Abstract
Background
Blastocystis has been described as the most common intestinal parasite in humans and has an increased impact on public health. However, the transmission of this parasite has not been conclusively determined.
Methods
To contribute to a better comprehension of the epidemiology of this infection, a cross-sectional survey aimed at providing the first documented data on the prevalence and risk factors associated with Blastocystis infection was carried out among three Orang Asli tribes (Proto-Malay, Negrito and Senoi) in selected villages at Negeri Sembilan, Perak and Pahang, Peninsular Malaysia. Faecal samples were examined by formalin-ether sedimentation and trichrome staining techniques.
Results
Of 500 individuals, 20.4% (102) were detected positive for Blastocystis; 13.3% (20/150) of Proto-Malays, 21.6% (30/139) of Negritos and 24.7% (52/211) of Senois were positive for Blastocystis, respectively. The positive cases showed a decrease with increasing age and most of the positive cases were observed in individuals less than 15 years old. Multivariate analysis confirmed that drinking untreated water and the presence of other family members infected with Blastocystis were significant risk factors of infection among the three tribes and overall population studied.
Conclusion
Essentially, the findings highlighted that Blastocystis infection is prevalent among Orang Asli communities in Malaysia. Further studies using molecular approaches to distinguish the subtype of Blastocystis is needed. The present study also revealed that this infection may be transmitted through waterborne and human-to-human contact. Therefore, interventions with the provision of clean water supply for the communities and health education especially to the parents are urgently required.
Keywords: Blastocystis, Waterborne, Human-to-human, Orang Asli, Malaysia
Background
Blastocystis is an anaerobic unicellular eukaryote that can inhabit the intestinal track of humans and many animals, it was first described in 1912 [1]. Numerous cross-sectional surveys have been carried out in different epidemiological settings and hence Blastocystis is known to be one of the most frequently found protozoan parasites in human faecal samples, especially in children and adults in developing countries [2,3]. Blastocystis prevalence in human often exceeds 5% in industrialized countries and can reach as high as 76% in developing countries [3,4]. However, prevalence data are largely dependent on the methods used for detection. The most common diagnostic technique used worldwide for identification of Blastocystis is the permanent stain. The use of xenic cultures in which Blastocystis is grown in vitro with non-specific microorganisms have been shown to be more sensitive in detecting Blastocystis but it is not commonly used in the diagnostic laboratory [5-7]. Several forms of Blastocystis are observed in faecal samples include vacuolar, multivacuolar, avacuolar, granular, amoeboid and cyst [2]. However, most laboratories recognize only the vacuolar form as the diagnostic stage since it can be easily distinguished from other protozoa.
Although its role in human disease has been widely debated in the literature during the two last decades, numerous recent in vivo and in vitro studies strongly suggest that Blastocystis is a pathogen [8-10]. Case reports have linked Blastocystis infection with various gastrointestinal and extraintestinal symptoms including diarrhea, abdominal pain, depression, fatigue, vomiting, constipation, anorexia, urticaria, skin rash, headaches and flatulence, but this parasite may also play a significant role in several chronic gastrointestinal illnesses such as irritable bowel syndrome and inflammatory bowel disease [11-14]. An epidemiological study on Blastocystis showed that the animal handlers have a higher risk of infection and that there is always the possibility of transmission between animals and human [15]. On the other hand, polymerase chain reaction (PCR)-based methodology has demonstrated that there might exist a human-to-human infection among small communities, suggesting that transmission also occurs through the faecal-oral route like other common gastrointestinal parasites [16,17]. Furthermore, transmission can be facilitated by the contamination of the environment, food or water with excreted cysts from the reservoir hosts [18]. Thus, the mode of transmission of the Blastocystis has not been conclusively determined [17].
In Malaysia, Blastocystis infection has been reported to have a high prevalence among urban and rural communities and also in the water of rivers from recreational areas [19-21]. However, knowledge of the epidemiology and risk factors of Blastocystis infection is also rather limited. Therefore, this study was carried out to determine the prevalence of Blastocystis and to investigate its association with the socioeconomic characteristics of three Orang Asli (Aborigine) tribes, namely Proto-Malay, Negrito and Senoi in Peninsular Malaysia.
Methods
Study area and population surveyed
A series of cross-sectional surveys were conducted in three different states of Peninsular Malaysia in Jelebu (2° 55’ N latitude, 102° 4’ E longitude), Gerik (5° 26’ N latitude, 101° 7’ E longitude) and Temerloh (3° 43’ N latitude, 102° 22’ E longitude) from June to December 2011 (Figure 1). Details of the studied villages and the populations sampled have been described elsewhere [22].
Figure 1.
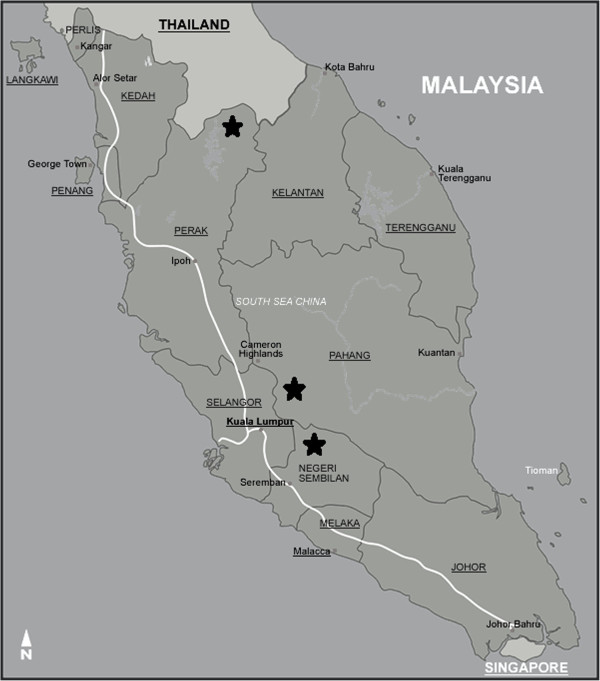
Map showing the location of the villages in Peninsular Malaysia involved in the study (stars).
Sample size
By using the formula provided by Kish [23], the expected sample size was calculated according to the following parameters: expected prevalence of Blastocystis at 4.4% and 17% [24,25], confidence interval of 9.5% and absolute precision (d) = 0.05 [26]. The required minimum sample size needed in this study was 65 individuals and the maximum would be 217 individuals in each tribe.
Questionnaire
A structured questionnaire was developed in English and then translated to Bahasa Melayu (the national language of Malaysia). The questionnaire was pre-tested among Orang Asli individuals who were admitted to Gombak Hospital, Selangor state. Trained research assistants interviewed participants in person, asking questions for demographic data (i.e., age, gender and education level), socioeconomic background (i.e., occupation, household income and educational status), behavioral risks (i.e., personal hygiene such as hand washing and food consumption), environmental sanitation and living condition characteristics (i.e., types of water supply, latrine system, sewage disposal system and presence of domestic animals). Participants were also asked if they had diarrhea and symptoms of gastroenteritis (i.e., vomiting, nausea, abdominal pain, watery stools and blood or mucus stools). For children, the questionnaire was completed by interviewing their parents or the guardian who had given informed consent.
