Abstract
Background
Preterm infants with periventricular brain injury (PBI) have a high incidence of atypical development and leg movements.
Objective
Determine whether kicking and treadmill stepping intervention beginning at 2 months corrected age (CA) in children with PBI improves motor function at 12 months CA when compared with control subjects.
Method
In a multi-center pilot study for a controlled clinical trial, sixteen infants with PBI were randomly assigned to home exercise consisting of kicking and treadmill stepping or a no-training control condition. Development was assessed at 2, 4, 6, 10, and 12 months CA with the Alberta Infant Motor Scale (AIMS). At 12 months children were classified as normal, delayed, or with cerebral palsy (CP).
Results
At 12 months CA 3 of 7 (43%) of the exercise group children walked alone or with one hand held versus 1 of 9 (11%) in the control group (p=.262), but no significant differences in AIMS scores were found at any age. Half of the subjects had CP or delay; the outcomes of these infants were not improved by exercise. Compliance with the home program was lower than requested and may have affected results.
Conclusion
Although not statistically significant with a small sample size, self-produced kicking and treadmill exercise may lower age at walking in infants with normal development following PBI, but improvements of the protocol to increase and document compliance are needed before a larger study is implemented.
Keywords: infant motor development, preterm infants, periventricular brain injury, exercise intervention
Introduction
Infants born preterm and with a very low birthweight demonstrate, on average, significant motor impairment persisting throughout childhood [1]. The presence of additional perinatal complications, such as perinatal brain injury or bronchopulmonary dysplasia (BPD), lowers standard scores on tests of motor development by half a standard deviation (SD) more [1].
Cerebral palsy (CP) in preterm infants is typically caused by white matter injury (WMI). WMI includes conditions such as intraventricular hemorrhage (IVH), periventricular hemorrhagic infarction, periventricular leukomalacia (PVL), and diffuse or focal noncystic injury [1–5]. Both sensory and motor pathways may be affected in these lesions [5]. The incidence of CP in children with severe WMI ranges from 50–85% [4]. These rates of CP compare with an incidence of 5–10% in all premature infants. Also common are cognitive and behavioral deficits affecting school performance in 25–50% [2,3]. In Illinois and other US states, children with perinatal brain injury are automatically eligible for early intervention services because of the recognized risk of poor developmental outcome associated with these lesions.
In addition to an increased risk of CP, numerous researchers have demonstrated a high incidence of atypical motor development in children with periventricular brain injuries. Kicking has altered characteristics in these children [6–9], and the quality of spontaneous kicking is related to age at walking in preterm infants [10]. Jeng and colleagues [11] showed a delay in onset of walking in children with WMI, which occurred on average at 14 months corrected age (CA). Hadders-Algra [12] identified a reduction of movement complexity and variability as a characteristic result of periventricular brain injuries, and Barbosa and colleagues [13–15] reported both delayed motor development and regressions in leg movements in infants such as these who were later diagnosed with CP. Specifically, infants who later developed CP were found over the course of monthly assessments through 4 months CA to regress in their ability to hold their legs in antigravity positions in supine and to perform spontaneous kicking movements with legs off the supporting surface [15].
Despite the high risk for poor motor outcome of children with periventricular brain injury, few studies have asked the question of whether intervention can alter outcomes. In fact, studies of early intervention for infants at high risk for motor dysfunction tend to show no or very limited treatment effects [16–18]. Of those with positive outcomes in the early weeks or months, sustained improvements have not yet been demonstrated by study of longer-term outcomes [19–22]. Questions that need addressing include whether it is possible to reduce the delay in motor development or the severity of CP in those who have sustained a permanent brain injury.
Because of the abundant descriptions of impaired leg movements in this population and the lack of research on the efficacy or effectiveness of early physical therapy to improve outcomes, this project was designed as a randomized, multi-institutional, and multidisciplinary pilot study of the effects of a home exercise intervention on motor function of preterm infants with periventricular brain injury. The overall objective was to:
Determine whether kicking and treadmill stepping intervention added to usual physical therapy beginning at 2 months CA improves motor function at 12 months CA in children with periventricular brain injury, as compared with outcomes of a control group receiving only usual physical therapy as prescribed by their personal caregivers.
The intervention program for this study was designed to address the regressions and delays in kicking and walking demonstrated by previous research and to supplement typical physical therapy by providing a program of task-specific activities [23] for lower extremity training in supine, sitting, standing, and walking. The theoretical basis of the program emphasized self-initiated movements in response to the presentation of attractive, commercially available toys. Success in contacting toys with the feet was expected to result in feedback for learning while providing exercise for strengthening leg muscles, developing endurance for physical activity, and stimulating development of anticipatory postural control in the head and trunk [24–27]. Use of different toys across the first year was intended to provide novelty to sustain the interest of the children and to encourage regular practice of leg movements in increasingly more demanding postural positions. In addition to kicking exercises, the program included treadmill stepping based on the successful application of this method for lowering the age at attainment of walking in children with Down syndrome [28] and a case report on treadmill training in an infant with WMI [29].
We hypothesized that infants receiving the early intervention program aimed at leg movements and locomotor performance would walk independently at an earlier age and would have better quality of movement and motor development at 12 months CA than infants receiving conventional physical therapy alone.
Methods
Subjects
The protocol for this study was approved by the Institutional Review Boards at each participating institution and parental assent was obtained for enrollment of subjects in the study. Subjects born in 2006–2007 were recruited from three neonatal intensive care units (NICUs). Inclusion criteria included the diagnosis of Grade III or IV IVH or PVL as visualized on ultrasound scan and reported in the medical record during the perinatal period. Children resulting from a multiple birth were eligible; both children were to be assigned to the same experimental group. Physicians in each NICU verified that subjects were expected to be healthy enough to be discharged home in order to begin a home exercise program at 2 months CA; no subjects were excluded for this reason but one infant was included because she was a twin to an eligible subject who had to be dropped from the study because she was never healthy enough to begin the program. A second subject remained in the hospital at 2 months CA and began the study at about 2.5 months CA after discharge home. Subjects were assigned to experimental groups (exercise or control) using a random numbers table for each site so as to assure that subjects from all 3 sites would appear approximately equal in the two experimental groups. Only the principal investigator and, for infants in the exercise group the physical therapist assigned to provide the exercise program, were aware of the group assignment. Following parental assent to participate while their infants were in the NICU, families were contacted again shortly before their children reached 2 months CA. If they were still interested in being involved in the study then the first assessment and training sessions were scheduled.
