Abstract
Extracellular hydrodynamic forces may be transmitted to the interior of cells through the alteration of integrin conformation and affinity. Integrin activation regulates leukocyte recruitment, cell activation, and transmigration. The cellular and molecular mechanisms for integrin activation are not precisely known, although intracellular calcium signaling is involved. Flow cytometry offers a versatile way to study intracellular calcium signaling in real-time. We report a novel method to generate defined shear by using a miniature Couette. Testing involved measuring shear induced intracellular calcium signals of human monoblastoid U937 cells in suspension. The Couette was connected externally to a flow cytometer and pressurized at 6 PSI (4.1 N/m2). Cells were subjected to well-defined shear between 0 and 1000 s−1 and delivered continuously within 10 s to a FACScan at 1 μl/s. Intracellular calcium levels and the percentage of cells activated increased as shear increased in duration and intensity.
INTRODUCTION
Leukocyte recruitment is regulated by chemokines (1), adhesion molecules (2), and physiological shear (3). Shear is generated from bifurcation of blood vessels (4), rapid changes in blood vessel diameters (5), and when cells are bound to the endothelium in blood flowing vessels (6). Extracellular forces can act on the cell membrane (7), adhesion molecules (8), and other surface receptors (9-10) to activate cellular processes such as growth and proliferation (11), protein synthesis, gene expression, migration (12), apoptosis (13), and regulation of integrin activation (14-15). Flow Cytometry offers a versatile approach to studying cell adhesion by measuring multiple cell signaling responses to physiological forces in real-time. Cytometry also compliments data obtained from atomic force microscopy (16), bioforce probes (17), parallel flow chambers (18), cone and plate viscometers (19), and magnetic bead micro-rheometer (20).
The use of a flow cytometer to study the effects of shear on cells is often done in conjunction with an external device; such as the cone and plate (21-23), parallel ring viscometer (24-25), turbidimetric aggregometer (26), or pushing cells through connecting tube with pressurized nitrogen (27-28). Examples of cells responding to shear have involved the measurement of cell aggregation and disaggregation rates for coupled adhesion molecules; VLA-4/VCAM-1 (15, 24), β2/L-selectin (29), LFA-1/ICAM-3, Mac-1/ICAM-3 (30), and P-selectin/PSGL-1 (23, 31-32). Aggregation rates depend on receptor-ligand bond coupling, applied force to the bonds, encounter rate, and contact area between cells. One method to unravel the complex shear/mechanical signaling pathway is to measure intracellular calcium response associated with shear. Changes to intracellular calcium are part of an intricate signaling pathway for cellular processes involving cell migration, metastasis, and cell growth, proliferation, and apoptosis.
To modulate cellular responses in real-time we have constructed a miniature Couette that was coupled to a flow cytometer. We measured intracellular calcium changes in real-time. The novelty of this device involved controlling the strength and duration of shear. One goal of constructing this device was to estimate the force applied to adhesion bonds between two bound cells (33-34). The Couette was tested by measuring how shear altered intracellular calcium levels in real-time. To do this the Couette was pressurized at 6 PSI (4.1 N/m2) and attached externally to a flow cytometer. Cells were transported to a flow cytometer within 10 s and with minimal transport induced shear. The novelty of the system allowed for well defined shear to be generated between 0 and 1000 s−1 and for the desired duration. Sample delivery time was comparable to other flow cytometric techniques. We used shear sensitive human monoblastoid U937 cells expressing VLA-4 integrin (15). Fluorescent calcium indicators Fura Red and Fluo-4 monitored relative levels of intracellular calcium. Fluorescence was detected with a FACScan flow cytometer. The relative fluorescence (intracellular calcium) was observed to increase as shear increased. We also observed that the percentage of cells activated increased as shear increased in duration and intensity.
MATERIALS AND METHODS
Materials
Fura Red AM (catalog number F3021), Fluo-4 AM (catalog number M14206), and CFSE (catalog number C1157) were purchased from Molecular Probes (Eugene, OR).
Cell Lines and Transfectant Construct
Human monoblastoid U937 cells were purchased from ATCC (Rockville, MD) and grown in RPMI 1640 with phenol red (Invitrogen Corporation, Grand Island, NY). Cell medium was supplemented with 10% heat inactivated fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 10 mM HEPES, pH 7.4, 100 μg/ml Ciprofloxacin, 2 mM L-glutamine, at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Site-directed mutants of formyl peptide receptor in the human monoblastoid line U937 constitutively expressing human VLA-4 integrin were prepared as described (35).
Cell Preparation and Detection
U937 cells (1×106cells/ml) were loaded with 6 μM Fura Red or 200 nM Fluo-4 for 30 min. at 37°C and gently mixed every 10 min. Cells were washed with HEPES buffer –HB - (110 mM NaCl, 10 mM KCI, 10 mM glucose, 1 mM, MgCl2, 1.5 mM Ca2+, and 30 mM HEPES, pH 7.4) and resuspended in HB that was supplemented with 0.1% Human Serum Albumin (Bayer, Elkhart, IN). We measured cell concentration using a hemocytometer and a stage microscope after cell preparation. All experiments were performed using HB.
Cells were kept on ice after staining and washing. Typically, 5 min. prior to each experiment 1 ml of 1×106cells fluorescently labeled cells were manually inserted into the Couette. The cells were allowed to rest for 5 min. while the inner cylinder was rotated slowly (20 s−1) to minimize cell settling. Calcium levels in sheared cells were measured in real-time while the Couette was pressurized at 6 PSI (4.1 N/m2). A FACScan flow cytometer with 488 nm Argon laser (BD Immunocytometry Systems, San Jose, CA. Installation date of optical filters, January 1989.) excited calcium sensitive dyes. Emission fluorescence was detected using 585 nm band pass and 650 nm long wavelength pass filter for Fura Red (FL2 and FL3, respectively) and 530 nm band pass filter for Fluo-4 (FL1). Voltage settings were 705 / 551 V (FL2/FL3) and 489 V (FL1) for Fura Red and Fluo-4 (and CFSE), respectively. No detector compensations were applied during data acquisition. Fura Red fluorescence decreases when this indicator is bound to free calcium while Fluo-4 fluorescence increases.
