Abstract
Objectives
Lymph node (LN) staging provides critical information in non-small cell lung cancer (NSCLC) patients. Lymphangiogenesis may be an important contributor to the pathophysiology of lymphatic metastases. We hypothesized that the presence of lymph node micrometastases positively correlates with VEGF-A/C/D and VEGF-receptor-3 (lymphangiogenic factors) expression in lymph nodes.
Methods
Forty NSCLC patients had pre-operative PET-CT and mediastinoscopy. RT-PCR assays for mRNA expression of epithelial markers (CK-7, CEACAM-5 and PLUNC) were performed in selected fluorodeoxyglucose (FDG)-avid lymph nodes. VEGF-A/C/D and VEGF-receptor-3 expression levels were measured in primary tumors and lymph nodes. Wilcoxon rank sum test was run for the association between the RT-PCR epithelial marker levels and VEGF expression levels in the LNs.
Results
RT-PCR for CK-7, CEACAM5 or PLUNC indicated lymph node micrometastatic disease in 19 of 35 patients (54%). There was a high correlation between detection of micrometastases and VEGF-A/C/D or VEGF-receptor-3 expression levels in lymph nodes. Median follow-up was 12.6 months.
Conclusions
RT-PCR analysis of FDG-avid lymph nodes results in up-staging of patients. Micrometastases correlate with the expression of VEGF in lymph nodes in NSCLC patients. This may reflect the role of lymphangiogenesis in promoting metastases.
Introduction
Lung cancer is the most frequent cause of cancer death in both men and women in the United States and will account for about 27% of all estimated cancer deaths in 2012 (1). The staging of lung cancer plays a critical role in efforts to combat this disease. Lymph node metastasis is the most important prognostic factor in locoregional lung cancer. However, the accurate identification of all lymph node disease in patients remains an elusive goal. This is reflected in the modest 5-year survival (73%) reported in the earliest stage of non-small cell lung cancer (NSCLC) – Stage IA by the International Association for the Study of Lung Cancer (IASLC) (2). Nearly 40% of node-negative patients will develop recurrent disease and die within 2 years (3). This is believed to be due to understaging of lung cancer patients i.e. under-recognition of micrometastases by standard hematoxylin and eosin (H&E) staining of lymph nodes. Thus, better staging methods are necessary to stratify patients, make therapeutic choices and evaluate the effectiveness of various treatment modalities in clinical trials. Intensive pathologic techniques such as serial sectioning, immunohistochemistry and RT-PCR are more sensitive in detecting these micrometastases (4–7). However, these techniques are labor intensive and expensive. Thus, they can practically be applied only to a limited number of lymph nodes in each patient. Multiple techniques for sentinel lymph node mapping have been studied as a means of selecting a few lymph nodes per patient for detailed pathologic analysis (8–11). Following a small 10-patient pilot study (12) to investigate the feasibility of a handheld gamma probe to detect lymph node FDG avidity in NSCLC surgical patients, we embarked on a larger study to assess its clinical utility.
Malignant cells metastasize from the primary tumor to other organs via either the lymphatic or vascular network. Indeed, tumor metastasis to regional lymph nodes often represents the first step of tumor dissemination and serves as a major prognostic indicator for the progression of human cancers (13). Improved therapy for lung cancer requires better fundamental understanding of the molecular mechanisms leading to lymphatic metastasis. Lymphangiogenesis has been suggested as an essential component in lymph node metastasis (14–16). The VEGF-C/VEGF-D/VEGFR-3 axis is the best validated signaling system for promoting lymphangiogenesis associated with solid tumors and the metastatic spread of tumor cells to lymph nodes (14). VEGF-A has also been shown to influence lymphangiogenesis (17).
This report focuses on the correlation of the presence of micrometastases with VEGF A, C, D and VEGF receptor-3 expression in the lymph nodes of a small cohort of patients that had lymph node mapping during their surgical resection.
Methods
Our Institutional Review Board (IRB) approved a radioguided lymph node mapping study on September 6, 2007 for 100 patients with resectable, clinical Stage I or II non-small cell lung cancer. Individual patient consent was obtained. Ancillary studies using molecular markers were written into the protocol. An exploratory subset of 40 patients was selected for correlation of lymph node epithelial marker expression with VEGF A, C, D and VEGF receptor-3 expression in the same lymph nodes using reverse transcriptase – polymerase chain reaction (RT-PCR) techniques.
