Abstract
Warfarin is a complex but highly effective treatment for reducing thromboembolic risk in atrial fibrillation (AF). We examined contemporary warfarin treatment rates in AF prior to the expected introduction of newer anticoagulants and the extent of practice-level variation in warfarin use. Within the National Cardiovascular Data Registry PINNACLE program between July 2008 and December 2009, we identified 9113 outpatients with AF from 20 sites who were at moderate to high risk for stroke (CHADS2 score >1) and would be optimally treated with warfarin. Using hierarchical regression models, the extent of site-level variation was quantified with the median rate ratio, which can be interpreted as the likelihood that 2 random practices would differ in treating ‘identical’ patients with warfarin. The overall rate of warfarin treatment was only 55.1% (5018/9913). Both untreated patients and treated patients had mean CHADS2 scores of 2.5 (P=0.38) and similar rates of heart failure, hypertension, diabetes mellitus, and prior stroke, suggesting an almost ‘random’ pattern of treatment. At the practice level, however, there was substantial variation in treatment, ranging from 25% to 80% (interquartile range for practices: 50% to 65%), with a median rate ratio of 1.31 (1.22, 1.55), P<0.001. In conclusion, within the PINNACLE registry, we found that warfarin treatment in AF was suboptimal, with large variations in treatment observed across practices. Our findings suggest important opportunities for practice-level improvement in stroke prevention for outpatients with AF and define a benchmark treatment rate prior to the introduction of newer anticoagulant agents.
Keywords: variation, warfarin, atrial fibrillation, outpatient
Introduction
The emergence of the National Cardiovascular Date Registry (NCDR) Practice Innovation and Clinical Excellence (PINNACLE) program for cardiac outpatients represents a unique data source to evaluate warfarin treatment patterns in a contemporary U.S. outpatient cohort.1,2 Results from a contemporary registry can provide important baseline treatment rates with warfarin prior to the introduction of newer anticoagulants, such as dabigatran and rivaroxaban. Accordingly, within the PINNACLE program, we examined (1) treatment rates with warfarin among outpatients with non-valvular atrial fibrillation (AF) who are at moderate to high risk for stroke, and (2) the extent of patient- and practice-level variation in warfarin use. The presence of significant site-level variation would identify opportunities for quality improvement, and our findings will provide important benchmark rates of warfarin treatment prior to the introduction of newer anticoagulants into routine practice.
METHODS
The PINNACLE program has been previously described.1,2 Briefly, in 2008, the American College of Cardiology Foundation’s NCDR launched PINNACLE (formerly known as the Improving Continuous Cardiac Care program, or IC3)—the first, national, prospective, office-based, cardiac quality improvement registry in the U.S. Academic and private practices were invited to participate in PINNACLE through the American College of Cardiology’s website, emails, brochures, and information webinars. Physicians or practice representatives (e.g., administrators) in interested practices underwent a series of educational training sessions prior to data submission.
Within participating practices, a variety of patient data were collected at the point of care, including patients’ symptoms, vital signs, comorbidities, and medications. In addition, data for established performance measures for coronary artery disease, heart failure, and AF were collected. Data collection was achieved through one of 2 mechanisms: a) paper forms completed at the time of clinic visits, or b) modification of a practice’s electronic medical record data collection system to comprehensively capture the requisite PINNACLE data elements. Data from practices are routinely submitted to the NCDR®, and data quality checks and analyses were performed at Saint Luke’s Mid America Heart Institute (Kansas City, MO), the primary analytical center for the PINNACLE program.
For the purposes of this study, of 136,796 patients enrolled into PINNACLE from July 1, 2008 through December 31, 2009, we included 18,393 patients with non-valvular AF. We further restricted the cohort to only those patients at moderate to high risk for stroke (i.e., a CHADS2 score >1), in whom warfarin therapy is considered a performance measure of high-quality care, and included patients from practices with at least 10 eligible patients (total of 9280 patients excluded).3,4 The final study sample was comprised of 9113 patients with non-valvular AF at moderate to high risk for stroke from 20 practices at 51 different office locations.
The co-primary outcomes were (1) the rate of warfarin treatment in patients with AF at moderate to high risk for stroke, and (2) the extent of practice-level variation in warfarin use. To minimize over-representation by patients with multiple visits, we included data from only the baseline enrollment visit of each patient.