Vortex
A Fischer Scientific Mini-Vortexer (Fischer Scientific, Hampton, NH) generated a maximum intracellular calcium response. The shear rate at 3200 RPM was estimated to be ~200 – 12000 s−1 for cells inside a vortexed 12 (outer diameter) ×75 mm tube. Fluid motion inside a vortexed tube was approximated to be that of fluid motion in a Couette viscometer. The maximum (SMax) and minimum (SMin) wall shear rate at a given rotational velocity is approximated (36) as
| Equation 1 |
where RI (ranging from ~5.4 to 2.5 mm) and Ro (5.5 mm) radii of the inner fluid and outer fluid surfaces and Ω is the angular speed of the inner cylinder.
Monoblastoid U937 cells were incubated at 37°C for 5 min. After 5 minutes cells were gently re-suspended before monitoring intracellular calcium with the FACScan flow cytometer. After 60 s, the sample was removed from the flow cytometer and vortexed for 10 s. Then data sampling was resumed.
Miniature Couette
The miniature Couette was composed of six sections; two concentric cylinders (samples were placed between cylinders), a motor to rotate inner cylinder, support frame to hold cylinders and motor, tubing to transport samples and pressurize sample volume, control to set rotational rate of inner cylinder, and water bath to maintain sample at 37°C. Samples were manually injected between two concentric cylinders through a port. (Automated sample injection was available.) The inner cylinder was 30 mm high by 19 mm wide (outer diameter) and composed of Teflon. Teflon minimized cellular reactivity and fluid surface tension. The outer Plexiglas cylinder was 31 mm high by 20 mm wide (inner diameter) and 5 mm thick. Plexiglas allowed for visual sample observations. Figure 1A shows the device. These materials gave optimal uniformity in fluid suspension and ease of sample removal from the miniature Couette. The Couette was enclosed by end caps, 25 mm diameter O-rings and six screws for each end cap. A metal shaft connected the inner Teflon cylinder to a 5 phase Vexta stepping motor (Oriental Motors, Torrance, CA) through a 5 mm hole in the center of the upper cap. An 8 mm O-ring was placed around the shaft to seal the end cap. A set screw in the shaft attached the shaft to the stepping motor. This “blender” configuration pressurized the sample region. Both lower end piece and stepping motor were attached to an external aluminum frame. A screw held the lower end cap to the external frame. A hole was drilled through both the Plexiglas end cap and lower aluminum frame to which a 0.01” ID (0.25mm) 15 cm long Peek tube (Western Analytical, Wildomar, CA) was attached for sample extraction to a flow cytometer.
Figure 1.
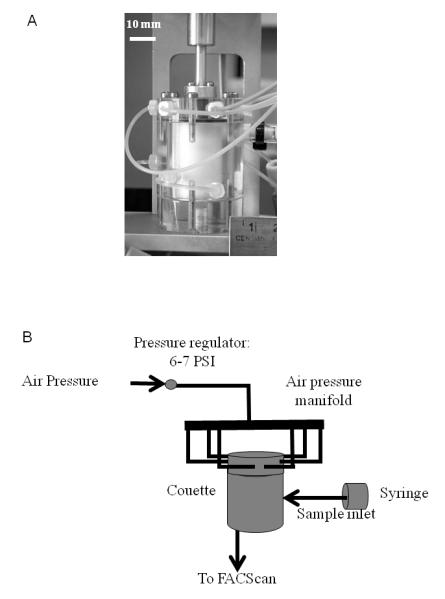
Schematic of Miniature Couette. A) A photograph of the miniature Couette shows a 19 mm diameter Teflon inner cylinder within a 20 mm diameter Plexiglas cylinder. White bar denotes 10 mm scale. B) A schematic of sample flow and air pressure regulation.
Six 0.02” (0.50mm) holes were drilled into the sides of the outer Plexiglas cylinder in a staggered configuration. This allowed samples to be injected into the Couette manually or under computer control. For simplicity, the data presented were obtained by manual injection through one port in which a 0.01” ID (0.25mm) Peek tube was attached. The Couette was pressurized to 6 PSI (4.1 N/m2) through six 0.02” (0.50 mm) ports that were drilled into the upper cap. Those ports were connected to 0.05” (1.27 mm) Peek tubing and attached to a manifold. An air pressure regulator was used in conjunction with a 0.2 μm filter (Pall Corporation, Eastern Hills, NY) to control and filter laboratory air. Air fluctuations were dampened with the regulator. The flow rate was adjusted to 1 μl/s and was comparable to flow cytometers. This flow rate helped to minimize transport induced shear and delivered to the cytometer within 10s. A schematic diagram is shown in Figure 1B.
Modifications to the Couette can be easily made. For example, the Couette can be pressurized at 20 PSI (13.8 N/m2) for sample delivery on the order of 1 s. For transit times less than one second the Couette can placed within millimeters of where fluorescently labeled cells intersect with a flow cytometer’s laser (flow cell). The Couette was designed with sample volumes of 1.0 and 2.0 ml volumes for 19 mm and 18 mm inner diameter cylinders, respectively. A decreased inner cylinder diameter gives a larger sample volume. For Newtonian fluids, the shear rate at the inner and outer radii of the sample region can be estimated using Equation 1 when the inner cylinder rotates with an angular speed Ω. During each experiment the miniature Couette was placed in a 37°C water bath, with the stepping motor above the water. The stepping motor was controlled with a custom built controller that measured the number of shaft rotations. Then the RPM rate was calculated by the controller. An RPM value was manually selected using the controller. RPM rates increased with increments of 5 RPM.
A 1 ml sample volume (19 mm inner cylinder) was used. Shear rate can be altered by switching to smaller or larger inner cylinders. For example, a 19 mm diameter cylinder gives a lower shear of 10 s−1 while an 18 mm diameter cylinder gives 5 s−1 for the same rotational rate. Injection ports were closed after 1 ml sample was added.
