Abstract
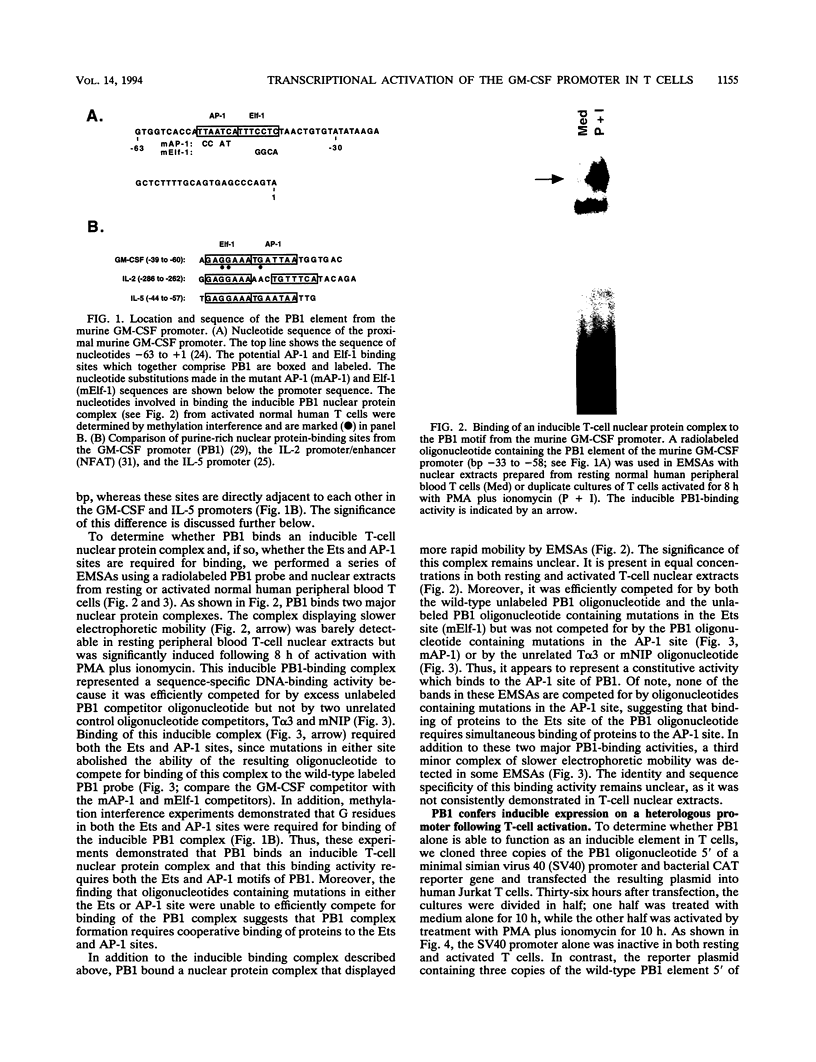
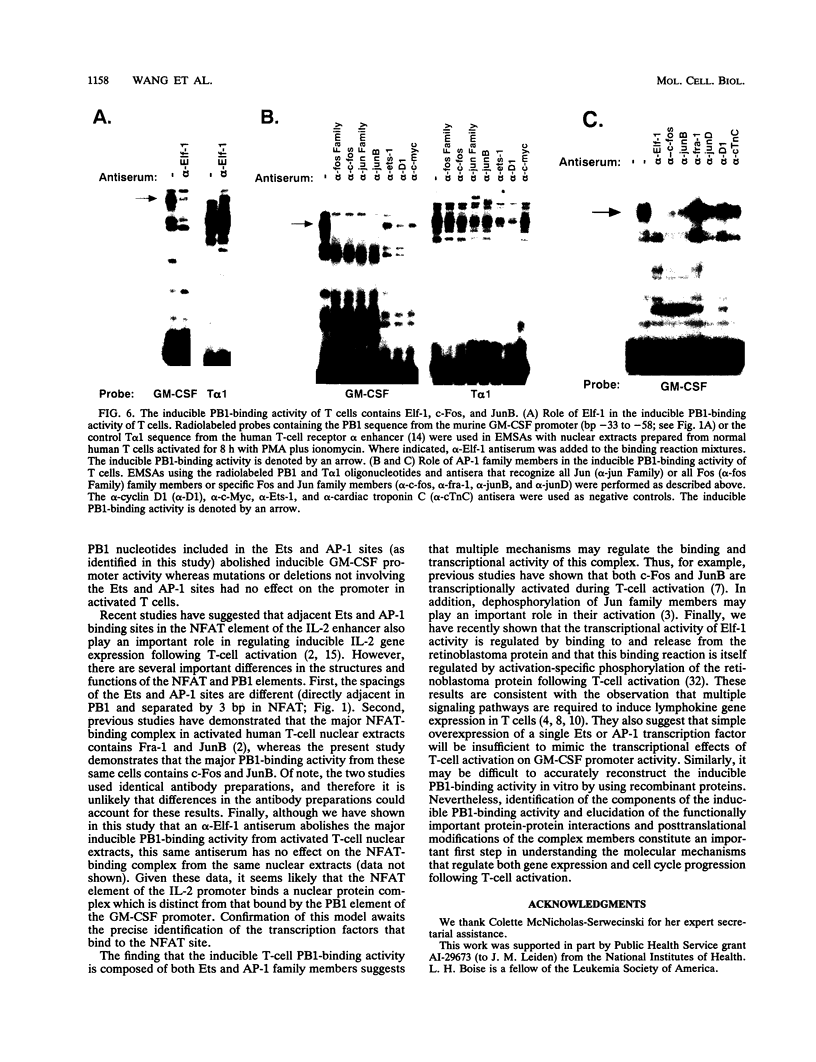
The granulocyte-macrophage colony-stimulating factor (GM-CSF) gene has been studied extensively as a model system of transcriptional induction during T-lymphocyte activation. The GM-CSF gene is not expressed in resting peripheral blood T cells but is rapidly induced at the transcriptional level following activation through the cell surface T-cell receptor. A highly conserved 19-bp element located immediately 5' of the human GM-CSF TATA box (bp -34 to -52), herein called purine box 1 (PB1), has been shown to bind a T-cell nuclear protein complex and to be required for transcriptional induction of the GM-CSF gene following T-cell activation. The PB1 sequence motif is highly conserved in both human and murine GM-CSF genes. In this report, we demonstrate that the PB1 element alone confers inducibility on a heterologous promoter following transfection into human Jurkat T cells. In addition, we identify a major PB1 nuclear protein-binding complex that is not present in resting peripheral blood T cells but is rapidly induced following T-cell activation. Sequence analysis revealed that PB1 is composed of adjacent binding sites for Ets and AP-1 transcription factors. In vitro mutagenesis experiments demonstrated that both the Ets and AP-1 sites are required for binding of the inducible PB1 nuclear protein complex and for the transcriptional activity of this element and the GM-CSF promoter in activated T cells. Using antibodies specific for different Ets and AP-1 family members, we demonstrate that the major inducible PB1-binding activity present in activated T-cell nuclear extracts is composed of the Elf-1, c-Fos, and JunB transcription factors. Taken together, these results suggest that cooperative interactions between specific Ets and AP-1 family members are important in regulating inducible gene expression following T-cell activation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K. Human and simian immunodeficiency retroviruses: activation and differential transactivation of gene expression. AIDS Res Hum Retroviruses. 1988 Jun;4(3):175–186. doi: 10.1089/aid.1988.4.175. [DOI] [PubMed] [Google Scholar]
- Boise L. H., Petryniak B., Mao X., June C. H., Wang C. Y., Lindsten T., Bravo R., Kovary K., Leiden J. M., Thompson C. B. The NFAT-1 DNA binding complex in activated T cells contains Fra-1 and JunB. Mol Cell Biol. 1993 Mar;13(3):1911–1919. doi: 10.1128/mcb.13.3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Chan J. Y., Slamon D. J., Nimer S. D., Golde D. W., Gasson J. C. Regulation of expression of human granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8669–8673. doi: 10.1073/pnas.83.22.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Durand D. B., Shaw J. P., Bush M. R., Replogle R. E., Belagaje R., Crabtree G. R. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988 Apr;8(4):1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann J., Golde D. W., Weisbart R. H., Gasson J. C. Granulocyte-macrophage colony-stimulating factor enhances phagocytosis of bacteria by human neutrophils. Blood. 1986 Sep;68(3):708–711. [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Ohashi T., Yamanishi K., Taniguchi T. Regulation of human interleukin-2 gene: functional DNA sequences in the 5' flanking region for the gene expression in activated T lymphocytes. Cell. 1986 Aug 1;46(3):401–405. doi: 10.1016/0092-8674(86)90660-4. [DOI] [PubMed] [Google Scholar]
- Gasson J. C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991 Mar 15;77(6):1131–1145. [PubMed] [Google Scholar]
- Gasson J. C., Weisbart R. H., Kaufman S. E., Clark S. C., Hewick R. M., Wong G. G., Golde D. W. Purified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophils. Science. 1984 Dec 14;226(4680):1339–1342. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Ho I. C., Yang L. H., Morle G., Leiden J. M. A T-cell-specific transcriptional enhancer element 3' of C alpha in the human T-cell receptor alpha locus. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6714–6718. doi: 10.1073/pnas.86.17.6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Valge-Archer V. E., Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992 Apr 30;356(6372):801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- Kovary K., Bravo R. Existence of different Fos/Jun complexes during the G0-to-G1 transition and during exponential growth in mouse fibroblasts: differential role of Fos proteins. Mol Cell Biol. 1992 Nov;12(11):5015–5023. doi: 10.1128/mcb.12.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M., Wang C. Y., Petryniak B., Markovitz D. M., Nabel G. J., Thompson C. B. A novel Ets-related transcription factor, Elf-1, binds to human immunodeficiency virus type 2 regulatory elements that are required for inducible trans activation in T cells. J Virol. 1992 Oct;66(10):5890–5897. doi: 10.1128/jvi.66.10.5890-5897.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T., June C. H., Thompson C. B. Multiple mechanisms regulate c-myc gene expression during normal T cell activation. EMBO J. 1988 Sep;7(9):2787–2794. doi: 10.1002/j.1460-2075.1988.tb03133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz D. M., Hannibal M., Perez V. L., Gauntt C., Folks T. M., Nabel G. J. Differential regulation of human immunodeficiency viruses (HIVs): a specific regulatory element in HIV-2 responds to stimulation of the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9098–9102. doi: 10.1073/pnas.87.23.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime H., Ewen M. E., Strom D. K., Kato J. Y., Hanks S. K., Roussel M. F., Sherr C. J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992 Oct 16;71(2):323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- McCune J. M. HIV-1: the infective process in vivo. Cell. 1991 Jan 25;64(2):351–363. doi: 10.1016/0092-8674(91)90644-e. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Otsuka T., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for granulocyte-macrophage colony stimulating factor: comparison of the mouse and human genes. EMBO J. 1985 Oct;4(10):2561–2568. doi: 10.1002/j.1460-2075.1985.tb03971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Shlomai J., Arai K., Arai N. Characterization of the mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) gene promoter: nuclear factors that interact with an element shared by three lymphokine genes--those for GM-CSF, interleukin-4 (IL-4), and IL-5. Mol Cell Biol. 1991 Dec;11(12):5894–5901. doi: 10.1128/mcb.11.12.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Niemeyer C. M., Sieff C. A., Mathey-Prevot B., Wimperis J. Z., Bierer B. E., Clark S. C., Nathan D. G. Expression of human interleukin-3 (multi-CSF) is restricted to human lymphocytes and T-cell tumor lines. Blood. 1989 Mar;73(4):945–951. [PubMed] [Google Scholar]
- Nimer S. D., Morita E. A., Martis M. J., Wachsman W., Gasson J. C. Characterization of the human granulocyte-macrophage colony-stimulating factor promoter region by genetic analysis: correlation with DNase I footprinting. Mol Cell Biol. 1988 May;8(5):1979–1984. doi: 10.1128/mcb.8.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimer S., Fraser J., Richards J., Lynch M., Gasson J. The repeated sequence CATT(A/T) is required for granulocyte-macrophage colony-stimulating factor promoter activity. Mol Cell Biol. 1990 Nov;10(11):6084–6088. doi: 10.1128/mcb.10.11.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker S. G., Hromas R., Kaushansky K. Transcriptional regulation of interleukin 3 gene expression in T lymphocytes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9650–9654. doi: 10.1073/pnas.87.24.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Wang C. Y., Ho I. C., Bohjanen P. R., Petryniak B., June C. H., Miesfeldt S., Zhang L., Nabel G. J., Karpinski B. cis-acting sequences required for inducible interleukin-2 enhancer function bind a novel Ets-related protein, Elf-1. Mol Cell Biol. 1992 Mar;12(3):1043–1053. doi: 10.1128/mcb.12.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Petryniak B., Thompson C. B., Kaelin W. G., Leiden J. M. Regulation of the Ets-related transcription factor Elf-1 by binding to the retinoblastoma protein. Science. 1993 May 28;260(5112):1330–1335. doi: 10.1126/science.8493578. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Clark S. C., Wong G. G., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. 1985 Mar 28-Apr 3Nature. 314(6009):361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Kwan L., Golde D. W., Gasson J. C. Human GM-CSF primes neutrophils for enhanced oxidative metabolism in response to the major physiological chemoattractants. Blood. 1987 Jan;69(1):18–21. [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]