Baseline characteristics between patients treated and not treated with warfarin were compared using t-tests for continuous variables and the chi-square test for categorical variables. Warfarin treatment rates were determined for each practice and examined with descriptive plots.
To examine the extent of practice-level variation in warfarin use, multivariable hierarchical regression models were constructed to determine the median rate ratio (RR). These were 2-level hierarchical models, with the practice modeled as a random effect and patient covariates as fixed effects.5 Because treatment rates exceeded 10%, we utilized log-binomial or modified Poisson regression models at all steps, which estimate a rate ratio directly 6,7. The resulting median RR can be interpreted as the likelihood that 2 random practices would differ in treating ‘identical’ patients with warfarin. The median RR is always 1 or greater, with a median RR of >1.20 suggesting significant practice-level variation.
In addition, we examined in these models whether patient-level predictors were stronger determinants of warfarin treatment than practice-level variation. This is possible because the median RR permits meaningful comparisons with the effect sizes of patient factors (e.g., age, sex) included in the hierarchical models, thus overcoming interpretational limitations that are inherent with the intra-class correlation coefficient.8,9 In these models, we included as covariates the following patient characteristics: age (<70, 70 to <80, ≥80), sex, insurance type (private, Medicare, public, none), congestive heart failure, hypertension, diabetes mellitus, peripheral arterial disease, concomitant use of thienopyridine therapy, and history of prior stroke or transient ischemic attack, coronary artery disease, or systemic embolism.
For each analysis, the null hypothesis was evaluated at a 2-sided significance level of 0.05 with 95% confidence intervals (CIs) calculated. All analyses were performed with SAS 9.2 (SAS Institute, Cary, NC) and R version 2.7.0 (Foundation for Statistical Computing, Vienna, Austria) 10.
RESULTS
Of 9113 patients with non-valvular AF at moderate to high risk for stroke and eligible for warfarin treatment, 5018 (55.1%) were treated with warfarin and 4095 (44.9%) were not. Baseline characteristics of those treated and not treated with warfarin are described in Table 1. Compared with untreated patients, patients treated with warfarin were similar in CHADS2 score, age, and rates of congestive heart failure, hypertension, diabetes mellitus, and prior stroke. However, patients treated with warfarin were more frequently male and were more likely to have dyslipidemia, peripheral arterial disease, and a prior history of systemic embolism. In contrast, patients not treated with warfarin were more likely to have private health insurance and prior coronary artery disease. Notably, the rate of percutaneous coronary intervention with a drug-eluting stent within the past year, for which thienopyridine therapy would be warranted, were similar for both groups.
Table 1.
Baseline Characteristics of Patients Treated and Not Treated with Warfarin.
| Covariates | Total Cohort (N = 9113) | Warfarin Therapy | P value | |
|---|---|---|---|---|
| Yes (N = 5018) | No (N = 4095) | |||
| Mean CHADS2 score | 2.5 ± 0.8 | 2.5 ± 0.8 | 2.5 ± 0.8 | 0.38 |
| Age ≥ 75 years | 76.5 ± 9.9 | 76.3 ± 9.5 | 76.8 ± 10.4 | 0.45 |
| Age Categories (years) | 0.07 | |||
| < 70 | 21.1% | 21.4% | 20.9% | |
| 70 to 79 | 35.9% | 36.9% | 34.7% | |
| ≥ 80 | 43.0% | 41.8% | 44.4% | |
| Insurance type | <0.001 | |||
| Private | 61.9% | 60.4% | 63.8% | |
| Medicare | 33.7% | 34.4% | 32.9% | |
| Other public | 1.3% | 1.5% | 1.1% | |
| None | 3.0% | 3.6% | 2.2% | |
| Men | 51.3% | 52.6% | 49.7% | 0.005 |
| White | 87.2% | 87.1% | 87.3% | 0.80 |
| Coronary artery disease* | 47.4% | 45.6% | 49.6% | <0.001 |
| Dyslipidemia** | 60.8% | 62.9% | 58.3% | <0.001 |
| Diabetes mellitus | 37.1% | 36.8% | 37.5% | 0.50 |
| Hypertension*** | 93.4% | 93.8% | 93.0% | 0.14 |
| Prior Stroke/TIA | 10.3% | 9.9% | 10.7% | 0.18 |
| Congestive heart failure | 32.1% | 32.5% | 31.6% | 0.35 |
| Peripheral arterial disease | 6.1% | 5.1% | 7.3% | <0.001 |
| Prior systemic embolism | 2.1% | 2.8% | 1.1% | <0.001 |
| Stable angina pectoris | 5.1% | 4.9% | 5.3% | 0.39 |
| PCI with DES in past 12 months | 4.0% | 4.0% | 4.0% | 0.93 |
History of coronary artery stenosis ≥70%, PCI, or coronary artery bypass surgery
Assessed by individual physician, most commonly due to LDL > 130
Assessed by individual physician, most commonly due to persistently elevated systolic BP (>140) or diastolic BP (>90)
Abbreviations: DES, drug-eluting stent; TIA, transient ischemic attack; PCI, percutaneous coronary intervention.