Flow Cytometry and Data Analysis
Flow cytometric analysis was done on a Becton-Dickinson FACScan flow cytometer (Becton-Dickinson Immunocytometry Systems, San Jose, CA). Percentage of events related to U937 cells were 95% and 70% for FACScan only and data obtained using the Couette, respectively. Difference was due fluidic changes when air pressure was used to control fluid flow to the flow cytometer. Data acquisition used CellQuest (Becton-Dickinson Immunocytometry Systems, San Jose, CA) and data were analyzed offline using Windows Multiple Document Interface Flow Cytometry Interface (Scripps, La Jolla, Ca). An elliptical forward and side scatter gate was used to selected U937 cell events. No analysis transformations of the data were made. Time and fluorescence information were obtained using an IDL (RSI, Boulder, CO.) based FacsQuery software, developed by Bruce Edwards (University of New Mexico, Albuquerque, NM). Further analysis was done using GraphPad Prism 4 (GraphPad, San Diego, CA) and PeakFitv4.12 (SeaSolve, San Jose, CA).
Bimodal Fit
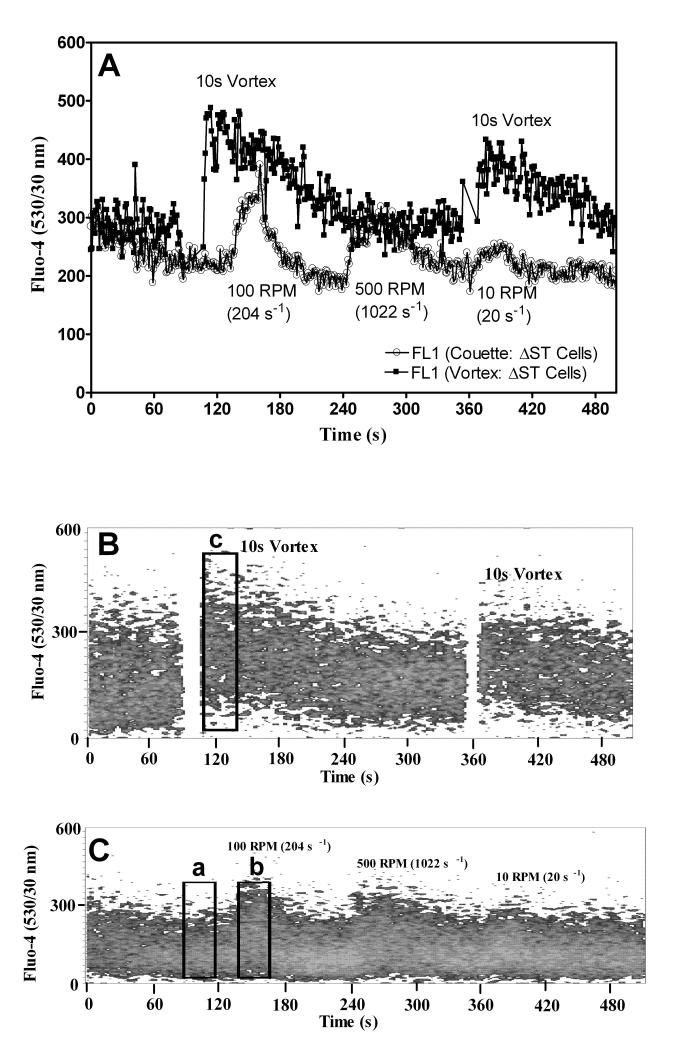
Sheared cells exhibit different degrees of intracellular calcium response that is a function of intensity and duration of applied shear. The distribution of cells that exhibited intracellular calcium changes ranged from a resting state to fully activated by shear. We analyzed the intracellular calcium distributions before and after the application of shear, as shown in Figure 5B and C. Region a of Figure 5C depicts resting state intracellular calcium level and Regions b and c are shear activated states. The analysis of resting state intracellular calcium levels used a one Gaussian curve fitted to the mean channel fluorescence distribution of region a. Region c represents the maximally excited state obtained by vortexing cells. This region was similarly fitted with a one Gaussian curve. Centroid and peak shape values were recorded for Regions a and c. Region b represents an intermediate level of shear activation obtained with the Couette. Two Gaussian curves are used to fit Region b. One fit used the peak centroid and shape values obtained from Region a (resting cells) and the other fit used peak centroid and shape values obtained from Region c (shear activated cells). Then a simultaneous two-Gaussian fit to the mean channel fluorescence distribution of Region b was done. The ratio of the total events under the two histograms (activated and resting) was taken to estimate the percentage of cells activated under shear (activated / (resting + activated) × 100.
Figure 5.
Cell calcium response induced with a Couette and vortexer. U937 cells were stained with Fluo-4. A 1 ml sample was injected into a Couette and placed in a 37°C water bath. Or a cytometry tube was attached to a FACScan. The flow rate was adjusted to 1 μl/s. A) A comparison of Fluo-4 response to vortexing (open circles, ○) and for shear at 100, 500, and 10 RPM generated using a Couette (circles, ●). B) Dot plot of Fluo-4 response versus time. Rectangular Region c represents the region of maximal shear response used to fit a Gaussian curve of Fluo-4 distribution. C) Dot plot of Couette Fluo-4 response versus time. Rectangular Region a represents the region used to fit a Gaussian curve to Fluo-4 distribution for the resting state and Region b represents the region used in a two Gaussian curve fit to extract percentage of cells activated due to shear. A black bar denotes the time scale of 60 s.
RESULTS
Uniformity of Real-time Sampling
Uniformity of Couette sample extraction was measured using Flow Check Fluorospheres (Beckman Coulter, Fullerton, CA). The rate of cell extraction was found to be comparable to a sample tube pressurized using a FACScan flow cytometer.