Field and laboratory procedures: Detection of Blastocystis
Following the administration of the questionnaire, a wide mouth screw-capped container pre-labelled with the individual’s name and code was distributed to each participant for the collection of a faecal sample the next day. Their ability to recognize their name was counter-checked. The participant was instructed to scoop a thumb sized faecal sample using a provided scoop into the container. Then, the container was placed in a zip-locked plastic bag. Parents and guardians were instructed to monitor their children during the sample collection in order to ensure that they placed their faecal samples into the correct container. All study participants were asked to provide a sufficiently large faecal sample (at least 10 grams) so that both formalin-ether sedimentation and trichrome staining techniques could be performed. This study had to rely on a single faecal sample collection because of the limitation of resources and the cultural belief of the aborigines against giving of their faecal samples.
Faecal samples were processed in the designated area of work in the study village within a maximum of six hours after collection by experienced laboratory technicians. Approximately 5 grams of each faecal sample were kept in a 15 ml centrifuge tube containing 3 ml polyvinyl alcohol (PVA). PVA-fixed samples were forwarded to the parasitological department of the Faculty of Medicine, Universiti Kebangsaan Malaysia. The samples were subjected to trichrome staining. Briefly, the smear cover slip was stained as follows: iodine alcohol (15 minutes), 70% alcohol (10 minutes), trichrome stain (10 minutes), acid alcohol (3 seconds), 95% alcohol (5 minutes), absolute alcohol (5 minutes) and wintergreen oil (5 minutes) [27]. The cover slip was mounted using DPX and examined under a light microscope at magnifications of 1000x. Additionally, another half of the samples were kept unfixed and stored at 4°C upon arrival at the laboratory for further analysis using formalin-ether sedimentation. Briefly, 2 grams of faecal sample were mixed with 7 ml of formalin and 3 ml of ether, centrifuged, stained with Lugol’s iodine and finally examined under a light microscope at magnifications of 400x [28]. The sample was reported as positive if cysts and/or vacuolar forms of Blastocystis were detected by any of these two techniques (Figure 2).
Figure 2.
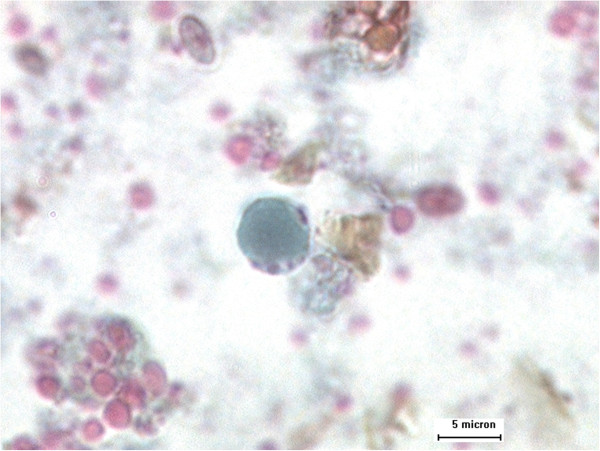
Vegetative stage of Blastocystis sp. (trichrome stain) in faecal sample.
Data management and analysis
Data was entered into a Microsoft Access and was cross-checked by the technical staff in order to ensure that data were entered correctly. Statistical analysis was performed using the SPSS version 20 (SPSS, Chicago, IL, USA). Only those individuals who had formalin-ether sedimentation and trichrome staining results together with complete questionnaire data were included in the final analyses.
For descriptive analysis, rate (percentage) was used to describe the characteristics of the studied population, including the prevalence of Blastocystis. A Chi-squares test (χ2) was used to test the associations between the variables. In the univariate analysis, the dependent variable was prevalence of Blastocystis, while the independent variables were demographic (gender and age group) and socioeconomic factors, behavioral risks, environmental sanitation, living condition characteristics and gastrointestinal symptoms. All variables that were significantly associated with the prevalence of Blastocystis in the univariate model were included in a logistic regression analysis to identify the risk factors for Blastocystis infection with control for the effects of possible confounders. For each statistically significant factor, an odds ratio (OR) and 95% confidence interval (CI) were computed by the univariate and multivariate logistic regression analyses. The level of statistical significance was set as p < 0.05. Furthermore, Mantel-Haenszel Chi-square test was used to test whether there is any confounding effect of the contingency tables.
Ethical issues
Prior to data collection, the study protocol (Reference Number: UKM 1.5.3.5/244/FF-165-2011) was reviewed and approved by the Ethics Committee of Universiti Kebangsaan Malaysia Medical Centre (UKMMC) and permission for field work was obtained from the Department of Orang Asli Development (JAKOA). Village meetings were held and village authorities and villagers were handed detailed explanations of the aims, procedures, potential risks and benefits of the study. During the meeting, they were also informed that their identity and personal information would be kept strictly confidential, and they could withdraw from the study at any point of time without citing reasons for doing so. If they agreed to participate, their consent was obtained in written form signature (or thumbprint for those who were illiterate) or parents were approached for consent on behalf of their children.
Results
Demographic and socioeconomic profiles
A total of 500 individuals aged from 2 to 74 years participated in this study with a median age of 18 years [interquartile range (IQR) 9–35]; and consisted of 150 (30%) Proto-Malays, 139 (28%) Negritos and 211 (42%) Senois.
Overall, the studied population came from a low socioeconomic background with more than half having less than 6 years of formal education. A high percentage of household income of less than RM500.00 (US$ 162.42) was predominantly seen in the Negrito (82.7%) and Senoi (60.7%) tribes. Most (95.3%) houses in Proto-Malay villages had piped water supplies and toilet facilities; conversely piped water supplies and safe sewage disposal facilities were lacking in the villages of the Negrito and Senoi tribes. General characteristics of each tribe, including their demographic and socioeconomic profiles are presented in Table 1.
Table 1.
General characteristics of the Orang Asli communities that participated in this study
| Characteristics/Tribes | Overall | Proto-Malay | Negrito | Senoi |
|---|---|---|---|---|
| |
n (%) |
n (%) |
n (%) |
n (%) |
|
Age groups (years) |
|
|
|
|
| <15 |
221 (44.2) |
59 (39.3) |
72 (51.8) |
90 (42.7) |
| ≥15 |
279 (55.8) |
91 (60.7) |
67 (48.2) |
121 (57.3) |
|
Gender |
|
|
|
|
| Male |
219 (43.8) |
66 (44.0) |
66 (47.5) |
87 (41.2) |
| Female |
281 (56.2) |
84 (56.0) |
73 (52.5) |
124 (58.8) |
|
Socioeconomic status |
|
|
|
|
| Father’s education (<6 years) |
222 (74.0) |
54 (66.7) |
68 (77.3) |
100 (76.3) |
| Mother’s education (<6 years) |
232 (56.2) |
53 (65.4) |
77 (87.5) |
102 (77.9) |
| Low monthly household income (<RM500) |
260 (52.0) |
17 (11.3) |
115 (82.7) |
128 (60.7) |
| Working mothers |
153 (51.0) |
45 (55.6) |
33 (37.5) |
75 (57.3) |
| Large family (≥8 members) |
171 (34.2) |
32 (21.3) |
63 (45.3) |
76 (36.0) |
| Supplied with piped water |
357 (71.4) |
143 (95.3) |
76 (54.7) |
138 (65.4) |
| Presence of toilet at household) | 308 (61.6) | 143 (95.3) | 48 (34.5) | 117 (55.5) |
RM = Malaysian Ringgit; (US$100 = RM307.85) [1st February 2013].
n = Number examined.