There was no relationship between the CHADS2 score and treatment rates. Among 5612 patients with a CHADS2 score of 2, 3086 (55.0%) were treated with warfarin. For the 2510 patients with a CHADS2 score of 3, 1399 (55.7%) were treated with warfarin. Finally, for the 991 patients with a CHADS2 score of ≥4, 533 (53.8%) were treated with warfarin. Table 2 depicts use of anti-platelet therapies for patients treated and not treated with warfarin. Notably, among the 4095 patients not treated with warfarin, 2082 (50.8%) were treated with aspirin alone, 180 (4.4%) with a thienopyridine alone, 414 (10.1%) with both aspirin and a thienopyridine, and 1419 (34.7%) with neither aspirin nor thienopyridine therapy.
Table 2.
Rates of Anti-Platelet Therapy for Patients Treated and Not Treated with Warfarin
| Antiplatelet Therapy | Total Cohort (N = 9113) | Warfarin Therapy | P value | |
|---|---|---|---|---|
| Yes (N = 5018) | No (N = 4095) | |||
| Aspirin | 3543 (38.9%) | 1461 (29.1%) | 2082 (50.8%) | <0.001 |
| Thienopyridine | 294 (3.2%) | 114 (2.3%) | 180 (4.4%) | <0.001 |
| Aspirin + Thienopyridine | 589 (6.5%) | 175 (3.5%) | 414 (10.1%) | <0.001 |
| None | 4687 (51.4%) | 3268 (65.1%) | 1419 (34.7%) | <0.001 |
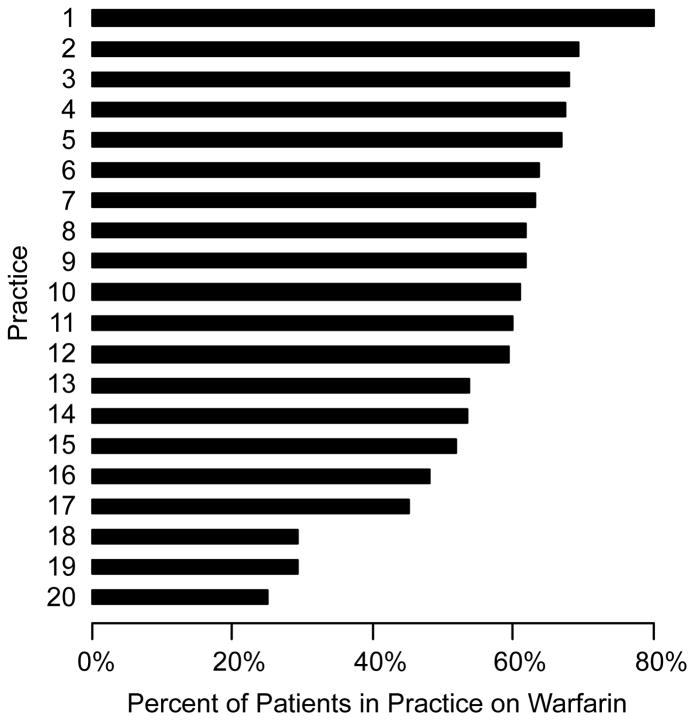
At the practice level, the median practice rate for warfarin treatment was 61%. There was significant variation in warfarin treatment, with a range of 25% to 80% and an inter-quartile range of 50% to 65% (Figure 1). The median RR was 1.31 (95% confidence interval: 1.22–1.55), P<0.001, suggesting moderate site-level variation in warfarin treatment across the practices in PINNACLE. There was no correlation between the number of AF patients at a practice and a practice’s rate of warfarin treatment (Spearman correlation of −0.14, p-value of 0.20). Moreover, practices with low warfarin treatment rates were not more likely to prescribe antiplatelet therapy than practices with high warfarin treatment rates (Spearman correlation of −0.10; p-value of 0.75) (Figure 2).