A handheld gamma probe was used to select the lymph nodes for these assays as reported previously (12). On the day of surgery, each patient had intravenous injection of 10 mCi of F18-Fluorodeoxyglucose (FDG) followed by mediastinoscopy and anatomic lung resection if all the sampled mediastinal lymph nodes were benign on frozen section analysis. Standard thoracoscopic or open lung resection, as appropriate for the individual patient’s tumor, was followed by complete thoracic lymphadenectomy. The lymph nodes were labeled using the ATS/Naruke lymph node map (18). All harvested lymph nodes were scanned with the gamma probe outside the thoracic cavity to measure the gamma radiation resulting from any accumulated FDG in individual nodes. Intrathoracic radioactivity measurements were abandoned early in the study because of their unreliability due to interfering signal from the heart and great vessels. The resected tumor was similarly scanned outside the thorax. We compared the radioactive signals from the lymph nodes to each other. The FDG avid (hot) nodes had more than twice the signal intensity of the coldest lymph node in the entire thoracic field for that particular patient. An equal number of FDG avid (hot) and non-FDG avid (cold) nodes were selected for detailed pathologic analysis.
All surgically removed lymph nodes were bisected and examined by routine H&E. The selected lymph nodes that were malignant on H&E staining required no further pathologic analysis. However, the selected lymph nodes that were not malignant on H&E staining were subjected to ultra-staging with multiple step sections, immunohistochemistry (IHC) using cytokeratin AE1/AE3 and RT-PCR for CK-7, CEACAM5 and PLUNC (epithelial markers). These nodes were processed according to a standard protocol. After formalin fixation and embedding in paraffin, step sections of each lymph node were taken at 30–40 micron intervals. The sections were stained with H&E and an average of ten serial sections were evaluated. IHC was performed with standard monoclonal mouse anti-human cytokeratin antibody clones AE1/AE3 (Dako Inc, Carpinteria, CA). Formalin fixed, paraffin embedded tissue was pretreated with Proteinase K for 5 minutes. The primary antibody was diluted 1:100 and then incubated on the slides for 30 minutes. All staining steps were performed on a Dako Autostainer machine. Detection was done using the Mouse Envision + system (also from Dako). The testing was performed in a CLIA certified clinical laboratory using prostate tissue as positive controls.
RNA extraction was performed on fresh primary tumors and an equal number of FDG avid and non-avid lymph nodes. Tissues were homogenized with Trizol reagent (Invitrogen Carlsbad, CA). RNA was then precipitated from the aqueous phase using isopropanol. For quality control, 260/280 ratios were examined to confirm preparation purity and an RNA aliquot was run on an Agilent 2100 bioanalyzer to confirm RNA integrity by generating an RNA Integrity Number (RIN) value. Human mRNAs for beta actin (ACTB), keratin 7 ( CK7), vascular endothelial growth factor A (VEGFA), C (VEGFC), D (VEGFD), VEGF receptor 3 (VEGFR3 or FLT4), carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5), and palate, lung and nasal epithelium associated protein (PLUNC) were quantified by RT-PCR-based TaqMan™ Gene Expression Assays (Applied Biosystems, Foster City, CA). The assay IDs were respectively, Hs99999903_m1, Hs00559840_m1, Hs00900055_m1, Hs1099203_m1, Hs01128659_m1, Hs01047677_m1, Hs00944025_m1, and Hs00213177_m1. Briefly, random primers and reagents provided with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) were used to reverse transcribe 2μg total RNA from primary lung tumors and lymph nodes. The cDNA were used as template in 44 cycle-PCR reactions on a 7900HT real-time PCR machine (Applied Biosystems). For each reaction, the quantification cycle (Cq) value, approximately inversely proportional to log2 value of the concentration of the analyte RNA, was obtained with SDS™ software (Applied Biosystems; version 2.3). The average of Cq values of triplicate PCR reactions was used for analysis. Cq values for the mRNAs of interest were normalized by subtracting the value for beta actin mRNA from them. Beta actin is a highly conserved gene frequently used as a loading control in PCR assays.