Prevalence and distribution of Blastocystis infection
The prevalence and distribution of Blastocystis infection are shown in Table 2. The overall prevalence of this infection was 20.4%. It is evident that, of 139 individuals studied from the Negrito tribe, 21.6% were infected with Blastocystis. However, individuals from the Senoi tribe had a relatively high prevalence of this infection which was 24.7%, whereby only 13.3% of the Proto-Malays reported positive for Blastocystis. Overall, the positive cases decreased with increasing age and most of the positive cases were observed in individuals of less than 15 years old. Similar findings were observed in the Proto-Malay, Negrito and Senoi tribes. The overall prevalence of Blastocystis was higher in females, with similar observations seen in all tribes. However, there was no significant difference of this infection between genders. With regards to tribe, it was observed that the Senoi presented a greater risk of Blastocystis infection than the Negrito and Proto-Malay tribes. On the other hand, there was no significant difference in the prevalence of Blastocystis infection among these three tribes.
Table 2.
Prevalence of Blastocystis infection among different Orang Asli tribes according to age groups and gender
| Overall | Proto-Malay | Negrito | Senoi | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
n |
n |
% |
n |
n |
% |
n |
n |
% |
n |
n |
% |
| positive |
tested |
positive |
positive |
tested |
positive |
positive |
tested |
positive |
positive |
tested |
positive |
|
|
Age groups (years) |
|
|
|
|
|
|
|
|
|
|
|
|
| <15 |
50 |
221 |
22.6 |
10 |
59 |
17.0 |
16 |
72 |
22.2 |
24 |
89 |
27.0 |
| ≥15 |
52 |
279 |
18.6 |
10 |
91 |
11.0 |
14 |
67 |
20.9 |
28 |
122 |
23.0 |
|
Gender |
|
|
|
|
|
|
|
|
|
|
|
|
| Male |
41 |
219 |
18.7 |
8 |
66 |
12.1 |
14 |
66 |
21.2 |
19 |
87 |
21.8 |
| Female |
61 |
281 |
21.7 |
12 |
84 |
14.3 |
16 |
73 |
21.9 |
33 |
124 |
26.6 |
| Total | 102 | 500 | 20.4 | 20 | 150 | 13.3 | 30 | 139 | 21.6 | 52 | 211 | 24.7 |
n = Number examined.
Co-infection with other intestinal parasites
For all positive faecal samples with Blastocystis (n = 102), 82.4% showed co-infection with one or more other parasites. Trichuris trichiura (53.9%) was the most common intestinal parasite found in conjunction with Blastocystis, followed by Ascaris lumbricoides (29.4%), Entamoeba histolytica/dispar/moshkovskii (26.5%), Entamoeba hartmanni (22.6%), Entamoeba coli (20.6%), Giardia intestinalis (17.7%), Iodamoeba butschlii (13.7%), Endolimax nana (6.9%), hookworm (5.9%) and Chilomastix mesnilii (2%). Furthermore, twelve samples were isolated from symptomatic subjects.
Risk factors for Blastocystis infection
Univariate analysis identified drinking untreated water (OR = 2.92; 95% CI = 1.84, 4.63; p < 0.001), not washing hands after playing with soil or gardening (OR = 1.58; 95% CI = 1.02, 2.45; p = 0.040) and the presence of other family members infected with Blastocystis (OR = 8.21; 95% CI = 5.07, 13.30; p < 0.001) as the main risk factors for the overall population studied (Table 3). In Proto-Malays, drinking untreated water (OR = 6.25; 95% CI = 1.52, 25.70; p = 0.019) and the presence of other family members infected with Blastocystis (OR = 21.48; 95% CI = 6.70, 68.82; p < 0.001) were identified as risk factors for Blastocystis (Table 4). In Negritos, drinking untreated water (OR = 2.79; 95% CI = 1.10, 7.03; p = 0.026) and the presence of other family members infected with Blastocystis (OR = 6.19; 95% CI = 2.55, 14.98; p < 0.001) were the predictors for Blastocystis infection (Table 4). Three risk factors were found to be positively significant in Senois; drinking untreated water (OR = 2.35; 95% CI = 1.10, 5.03; p = 0.025), not washing hands after playing with soil or gardening (OR = 2.00; 95% CI = 1.06, 3.78; p = 0.031) and the presence of other family members infected with Blastocystis (OR = 6.11; 95% CI = 3.10, 12.03; p < 0.001) (Table 4).
Table 3.
Potential risk factors associated with Blastocystis infection among the overall population studied (univariate analysis, n = 500)
| Variables | No. examined | Infected (%) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Age (years) |
|
|
|
|
| <15 |
221 |
22.6 |
1.28 (0.83,1.97) |
0.272 |
| ≥15 |
279 |
18.6 |
1 |
|
| Gender |
|
|
|
|
| Female |
281 |
21.7 |
1.20 (0.77,1.87) |
0.411 |
| Male |
219 |
18.7 |
1 |
|
| Drinking untreated water |
|
|
|
|
| Yes |
232 |
29.4 |
2.92 (1.84,4.63) |
<0.001a,b |
| No |
265 |
12.5 |
1 |
|
| Bathing and washing in the river |
|
|
|
|
| Yes |
143 |
23.8 |
1.33 (0.83,2.12) |
0.236 |
| No |
357 |
19.0 |
1 |
|
| Not washing hands after playing with soil or gardening |
|
|
|
|
| Yes |
197 |
25.0 |
1.58 (1.02,2.45) |
0.040a |
| No |
303 |
17.4 |
1 |
|
| Presence of domestic animals |
|
|
|
|
| Yes |
309 |
20.1 |
0.96 (0.62,1.49) |
0.862 |
| No |
191 |
20.8 |
1 |
|
| Indiscriminate defecation |
|
|
|
|
| Yes |
192 |
22.9 |
1.28 (0.82,1.99) |
0.270 |
| No |
308 |
18.8 |
1 |
|
| Sewage disposal |
|
|
|
|
| Outdoor |
240 |
22.1 |
1.22 (0.79,1.87) |
0.369 |
| Common drainage |
260 |
18.8 |
1 |
|
| Eating with hands |
|
|
|
|
| Yes |
328 |
20.2 |
0.96 (0.60,1.52) |
0.859 |
| No |
172 |
20.9 |
1 |
|
| Consuming raw vegetables |
|
|
|
|
| Yes |
238 |
21.1 |
1.08 (0.70,1.68) |
0.717 |
| No |
262 |
19.8 |
1 |
|
| Eating fresh fruits |
|
|
|
|
| Yes |
395 |
18.4 |
0.63 (0.39,1.01) |
0.055 |
| No |
105 |
26.4 |
1 |
|
| Father’s education |
|
|
|
|
| Non-educated (<6 yrs) |
222 |
24.3 |
1.47 (0.76,2.83) |
0.247 |
| Educated (>6 yrs) |
78 |
17.9 |
1 |
|
| Mother’s education |
|
|
|
|
| Non-educated (<6 yrs) |
232 |
25.0 |
1.93 (0.93,4.03) |
0.075 |
| Educated (>6 yrs) |
68 |
14.7 |
1 |
|
| Working mothers |
|
|
|
|
| Yes |
153 |
23.8 |
1.15 (0.67,1.97) |
0.625 |
| No |
147 |
21.5 |
1 |
|
| Household members |
|
|
|
|
| ≥8 |
171 |
21.6 |
1.12 (0.71,1.77) |
0.621 |
| <8 |
329 |
19.8 |
1 |
|
| Household monthly income |
|
|
|
|
| <RM500 |
260 |
23.5 |
1.49 (0.96,2.32) |
0.077 |
| >RM500 |
240 |
17.1 |
1 |
|
| Other family members infected with Blastocystis |
|
|
|
|
| Yes |
116 |
50.9 |
8.21 (5.07,13.30) |
<0.001a,b |
| No | 384 | 11.2 | 1 |
RM = Malaysian Ringgit; (US$100 = RM307.85) [1st February 2013].