Intervention
Children assigned to the exercise group received monthly visits from an exercise physical therapist who provided them with a series of 4 toys to facilitate kicking: a mobile with Velcro tethers attached to the ankles such that kicking or other leg movements created toy movement that rewarded the infant with interesting visual and auditory feedback (from 2–4 months CA), a play gym with toys suspended from an overhead bar which when kicked produced lights and sounds (from 4–6 months CA), a toy piano to “play” with the feet (from 5–10 months CA), and a blow-up ball attached by a tether to a plastic base1 for kicking practice that also produced lights and sounds when moved (from 8–12 months CA). During monthly exercise visits, therapists issued toys according to the age schedule devised for the study, showed parents how to set up and use the toys, explained the theory behind the kicking and treadmill exercise, and reviewed the expectations for performing the exercise. Children were positioned in an infant bath seat for mobile training, were placed in supine for work with the play gym, and sat on the parent’s lap for playing the piano. Depending on the child’s motor skills, kicking the tethered ball could be done in either sitting or standing. Although parents were shown how the toys worked to produce interesting feedback and occasionally moved the infants’ legs to encourage them to kick, the infants were allowed to explore and play with the toys without additional handling. Parents were asked to perform the kicking exercises for 8 minutes per day/5 days per week.
At 4 months CA, stepping practice with the parent suspending the child over a portable treadmill2 moving at a speed from 0–.6 m/s (to be increased by the parent according to the child’s stepping capabilities) was added. Parents were asked to perform treadmill stepping for 8 minutes per day/5 days a week for the duration of the study. They were given a diary to record exercise compliance (dates of exercise, total time for each exercise session, number of continuous steps taken on the treadmill, and any comments about behavior) that was collected and replaced with a new diary at each monthly visit from the exercise physical therapist. Children in both groups were also allowed to participate in any intervention prescribed by their personal caregivers. At each visit the therapist inquired about whether any physical therapy was being received.
Assessment
Infants in both groups were visited 5 times during the course of the study for testing of motor development by one of three physical therapists blinded to experimental group assignment. Parents were asked to avoid revealing their group assignment during these visits. AIMS test sessions and the children’s performance with the toys and treadmill were videotaped for later review. Motor Development was tested at 2 (study entry), 4, 6, 10, and 12 months CA with the Alberta Infant Motor Scale [30]. Rater reliability of each therapist was assessed from scoring two videotaped AIMS tests compared with scores by an experienced investigator. Agreement on the dichotomous item scores assessed with kappa analysis varied from .18 to .92. Low reliability on the part of a tester required additional evaluation to obtain a kappa of .90.
At 12 months CA all children were assessed by a physician blinded to group assignment and to AIMS performance until after the examination was completed. Diagnosis was performed by a pediatric rehabilitation medicine physician, boarded in pediatrics and in physical medicine and rehabilitation, with 25 years experience with children with CP. After administering a standard protocol to assess reflexes, postural tone, and movement quality, the physician judged whether the child did or did not have CP, and was then given information on the child’s AIMS performance. The physician assigned to those with CP a functional level based on the Gross Motor Function Classification System (GMFCS) categories for children before the 2nd birthday [31; www.canchild.ca].
Statistical Analysis
Parent diaries for children in the exercise group were reviewed to determine days per month and length of time per session they engaged their infants in the prescribed activities. For analysis of motor development the raw scores from AIMS testing were transformed into Z scores based on means and SDs for performance of the AIMS normative sample of Canadian infants within the same age group [30]. For any missing data points, a mean of the adjacent two data points, if available, was used to estimate the missing Z score. If not, the last observation carried forward was applied. In this manner, two of a total of 80 data points were substituted for missing data. Because the outcomes for AIMS Z scores were not normally distributed, and positive and negative outliers existed at several ages, medians rather than means were explored to assess the developmental trend within both control and exercise groups.
Equivalence of the exercise and control groups was assessed at study outset using the Fisher exact test for dichotomous variables and the t test to compare gestational age at birth and AIMS scores at 2 months CA. We used the Kruskal-Wallis nonparametric test to assess for significant differences between groups at each month. Given no significant differences found between control and exercise groups at study entry, a longitudinal ranked data analysis was performed to test for group and month effect at 4, 6, 10, and 12 months CA, with or without group by month interaction terms, using the method described by Brunner and colleagues [32] and the corresponding online resources (http://www.ams.med.uni-goettingen.de/de/sof/ld/makros.html). The statistical analysis used SAS, Version 9.2, published by the SAS Institute, Inc., Cary, NC, USA. Performance on individual AIMS items at 12 months CA was reviewed from test forms and verified from videotapes of testing sessions to determine which children were walking alone, walking with assistance or cruising along furniture, or not walking. The Fisher exact test was used to compare the number of children walking/not walking in each group. All presented probability values were two-tailed with statistical significance defined as p < 0.05.
Results
Subjects
Eighteen subjects with periventricular brain injury were initially recruited from the three NICUs. One subject was withdrawn after parental assent was obtained but prior to beginning of data collection and training because the family changed their mind about participation; a second subject died during the course of the study. The latter infant was too ill to ever begin study participation, but her twin completed the study. Thus, 16 subjects randomly assigned to one of two experimental groups (7 exercise, 9 control) completed the study. No attrition occurred after any child began the exercise or testing program, although one subject could not be scheduled for the final AIMS test until 16 months CA.
The sample was diverse in terms of gender and ethnicity. There were 8 males and 8 females, all born prematurely. The range of gestational age at birth was from 23–32 weeks with a mean of 27 weeks (SD 2.9 weeks). Nine subjects were African-American, 5 white (one Middle-Eastern), and 2 Hispanic. Table 1 presents information comparing the equivalence of the groups on conditions that might affect the outcomes of the study. There were no statistically significant differences between groups in gestational age at birth, type of brain insult, BPD, motor development at study entry, or participation in early physical therapy.
Table 1.