Figure 1. Variation in Treatment Rates with Warfarin Across Practices.
The median practice treatment rate with warfarin was 61%, with a range from 25% to 80% and an inter-quartile range of 50% to 65%.
Figure 2. Practice Rate of Antiplatelet Therapy.
There was little correlation between a practice’s rate of warfarin treatment and its rate of antiplatelet (aspirin or thienopyridines) therapy.
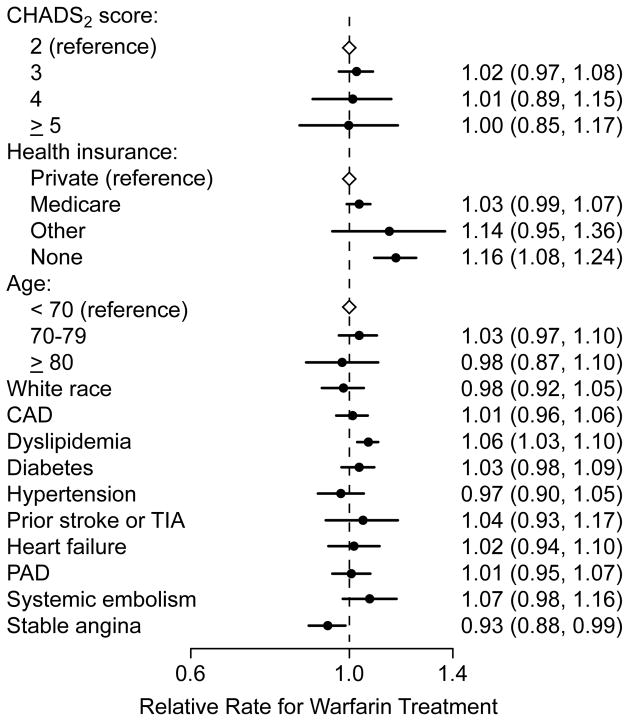
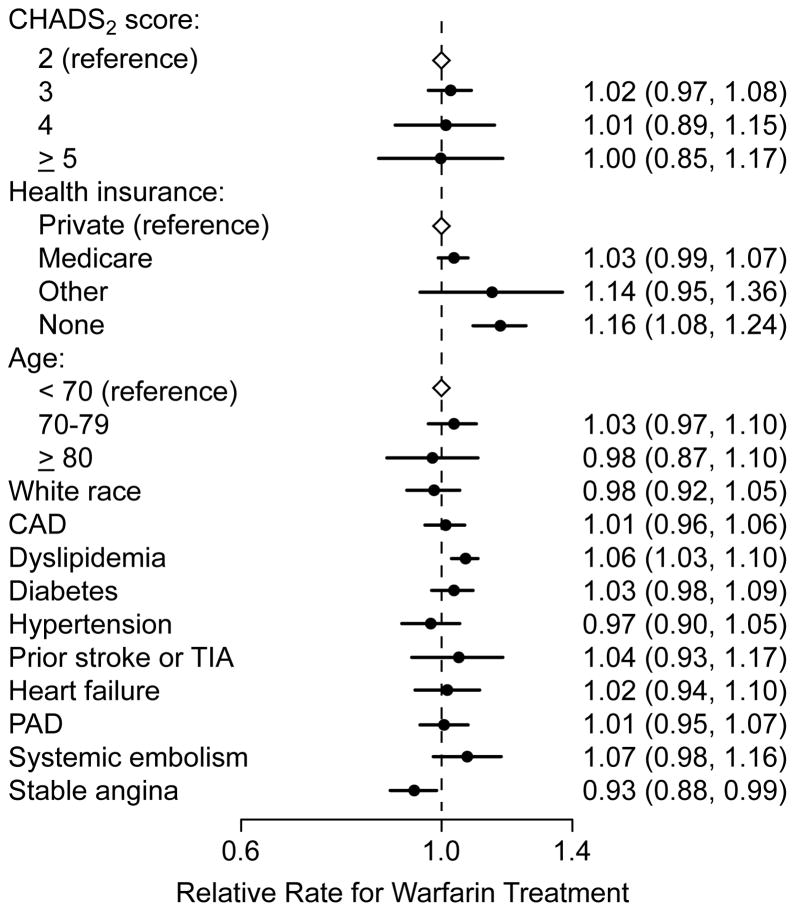
There were few patient-level predictors associated with warfarin treatment. Overall CHADS2 score, age, hypertension, diabetes mellitus, heart failure, coronary artery disease, and prior history of stroke or transient ischemic attack were each not associated with warfarin treatment. Treatment with aspirin or thienopyridine therapy was associated with not receiving warfarin treatment, but whether the use of anti-platelet therapy was the result or cause for not initiating warfarin treatment is unclear. Notably, in the multivariable hierarchical model, the effect size of the median RR was greater than those of any of the patient-level predictors for warfarin use (Figure 3), suggesting that site-level variation explained a larger proportion of the variance in warfarin treatment than patient factors.
Figure 3. Patient Predictors of Warfarin Treatment.
Patient characteristics were not strong predictors of warfarin treatment, including components of the CHADS2 score. The median rate ratio for site-level variation of 1.31 was larger than the estimates of effect for any of the patient factors, suggesting that practice level variation explained a larger proportion of the variance in warfarin treatment than individual patient factors.
Finally, we examined what proportion of untreated patients was initiated on warfarin during the subsequent visit. We found that only 232 (5.7%) of the 4095 untreated patients were initiated on warfarin after the first visit, and our study findings were similar when the analyses were repeated with these patients re-classified as warfarin treated patients (results not shown).
DISCUSSION
In this prospective, multi-site cohort of 9113 cardiac outpatients with non-valvular AF, we found that warfarin use in patients at moderate to high risk for stroke was only 55%. There was substantial variation across practices in warfarin treatment rates, with a median rate of 61% and a range from 25% to 80%. In fully adjusted models, practice-level variation explained a greater amount of the observed variance in warfarin treatment than any patient characteristic. Our findings therefore suggest that warfarin treatment will remain suboptimal unless the characteristics of high-performing practices can be identified and generalized to those practices with the lowest rates.