Reference group marked as OR = 1 [OR = Odds Ratio].
CI = Confidence interval.
a Significant association (p < 0.05).
b Variables were confirmed by multivariate analysis as significant risk factors.
Table 4.
Associations between Blastocystis infection and risk factors among different Orang Asli tribes
| |
Proto-Malay (n = 150) |
Negrito (n = 139) |
Senoi (n = 211) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | No. examined | Infected (%) |
OR |
p-value | No. examined | Infected (%) |
OR |
p-value | No. examined | Infected (%) |
OR |
p-value |
| (95% CI) | (95% CI) | (95% CI) | ||||||||||
|
Age (years) | ||||||||||||
| <15 |
59 |
16.9 |
1.65 (0.64,4.26) |
0.294 |
72 |
22.2 |
1.08 (0.48,2.43) |
0.849 |
90 |
26.7 |
1.21 (0.64,2.67) |
0.557 |
| ≥15 |
91 |
11.0 |
1 |
|
67 |
20.9 |
1 |
|
121 |
23.1 |
1 |
|
|
Gender | ||||||||||||
| Female |
84 |
14.3 |
1.21 (0.46,3.15) |
0.699 |
73 |
21.9 |
1.04 (0.46,2.34) |
0.920 |
124 |
26.6 |
1.30 (0.68,2.48) |
0.428 |
| Male |
66 |
12.1 |
1 |
|
66 |
21.2 |
1 |
|
87 |
21.8 |
1 |
|
|
Drinking untreated water | ||||||||||||
| Yes |
9 |
44.4 |
6.25 (1.52,25.70) |
0.019a,b |
79 |
28.0 |
2.79 (1.10,7.03) |
0.026a,b |
144 |
29.2 |
2.35 (1.10,5.03) |
0.025a,b |
| No |
141 |
11.3 |
1 |
|
60 |
12.3 |
1 |
|
67 |
14.9 |
1 |
|
|
Bathing and washing in the river | ||||||||||||
| Yes |
7 |
14.3 |
1.09 (0.12,9.54) |
0.939 |
63 |
28.6 |
2.13 (0.94,4.86) |
0.068 |
73 |
20.5 |
0.71 (0.36,1.40) |
0.315 |
| No |
143 |
13.3 |
1 |
|
76 |
15.8 |
1 |
|
138 |
26.8 |
1 |
|
|
Not washing hands after playing with soil or gardening | ||||||||||||
| Yes |
27 |
11.5 |
0.82 (0.22,3.04) |
0.767 |
91 |
22.0 |
1.07 (0.46,2.52) |
0.876 |
79 |
32.9 |
2.00 (1.06,3.78) |
0.031a |
| No |
123 |
13.7 |
1 |
|
48 |
20.8 |
1 |
|
132 |
19.7 |
1 |
|
|
Presence of domestic animals | ||||||||||||
| Yes |
120 |
14.2 |
1.49 (0.41,5.44) |
0.548 |
70 |
16.7 |
0.61 (0.26,1.45) |
0.261 |
119 |
27.7 |
1.45 (0.77,2.81) |
0.237 |
| No |
30 |
10.0 |
1 |
|
69 |
24.7 |
1 |
|
92 |
20.7 |
1 |
|
|
Indiscriminate defecation | ||||||||||||
| Yes |
7 |
14.3 |
1.09 (0.12,9.54) |
0.939 |
91 |
23.1 |
1.30 (0.54,3.11) |
0.555 |
94 |
23.4 |
0.89 (0.47,1.67) |
0.708 |
| No |
143 |
13.3 |
1 |
|
48 |
18.8 |
1 |
|
117 |
25.6 |
1 |
|
|
Sewage disposal | ||||||||||||
| Outdoor |
21 |
9.5 |
0.65 (0.14,3.03) |
0.580 |
106 |
21.7 |
1.03 (0.40,2.67) |
0.953 |
113 |
24.8 |
1.02 (0.54,1.90) |
0.961 |
| Common drainage |
129 |
14.0 |
1 |
|
33 |
21.2 |
1 |
|
98 |
24.5 |
1 |
|
|
Eating with hands | ||||||||||||
| Yes |
70 |
12.0 |
0.79 (0.31,2.04) |
0.631 |
105 |
22.4 |
1.25 (0.46,3.40) |
0.657 |
153 |
22.6 |
0.67 (0.34,1.32) |
0.247 |
| No |
80 |
14.7 |
1 |
|
34 |
18.8 |
1 |
|
58 |
30.4 |
1 |
|
|
Consuming raw vegetables | ||||||||||||
| Yes |
63 |
12.7 |
0.91 (0.35,2.37) |
0.846 |
113 |
24.6 |
3.74 (0.83,16.89) |
0.068 |
62 |
23.1 |
0.88 (0.46,1.76) |
0.724 |
| No |
87 |
13.8 |
1 |
|
26 |
8.0 |
1 |
|
149 |
25.3 |
1 |
|
|
Eating fresh fruits | ||||||||||||
| Yes |
129 |
14.7 |
3.46 (0.44,27.28) |
0.213 |
122 |
19.7 |
0.45 (0.15,1.34) |
0.142 |
144 |
21.0 |
0.62 (0.33,1.17) |
0.139 |
| No |
21 |
4.8 |
1 |
|
17 |
35.3 |
1 |
|
67 |
29.9 |
1 |
|
|
Father’s education | ||||||||||||
| Non-educated (<6 yrs) |
54 |
14.8 |
1.00 (0.27,3.67) |
1.000 |
68 |
26.5 |
2.04 (0.53,7.79) |
0.290 |
100 |
28.0 |
1.33 (0.52,3.44) |
0.551 |
| Educated (>6 yrs) |
27 |
14.8 |
1 |
|
20 |
15.0 |
1 |
|
31 |
22.6 |
1 |
|
|
Mother’s education | ||||||||||||
| Non-educated (<6 yrs) |
53 |
18.9 |
3.02 (0.61,14.89) |
0.158 |
77 |
22.1 |
0.50 (0.13,1.90) |
0.298 |
102 |
30.4 |
2.73 (0.88,8.50) |
0.075 |
| Educated (>6 yrs) |
28 |
7.1 |
1 |
|
11 |
36.4 |
1 |
|
29 |
13.8 |
1 |
|
|
Working mothers | ||||||||||||
| Yes |
45 |
15.6 |
1.14 (0.33,3.95) |
0.834 |
33 |
25.8 |
1.18 (0.43,3.25) |
0.753 |
75 |
28.0 |
1.17 (0.53,2.56) |
0.701 |
| No |
36 |
13.9 |
1 |
|
55 |
22.8 |
1 |
|
56 |
25.0 |
1 |
|
|
Household members | ||||||||||||
| ≥8 |
32 |
12.5 |
0.91 (0.28,2.94) |
0.876 |
63 |
25.4 |
1.51 (0.67,3.39) |
0.320 |
76 |
22.4 |
0.82 (0.42,1.60) |
0.565 |
| <8 |
118 |
13.6 |
1 |
|
76 |
18.4 |
1 |
|
135 |
25.9 |
1 |
|
|
Household monthly income | ||||||||||||
| <RM500 |
17 |
17.6 |
1.46 (0.38,5.62) |
0.578 |
115 |
24.3 |
3.54 (0.78,16.01) |
0.083 |
128 |
23.4 |
0.85 (0.45,1.60) |
0.613 |
| >RM500 |
133 |
12.8 |
1 |
|
24 |
8.3 |
1 |
|
83 |
26.5 |
1 |
|
|
Other family members infected with Blastocystis | ||||||||||||
| Yes |
18 |
61.1 |
21.48 (6.70,68.82) |
<0.001a,b |
33 |
48.5 |
6.19 (2.55,14.98) |
<0.001a,b |
65 |
49.2 |
6.11 (3.10,12.03) |
<0.001a,b |
| No | 132 | 6.8 | 1 | 106 | 13.2 | 1 | 146 | 13.7 | 1 | |||
RM = Malaysian Ringgit; (US$100 = RM307.85) [1st February 2013].