Descriptive statistics such as frequencies and relative frequencies were computed for categorical variables. Numeric variables were summarized using simple descriptive statistics such as the mean, standard deviation, median, range, etc. The Wilcoxon rank sum test was used to correlate the lymph node micrometastatic status to normalized VEGF numeric variables. Box plots were also provided to show the differences in VEGF expression according to lymph node micrometastatic status. A 0.05 nominal significance level was used in all testing. The expression of epithelial markers in lymph nodes was used to upstage individual patients. All statistical analyses were done using SAS (version 9.3).
Results
The demographic characteristics of the forty patients in this study are shown in table 1. The nodal stage distribution of patients by routine H&E, immunohistochemistry (IHC) and quantitative real-time polymerase chain reaction (RT-PCR) are shown in table 2. Immunohistochemistry and RT-PCR resulted in up-staging of patients, culminating in positive N1 and N2 lymph nodes in 45% and 15% of patients respectively by RT-PCR.
Table 1.
Patient Demographic, Surgical and Pathologic Characteristics
| Demographic or Characteristic | No. of Patients (N=40) | % |
|---|---|---|
|
| ||
| Age (years) | ||
| Median | 71 | |
| Range | 52 – 84 | |
|
| ||
| Sex | ||
| Male | 7 | 17.5 |
| Female | 33 | 82.5 |
|
| ||
| Clinical follow-up (months) | ||
| Median | 12.6 | |
| Range | 3.7–31.6 | |
|
| ||
| Clinical Stage | ||
| I | 36 | 90 |
| II | 4 | 10 |
|
| ||
| Sex | ||
| Male | 7 | 17.5 |
| Female | 33 | 82.5 |
|
| ||
| Operation performed | ||
| Lobectomy | 39 | 97.5 |
| Pneumonectomy | 1 | 2.5 |
|
| ||
| Tumor histology | ||
| Adenocarcinoma | 29 | 72.5 |
| Squamous cell carcinoma | 9 | 22.5 |
| Adenosquamous carcinoma | 1 | 2.5 |
| Large Cell Carcinoma | 1 | 2.5 |
Table 2.
Lymph Nodal Staging Distribution by Various Modalities
| Staging Modality | No. of Patients in N Categories | ||
|---|---|---|---|
| NO | N1 | N2 | |
| Routine H & E | 35 | 4 | 1 |
| Immunohistochemistry | 33 | 6 | 1 |
| Quantitative RT-PCR | 16 | 18 | 6 |
H & E = Hematoxylin & Eosin; RT-PCR = reverse transcriptase-polymerase chain reaction
In the pathological examination of the excised nodes: 5 patients had proven metastatic disease in the studied LNs on H&E, while IHC identified LN disease in 2 of the 35 patients without H&E evidence for metastatic disease. The RT-PCR analysis suggested additional metastatic disease in 19 of 35 patients (54%).
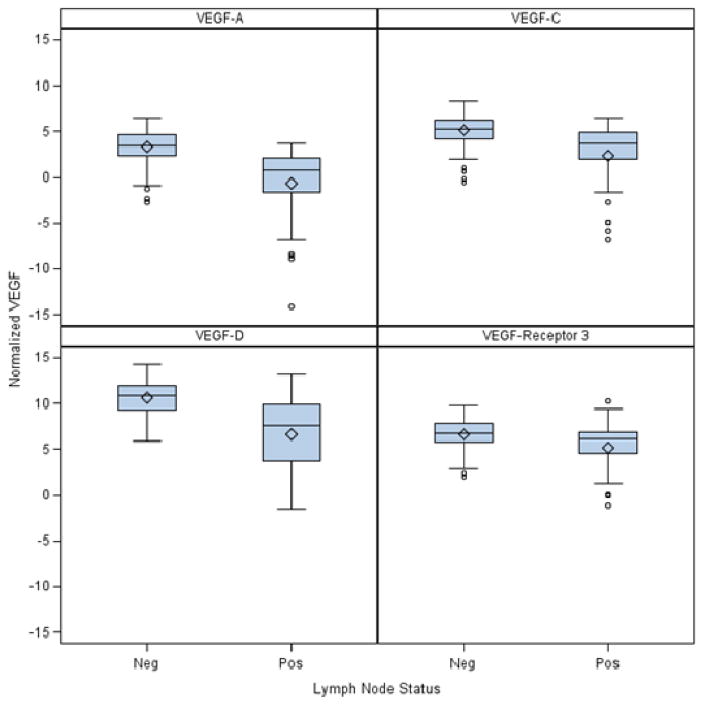
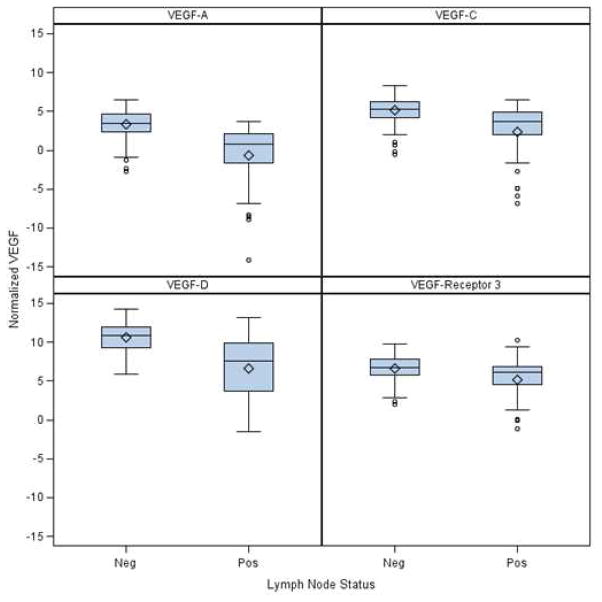
189 lymph nodes were evaluated by RT-PCR from the 40 patients. There was a highly positive correlation between RT-PCR detection of micrometastases and VEGF A, C, D or VEGF-receptor 3 expression levels in LNs (Table 4). Box plots are also provided to show the differences in location and scale of VEGF expression between malignant (positive) and non-malignant (negative) lymph node groups.