Testing Responsiveness of U937 cells
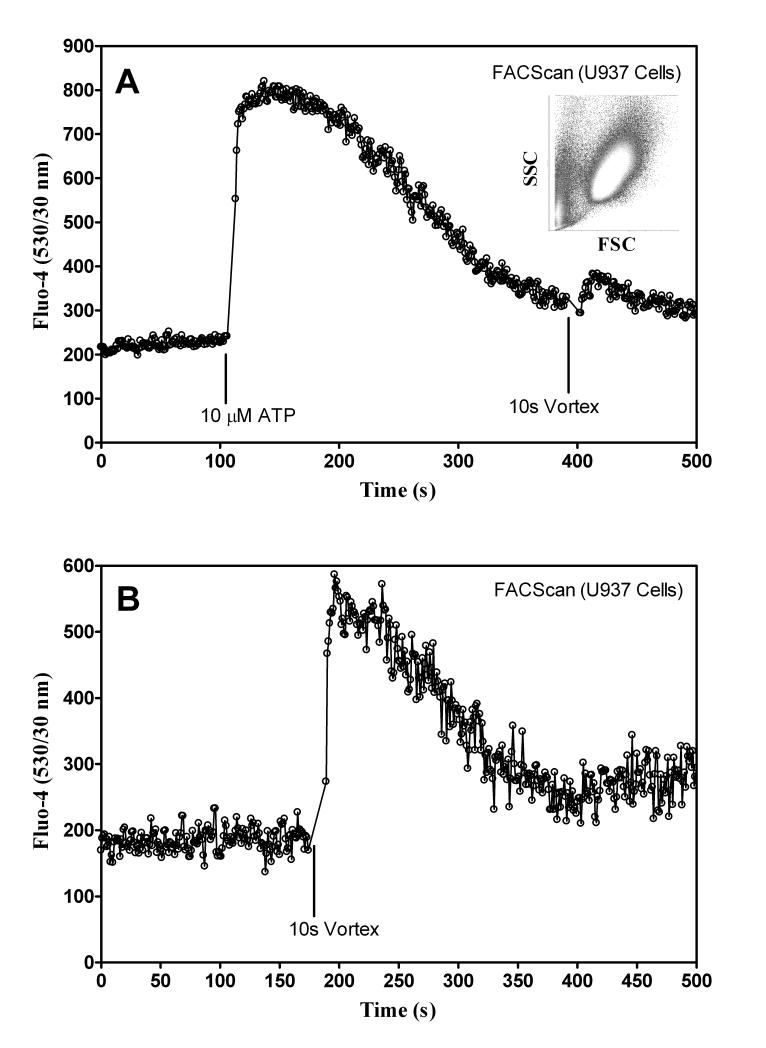
Two methods were used to measure cell responsiveness. The first exposed U937 cells to shear from a Fischer Scientific Mini-Vortexer at 3200 RPM for 10 s. The second method obtained calcium signaling without shear by adding 10 μM ATP to activate the nucleotide receptors constitutively expressed on U937 cells (P2Y2, and P2Y6) (37-39). Typical results for healthy cells are shown in Figure 2A and B, where an increase in Fluo-4 signal indicated intracellular activation.
Figure 2.
Testing responsiveness of U937 Cells. A) U937 cells were stained with Fluo-4. Then cells were sampled with the FACScan for 120 s, 10 μM ATP was added, and sampling resumed. At 380 s the sample was vortexed for 10 s and then sampling resumed. Inset shows typical raw forward and side scatter data used as a gate for final plots. B) U937 cells sampled with FACScan only and vortexed for 10 s.
Real-time Flow Using Cells
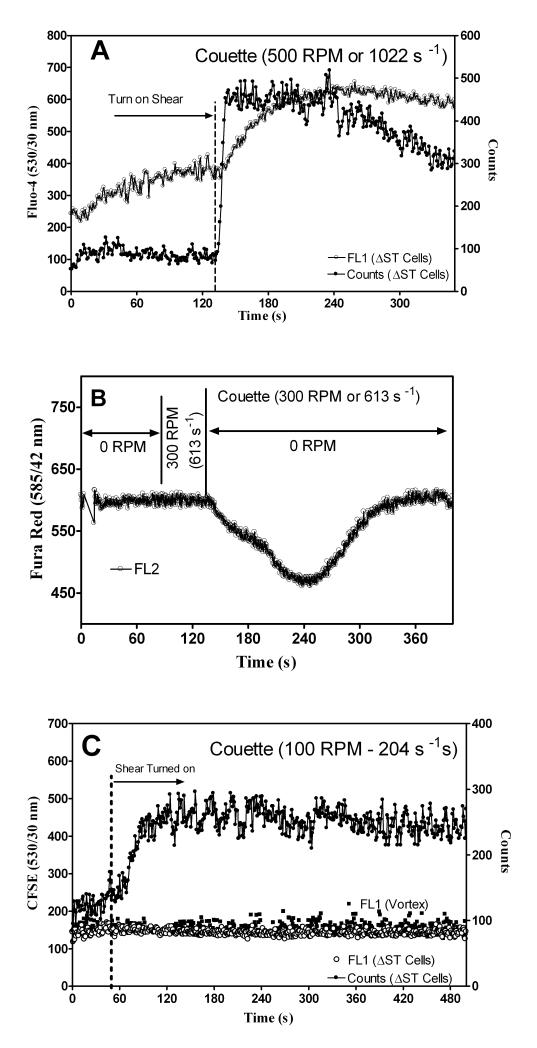
To determine if changes in FL1 (Fluo-4) signal were due to shear activated U937 cells, three different staining techniques were used. Figures 3A, B, and C show cell staining with Fluo-4 (upper figure), Fura Red (center figure), and CFSE (lower figure), respectively. Fura Red fluorescence decreases when the indicator is bound to free calcium while Fluo-4 increases. Both calcium indictors showed that the intracellular calcium levels change in response to shear. Figure 3C shows data obtained for U937 cells stained with CFSE (label intracellular and cell-surface proteins), which is not sensitive to shear. The decreased Fura Red and the increased Fluo-4 signal verified that cells responded to shear and the data was not a mechanical artifact originating from the miniature Couette.
Figure 3.
Characterization of calcium response in the Couette. A) U937 cells were stained with Fluo-4 calcium indicator. A 1 ml sample was injected into the miniature Couette and kept in 37°C water bath. The flow rate was adjusted to 1 μl/s before the inner cylinder was rotated at 500 RPM (1022 s−1) and U937 cell count rate (filled circles, ●) and fluorescence (circles, ○) were recorded. B) U937 cell were stained with Fura Red calcium indicator. A 1 ml sample was injected into the miniature Couette and the flow rate was adjusted to 1 μl/s. Then the inner cylinder rotated at 300 RPM (613 s−1) for 30 s and turned off (0 RPM). U937 cell fluorescence (circles, ○) was recorded. C) U937 cells were stained with CFSE (labeled intracellular and cell-surface proteins), vortexed for 10 s, and sampled with a FACScan (filled squares, ■). Cells were also sheared continuously at 100 RPM with the Couette (circles, ○). Count rates for cells sheared at 100 RPM in Couette are shown (filled circles, ●).