Reference group marked as OR = 1 [OR = Odds Ratio].
CI = Confidence interval.
a Significant association (p < 0.05).
b Variables were confirmed by multivariate analysis as significant risk factors.
Logistic regression analysis of the overall population studied again confirmed that drinking untreated water (OR = 2.75; 95% CI = 1.66, 4.55; p < 0.001) and the presence of other family members infected with Blastocystis (OR = 7.96; 95% CI = 4.84, 13.08; p < 0.001) as the real risk factors for Blastocystis infection. Similar observations were also identified in the Proto-Malay, Negrito and Senoi tribes.
Discussion
The epidemiological studies on Blastocystis infection have been inconclusive in several aspects, particularly regarding the source of infection and mode of transmission [29,30]. Several studies have indicated human-to-human and animal-to-animal transmission models and it has been revealed that the cyst form was the only transmissible stage of this parasite [31,32]. A recent study by Lee et al.[33] provided molecular evidence supporting zoonotic transmission of Blastocystis in a rural community in Nepal. On the other hand, the prevalence of Blastocystis infection might be varied due to the laboratory method used for detection, age group and hygienic condition of the population studied [34]. However, high prevalence reported from developing countries confirmed that poor hygiene was involved in the transmission of the disease.
In the present study, the overall prevalence of Blastocystis among Orang Asli in Malaysia was 20.4%, which differs from previous local reports [19,24,35,36] but in agreement with the latest report by Abdulsalam et al.[21]. Comparing our findings with studies from other countries showed that the prevalence reported by the present study was similar to those reported in Jordan (25%), Brazil (26.5%), Colombia (22.4%) and Nepal (26.1%) [37-40]. However, the prevalence rate of the infections observed in this study is higher than reported in Thailand and India [34,41]. In contrast, considerably higher prevalence rates of 31%, 48.7%, 32% and 40.7% were reported among Egyptians, Filipinos, Pakistanian and Argentinean individuals, respectively [12,42-44]. Our findings showed no significant difference in the prevalence of Blastocystis infection according to age and gender of the participants, and this is consistent with the results of previous reports [18,19,21,40]. However, a study carried out by Li et al.[45] demonstrated that individuals aged 60 years and above had the highest prevalence of Blastocystis in Shanghai municipality and Yangjia country, which is similar to a finding reported from Glasgow [7]. Furthermore, Al-Fellani et al.[46] reported males were more frequently infected (28.98%) with Blastocystis than females (23.89%) and the difference was statistically significant. Likewise, several studies also reported significantly higher prevalence in male than female subjects [37,47].
This present study demonstrated that the prevalence rates of this infection were higher in Negritos and Senois than the Proto-Malay tribe. This could be attributed to the lack of basic amenities in the Negrito and Senoi tribes where almost 50% of the communities are not accessed to piped water supply and safe toilet facilities. It indicates that the poor provision of basic amenities plays an important role in the transmission of Blastocystis in Senois and Negritos. Similar findings were seen in Entamoeba histolytica/dispar/moshkovskii infection carried out in the same communities which reported a high prevalence rate of this infection among members of the Negritos who have poor housing conditions and basic amenities [48]. However, this finding contradicted previously reported prevalence of Giardia intestinalis infection carried out in the same communities which demonstrated a high prevalence rate of giardiasis among members of the Proto-Malay tribe who have a better housing condition and basic amenities [22]. Other family members infected with giardiasis were identified as a risk factor of the infection and human-to-human transmission was postulated as a main mode of transmission of giardiasis in the communities [22].
Waterborne transmission has been speculated as the mode of transmission of Blastocystis in several studies, especially those conducted in tropical countries and in travelers who just returned from these countries [37,49]. These travelers also gave a history of drinking untreated water while they were abroad [49]. On further analysis of risk factors using multivariate analysis, we found that those who drink untreated water or unboiled water were statistically significant for the Proto-Malay (p = 0.019), Negrito (p = 0.026) and Senoi tribes (p = 0.025). Moreover, drinking untreated water was also found to be a significant predictor of acquiring Blastocystis infection in the overall population studied (p < 0.001). It is interesting to note that although the prevalence rate of Blastocystis was low in Proto-Malays as compared to Senois and Negritos, and almost 95% of the population in Proto-Malay villages have access to piped water supply, individuals from the Proto-Malay tribe who drink untreated water or unboiled water have a higher risk of acquiring this infection as compared to individuals in Senoi and Negrito tribes. During the visits to the villages, we observed that most of the Proto-Malays preferred to drink unboiled water because it was tastier than boiled water. The cysts of Blastocystis are rather small compared to the cysts of other common protozoa such as Giardia intestinalis and Entamoeba histolytica[29]. Thus, it is likely that the cysts of Blastocystis could escape the conventional water filtration techniques [29]. Furthermore, cysts of Blastocystis were resistant to chlorine at the standard concentration used in tap water [50] and able to survive in water for up to 19 days at a normal temperature, but are fragile at extremes of heat and cold, and in common disinfectants [51,52]. All these characteristics made untreated water a suitable source of infection of this protozoa. Besides that, post-treatment contamination of drinking water that is usually stored in a bucket for household use and the common practice of drinking unboiled water might be the possible reason for the high risk. The unboiled or unfiltered drinking water might have been contaminated with Blastocystis cysts from faecal shedding, which may lead to Blastocystis infection as suggested by a group in Thailand and Nepal [18,40]. During the visit to the villages, we observed that 28.6% of the households from the three tribes used water from the wells, streams and rain with or without further treatment by filtration or boiling. Therefore, the present study provides baseline data for the role of waterborne transmission in Blastocystis infection among the Proto-Malay, Negrito and Senoi tribes. However, there is a need for further investigations to assess whether the reported association of Blastocystis infection and drinking untreated water is a causal association.