Comparison of Exercise and Control Groups in Medical Conditions, Development on the AIMS at Study Entry, and Other Physical Therapy
| Condition | Exercise Group (n=7) | Control Group (n=9) | Probability |
|---|---|---|---|
| Mean GA at Birth (SD) | 25.9 weeks (2.41) | 28.1 weeks (3.06) | 0.133a |
| Frequency of IVH Grade III or IV | 6 | 6 | 0.585b |
| Frequency of PVL | 1 | 3 | 0.585b |
| Frequency of BPD | 6 | 3 | .060b |
| Mean AIMS Z score at Study Entry (SD) | −0.67 (1.69) | −0.41 (1.31) | 0.734a |
| Frequency of PT Beginning By 5 months CA | 1 | 4 | 0.308b |
Abbreviations: Standard deviation (SD), Gestational age (GA), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), bronchopulmonary dysplasia (BPD, chronic lung disease), Alberta Infant Motor Scale (AIMS), corrected age (CA), physical therapy (PT)
t test
Fisher exact test
Physical Therapy
The intervention to facilitate development of leg movements in the exercise group was intended to be a supplement to usual physical therapy, not the sole exercise intervention. Because children with perinatal brain injury in Illinois are automatically qualified for early intervention services, it was our expectation that all of the subjects in this study would receive other physical therapy. We were surprised to discover, however, that only 7 of the 16 children in the study received any physical therapy before 12 months CA, and only 5 received physical therapy that began by 5 months CA (1 in the exercise group and 4 in the control group; see Tables 1 and 2).
Table 2.
Description of Subjects Arranged in Order of AIMS Outcome and Experimental Group Assignment
| Subject Number & Group | 104E | 110E | 108E | 301E | 103E | 102E | 111E | 203C | 106C | 101C | 107C | 202C | 109C | 302C | 201C | 303C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IVH grade or PVL | III | III | III | III | III R; IV L | III | IV R; III L | III | PVL | III | PVL | PVL | IV | III | III | III |
| BPD | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | No | No | No | Yes | Yes |
| Birth weight (grams) | 1680 | 1120 | 690 | 885 | 637 | 870 | 530 | 964 | 2052 | 1370 | 1820 | 1106 | 680 | 958 | 835 | 709 |
| GA at birth | 30 | 27 | 23 | 27 | 24 | 26 | 24 | 26 | 32 | 29 | 31 | 28 | 26 | 32 | 25 | 24 |
| CA in Months at PT Onset | --- | --- | --- | 8 | 11 | --- | 2 | 3 | 3 | --- | --- | 2 | 13 | --- | --- | 5 |
| 2 mo AIMS Z | 2.40 | 0.08 | −1.98 | −1.98 | 0.36 | −2.01 | −1.57 | −0.74 | 0.87 | 1.38 | 0.87 | −0.15 | --- | −2.91 | −0.74 | −2.31 |
| 12 mo AIMS Z | 0.80 | 0.53 | 0.24 | −1.68 | −3.42 | −4.96 | −5.39 | 0.53 | 0.24 | 0.10 | 0.10 | −0.35 | −3.70 | −3.80 | −5.09 | −10.10 |
| Highest motor skill at 12 mo CA | Walks alone | Walks alone | Walks with one hand held | Creeps, takes steps with trunk support | Sits, stands with support | Rolls, does not sit alone | Does not roll or sit alone | Walks alone | Creeps, cruises along furniture | Creeps, takes steps with trunk support | Cruises, takes steps with trunk support | Creeps, cruises along furniture | Rolls, sits alone | Sits, stands with support | Walks at 16 mo | Does not roll or sit alone |
| GMFCS | --- | --- | --- | --- | II | IV | V | --- | --- | --- | --- | --- | II | II | --- | V |
E, exercise group; C, control group; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; R, right; L, left; BPD, bronchopulmonary dysplasia; GA, gestational age in weeks; PT, physical therapy; CA, corrected age; AIMS, Alberta Infant Motor Scale; GMFCS, Gross Motor Function Classification System
For those children who received physical therapy, typical intervention provided in Illinois occurred for one hour once a week in the child’s home and was family-focused and routines-based, i.e. involves providing families with suggestions to be incorporated into daily activities in which parents and infants engage. When children began physical therapy, we observed a treatment session in order to characterize the intervention that occurred. The majority of observed physical therapy sessions focused on training in meeting age-appropriate developmental milestones and education of caregivers in assisting their child to meet these milestones. Only one child, who had more severe functional limitations, had assistive devices that included orthotics and positioning devices for sitting and standing. All observed sessions included education of caregivers in daily activities and exchange of information with caregivers about the child’s current development and health status.
Compliance with the Exercise Protocol
Review of the diaries kept by families of children in the exercise group revealed that compliance with keeping the diary was highly variable; no one, however, reported adhering to the request to perform the exercise 5 days a week. The typical performance was 2–3 days per week and it appeared that the scheduling of a visit by the exercise therapist tended to increase compliance in the week before and the week after the visit. The typical length of the exercise session was about 5 minutes. Compliance was also better in the first 7–8 months of the study and tended to be minimal in the last 2–3 months. Parents typically quit using the treadmill if their children became independent walkers, but one mother had to stop using the treadmill because she had back surgery and could no longer hold the child for stepping exercise.
Disability Outcomes at 12 Months CA
A wide range of development and disability was demonstrated in the subjects (Table 2); as anticipated, our recruitment process resulted in half of the subjects showing impaired motor performance at 12 months CA. On the other hand, 8 children had performance on the AIMS that was at or above the median, i.e. 50th percentile of the Canadian age norms. Upon study entry all 8 of these children had AIMS scores above the 25th percentile (5 were above the 50th percentile), showing early recovery from perinatal brain injury.
The exercise group (n=7) had 3 children with typical development (AIMS scores at or above the 50th percentile and no abnormal neurologic findings), 3 with CP, and 1 with delayed motor development (AIMS score < 10th percentile but no CP). The control group (n=9) had 5 children with typical development, 3 with CP and 1 with delayed motor development (no CP). Thus, there were more children in the (larger) control group with normal outcome, but CP and delayed development occurred equally in the two groups. When categorized by severity of CP using the Gross Motor Function Classification System (GMFCS), outcomes were as follows (see also Table 2):
Walking Performance at 12 Months
Three children in the exercise group (43%) either walked independently (N=2) or walked with one hand held (N=1), while only one (11%) of the children in the control group walked independently (p=.262). The rest of the children within the range of typical development on the AIMS could either creep, cruise along furniture, or take steps when supported at the trunk. As would be expected, the children not walking had AIMS scores near the 50th percentile, while those who were walking at 12 months CA all scored at about the 75th percentile; no ceiling effect, however, was observed. None of the children with delayed development on the AIMS or with CP (N=8) were able to walk at 12 months CA.