Real-time Calcium Response Induced by Shear
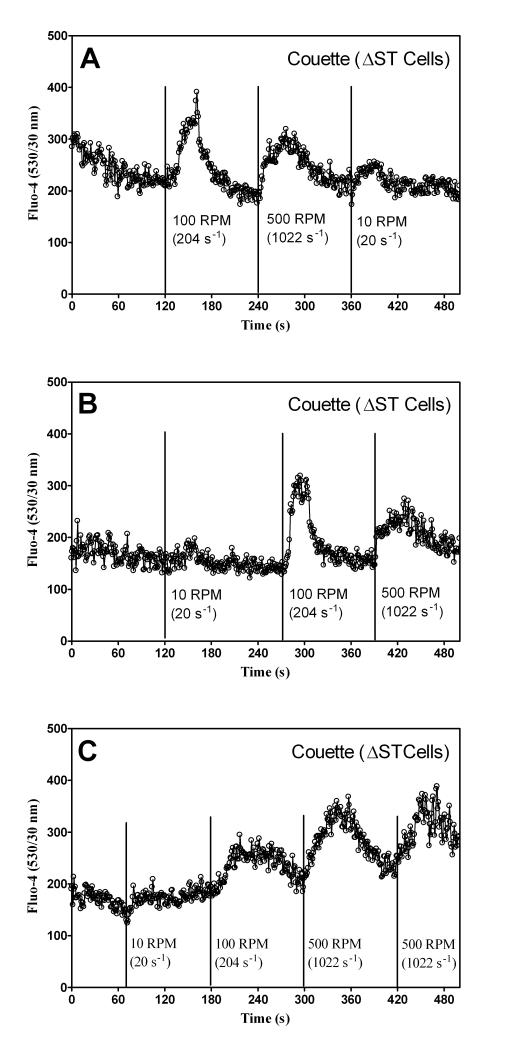
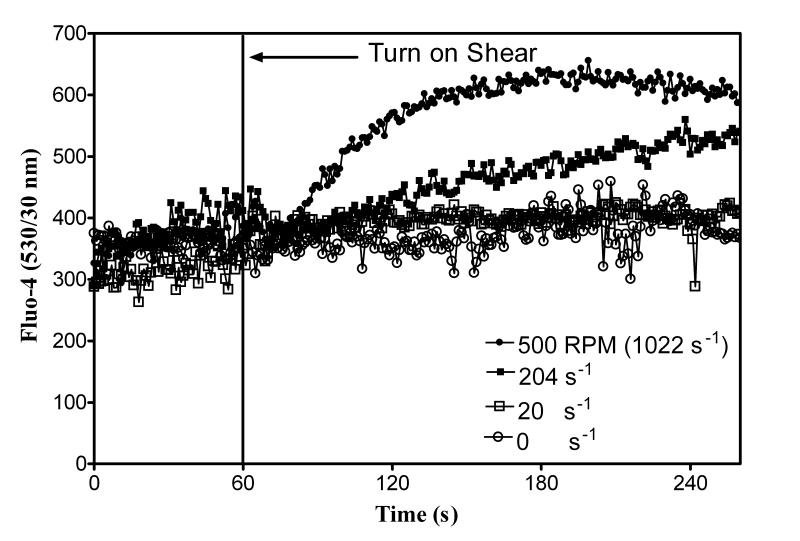
U937 cells were stained with Fluo-4 because of its strong fluorescence intensity. A 1 ml sample was injected into the Couette and allowed to rest for 5 min. Then the Couette was pressurized and flow rate adjusted to 1 μl/s without shear for 60 to 120 s. Figures 4A, B, and C show cellular responses when shear was applied for 30 s at 10 (20 s−1), 100 (204 s−1), and 500 (1022 s−1) RPM, respectively. The data in Figures 5A, B, and C show a comparison of the relative intracellular calcium between the Couette and the maximum calcium response to vortexing. Figure 6 shows intracellular calcium response to continuous shear. After 60 s, shear was applied continuously at 0 s−1, 20 s−1, 204−1, and 1022 s−1. Cell settling was minimized during a 5 min. rest prior to shear by rotating the inner cylinder at 20 s−1. A shear rate ~100 s−1 was required before a calcium response was observed.
Figure 4.
U937 cell calcium response to pulsed shear. U937 cells were stained with Fluo-4. A 1 ml sample was injected into the Couette and placed in a 37°C water bath. The flow rate was adjusted to 1 μl/s. A) Cells were sheared at 100 (204 s−1), 500 (1022 s−1), and 10 (20 s−1) RPM sequentially. B) Cells were sheared at 10 (20 s−1), 100 (204 s−1), and 500 (1022 s−1) RPM sequentially. C) Cells were sheared at 10 (20 s−1), 100 (204 s−1), 500 (1022 s−1), and 500 (1022 s−1) RPM sequentially.
Figure 6.
U937 calcium response to continuous shear. U937 cells were stained with Fluo-4. A 1 ml sample was injected into a miniature Couette and was placed in a 37°C water bath. Cells were incubated in the Couette for 5 min. The flow rate was adjusted to 1 μl/s. Vertical line indicates time when shear was applied. After 60 s continuous shear was applied at 0 (circles, ○), 10 (20 s−1; squares, □), 100 (204 s−1; filled squares, ■), and 500 (1022 s−1; filled circles, ●) RPM. Data are normalized to the first 60 s without shear.
DISCUSSION
A novel miniature Couette was constructed to generate uniform shear fields for cells in suspension and for real-time analysis with a flow cytometer. Regulated air pressure pushed cells with minimal shear from a Couette to a FACScan flow cytometer. We observed 10s cell transit times for flow rates of 1 μl/s and with the sample volume pressurized at 6 PSI (4.1 N/m2).
Sample Uniformity and Delivery Time
Flow check Fluorosphere beads and U937 cells labeled with Fluo-4 confirmed the uniform rate of sample extraction from the Couette. Figures 3A, B, and C showed that changes to Fluo-4 fluorescence were due to shear and not a miniature Couette artifact. This was especially evident for Fura Red in which decreases in fluorescence occurs when Fura Red is bound to free calcium (generated by shear). Finally, we also observed constant fluorescence from non-shear/calcium sensitive CFSE labeled cells. If the reduction in Fura Red signal were due to changes in the flow cytometer fluid sheath geometry, the forward and side scatter signal (used for cell identification in flow cytometers; see Figure 2A inset) would change. No changes were observed when normal FACScan operation was compared to Couette experiments (data not shown). Figures 3B and C show that sample delivery time was ~10 s from when the shear was turned on (measured from start of shear to when counts were recorded).