Despite being a performance measure for AF, significant barriers exist in initiating warfarin therapy, compared to medications for other cardiac conditions, such as beta-blockers or statins. These barriers include patient refusal because of concerns about bleeding risk, the need for routine monitoring, and physician factors (e.g., time allotted for outpatient clinic visits).11,12 As it is unlikely that differences in practice rates of patient contraindications to warfarin therapy or refusal to take warfarin could account for the wide range of practice treatment rates (from 25% to 80%), the presence of substantial site-level variation in this cohort likely reflects actual differences in the quality of care across practices. Of equal concern, this variation was not explained by higher rates of antiplatelet therapy in practices with low warfarin treatment rates. However, the reasons for why some practices are able to achieve high warfarin treatment rates remain unclear. Because our cohort included patients from only 20 practices, our study would be severely under-powered in analyses of the relationship between specific practice characteristics and practice rates of warfarin treatment. Future studies are therefore needed to clarify which provider or practice factors—such as the presence of an anticoagulation clinic or anticoagulation protocols, collaborative office practices with anticoagulation pharmacists and/or nurses, average clinic time for outpatient visits at a practice, physician views about warfarin use—may be responsible for the large observed variations in warfarin use across practices.
With the introduction of newer anticoagulants such as dabigatran and likely rivaroxaban in clinical practice, our findings also provide an important benchmark rate for anticoagulant use in contemporary outpatient practice in the U.S. If indeed barriers to initiating warfarin treatment include lack of office clinician time in a busy outpatient clinic and low levels of reimbursement for patient education and monitoring of anticoagulation levels, it is likely that treatment rates with effective anticoagulants in AF patients at moderate to high risk of stroke will increase after the introduction of these newer agents which do not require monitoring of drug levels or adjusting of doses. Future studies within PINNACLE will need to examine whether treatment rates increase overall at the patient level and whether practice level variation narrows after the introduction of these novel anticoagulants into clinical practice.