It is interesting to note that in the present study, the presence of other family members infected with Blastocystis has been identified as the significant risk factors (p < 0.001) among Proto-Malays, Negritos, Senois and overall population, respectively. This evidence suggests infection can occur within families and human-to-human transmission of Blastocystis is another possible mode of transmission identified in the present study. Sharing of poorly maintained sanitary facilities with a household may facilitate human-to-human transmission [40]. Therefore, maintaining clean sanitary facilities and practices are important to avoid transmission of Blastocystis to others. Furthermore, screening of other family members should also be recommended as one of the strategies in controlling Blastocystis infection in all tribes of Orang Asli communities as an infected family member appears to be an important risk factor for this infection. A family outbreak of Blastocystis infection with gastroenteritis has been reported [53]. Thus, the possibility of a Blastocystis outbreak is of concern. Likewise, a study done by Pipatsatitpong et al.[54] demonstrated that orphans who lived in the same room as childcare workers who were infected with Blastocystis had a higher risk of acquiring blastocystosis than those who lived in the rooms without infected childcare workers. Transmission of Blastocystis has also been reported among family members [55,56] and among mentally deficient persons in institutions [57,58].
In the present study, we have been using both concentration technique and trichrome staining. Some authors suggested that concentration techniques were unsuitable to detect Blastocystis from faecal samples because the parasite could be easily disrupted [59]. In contrast, several other studies have reported that concentration techniques are useful [60,61]. Our results showed that trichrome stain was suitable for the diagnosis of Blastocystis infection. It is noted that all other reports on the prevalence of Blastocystis in Malaysia used different method which was in vitro cultivation. However, cultivation of the organism plays a minor role especially as results are available only after 48 to 72 hours [62]. This may not make them appropriate for comparison with this study. Nonetheless, these results could serve as baseline information on the prevalence and risk factors of Blastocystis among Orang Asli in Malaysia.
The present study has several limitations. Firstly, the findings were based on formalin-ether sedimentation and trichrome staining techniques. We were unable to culture in Jones’ medium due to limited faecal samples. By virtue of this fact, if in vitro cultivation was employed as a diagnostic tool, the prevalence of Blastocystis may be higher than the current reported prevalence due to its sensitivity. Secondly, the inconsistency between observation and response given by the subjects. These inconsistencies could be caused by differences in educational background among the Orang Asli.
Conclusion
In conclusion, this is the first study to comprehensively provide the prevalence and risk factors of Blastocystis infection among the Proto-Malay, Negrito and Senoi tribes. Our postulation concurs with the study carried out previously which reported that the transmission to humans was through drinking untreated water. Furthermore, the present study also observes human-to-human transmission appears to be one of the modes of transmission of Blastocystis in these communities. Therefore, it is suggested that an intervention strategy should be taken to protect the community and to advise them to drink boiled water. Screening and giving treatment to the infected family members on the basis of one affected member would appear to be justified in order to avoid or reduce the Blastocystis infection among Orang Asli.
Competing interests
The authors’ declare that they have no competing interests.
Authors’ contributions
TSA was involved in all phases of the study, including study design, data collection, data analysis and write up of the manuscript; NM and MKAG supervised the study, and revised the manuscript; NM and MKAG were involved in the statistical analysis of data; SNA and FMS were involved in the collection and laboratory examination of samples. All authors read and approved the final manuscript. TSA and NM are the guarantors of the paper.
Financial support
This work was supported in part by the UKMMC Fundamental Research Grant (FF-165-2011) and Special Research University Grant (UKM-GUP-2011-316) from Universiti Kebangsaan Malaysia.
Contributor Information
Tengku Shahrul Anuar, Email: tsatab@lycos.com.
Mohamed Kamel Abdul Ghani, Email: mkamal@medic.ukm.my.
Siti Nor Azreen, Email: azreenm@gmail.com.
Fatmah Md Salleh, Email: fmdsalleh@yahoo.com.
Norhayati Moktar, Email: syahbasree@yahoo.com.
Acknowledgements
We gratefully acknowledge the Ministry of Rural and Regional Development Malaysia for granting us permission to conduct this research. We also thank all the participants from the Parit Gong village, Pasu village, Pian village, Bagan Balak village, Sungai Banun village, Desa Damai village, Sungai Raba village, and Pengkalan Permai village for their commitment and contribution in providing their faecal samples.
References
- Brumpt E. Colite a Tetramitus mesnili (Wenyon 1910) et colite a Trichomonas intestinalis Leuchart 1879. Blastocystis hominis n. sp. et forms voisines. Bull Soc Pathol Exot. 1912;5:725–730. [Google Scholar]
- Stenzel DJ, Boreham PFL. Blastocystis hominis revisited. Clin Microbiol Rev. 1996;9:563–584. doi: 10.1128/cmr.9.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KSW. New insights on classification, identification and clinical relevance of Blastocystis spp. Clin Microbiol Rev. 2008;21:639–665. doi: 10.1128/CMR.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogruman-Al F, Simsek Z, Boorom K, Ekici E, Sahin M, Tuncer C, Kustimur S, Altinbas A. Comparison of methods for detection of Blastocystis infection in routinely submitted stool samples and also in IBS/IBD patients in Ankara, Turkey. PLoS ONE. 2010;5:e15484. doi: 10.1371/journal.pone.0015484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman V, Khan KZ. A comparison of direct microscopy with culture for the diagnosis of Blastocystis hominis. Southeast Asian J Trop Med Public Health. 1994;25:792–793. [PubMed] [Google Scholar]
- Leelayoova S, Taamasri P, Rangsin R, Naaglor T, Thathaisong U, Mungthin M. In-vitro cultivation: a sensitive method for detecting Blastocystis hominis. Ann Trop Med Parasitol. 2002;96:803–807. doi: 10.1179/000349802125002275. [DOI] [PubMed] [Google Scholar]