Count Rate and Shear
After cells had rested for 5 min. the flow rate was adjusted to 1 μl/s. Upon application of shear the count rates increased (Figure 3C). The larger than average initial count rate was likely the result of cell settling. This was minimized by slowly rotating the inner cylinder while the cells rested prior to each experiment. The effect of cell settling was further minimized by the addition of a 0.2” extraction port located in the lower end cap. Although count rates were not always uniform the important parameter, fluorescence, remained constant unless a shear response occurred.
Shear and Intracellular Calcium
Figures 4 and 6 show that shear modulated intracellular calcium and the response was proportional to the intensity and duration of applied shear. Maximum calcium responses were achieved for short shear pulses greater than 200 s−1. When cells were continuously sheared (Figure 6) the intracellular calcium response was dependent on the shear rate; low shear (204 s−1) gave a slower intracellular calcium response than those for high shear (1022 s−1). A bimodal fit analysis, described in Materials and Methods, showed that the percentage of cells activated by the Couette increased with shear. The shape of the calcium response was observed to change for different experiments. Table 2 shows the percentage of cells activated in the Couette; higher shear induced a larger fraction of all or none calcium response (40). Figure 6 illustrates that continuous application of low and high shear elevated intracellular calcium to similar levels after several minutes. The decreased Fura Red signal can be due to dye leakage during long experiments. However, cross checking the data obtained with Fluo-4 showed this effect to be minimal. To improve dye retention cells can be incubated with a calcium indicator and Probenecid (Molecular Probe, Eugene, OR).
Table 2.
Percentage of Cells Activated by Shear
| RPM | Shear Rate (1/s) |
Percentage of Cells Activated (Activated/( Resting + Activated) × 100 |
|---|---|---|
| 10 | 20 | 22±5 |
| 100 | 204 | 48±15 |
| 500 | 1022 | 49±14 |
Comparison With Other Shear Devices
Currently there are several techniques to generate shear in conjunction with a flow cytometer. First, magnetic stir bars can be placed inside of flow cytometer sample tube (24). This method is the simplest way to generate shear. However, the shear field is not defined. Another technique pushes samples through tubes (27-28). This technique is limited by non-uniformity, duration, and strength of the shear field. A third technique uses a cone and plate or parallel ring viscometer (15, 21, 24, 29). This device is external to a flow cytometer and requires a means to transport cells from this device to a flow cytometer. One approach involves manually remove cells and placing them in paraformaldehyde (41). This introduces additional shear through sampling handling and has limited real-time potential. A second approach can remove cells by using a peristaltic pump or syringe pump (21). Most external devices introduce additional shear through sample handling and have limited real-time capability. A miniature Couette was designed to overcome the above limitations by moving cells to a flow cytometer with minimal sample handling and within a defined shear field.
In addition, the miniature Couette can facilitate real-time studies of assays involving cellular interactions in a suspension. These include interactions between: 1) cells expressing a receptor and a soluble ligand pair; 2) two different cell lines expressing receptor and ligand pair (aggregation); 3) two cell lines expressing receptor and ligand, where a small molecule can be added to block the receptor/ligand binding (disaggregation); and 4) cell/cellular receptor expression and fluid shear response. Additional shear sensitive cells, such as platelets (42), can be studied with this system.
Cell Signaling
Shear induced intracellular calcium response can be placed in context for the regulation VLA-4 integrin affinity in real-time by using a vortex mixer (25). Even though the shear field generated using a vortexer is poorly defined, the intracellular Ca2+ increase corresponds to VLA-4 activation/higher affinity (15). The VLA-4 integrin can be activated by changes to intracellular calcium through the use of Ca2+ ionophores (ionomycin and A23187), in the absence of shear. Thus, regulation of integrin activity is controlled by intracellular calcium levels. An example of intracellular signaling is seen in Figure 2A. Shear induced a decreased intracellular calcium response when it was applied after the addition of ATP. The decrease was due to the depletion of intracellular calcium stores. Both ATP and shear activate the intracellular signaling pathways that involve the production of IP3. IP3 is used in the mobilization of calcium from the endoplasmic reticulum (43). Further, VLA-4 activation and shear induced intracellular calcium response is abolished using a calcium chelator BAPTA-AM.
Shear can activate surface receptors that are sensitive to mechanical stress (9, 44). The activation of these sensors allows for a mechanical stimulus to be transferred to inside the cell in a process called outside-in signaling. Integrin molecules are mechanosensors. For example, integrins can sense changes to blood flow (45-47). Intracellular calcium signaling serves a special role in the regulation of the integrin activation (48-50), since calcium levels control integrin activation (24). Monitoring calcium and integrin changes are accessible in real-time with the miniature Couette.
Table I.