There has been one death from disease and two other recurrences among patients who had micrometastatic disease detected in their lymph nodes by RT-PCR. In the group of patients without such disease detected in their lymph nodes, there has been only one recurrence so far. The difference in the overall and recurrence free survival between these two groups has not reached statistical significance during our limited follow-up period. This will be reassessed when the median follow-up reaches 24 months.
Discussion
Increased understanding of the complex biology of lung cancer has led to advances in management of the disease. Accurate staging of individual patients remains a critical need. It guides the choice of therapy and stratifies patients appropriately for clinical trial of novel interventions. It also facilitates the comparison of treatment outcomes. Standard methods of evaluating thoracic lymph nodes (hematoxylin-eosin staining) can miss micrometastases (6, 7, 13, 19–21). Intensive pathologic techniques such as serial sectioning, immunohistochemistry and RT-PCR are more sensitive in detecting these micrometastases (4, 4–7). However, these techniques are labor intensive and expensive. Thus, they can practically be applied only to a limited number of lymph nodes in each patient. We used a handheld gamma probe to select lymph nodes for measurement of the expression of mRNA for epithelial markers by RT-PCR.
Due to the greater sensitivity of RT-PCR for micrometastatic lymph node disease detection, we upstaged 19 of 35 patients (54%) using this technique compared to routine pathology (H&E). This is consistent with other RT-PCR based studies (19, 22, 23). The data was reported on a “per patient” basis and not a “per node” basis. This is because decisions on adjuvant therapy would be based on the presence or absence of any lymph node metastases regardless of the number of lymph nodes involved. We also wish to assess survival based on the presence or absence of any micrometastatic disease in the lymph nodes (i.e. two groups).
Concerns have been expressed that RT-PCR may be overly sensitive and may include false positives from mesothelial or endothelial cells within lymph nodes. The prognostic significance of RT-PCR detection of tumor-specific molecular markers has been shown by others (4, 23, 24). We selected CK-7, CEACAM 5 and PLUNC as the epithelial markers of interest based on literature review (4, 22, 23). The positive threshold for the expression levels of these markers in the lymph nodes was based on the minimal expression of the same markers in primary tumors. RT-PCR is an imperfect ‘gold standard’ for the presence of lymph node micrometastasis. Since, there is no readily available method to verify its accuracy, we have to depend on the recurrence free survival of the two groups defined by the presence or absence of RT-PCR detected nodal disease. Our median follow-up is still relatively short (12.6 months). Thus, it seems early to assess the survival impact of molecularly detected lymph node micrometastasis in our patient cohort. If the prognostic value of molecularly detected micrometastases is proven, it would be appropriate to run clinical trials to assess the benefit of adjuvant therapy in such patients. Chemotherapy is the logical choice but the value of innovative intraoperative interventions, radiotherapy and targeted agents in such patients could be investigated.