Longitudinal Performance on the AIMS
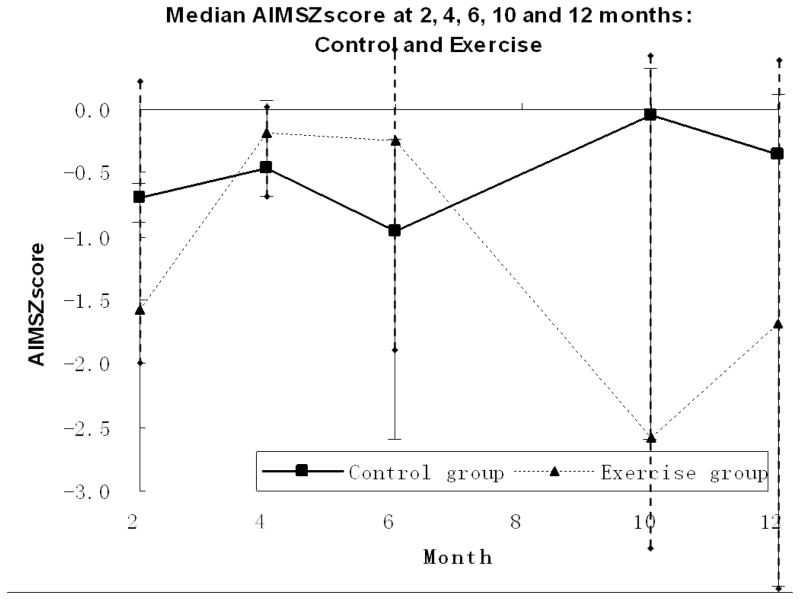
At study entry (2 months CA) the mean performance on the AIMS of both groups was in the low average range with a Z score of −0.67 SD below the mean for the exercise group and −0.41 SD for the control group, but the difference was not statistically significant so the groups were deemed to be equivalent at study onset (p = .73). Figure 1 shows the median AIMS Z scores at each monthly assessment by group and Table 2 provides details on the characteristics and performance of individual children.
Figure 1.
Median Z Scores on the AIMS by Age for Exercise (n=7) and Control (n=9) Groups
The longitudinal ranked data analysis for the AIMS scores at 4, 6, 10, and 12 months CA showed no statistically significant effects for group, month, or intervention group by month interaction effects (Table 3). A test of the main effect for group resulted in a non-significant effect for intervention with p = 0.19. Thus the statistical tests did not show an advantage for the exercise group on overall motor development on the AIMS at any point in time. A significant main effect with p = 0.02 for month was found, reflecting the general decline in AIMS Z scores across time. As shown in Figure 2, this result is due to the performance of the children with CP whose scores in both groups declined further relative to age norms at each successive measurement. Thus, there was no evidence that the intervention helped children with CP.
Table 3.
Tests of Group, Age, and Intervention Group By Age Effects
| Tests of Fixed Effects for Outcome AIMS Score | |||
|---|---|---|---|
| Factor | d.f. | value | p-value |
| Intervention | 1 | 0.0476 | 0.8273 |
| Month | 1.4097 | 2.2724 | 0.1204 |
| Intervention * Month | 1.4097 | 2.1525 | 0.1324 |
Control group n=9; exercise group n=7
Figure 2.
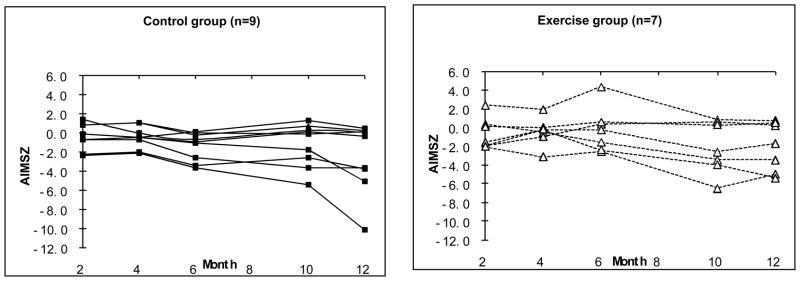
Scores on the AIMS by Age for Individual Exercise and Control Group Children
Discussion
In summary, the intervention used in this small pilot study did not result in statistically significant differences in overall motor development between groups on the AIMS at any age. Although the results were not statistically significant, we believe it may be clinically significant that children who recovered from brain injury with typical motor development at 12 months CA walked earlier and therefore had AIMS standard scores above the median for age norms if they experienced the exercise program. Outcomes of children with CP did not appear to be altered by the intervention.
Recruitment and Outcomes
Although we are unable to draw definitive conclusions from a pilot study involving only 16 subjects, the results of this project provide support for the feasibility of continued research on leg exercise effects on recovery from periventricular brain injury. First, we were successful in recruiting a group of high-risk subjects from three collaborating institutions of whom 50% had CP or delayed development at 12 months CA. Many studies of intervention for high-risk infants have not had significant numbers of children with the outcome of CP to provide sufficient statistical power to analyze effects on reduction of the incidence or severity of CP (e.g., Piper et al. 1986 [18]). Our results provide support for a larger study of children with documented injury that would be likely to provide such power. Unfortunately, however, the length of time required to recruit the sample was about one year, so multi-center studies will be needed in order to recruit large samples of subjects for this type of work.
Despite the presence in the control group of more children with early physical therapy outside of the project (Table 2), effects on age at walking favoring the exercise group may have been present. Although more children with motor performance at or above the median at 12 months CA were found in the control group, 100% of the children with typical development in the exercise group were walking alone or with one hand held at 12 months CA, while only 20% of the children with typical motor development in the control group walked alone. After an average of 10 months of treadmill exercise, Ulrich and colleagues reported a lowering of the age of independent walking averaging 4 months in children with Down syndrome [28]. Given reports of a mean of 14 months for independent walking in children with WMI [11], it is possible that our intervention could help those children with the potential to recover to obtain this milestone near the mean age for typically developing children.