- Suresh K, Smith H. Comparison of methods for detecting Blastocystis hominis. Eur J Clin Microbiol Infect Dis. 2004;23:509–511. doi: 10.1007/s10096-004-1123-7. [DOI] [PubMed] [Google Scholar]
- Stark D, van Hal S, Marriott D, Elis J, Harkness J. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int J Parasitol. 2007;37:11–20. doi: 10.1016/j.ijpara.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Boorom KF, Smith H, Nimri L, Viscogliosi E, Spanakos G, Parkar U, Li LH, Zhou XN, Ok UZ, Leelayoova S, Jones MS. Oh my aching gut: irritable bowel syndrome, Blastocystis and asymptomatic infection. Parasit Vectors. 2008;1:40. doi: 10.1186/1756-3305-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold CR, Nielsen HV, Molbak K, Smith HV. Pursuing the clinical significance of Blastocystis–diagnostic limitations. Trends Parasitol. 2009;25:23–29. doi: 10.1016/j.pt.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Giacometti A, Cirioni O, Fiorentini A, Fortuna M, Scalise G. Irritable bowel syndrome in patients with Blastocystis hominis infection. Eur J Clin Microbiol Infect Dis. 1999;18:436–439. doi: 10.1007/s100960050314. [DOI] [PubMed] [Google Scholar]
- Yakoob J, Jafri W, Jafri N, Khan R, Islam M, Beg MA, Zaman V. Irritable bowel syndrome: in search of an etiology: role of Blastocystis hominis. Am J Trop Med Hyg. 2004;70:383–385. [PubMed] [Google Scholar]
- Dogruman-Al F, Kustimur S, Yoshikawa H, Tuncer C, Simsek Z, Tanyuksel M, Araz E, Boorom K. Blastocystis subtype in irritable bowel syndrome and inflammatory bowel disease in Ankara, Turkey. Mem Inst Oswaldo Cruz. 2009;104:724–727. doi: 10.1590/S0074-02762009000500011. [DOI] [PubMed] [Google Scholar]
- Jones MS, Whipps CM, Ganac RD, Hudson NR, Boroom K. Association of Blastocystis subtype 3 and 1 with patients from an Oregon community presenting with chronic gastrointestinal illness. Parasitol Res. 2009;104:341–345. doi: 10.1007/s00436-008-1198-7. [DOI] [PubMed] [Google Scholar]
- Salim HR, Kumar GS, Vellayan S, Mak JW, Anuar AK, Init I, Vennila GD, Saminathan R, Ramakrishnan K. Blastocystis in animal handlers. Parasitol Res. 1999;85:1032–1033. doi: 10.1007/s004360050677. [DOI] [PubMed] [Google Scholar]
- Suresh K, Salim HR, Jamaiah I, Anuar AK. Blastocystis hominis in high-rise flat dwellers in Kuala Lumpur, Malaysia. Trans R Soc Trop Med Hyg. 2001;95:377–378. doi: 10.1016/S0035-9203(01)90186-5. [DOI] [PubMed] [Google Scholar]
- Abe N, Wu Z, Yoshikawa H. Molecular characterization of Blastocystis isolates from birds by PCR with diagnostic primers and restriction fragment length polymorphism analysis of the small subunit ribosomal RNA gene. Parasitol Res. 2003;89:393–396. doi: 10.1645/0022-3395(2003)089[0393:ESHIIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Leelayoova S, Siripattanapipong S, Thathaisong U, Naaglor T, Taamasri P, Piyaraj P, Mungthin M. Drinking water: a possible source of Blastocystis spp. subtype 1 infection in school children of a rural community in central Thailand. Am J Trop Med Hyg. 2008;79:401–406. [PubMed] [Google Scholar]
- Noor Azian MY, San YM, Gan CC, Yusri MY, Nurulsyamzawaty Y, Zuhaizan AH, Maslawaty MN, Norparina I, Vythilingam I. Prevalence of intestinal protozoa in an aborigine community in Pahang, Malaysia. Trop Biomed. 2007;24:55–62. [PubMed] [Google Scholar]
- Ithoi I, Azman J, Mak JW, Wan Yusoff WS, Rohela M. Occurrence of Blastocystis in water of two rivers from recreational areas in Malaysia. J Parasitol Res. 2011. [DOI] [PMC free article] [PubMed]
- Abdulsalam AM, Ithoi I, Al-Mekhlafi HM, Ahmed E, Surin J, Mak JW. Drinking water is a significant predictor of Blastocystis infection among rural Malaysian primary schoolchildren. Parasitology. 2012;139:1014–1020. doi: 10.1017/S0031182012000340. [DOI] [PubMed] [Google Scholar]
- Anuar TS, Al-Mekhlafi HM, Abdul Ghani MK, Osman E, Mohd Yasin A, Nordin A, Nor Azreen S, Md Salleh F, Ghazali N, Bernadus M, Moktar N. Giardiasis among different tribes of Orang Asli in Malaysia: Highlighting the presence of other family members infected with Giardia intestinalis as a main risk factor. Int J Parasitol. 2012;42:871–880. doi: 10.1016/j.ijpara.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Kish L. Survey sampling. John Wiley & Sons, Inc, New York; 1968. London. [Google Scholar]
- Sinniah B, Rajeswari B. Blastocystis hominis infection, a cause of human diarrhea. Southeast Asian J Trop Med Public Health. 1994;25:490–493. [PubMed] [Google Scholar]
- Rajah SH, Suresh KG, Vellayan S, Mak JW, Khairul Anuar A, Init I, Vennila GD, Saminathan R, Ramakrishnan K. Blastocystis in animal handlers. Parasitol Res. 1999;85:1032–1033. doi: 10.1007/s004360050677. [DOI] [PubMed] [Google Scholar]
- Lwanga SK, Lemeshow S. Sample size determination in health studies: A practical manual. World Health Organization, Geneva, Switzerland; 1991. [Google Scholar]
- Salleh FM, Anuar TS, Yasin AM, Moktar N. Wintergreen oil: A novel method in Wheatley’s trichrome staining technique. J Microbiol Methods. 2012;91:174–178. doi: 10.1016/j.mimet.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Fleck SL, Moody AH. Diagnostic Technique in Medical Parasitology 11th ed. Cambridge U.P, Cambridge; 1993. pp. 10–14. [Google Scholar]
- Leelayoova S, Rangsin R, Taamasri P, Naaglor T, Thathaisong U, Mungthin M. Evidence of waterborne transmission of Blastocystis hominis. Am J Trop Med Hyg. 2004;70:658–662. [PubMed] [Google Scholar]
- Yan Y, Su S, Ye J, Lai X, Lai R, Liao H, Chen G, Zhang R, Hou Z, Luo X. Blastocystis sp. subtype 5: a possibly zoonotic genotype. Parasitol Res. 2007;101:1527–1532. doi: 10.1007/s00436-007-0672-y. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Morimoto K, Wu Z, Singh M, Hashimoto T. Problems in speciation in the genus Blastocystis. Trends Parasitol. 2004;20:251–255. doi: 10.1016/j.pt.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Kuo HY, Chiang DH, Wang CC, Chen TL, Fung CP, Lin CP, Cho WL, Liu C. Clinical significance of Blastocystis hominis: experience from a medical center in northern Taiwan. J Microbiol Immunol Infect. 2008;41:222–226. [PubMed] [Google Scholar]
- Lee LI, Chye TT, Karmacharya BM, Govind SK. Blastocystis sp.: waterborn zoonotic organism, a possibility? Parasit Vectors. 2012;5:130. doi: 10.1186/1756-3305-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaicharoen R, Ngrenngarmlert W, Wongjindanon N, Sripochang S, Kiatfuengfoo R. Infection of Blastocystis hominis in primary schoolchildren from Nakhon Pathom province, Thailand. Trop Biomed. 2006;23:117–122. [PubMed] [Google Scholar]