Malignant cells metastasize from the primary tumor to other organs via either the lymphatic or vascular network. Indeed, tumor metastasis to regional lymph nodes often represents the first step of tumor dissemination and serves as a major prognostic indicator for the progression of human cancers (13). It is currently believed that lymphatics provide the major route of lung cancer metastases. However, the exact molecular mechanisms remain unclear. There is experimental evidence that tumors can induce the formation of new lymphatic vessels (lymphangiogenesis) even before they metastasize to lymph nodes, and that metastastic tumor cells continue to induce lymphatic vessel growth within sentinel lymph nodes, possibly promoting their further metastatic dissemination (14). The VEGF-C/VEGF-D/VEGFR-3 axis is the best validated signaling system for promoting lymphangiogenesis associated with solid tumors and the metastatic spread of tumor cells to lymph nodes (14). The secreted glycoproteins VEGF-C or VEGF-D activate VEGFR-3, a cell surface receptor tyrosine kinase on lymphatic endothelium, leading to growth of lymphatic vessels (25). Over-expression of VEGF-C and/or VEGF-D by tumor cells increases peritumoral and/or intratumoral lymphangiogenesis, promotes metastasis to local lymph nodes and may facilitate distant organ metastasis. The role of VEGF-A in angiogenesis via activation of its receptors, VEGFR-1 and VEGFR-2, has been extensively documented, but it has also been shown to influence lymphangiogenesis (17). As a means of exploring the role of lymphangiogenesis in the occurrence of nodal micrometastases, we measured VEGF A, C, D and VEGF receptor-3 expression in LNs. The quantification cycle (Cq) values for the mRNAs of interest were normalized by subtracting the value for beta actin mRNA from them (Table 4). Beta-actin is a highly conserved gene frequently used as a loading control in PCR assays. Actins are proteins that are involved in cell motility, structure and integrity. Thus, all the lymph nodes were expected to express beta-actin. Note that lower Cq values reflect higher mRNA expression levels. The correlation analysis was performed with the VEGF Cq values as continuous variables and the lymph node status as categorical values, either positive (malignant) or negative (non-malignant). Our study showed a highly positive correlation between the expression of VEGF A, C, D and VEGF receptor-3 in lymph nodes and the presence of micrometastases in those same nodes. This is consistent with the lymphangiogenesis literature. It would be worthwhile to investigate whether anti-lymphangiogenic treatment can prevent lymphatic and distant metastasis of NSCLC.
Limitations of our study include the fact that not all lymph nodes had IHC and RT-PCR. Performing such analysis on every single node would be too laborious and expensive. Also, our short clinical follow-up precludes survival analysis at this time.
Conclusion
IHC and RT-PCR for epithelial markers can be used to identify non-small cell lung cancer patients with lymph node micrometastatic disease. The presence of micrometastases was associated with higher VEGF A, C, D and VEGF receptor-3 expression in LNs. The impact of these findings on survival will be determined with further follow-up.
Figure 1.
Box plots of VEGF expression (measured as RT-PCR Cq levels) grouped by lymph node micrometastatic status.
Note: Lower Cq values reflect higher mRNA expression levels
Table 3.
