. Likewise, peptides post-translationally modified as through conversion of arginine to citrulline can give rise to T-cell activation in the joints of patients with rheumatoid arthritis.12
Some self-proteins are never exposed to the immune system except under pathological conditions. A case of mistaken identity (self-protein perceived by the immune system as foreign) in the absence of central tolerance may follow. Sympathetic ophthalmia is one example. Following a piercing injury to one eye, exposed ocular proteins that are normally sequestered, induce T-cell mediated granulomatous uveitis including in the contralateral eye of the patient. Unless given immunosuppressive drugs, total blindness can follow as a result of a vicious T-cell immune attack on the normal eye. By exposing self-peptides in the HLA-B*57:01 groove not normally associated with this allele, abacavir initiates an autoimmune attack, whereby T-cells perceive “new” self-peptides as foreign in the absence of central tolerance.5
This false sense of non-self identity is not restricted to abacavir. Illing et al showed that the anticonvulsant carbamazepine, linked to HLA-B*15:02 hypersensitivity, binds to that allele and alters its associated peptide set as well.5 Given rapidly evolving HLA typing methods and the awareness engendered by the current study, many examples of known drug-HLA associated disease13 will be linked to similar mechanisms, mandating broad HLA pre-screening to obviate such drug reactions. By extension, one might predict that other HLA-drug interactions in conjunction with organ-specific metabolic processes could limit altered peptide display to a specific parenchymal tissue, thereby restricting inflammation to one organ (i.e. liver, kidney, bone marrow, etc.). Environmental toxins and other chemicals may target HLA as well. The extreme polymorphism14 of the HLA-locus (4,269 HLA-A, -B and -C molecules at last count) thought to be driven by selective forces from infectious pathogens15 affords ample opportunity for drug-HLA interactions. Analogous drug interactions probably exist for class II MHC molecule (DR, DP and DQ) which bind peptides recognized by CD4 T-cells. The field of pharmacogenetics is going to expand, and in so doing, redefine the basis of many forms of autoimmunity.
References
- 1.Hetherington S, et al. Clin Ther. 2001;23:1603–1614. doi: 10.1016/s0149-2918(01)80132-6. [DOI] [PubMed] [Google Scholar]
- 2.Mallal S, et al. Lancet. 2002;359:727–732. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 3.Hetherington S, et al. Lancet. 2002;359:1121–1122. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- 4.Mallal S, et al. NEJM. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 5.Illing PT, et al. Current issue. 2012 [Google Scholar]
- 6.Kim ST, et al. Frontiers in T Cell Biology. 2012;3:article 76. doi: 10.3389/fimmu.2012.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith KA. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 8.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Immunity. 1996;6:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 9.Madden DR, Gorga JC, Strominger JL, Wiley DC. Nature. 1991;353:321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- 10.Chessman D, et al. Immunity. 2008;28:822–832. doi: 10.1016/j.immuni.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 11.von Boehmer Immunol Today. 1992;11:454–458. doi: 10.1016/0167-5699(92)90075-I. [DOI] [PubMed] [Google Scholar]
- 12.Klareskog L, Rönnelid J, Lundberg K, Padyukov L, Alfredsson L. Annu Rev Immunol. 2008;26:651–675. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 13.Alfirevic A, Pirmohamed M. Pharmaceuticals. 2011;4:69–90. [Google Scholar]
- 14.Robinson J, Mistr K, McWilliam H, Lopez R, Parham P, Marsh SG. Nucleic Acids Research. 2011;39(Suppl 1):D1171–6. doi: 10.1093/nar/gkq998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty PC, Zinkernagel RM. Lancet. 1975;i:1406–1409. doi: 10.1016/s0140-6736(75)92610-0. [DOI] [PubMed] [Google Scholar]