- Menon BS, Abdullah M, Mahamud F, Singh B. Brief report: Intestinal parasites in Malaysian children with cancer. J Trop Pediatr. 1999;45:241–242. doi: 10.1093/tropej/45.4.241. [DOI] [PubMed] [Google Scholar]
- Mohamed Kamel AG, Hartini Y. Blastocystis hominis: its presence in the faecal samples of Orang Asli children at Pos Lenjang, Pahang, Malaysia. Sains Malaysiana. 2011;40:1123–1127. [Google Scholar]
- Nimri LF. Evidence of an epidemic of Blastocystis hominis infections in preschool-children in Northern Jordan. J Clin Microbiol. 1993;31:2706–2708. doi: 10.1128/jcm.31.10.2706-2708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento SA, Moitinho MD. Blastocystis hominis and other intestinal parasites in a communitu of Pitannga City Parana State, Brazil. Rev Inst Med Trop São Paulo. 2005;47:213–217. doi: 10.1590/s0036-46652005000400007. [DOI] [PubMed] [Google Scholar]
- Boeke CE, Mora-Plazas M, Forero Y, Villamor E. Intestinal protozoan infections in relation to nutritional status and gastrointestinal morbidity in Colombian schoolchildren. J Trop Pediatr. 2010;56:299–306. doi: 10.1093/tropej/fmp136. [DOI] [PubMed] [Google Scholar]
- Lee IL, Tan TC, Tan PC, Nanthiney DR, Biraj MK, Surendra KM, Suresh KG. Predominance of Blastocystis sp. subtype 4 in rural communities, Nepal. Parasitol Res. 2012;110:1553–1562. doi: 10.1007/s00436-011-2665-0. [DOI] [PubMed] [Google Scholar]
- Rayan P, Verghese S, McDonnell P. Geographical location and age affects the incidence of parasitic infestations in schoolchildren. Indian J Pathol Microbiol. 2010;53:498–504. doi: 10.4103/0377-4929.68292. [DOI] [PubMed] [Google Scholar]
- El-Masry NA, Bassily S, Farid Z, Aziz AG. Potential clinical significance of Blastocystis hominis in Egypt. Trans R Soc Trop Med Hyg. 1990;84:695. doi: 10.1016/0035-9203(90)90152-5. [DOI] [PubMed] [Google Scholar]
- Baldo ET, Belizario VY, De Leon WU, Kong HH, Chung DI. Infection status of intestinal parasites in children living in residential institutions in Metro Manila, the Philippines. Korean J Parasitol. 2004;42:67–70. doi: 10.3347/kjp.2004.42.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa MI, Navone GT, Orden AB, Torres MF, Castro LE, Oyhenart EE. Socio-environmental conditions, intestinal parasitic infections and nutritional status in children from a suburban neighborhood of La Plata, Argentina. Acta Trop. 2011;118:184–189. doi: 10.1016/j.actatropica.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Li LH, Zhang XP, Lv S, Zhang L, Yoshikawa H, Zhiliang W, Steinmann P, Utzinger J, Tong XM, Chen SH, Zhou XN. Cross-sectional surveys and subtype classification of human Blastocystis isolates from four epidemiological settings in China. Parasitol Res. 2007;102:83–90. doi: 10.1007/s00436-007-0727-0. [DOI] [PubMed] [Google Scholar]
- Al-Fellani MA, Khan AH, Al-Gazoui RM, Zaid MK, Al-Ferjani MA. Prevalence and clinical features of Blastocystis hominis infection among patients in Sebha, Libya. Sultan Qaboos Univ Med J. 2007;7:35–40. [PMC free article] [PubMed] [Google Scholar]
- Wang KX, Li CP, Wang J, Cui YB. Epidemiological survey of Blastocystis hominis in Huainan City, Anhui Province, China. World J Gastroenterol. 2002;8:928–932. doi: 10.3748/wjg.v8.i5.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrul Anuar T, Al-Mekhlafi HM, Abdul Ghani MK, Osman E, Mohd Yasin A, Nordin A, Nor Azreen S, Md Salleh F, Ghazali N, Bernadus M, Moktar N. Prevalence and risk factors associated with Entamoeba histolytica/dispar/moshkovskii infection among three Orang Asli ethnic groups in Malaysia. PLoS One. 2012;7:e48165. doi: 10.1371/journal.pone.0048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain KC, Noble MA, Freeman HJ, Barteluk RL. Epidemiology and clinical features associated with Blastocystis hominis infection. Diagn Microbiol Infect Dis. 1987;8:235–244. doi: 10.1016/0732-8893(87)90055-1. [DOI] [PubMed] [Google Scholar]
- Zaki M, Zaman V, Sheikh NA. Resistance of Blastocystis hominis cysts to chlorine. J Pakistan Med Assoc. 1996;46:178–179. [PubMed] [Google Scholar]
- Zaman V, Howe J, Ng M. Ultrastructure of Blastocystis hominis cysts. Parasitol Res. 1995;81:465–469. doi: 10.1007/BF00931787. [DOI] [PubMed] [Google Scholar]
- Moe KT, Singh M, Howe J, Ho LC, Tan SW, Ng GC, Chen XQ, Yap EH. Observation on the ultrastructure and viability of the cystic stage of Blastocystis hominis from human feces. Parasitol Res. 1996;82:439–444. doi: 10.1007/s004360050142. [DOI] [PubMed] [Google Scholar]
- Guglielmetti P, Cellesi C, Figura N, Rossolini A. Family outbreak of Blastocystis hominis associated gastroenteritis. Lancet. 1989;2:1394. doi: 10.1016/s0140-6736(89)92000-x. [DOI] [PubMed] [Google Scholar]
- Pipatsatitpong D, Rangsin R, Leelayoova S, Naaglor T, Mungthin M. Incidence and risk factors of Blastocystis infection in an orphanage in Bangkok, Thailand. Parasit Vectors. 2012;5:37. doi: 10.1186/1756-3305-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanad MM, Darwish RM, Yousef SM, Nassef NE. Blastocystis hominis: laboratory identification and clinical relevance. J Trop Med. 1991;1:61–70. [Google Scholar]
- Torres P, Miranda JC, Flores L, Riquelme J, Franjola R, Perez J, Auad S, Hermosilla C, Riquelme S. Blastocystosis and other intestinal protozoan infections in human riverside communities from Valdivia River Basin, Chile. Rev Inst Med Trop São Paulo. 1992;34:557–564. doi: 10.1590/S0036-46651992000600010. [DOI] [PubMed] [Google Scholar]
- Yamada M, Matsumoto Y, Tegoshi T, Yoshida Y. The prevalence of Blastocystis hominis infection in humans in Kyoto City. Jpn J Trop Med Hyg. 1987;15:158–159. [Google Scholar]
- Hunt ALC, Goldsmid JM, Pasha M. Enteric pathogens in mentally handicapped patients in hospital. Med J Aust. 1990;152:277–278. doi: 10.5694/j.1326-5377.1990.tb120934.x. [DOI] [PubMed] [Google Scholar]
- Miller RA, Minshew BH. Blastocystis hominis: an organism in search of a disease. Rev Infect Dis. 1988;10:930–938. doi: 10.1093/clinids/10.5.930. [DOI] [PubMed] [Google Scholar]
- Hussain Qadri SM, Al-Okaili GA, Al-Dayel F. Clinical significance of Blastocystis hominis. J Clin Microbiol. 1989;27:2407–2409. doi: 10.1128/jcm.27.11.2407-2409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldeen WE, Hale D. Use of Hemo-De to eliminate toxic agents used for concentration and trichrome staining of intestinal parasites. J Clin Microbiol. 1992;30:1893–1895. doi: 10.1128/jcm.30.7.1893-1895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002;15:329–341. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