Association between Lymph Node micrometastatic status and normalized VEGF RT-PCR quantification cycles (Cq levels) in LNs
| Normalized Variable | Statistic | Lymph Node micrometastatic status | |||
|---|---|---|---|---|---|
|
| |||||
| Negative | Positive | Overall | P-value | ||
|
| |||||
| VEGF-A | Mean (SD) /N | 3.4 (1.7) /148 | −0.7 (4.1) /41 | 2.5 (2.9) /189 | <.0001 |
| Median (Range) | 3.5 (−2.7,6.5) | 0.8 (−14.1, 3.7) | 2.9 (−14.1,6.5) | ||
|
| |||||
| VEGF-C | Mean (SD)/N | 5.1 (1.7)/148 | 2.3 (3.6) / 41 | 4.5 (2.5) /189 | <.0001 |
| Median (Range) | 5.3 (−0.5, 8.3) | 3.7 (−6.9,6.5) | 5 (−6.9,8.3) | ||
|
| |||||
| VEGF-D | Mean (SD)/N | 10.6 (1.8) /148 | 6.7(4.1)/41 | 9.7 (3) /189 | <.0001 |
| Median (Range) | 10.9 (5.9, 14.3) | 7.6 (−1.5, 13.2) | 10.5 (−1.5, 14.3) | ||
|
| |||||
| VEGF-Receptor 3 | Mean (SD)/N | 6.7 (1.5)/148 | 5.2 (2.8) / 41 | 6.4 (2) / 189 | <.0025 |
| Median (Range) | 6.8 (2.1,9.8) | 6.1 (−1.1, 10.3) | 6.6 (−1.1, 10.3) | ||
VEGF = Vascular Endothelial Growth Factor; SD = Standard Deviation; N = Sample Number
Note: Lower Cq values reflect higher mRNA expression levels
Acknowledgments
Supported by;
1. The National Cancer Institute (NCI) 1 K23 CA122182 Grant
2. The Thoracic Surgery Foundation for Research and Education (TSFRE) MCSDA Award
Footnotes
Presented at the American Association for Thoracic Surgery Annual Meeting, San Francisco, May 1, 2012
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007 Aug;2(8):706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 3.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997 Jun;111(6):1710–7. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 4.Melfi FM, Lucchi M, Davini F, Viti A, Fontanini G, Boldrini L, et al. Intraoperative sentinel lymph node mapping in stage I non-small cell lung cancer: Detection of micrometastases by polymerase chain reaction. Eur J Cardiothorac Surg. 2008 Jul;34(1):181–6. doi: 10.1016/j.ejcts.2008.03.059. [DOI] [PubMed] [Google Scholar]
- 5.Nwogu C. Sentinel node and positron emission tomography mapping in lung cancer. Semin Thorac Cardiovasc Surg. 2009 Winter;21(4):323–6. doi: 10.1053/j.semtcvs.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Rusch VW, Hawes D, Decker PA, Martin SE, Abati A, Landreneau RJ, et al. Occult metastases in lymph nodes predict survival in resectable non-small-cell lung cancer: Report of the ACOSOG Z0040 trial. J Clin Oncol. 2011 Nov 10;29(32):4313–9. doi: 10.1200/JCO.2011.35.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faries MB, Morton DL. Staging of regional nodes in pulmonary malignancies. Ann Surg Oncol. 2012 Mar;19(3):703–5. doi: 10.1245/s10434-011-2146-9. [DOI] [PubMed] [Google Scholar]
- 8.Little AG, DeHoyos A, Kirgan DM, Arcomano TR, Murray KD. Intraoperative lymphatic mapping for non-small cell lung cancer: The sentinel node technique. J Thorac Cardiovasc Surg. 1999 Feb;117(2):220–4. doi: 10.1016/S0022-5223(99)70415-0. [DOI] [PubMed] [Google Scholar]
- 9.Liptay MJ, Masters GA, Winchester DJ, Edelman BL, Garrido BJ, Hirschtritt TR, et al. Intraoperative radioisotope sentinel lymph node mapping in non-small cell lung cancer. Ann Thorac Surg. 2000 Aug;70(2):384–9. doi: 10.1016/s0003-4975(00)01643-x. [DOI] [PubMed] [Google Scholar]
- 10.Lardinois D, Brack T, Gaspert A, Spahr T, Schneiter D, Steinert HC, et al. Bronchoscopic radioisotope injection for sentinel lymph-node mapping in potentially resectable non-small-cell lung cancer. Eur J Cardiothorac Surg. 2003 May;23(5):824–7. doi: 10.1016/s1010-7940(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 11.Meyer A, Cheng C, Antonescu C, Pezzetta E, Bischof-Delaloye A, Ris HB. Successful migration of three tracers without identification of sentinel nodes during intraoperative lymphatic mapping for non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2007 Apr;6(2):214–8. doi: 10.1510/icvts.2006.141911. [DOI] [PubMed] [Google Scholar]