Estimated Shear in Miniature Couette
| RPM | Shear Rate at Inner Radius (1/s) |
Shear Rate at Outer Radius (1/s) |
|---|---|---|
| 10 | 21 | 19 |
| 100 | 215 | 193 |
| 1000 | 2150 | 1930 |
LITERATURE CITED
- 1.Schramm R, Thorlacius H. Neutrophil recruitment in mast cell-dependent inflammation: inhibitory mechanisms of glucocorticoids. Inflamm Res. 2004;53:644–52. doi: 10.1007/s00011-004-1307-8. [DOI] [PubMed] [Google Scholar]
- 2.Kluger MS. Vascular endothelial cell adhesion and signaling during leukocyte recruitment. Adv Dermatol. 2004;20:163–201. [PubMed] [Google Scholar]
- 3.Kim MB, Sarelius IH. Regulation of leukocyte recruitment by local wall shear rate and leukocyte delivery. Microcirculation. 2004;11:55–67. doi: 10.1080/10739680490266199. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji T, Suzuki J, Shimamoto R, Yamazaki T, Nakajima T, Nagai R, Komatsu S, Ohtomo K, Toyo-oka T, Omata M. Vector Analysis of the Wall Shear Rate at the Human Aortoiliac Bifurcation Using Cine MR Velocity Mapping. American Roentgen Ray Society. 2002;178:995–9. doi: 10.2214/ajr.178.4.1780995. [DOI] [PubMed] [Google Scholar]
- 5.Reneman RS, Arts T, Hoeks APG. Wall Shear Stress - an Important Determinant of Endothelial Cell Function and Structure - in the Arterial System in vivo Discrepancies with Theory. J Vasc Res. 2006;43:251–69. doi: 10.1159/000091648. [DOI] [PubMed] [Google Scholar]
- 6.Traub O, Berk BC. Laminar Shear Stress Mechanisms by Which Endothelial Cells Transduce an Atheroprotective Force. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18:677–85. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 7.Butler PJ, Tsou TC, Li JY, Usami S, Chien S. Rate sensitivity of shear-induced changes in the lateral diffusion of endothelial cell membrane lipids: a role for membrane perturbation in shear-induced MAPK activation. FASEB J. 2002;16:216–18. doi: 10.1096/fj.01-0434fje. [DOI] [PubMed] [Google Scholar]
- 8.Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26:17–27. doi: 10.1016/j.immuni.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 9.AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM. Ciliary Polycystin-2 Is a Mechanosensitive Calcium Channel Involved in Nitric Oxide Signaling Cascades. Circulation Research. 2009;104:860–9. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin J, Kuebler WM. Mechanotransduction by TRP Channels: General Concepts and Specific Role in the Vasculature. Cell Biochem Biophys. 2010;56:1–18. doi: 10.1007/s12013-009-9067-2. [DOI] [PubMed] [Google Scholar]
- 11.Kimiko Y, Tomono T, Takayuki A, Norihiko O, Takaaki S, Akira K, Joji A. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. Journal of Applied Physiology. 2003;95:2081–8. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 12.Cuvelier SL, Paul S, Shariat N, Colarusso P, Patel KD. Eosinophil adhesion under flow conditions activates mechanosensitive signaling pathways in human endothelial cells. Journal of Experimental Medicine. 2005;202:865–76. doi: 10.1084/jem.20041315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Haylor JL, Ong ACM. Polycystin-2 expression is increased following experimental ischaemic renal injury. Nephrol Dial Transplant. 2002;17:2138–44. doi: 10.1093/ndt/17.12.2138. [DOI] [PubMed] [Google Scholar]
- 14.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–47. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zwartz GJ, Chigaev A, Dwyer DC, Foutz TD, Edwards BS, Sklar LA. Real-time analysis of very late antigen-4 affinity modulation by shear. J Biol Chem. 2004;279:38277–86. doi: 10.1074/jbc.M402944200. [DOI] [PubMed] [Google Scholar]
- 16.Leckband D. Nanomechanics of adhesion proteins. Curr Opin Struct Biol. 2004;14:524–30. doi: 10.1016/j.sbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Perret E, Leung A, Feracci H, Evans E. Trans-bonded pairs of E-cadherin exhibit a remarkable hierarchy of mechanical strengths. PNAS. 2004;101:16472–77. doi: 10.1073/pnas.0402085101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang S, Dong C. Integrin VLA-4 enhances sialyl-Lewisx/a-negative melanoma adhesion to and extravasation through the endothelium under low flow conditions. Am. J. Physiol Cell Physiol. 2008;295:C701–7. doi: 10.1152/ajpcell.00245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynam E, Sklar LA, Taylor AD, Neelamegham S, Edwards BS, Smith CW, Simon SI. Beta2-integrins mediate stable adhesion in collisional interactions between neutrophils and ICAM-1-expressing cells. Journal of Leukocyte Biology. 1998;64:622–30. doi: 10.1002/jlb.64.5.622. [DOI] [PubMed] [Google Scholar]
- 20.Bausch AR, Ziemann F, Boulbitch AA, Jacobson K, Sackmann E. Local measurements of viscoelastic parameters of adherent cell surfaces by magnetic bead microrheometry. Biophys J. 1998;75:2038–49. doi: 10.1016/S0006-3495(98)77646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards BS, Kuckuck FW, Prossnitz ER, Okun A, Ransom JT, Sklar LA. Plug flow cytometry extends analytical capabilities in cell adhesion and receptor pharmacology. Cytometry. 2001;43:211–6. doi: 10.1002/1097-0320(20010301)43:3<211::aid-cyto1052>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Shankaran H, Neelamegham S. Effect of secondary flow on biological experiments in the cone-plate viscometer: methods for estimating collision frequency, wall shear stress and inter-particle interactions in non-linear flow. Biorheology. 2001;38:275–304. [PubMed] [Google Scholar]
- 23.Xiao Z, Goldsmith HL, McIntosh FA, Shankaran H, Neelamegham S. Biomechanics of P-selectin PSGL-1 bonds: shear threshold and integrin-independent cell adhesion. Biophys J. 2006;90:2221–34. doi: 10.1529/biophysj.105.065789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chigaev A, Zwartz G, Graves SW, Dwyer DC, Tsuji H, Foutz TD, Edwards BS, Prossnitz ER, Larson RS, Sklar LA. Alpha4beta1 integrin affinity changes govern cell adhesion. J Biol Chem. 2003;278:38174–82. doi: 10.1074/jbc.M210472200. [DOI] [PubMed] [Google Scholar]