Theory and Exercise Protocol
Several previous studies of interventions to promote motor development in high-risk infants have been based on the theory of Neuro-Developmental Treatment (NDT), which emphasizes facilitation of postural control and movement [18, 20–21]. At best, these studies demonstrated only short-term effects to date. Newer approaches under study emphasize self-produced movement and coaching of parents to encourage movement exploration and variety [33–34]. Our study is believed to be the first to attempt a task-oriented approach focused on locomotion in a home-based exercise program carried out by parents of children with periventricular brain injury. Unfortunately, we do not find any convincing evidence that the exercise intervention helped those infants who turned out to have CP. These children in both groups fell further and further behind their age peers as the year progressed. It is likely that children with impaired movement will need use of additional facilitation techniques or behavioral shaping of performance to produce more activity of a sustained nature with a greater variety of movement than can be produced under self-initiated and controlled conditions. Behavioral shaping has been successfully used with typically developing infants [6, 24] and in constraint-induced movement therapy for children with CP [25], but it is unknown if these techniques could be useful in early infancy for children with CP.
Parental Compliance
A second issue that may have affected the outcomes is compliance with the exercise regimen. Parents were most likely to do the exercises only 2–3 times per week rather than the 5 times per week requested. Compliance with the protocol fell off even more in the latter part of the year. We believe that this may be because in the early part of the program, parents simply needed to set the children up for playing with the mobile or play gym, and then the children worked on their own. On the other hand, the two toys used in the latter part of the first year (piano and tether ball) required more participation on the part of the parents who had to physically support their children during the activities. It is likely that the introduction of more demanding toys, coupled with visits that only occurred monthly, was insufficient for maintaining adherence to the exercise program, especially for families with multiple life challenges. It is also possible that if children were perceived by their parents as doing well, the families had less incentive to continue to perform the exercise. Finally, as children became heavier it was more difficult for families to hold the children over the treadmill; support harnesses are likely to be necessary to ensure compliance with this aspect of the program at later ages and children with CP will likely need manual assistance for reciprocation of the legs.
Limitations of the Study
The sample in this pilot study was small, and no definitive conclusions can be drawn. The lack of results is disappointing and may be because the treatment was not delivered as designed so that the dosage was insufficient to produce significant change in overall motor development. Although research has not demonstrated that early physical therapy alters the outcome of children such as those in this study, we also view the lack of participation of families in a regular routines-based physical therapy program as a major limitation of this research, particularly for the children with CP who might have been able to more effectively access the toys and take steps on the treadmill if they had experienced more active exercise to promote postural control and movement exploration. Unfortunately, many barriers to obtaining physical therapy exist for children with cerebral palsy, [35] and Illinois is a state with a poor record of enrolling children in early intervention before their first birthday (19th among U.S. states in 2007–2008) [36]. A means for either insuring that outside physical therapy is available to interested parents or providing a full therapy program for all subjects within the experiment would be needed for an adequate evaluation of the effectiveness of our task-specific lower extremity training program.
Quality of the measurements used is always a question in clinical trials and it is possible that use of more sensitive tools for measuring motor development might have improved the results of the study. We used the AIMS because of its excellent population-based age standards and the use of item criteria that emphasize the postural control and quality of spontaneously generated functional movements. Although the AIMS norms we used were based on the performance of children born after at least 38 weeks gestational age, Pin and colleagues [37] have provided an elegant demonstration of the sensitivity of the AIMS items to differences in preterm infant motor development that typically result in lower scores for preterm than for full term infants [37–39]. Other research has suggested, however, that the norms may need revision for both preterm and full term infants, [38–40] perhaps because the Back-to-Sleep movement altered the timing of attainment of early gross motor skills [40]. Contrary to expectations, however, of the 10 children without neurologic findings in our study, 8 performed at or even above the mean when compared with the Canadian performance norms. We conjecture that this finding might be because our children were assessed after the delayed development related to Back-to-Sleep recommendations was well known, and parents are now advised to provide frequent play in prone for their children.
Another test such as the Gross Motor Function Measure [41] that was designed for use with children with CP might prove more valuable for detecting differences between groups in a larger study. Finally, it is known that GMFCS ratings are not stable in children with CP as young as those in this study [42–43]. Although Gorter and colleagues [43] do recommend classification early on, they suggest that a more reliable classification can be made at or after 2 years of age.
We asked testing physical therapists at the end of the study whether group assignment had become known to them during the course of their interactions with families in the study. In 7 of 16 cases the study assignment was revealed (5 in the exercise group, 2 in the control group). This was typically via mention of the exercise toys when the testing therapist visited (“Oh, you didn’t need to bring your treadmill up the stairs; you could have used ours” - by a mother in the exercise group; “How come we don’t have these toys to use?” - by a father in the control group who walked in during testing). This would clearly be a major problem in a large-scale clinical trial and it will be necessary to find a better means than reminding parents to control for this potential biasing influence on test results.
Suggestions for Protocol Revision
Given the limitations of the study and what we have learned from the development and outcomes of the infants in the study, we propose the following possible revisions to the protocol in further pilot work before initiating a large clinical trial.
Exercise materials and training
The kicking protocol should include replacing the inflatable tether ball or adding toys that are more interesting for the children to use from 8–12 months CA. We could also add more toys to choose from for kicking practice at all ages to keep the program novel for both infants and parents. Including foot reaching activities like those used by Heathcock and Galloway [44] in a study with preterm infants would be useful to increase the variety of leg movements elicited. Furthermore, it is likely that we need to add activities to promote use of acquired leg movements in antigravity positions to encourage crawling and creeping on hands and knees. For children who cannot produce more than 80% alternating steps on the treadmill by 7 months CA [45], a partial body weight support harness should be provided and parents trained in assisting reciprocating movements of the legs when walking on the treadmill.
We did not assess learning of the kicking paradigm. It would be useful to assess learning to produce increased numbers of foot contacts with the toys using methods such as those of Heathcock and Galloway [44] and institute a behavioral shaping program for children who do not appear to be making the connection between their movements and feedback from the toys. Alternatively, more assistance to movement could be provided by parents when weakness or poverty of movement appears to be the problem. For example, tethers could be added to the play gym to provide partial support of the legs to facilitate holding legs up during kicking, or part of the weight of the legs could be taken manually as children try to play the piano with their feet. Activities to promote foot reaching used by Heathcock and Galloway [44] provided support to assist antigravity control of the legs, which might be especially helpful for the children with CP. Although assistance to movements might be necessary for these particularly high-risk infants, alterations to the protocol should keep in mind the principles described as influential in successful clinical trials by Schertz and Gordon [46]: activities must be task-oriented, structured, motivating, and intensive.