Our study findings should be interpreted in light of the following limitations. Although our study enrolled over 9113 patients with AF, PINNACLE practices may be highly motivated for quality improvement; therefore, warfarin treatment rates may be even lower in practices not participating in PINNACLE. Second, while practices were asked to submit data on all patients with AF, the PINNACLE program has no way of determining whether data on some patients were excluded from the program. However, to the extent that participating practices depend upon the PINNACLE program to report for their pay-for-performance measures, it is in the practices’ economic interests to submit complete data on all their cardiac patients. Third, the PINNACLE registry did not systematically collect information on potential contraindications to anticoagulant or antiplatelet therapy. However, rates of contraindications have been reported to be only about 6% in other registries.13 Moreover, rates of contraindications to anticoagulant therapy are not expected to vary widely across practices and therefore would be unlikely to account for the significant variation observed across practices. And finally, while treatment with warfarin is viewed as a metric of quality care, this current study was not designed to examine clinical outcomes, which also may vary substantially with treatment patterns.
Acknowledgments
Sponsorship
The PINNACLE Registry™ is an initiative of the American College of Cardiology Foundation, MedAxiom and Spirit of Women. The Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership is a Founding Sponsor of the PINNACLE Registry.
Footnotes
Author Disclosures
- Dr. Chan is supported by a Career Development Grant Award (K23HL102224) from the NHLBI.
- Drs. Chan and Spertus and Ms. Tang are affiliated with the Mid America Heart Institute, which is the major analytic center for the PINNACLE program and receives funding from the American College of Cardiology for this role.
- The efforts and cooperation of the cardiology practices currently enrolled in PINNACLE are greatly appreciated by the authors and by the NCDR PINNACLE Work Group.
- Dr. Maddox is supported by a Health Services Research and Development Career Development Award from the U.S. Department of Veterans Affairs.
Official NCDR Disclaimer
This research was supported by the ACC Foundation’s National Cardiovascular Data Registry (NCDR). Although the manuscript underwent internal review by an NCDR Research and Publications committee, the views expressed in this manuscript represent those of the authors, and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com.
References
- 1.Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang F, Jones PG, Breeding T, Thrutchley D, Rumsfeld JS, Spertus JA. Cardiac Performance Measure Compliance in Outpatients The American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) Program. J Am Coll Cardiol. 2010;56:8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan PS, Oetgen WJ, Spertus JA. The Improving Continuous Cardiac Care (IC(3)) program and outpatient quality improvement. Am J Med. 2010;123:217–9. doi: 10.1016/j.amjmed.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Estes NA, 3rd, Halperin JL, Calkins H, Ezekowitz MD, Gitman P, Go AS, McNamara RL, Messer JV, Ritchie JL, Romeo SJ, Waldo AL, Wyse DG. ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation): developed in collaboration with the Heart Rhythm Society. Circulation. 2008;117:1101–20. doi: 10.1161/CIRCULATIONAHA.107.187192. [DOI] [PubMed] [Google Scholar]
- 4.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein H. Multilevel Statistical Models. London and New York: Edward Arnold; Wiley; 1995. pp. 21–78. [Google Scholar]
- 6.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 7.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160:301–5. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 8.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161:81–8. doi: 10.1093/aje/kwi017. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein H, Browne W, Rasbash J. Partitioning variation in multilevel models. Understanding Statistics. 2002;1:223–232. [Google Scholar]
- 10.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing V; Austria: 2008. URL http://www.R-project.org. [Google Scholar]
- 11.Howitt A, Armstrong D. Implementing evidence based medicine in general practice: audit and qualitative study of antithrombotic treatment for atrial fibrillation. BMJ. 1999;318:1324–7. doi: 10.1136/bmj.318.7194.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–6. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Meinertz T, Kirch W, Rosin L, Pittrow D, Willich SN, Kirchhof P. Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol. 2011 doi: 10.1007/s00392-011-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]