- 25.Zwartz G, Chigaev A, Foutz T, Larson RS, Posner R, Sklar LA. Relationship between molecular and cellular dissociation rates for VLA-4/VCAM-1 interaction in the absence of shear stress. Biophys J. 2004;86:1243–52. doi: 10.1016/S0006-3495(04)74198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagi Y, Andoh A, Inatomi O, Bamba S, Tsujikawa T, Fujiyama Y, Mitsuyama K, Yoshida T. Modulation of platelet aggregation responses by leukocytapheresis therapy in patients with active ulcerative colitis. J Gastroenterol. 2006;41:540–6. doi: 10.1007/s00535-006-1797-y. [DOI] [PubMed] [Google Scholar]
- 27.Tárnok A, Ulrich H. Characterization of pressure-induced calcium response in neuronal cell lines. Cytometry. 2001;43:175–81. doi: 10.1002/1097-0320(20010301)43:3<175::aid-cyto1046>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Tárnok A. Improved kinetic analysis of cytosolic free calcium in pressure-sensitive neuronal cells by fixed-time flow cytometry. Cytometry. 1996;23:82–9. doi: 10.1002/(SICI)1097-0320(19960101)23:1<82::AID-CYTO13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 29.Simon SI, Chambers JD, Butcher E, Sklar LA. Neutrophil aggregation is beta 2-integrin- and L-selectin-dependent in blood and isolated cells. J Immunol. 1992;149:2765–71. [PubMed] [Google Scholar]
- 30.Neelamegham S, Taylor AD, Shankaran H, Smith CW, Simon SI. Shear and time-dependent changes in Mac-1, LFA-1, and ICAM-3 binding regulate neutrophil homotypic adhesion. J Immunol. 2000;164:3798–805. doi: 10.4049/jimmunol.164.7.3798. [DOI] [PubMed] [Google Scholar]
- 31.Guyer DA, Moore KL, Lynam EB, Schammel CM, Rogelj S, McEver RP, Sklar LA. P-selectin glycoprotein ligand-1 (PSGL-1) is a ligand for L-selectin in neutrophil aggregation. Blood. 1996;88:2415–21. [PubMed] [Google Scholar]
- 32.Edwards BS, Curry MS, Tsuji H, Brown D, Larson RS, Sklar LA. Expression of P-selectin at low site density promotes selective attachment of eosinophils over neutrophils. J Immunol. 2000;165:404–10. doi: 10.4049/jimmunol.165.1.404. [DOI] [PubMed] [Google Scholar]
- 33.Tandon P, Diamond SL. Kinetics of beta2-integrin and L-selectin bonding during neutrophil aggregation in shear flow. Biophys J. 1998;75:3163–78. doi: 10.1016/S0006-3495(98)77758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon SI, Goldsmith HL. Leukocyte adhesion dynamics in shear flow. Ann Biomed Eng. 2002;30:315–32. doi: 10.1114/1.1467677. [DOI] [PubMed] [Google Scholar]
- 35.Kew RR, Peng T, DiMartino SJ, Madhavan D, Weinman SJ, Cheng D, Prossnitz ER. Undifferentiated U937 cells transfected with chemoattractant receptors: a model system to investigate chemotactic mechanisms and receptor structure/function relationships. J Leukoc Biol. 1997;61:329–37. doi: 10.1002/jlb.61.3.329. [DOI] [PubMed] [Google Scholar]
- 36.Apelblat A, Healy JC, Joly M. Shear rate in the Couette viscometer with a narrow annular gap between cylinders. A new approximate formula. Rheol. Acta. 1975;14:976–8. [Google Scholar]
- 37.Jin J, Dasari VR, Sistare FD, Kunapuli SP. Distribution of P2Y receptor subtypes on haematopoietic cells. Br J Pharmacol. 1998;123:789–94. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chorna NE, Chevres M, Santos-Berrios C, Orellano EA, Erb L, González FA. P2Y2 receptors induced cell surface redistribution of alpha(v) integrin is required for activation of ERK 1/2 in U937 cells. J Cell Physiol. 2007;211:410–22. doi: 10.1002/jcp.20946. [DOI] [PubMed] [Google Scholar]
- 39.Yebdri BF, Kukulski F, Tremblay A, Sévigny J. Concomitant activation of P2Y(2) and P2Y(6) receptors on monocytes is required for TLR1/2-induced neutrophil migration by regulating IL-8 secretion. Eur J Immunol. 2009;39:2885–94. doi: 10.1002/eji.200939347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards BS, Kuckuck FW, Prossnitz ER, Ransom JT, Sklar LA. HTPS flow cytometry: a novel platform for automated high throughput drug discovery and characterization. J Biomol Screen. 2001;6:83–90. doi: 10.1177/108705710100600204. [DOI] [PubMed] [Google Scholar]
- 41.Owen JT, McCarty OJ, Jadhav S, Burdick MM, Bell WR, Konstantopoulos K. Fluid Shear Regulates the Kinetics and Molecular Mechanisms of Activation-Dependent Platelet Binding to Colon Carcinoma Cells. Biophysical Journal. 2002;83:836–48. doi: 10.1016/S0006-3495(02)75212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White-Adams TC, Berny MA, Patel IA, Tucker EI, Gailani D, Gruber A, McCarty OJ. Laminin promotes coagulation and thrombus formation in a factor XII-dependent manner. J Thromb Haemost. 2010;8:1295–301. doi: 10.1111/j.1538-7836.2010.03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song Z, Vijayaraghavan S, Sladek CD. ATP increases intracellular calcium in supraoptic neurons by activation of both P2X and P2Y purinergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;292:R423–31. doi: 10.1152/ajpregu.00495.2006. [DOI] [PubMed] [Google Scholar]
- 44.Barakat AI. A model for shear stress-induced deformation of a flow sensor on the surface of vascular endothelial cells. J Theor Biol. 2001;210:221–36. doi: 10.1006/jtbi.2001.2290. [DOI] [PubMed] [Google Scholar]
- 45.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–75. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 46.Martinez-Lemus LA, Wu X, Wilson E, Hill MA, Davis GE, Davis MJ, Meininger GA. Integrins as unique receptors for vascular control. J Vasc Res. 2003;40:211–33. doi: 10.1159/000071886. [DOI] [PubMed] [Google Scholar]
- 47.Lele TP, Thodeti CK, Ingber DE. Force meets chemistry: analysis of mechanochemical conversion in focal adhesions using fluorescence recovery after photobleaching. J Cell Biochem. 2006;97:1175–83. doi: 10.1002/jcb.20761. [DOI] [PubMed] [Google Scholar]
- 48.Ashida N, Takechi H, Kita T, Arai H. Vortex-mediated mechanical stress induces integrin-dependent cell adhesion mediated by inositol 1,4,5-trisphosphate-sensitive Ca2+ release in THP-1 cells. J Biol Chem. 2003;14278:9327–31. doi: 10.1074/jbc.M212316200. [DOI] [PubMed] [Google Scholar]
- 50.Chigaev A, Waller A, Amit O, Sklar LA. Galphas-coupled receptor signaling actively down-regulates alpha4beta1-integrin affinity: a possible mechanism for cell de-adhesion. BMC Immunol. 2008;9:26–42. doi: 10.1186/1471-2172-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]