Because the focused intervention provided in this study was intended to be a supplement to other physical therapy, we should assure that conventional routines-based physical therapy is available for all children in the study in order to adequately evaluate the efficacy of our locomotor-focused training program. Descriptions of the physical therapy provided can be obtained using either the method described by Blauw-Hospers and colleagues [34] or Hashimoto and McCoy [47].
Parental compliance
As Ulrich suggests [48], much more activity is likely to be needed than the average of 5 minutes per session 3 times a week provided by the typical parents in this study in order to have a significant effect on learning and outcomes. In the Heathcock and Galloway [44] study, parents were asked to provide 10 minutes of foot reaching practice for preterm infants 5 days/week. Under this regimen it took 8 weeks of practice for their experimental group to obtain significant learning effects, so both increased intensity and increased frequency are likely to be needed in our work. To that end, home visits could be initiated weekly for the first two months of the study, and biweekly thereafter, to provide more instruction in the exercise program and to provide feedback about the child’s performance to encourage parent participation. The concepts embodied in pragmatic clinical trial designs would also suggest that parents should be involved in developing procedures that would improve their ability to follow the exercise prescription [49]. Better measures of compliance, possibly including instrumentation, should also be identified.
The internal validity of the study must be improved by maintaining blinding of testing therapists. Possible strategies include calling each family before visits for testing to remind them to not reveal group assignment and developing a handout for families that describes typical situations in which a parent accidentally reveals group assignment to encourage families to be thoughtful in avoiding these situations.
Research Design and Measurement
The results of this study show that some children will recover from periventricular brain injury with typical gross motor development at 12 months CA, while others will be delayed or have CP of varying degrees of severity. An approach to dealing with the problem of inability to diagnose developmental outcomes before random assignment to study groups might include 1) use of early assessments with greater validity than the AIMS, such as the Test of Infant Motor Performance (TIMP) [14, 50], in order to stratify children into groups for random assignment to intervention at 4 months CA before treadmill training is added to the protocol, and 2) after stratified random assignment to groups, adapting the assessment strategy based on emerging aspects of development. This is an example of an adapted clinical trial design using prognostic biomarkers [51]. In order to make this work, all children upon study entry would receive the first toy for kicking training until random assignment and the beginning of treadmill training occurs at 4 months CA. An advantage of this approach is that compliance with the exercise protocol could be monitored and used to eliminate families from participation before random assignment takes place, if they are unable to meet the requirements for exercise training frequency and intensity.
The second strategy would be to improve the measurement approach by assessing all children with typical development on the TIMP using the AIMS, beginning at 4 months CA and continuing to use the AIMS (with new norms for North American children if necessary) and age at walking as outcome measures. Assessment after 12 months CA would be switched to another standardized gross motor assessment with appropriate norms for preschoolers. For children with delayed development on the TIMP by 4 months CA, we could use the Gross Motor Function Measure-88 (rather than the GMFM-66 because the latter has less sensitivity to change in low functioning children) [41] in addition to the AIMS to document developmental change. Ultimately, the study would also need to continue follow-up to ages beyond 12 months CA, including re-evaluation of the diagnosis of CP and reassignment on the GMFCS at 2 or 3 years for use in comparing outcomes of exercise versus no exercise for those children who develop CP. Given these revisions to the protocol, the study would need to be powered to allow for sufficient numbers of subjects for separate assessment of outcome groups with and without CP.
Suggestions for Further Research
In general, more research on interventions for infants with perinatal brain injury is needed because these children have high risk for adverse outcomes, yet are frequently excluded entirely from studies of early intervention. In this project, only short-term effects of the program were studied and it would be important to study long-term outcomes after improving the study protocol. DeKieviet and colleagues [1] showed that motor development delays are often modulated in the early years but then were more salient as children were tested at school age. Effects of early training in “making things happen” might also be of assistance in ameliorating cognitive and sensory deficits for which these children remain at high risk, so further study should also measure these types of outcomes. Finally, because BPD was a common co-morbidity in this group of children, assessment of exercise effects on pulmonary function is another avenue for research. In summary, although no effects of exercise on AIMS performance were found in this small interdisciplinary pilot study, the possibility that earlier walking was facilitated by the intervention bears further study with an improved protocol for assigning subjects to experimental groups, ensuring compliance, and measuring outcomes.
Acknowledgments
This project was supported by the University of Illinois at Chicago Center for Clinical and Translational Science, Award Number UL1RR029879 from the National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the U.S. National Institutes of Health. The study sponsor played no role in the study design; collection, analysis and interpretation of the data; nor the writing or decision to submit the manuscript.
We appreciate advice on the exercise protocol provided by Gay Girolami, George Hornby, and Dale Ulrich, and the technical assistance with equipment design of Jim Boynewicz and with data management of Kristin Rankin, Andrew Cooper, and Nour Sayes.
Footnotes
In September 2010 Fisher-Price issued a recall for this toy because the valve for inflation could come out and pose a choking hazard. Safe balls that are now available have a YELLOW dot on them and only these toys should be used with children.
Carlin’s Creations, 27366 Oak Drive, Sturgis, MI 49091, USA.
Conflict of interest: The lead author, SK Campbell, is part owner of Infant Motor Performance Scales, LLC (IMPS), the publisher of the Test of Infant Motor Performance (TIMP). The TIMP was used in this study but is not included in the original data presented in this manuscript. IMPS, LLC, provided no funding for this study and the University of Illinois at Chicago deemed the conflict to be suitably handled by recognition of this ownership in the parental assent form approved by the university’s Institutional Review Board. Co-author Laura Zawacki presents workshops on the TIMP for IMPS, LLC. Other coauthors have no conflicts of interest to declare.