- 12.Nwogu C, Fischer G, Tan D, Glinianski M, Lamonica D, Demmy T. Radioguided detection of lymph node metastasis in non-small cell lung cancer. Ann Thorac Surg. 2006 Nov;82(5):1815, 20. doi: 10.1016/j.athoracsur.2006.05.104. discussion 1820. [DOI] [PubMed] [Google Scholar]
- 13.Jiao X, Krasna MJ. Clinical significance of micrometastasis in lung and esophageal cancer: A new paradigm in thoracic oncology. Ann Thorac Surg. 2002 Jul;74(1):278–84. doi: 10.1016/s0003-4975(01)03376-8. [DOI] [PubMed] [Google Scholar]
- 14.Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann N Y Acad Sci. 2008;1131:225–34. doi: 10.1196/annals.1413.020. [DOI] [PubMed] [Google Scholar]
- 15.Chang C, Wang P, Cheng H, Li L, Yang H, Zhang L. The relationship of VEGF-C, VEGF-D and VEGFR-3 with lymph node metastasis of non-small cell lung cancer. Chin J Clin Oncol. 2009;36(16) [cited 20 October 2009] [Google Scholar]
- 16.Massi D, Gököz Ö. The biological significance of lymphangiogenesis in human tumours. Diagnostic Histopathology. 2010 Jun;16(6):295–305. [Google Scholar]
- 17.Wissmann C, Detmar M. Pathways targeting tumor lymphangiogenesis. Clin Cancer Res. 2006 Dec 1;12(23):6865–8. doi: 10.1158/1078-0432.CCR-06-1800. [DOI] [PubMed] [Google Scholar]
- 18.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997 Jun;111(6):1718–23. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 19.Faries MB, Bleicher RJ, Ye X, Essner R, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for primary and metastatic pulmonary malignant neoplasms. Arch Surg. 2004 Aug;139(8):870–6. doi: 10.1001/archsurg.139.8.870. [DOI] [PubMed] [Google Scholar]
- 20.Gu CD, Osaki T, Oyama T, Inoue M, Kodate M, Dobashi K, et al. Detection of micrometastatic tumor cells in pN0 lymph nodes of patients with completely resected nonsmall cell lung cancer: Impact on recurrence and survival. Ann Surg. 2002 Jan;235(1):133–9. doi: 10.1097/00000658-200201000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein NS, Mani A, Chmielewski G, Welsh R, Pursel S. Immunohistochemically detected micrometastases in peribronchial and mediastinal lymph nodes from patients with T1, N0, M0 pulmonary adenocarcinomas. Am J Surg Pathol. 2000 Feb;24(2):274–9. doi: 10.1097/00000478-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Pulte D, Li E, Crawford BK, Newman E, Alexander A, Mustalish DC, et al. Sentinel lymph node mapping and molecular staging in nonsmall cell lung carcinoma. Cancer. 2005 Oct 1;104(7):1453–61. doi: 10.1002/cncr.21325. [DOI] [PubMed] [Google Scholar]
- 23.Benlloch S, Galbis-Caravajal JM, Alenda C, Peiro FM, Sanchez-Ronco M, Rodriguez-Paniagua JM, et al. Expression of molecular markers in mediastinal nodes from resected stage I non-small-cell lung cancer (NSCLC): Prognostic impact and potential role as markers of occult micrometastases. Ann Oncol. 2009 Jan;20(1):91–7. doi: 10.1093/annonc/mdn538. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto T, Kobayashi Y, Ishikawa Y, Tsuchiya S, Okumura S, Nakagawa K, et al. Prognostic value of genetically diagnosed lymph node micrometastasis in non-small cell lung carcinoma cases. Cancer Research. 2000 Nov 11;60(22):6472–8. [PubMed] [Google Scholar]
- 25.Stacker SA, Achen MG. From anti-angiogenesis to anti-lymphangiogenesis: Emerging trends in cancer therapy. Lymphat Res Biol. 2008;6(3–4):165–72. doi: 10.1089/lrb.2008.1015. [DOI] [PubMed] [Google Scholar]