References
- 1.de Kieviet JF, Piek JP, Aarnoudse-Moens CS, Oosterlaan J. Motor development in very preterm and very low-birth-weight children from birth to adolescence. A meta-analysis. JAMA. 2009;302:2235–2242. doi: 10.1001/jama.2009.1708. [DOI] [PubMed] [Google Scholar]
- 2.Babcock MA, Kostova FV, Ferriero DM, Johnston MV, Brunstrom JE, Hagberg H, et al. Injury to the preterm brain and cerebral palsy: Clinical aspects, molecular mechanisms, unanswered questions, and future research directions. J Child Neurol. 2009;24:1064–1084. doi: 10.1177/0883073809338957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Back SA. Perinatal white matter injury: The changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Mental Retard Dev Dis Res Rev. 2006;12:129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 4.Roze E, Van Braeckel KNJA, van der Veere CN, Maathuis CGB, Martijn A, Bos AF. Functional outcome at school age of preterm infants with periventricular hemorrhagic infarction. Pediatr. 2009;123:1493–1500. doi: 10.1542/peds.2008-1919. [DOI] [PubMed] [Google Scholar]
- 5.Hoon AH, Jr, Lawrie WT, Jr, Melhem ER, Reinhardt EM, Van Zijl PC, Solaiyappan M, et al. Diffusion tensor imaging of periventricular leukomalacia shows affected sensory cortex white matter pathways. Neurol. 2002;59:752–756. doi: 10.1212/wnl.59.5.752. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y-P, Fetters L, Holt KG, Saltzman E. Making the mobile move: Constraining task and environment. Infant Behav Dev. 2002;25:195–220. [Google Scholar]
- 7.Droit S, Boldrini A, Cioni G. Rhythmical leg movements in low-risk and brain-damaged preterm infants. Early Hum Dev. 1996;44:201–213. doi: 10.1016/0378-3782(95)01709-7. [DOI] [PubMed] [Google Scholar]
- 8.Fetters L, Chen U-p, Jonsdottir J, Tronick EZ. Kicking coordination captures differences between full-term and premature infants with white matter disorder. Hum Movement Sci. 2004;22:729–748. doi: 10.1016/j.humov.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Vaal J, van Soest AJ, Hopkins B, Sie LT, van der Knaap MS. Development of spontaneous leg movements in infants with and without periventricular leukomalacia. Exp Brain Res. 2000;135:94–105. doi: 10.1007/s002210000508. [DOI] [PubMed] [Google Scholar]
- 10.Jeng S-F, Chen L-C, Tsou K-I, Chen WJ, Luo H-J. Relationship between spontaneous kicking and age of walking attainment in preterm infants with very low birth weight and full-term infants. Phys Ther. 2004;84:159–172. [PubMed] [Google Scholar]
- 11.Jeng S-F, Yau K-IT, Liao H-F, Chen L-C, Chen P-S. Prognostic factors for walking attainment in very low-birthweight preterm infants. Early Hum Dev. 2000;59:159–173. doi: 10.1016/s0378-3782(00)00088-8. [DOI] [PubMed] [Google Scholar]
- 12.Hadders-Algra M. Reduced variability in motor behaviour: An indicator of impaired cerebral connectivity? Early Hum Dev. 2008;84:787–789. doi: 10.1016/j.earlhumdev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa VM, Campbell SK, Berbaum M. Discriminating infants from different developmental outcome groups using the Test of Infant Motor Performance (TIMP) item responses. Pediatr Phys Ther. 2007;19:28–39. doi: 10.1097/PEP.0b013e31802f65f9. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa VM, Campbell SK, Sheftel D, Singh J, Beligere N. Longitudinal performance of infants with cerebral palsy on the Test of Infant Motor Performance and on the Alberta Infant Motor Scale. Phys Occup Ther in Pediatr. 2003;23(3):7–29. [PubMed] [Google Scholar]
- 15.Barbosa VM, Campbell SK, Smith E, Berbaum M. Comparison of Test of Infant Motor Performance (TIMP) item responses among children with cerebral palsy, developmental delay, and typical development. AJOT. 2005;59:446–456. doi: 10.5014/ajot.59.4.446. [DOI] [PubMed] [Google Scholar]
- 16.Badr LK, Garg M, Kamath M. Intervention for infants with brain injury: Results of a randomized controlled study. Infant Behav Dev. 2006;29:80–90. doi: 10.1016/j.infbeh.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blauw-Hospers CH, Hadders-Algra M. A systematic review of the effects of early intervention on motor development. Dev Med Child Neurol. 2005;47:421–432. doi: 10.1017/s0012162205000824. [DOI] [PubMed] [Google Scholar]
- 18.Piper MC, Kunos VI, Willis DM, Mazer BL, Ramsay M, Silver KM. Early physical therapy effects on the high risk infant: A controlled trial. Pediatr. 1986;78:216–224. [PubMed] [Google Scholar]
- 19.Als H, Duffy FH, McAnulty GB, Rivkin MJ, Vajapeyam S, Mulkern RV, et al. Early experience alters brain function and structure. Pediatr. 2004;113:846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 20.Girolami G, Campbell SK. Efficacy of a Neuro-Developmental Treatment program to improve motor control of preterm infants. Pediatr Phys Ther. 1994;6(4):175–184. [Google Scholar]
- 21.Lekskulchai R, Cole J. Effect of a developmental program on motor performance in infants born preterm. Australian J Physiother. 2001;47:169–176. doi: 10.1016/s0004-9514(14)60264-6. [DOI] [PubMed] [Google Scholar]
- 22.White-Traut RC, Nelson MN, Silvestri JM, Vasan U, Littau S, Meleedy-Rey P, et al. Effect of auditory, tactile, visual, and vestibular intervention on length of stay, alertness, and feeding progression in preterm infants. Dev Med Child Neurol. 2002;44:91–97. doi: 10.1017/s0012162201001736. [DOI] [PubMed] [Google Scholar]
- 23.Malouin F, Richards CL. Assessment and training of locomotion after stroke: Evolving concepts. In: Refshauge K, Ada L, Ellis E, editors. Science-based rehabilitation: Theories into practice. New York: Butterworth Heinemann; 2005. pp. 185–222. [Google Scholar]
- 24.Angulo-Kinzler RM, Ulrich B, Thelen E. Three-month-old infants can select specific leg motor solutions. Motor Control. 2002;6:52–68. doi: 10.1123/mcj.6.1.52. [DOI] [PubMed] [Google Scholar]
- 25.Gordon AM, Magill RA. Motor learning: Application of principles to pediatric rehabilitation. In: Campbell SK, Palisano R, Orlin M, editors. Physical therapy for children. Philadelphia, PA: Elsevier; 2012. pp. 151–175. [Google Scholar]
- 26.Green EM, Mulcahy CM, Pountney TE. An investigation into the development of early postural control. Dev Med Child Neurol. 1995;37:437–448. doi: 10.1111/j.1469-8749.1995.tb12027.x. [DOI] [PubMed] [Google Scholar]
- 27.Thelen E. Three-month-old infants can learn task specific patterns of interlimb coordination. Psychol Sci. 1994;5:280–285. [Google Scholar]
- 28.Ulrich DA, Ulrich BD, Angulo-Kinzler RM, Yun J. Treadmill training of infants with Down syndrome: Evidence-based developmental outcomes. Pediatr. 2001 doi: 10.1542/peds.108.5.e84. http://www.pediatrics.org/cgi/content/full/108/5/e84. [DOI] [PubMed]
- 29.Bodkin AW, Baxter RS, Heriza CB. Treadmill training for an infant born preterm with a Grade III intraventricular hemorrhage. Phys Ther. 2003;83:1107–1118. [PubMed] [Google Scholar]
- 30.Piper M, Darrah J. Motor assessment of the developing infant. Philadelphia, PA: WB Saunders; 1994. [Google Scholar]
- 31.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 32.Brunner E, Domhof S, Langer F. Nonparametric analysis of longitudinal data in factorial experiments. New York: Wiley; 2002. [Google Scholar]
- 33.Blauw-Hospers CH, de Graaf-Peters VB, Dirks T, Bos AF, Hadders-Algra M. Does early intervention in infants at high risk for a developmental motor disorder improve motor and cognitive development? Neurosci Biobehav Rev. 2007;31:1201–1212. doi: 10.1016/j.neubiorev.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Blauw-Hospers CH, Dirks T, Hulshof LJ, Hadders-Algra M. Development of a quantitative tool to assess the content of physical therapy for infants. Pediatric Phys Ther. 2010;22:189–198. doi: 10.1097/PEP.0b013e3181dbd5f1. [DOI] [PubMed] [Google Scholar]
- 35.Cada EA, O’Shea RK. Identifying barriers to occupational and physical therapy services for children with cerebral palsy. J Pediatr Rehab Med. 2008;1:127–135. [PubMed] [Google Scholar]
- 36.Illinois Department of Human Services. Part C State Annual Performance Report for (FFY07) Springfield, IL: [Accessed April 2, 2011]. Early Intervention Illinois Annual Performance Report. Indicator #5 - Participation in Early Intervention for Children Under the Age of One. OMB No: 1820-0578. at http://www.dhs.state.il.us/page.aspx?item=42455. [Google Scholar]
- 37.Pin TW, Eldridge B, Galea MP. Motor trajectories from 4 to 18 months corrected age in infants born at less than 30 weeks of gestation. Early Hum Dev. 2010;86:573–580. doi: 10.1016/j.earlhumdev.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 38.van Haastert IC, de Vries LS, Helders P, Jongmans MJ. Early gross motor development of preterm infants according to the Alberta Infant Motor Scale. J Pediatr. 2006;149:617–622. doi: 10.1016/j.jpeds.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 39.Formiga CKMR, Linhares MBM. Motor development curve from 0 to 12 months in infants born preterm. [Accessed December 20, 2010];Acta Paediatr. 2010 doi: 10.1111/j.1651-2227.2010.02002. [DOI] [PubMed] [Google Scholar]
- 40.Fleuren KMW, Smit LS, Stijnen T, Hartman A. New reference values for the Alberta Infant Motor Scale need to be established. Acta Paediatr. 2007;96:424–427. doi: 10.1111/j.1651-2227.2007.00111.x. [DOI] [PubMed] [Google Scholar]
- 41.Russell D, Rosenbaum P, Avery L, Lane M. Gross Motor Function Measure (GMFM-66 and GMFM-88): User’s manual. London: MacKeith Press; 2002. [Google Scholar]
- 42.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the Gross Motor Function Classification System. Dev Med Child Neurol. 2006;48:424–428. doi: 10.1017/S0012162206000934. [DOI] [PubMed] [Google Scholar]
- 43.Gorter JW, Ketelaar M, Rosenbaum P, Helders PJM, Palisano R. Use of the GMFCS in infants with CP: the need for reclassification at age 2 years or older. Dev Med Child Neurol. 2008;51:46–52. doi: 10.1111/j.1469-8749.2008.03117.x. [DOI] [PubMed] [Google Scholar]
- 44.Heathcock JC, Galloway JC. Exploring objects with feet advances movement in infants born preterm: A randomized controlled trial. Phys Ther. 2009;89:1027–1038. doi: 10.2522/ptj.20080278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo H-J, Chen P-S, Hsieh W-S, Lin K-H, Lu T-W, Chen WJ, et al. Associations of supported treadmill stepping with walking attainment in preterm and full-term infants. Phys Ther. 2009;89:1215–1225. doi: 10.2522/ptj.20080369. [DOI] [PubMed] [Google Scholar]
- 46.Schertz M, Gordon AM. Changing the model: a call for a re-examination of intervention approaches and translational research in children with developmental disabilities. Dev Med Child Neurol. 2009;51:6–7. doi: 10.1111/j.1469-8749.2008.03220.x. [DOI] [PubMed] [Google Scholar]
- 47.Hashimoto M, McCoy SW. Validation of an activity-based data form developed to reflect interventions used by pediatric physical therapists. Pediatr Phys Ther. 2009;21:52–61. doi: 10.1097/PEP.0b013e318196ecad. [DOI] [PubMed] [Google Scholar]
- 48.Ulrich BD. Opportunities for early intervention based on theory, basic neuroscience, and clinical science. Phys Ther. 2010;90:1868–1880. doi: 10.2522/ptj.20100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luce BR, Kramer JM, Goodman SN, Connor JT, Tunis S, Whicher D, Schwartz JS. Rethinking randomized clinical trials for comparative effectiveness research: The need for transformational change. Ann Int Med. 2009;151:206–209. doi: 10.7326/0003-4819-151-3-200908040-00126. [DOI] [PubMed] [Google Scholar]
- 50.Campbell SK, Levy P, Zawacki L, Liao P-j. Population-based age standards for interpreting results on the Test of Infant Motor Performance. Pediatr Phys Ther. 2006;18:119–125. doi: 10.1097/01.pep.0000223108.03305.5d. [DOI] [PubMed] [Google Scholar]
- 51.Chow S-C, Chang M. Adaptive design methods in clinical trials – a review. [Accessed December 20, 2010];Orphanet J Rare Diseases. 2008 3:11. doi: 10.1186/1750-1172-3-11. at http://www.ojrd.com/content/3/1/11. [DOI] [PMC free article] [PubMed] [Google Scholar]