Abstract
This article summarizes presentations of a symposium examining the potential impact of activity-based therapies (ABT) in promoting neurological and functional recovery after spinal cord injury (SCI). The symposium addressed 3 key questions concerning activity-based therapy in SCI: (1) What clinical approaches are used? (2) Is there empirical evidence supporting efficacy of ABT in promoting neurological recovery and improving overall function, health, and quality of life? (3) What are the issues related to long-term viability of ABT?
Keywords: activity-based therapy, fitness and health, functional recovery, neurologic recovery, quality of life, spinal cord injury, therapeutic exercise
Activity-based therapy (ABT) refers to “interventions that target activation of the neuromuscular system below the level of the lesion, with the goal of retraining the nervous system to recover a specific motor task.”1 Intense physical activity has been shown to improve physiological function and health outcomes in individuals with chronic (> 1 year postinjury) spinal cord injury (SCI). The effects of intense activity on neurological and functional recovery, however, have not been fully investigated nor verified.
This article summarizes presentations of a symposium examining the potential impact of ABT in promoting neurological and functional recovery after SCI. The symposium addressed 3 key questions concerning ABT in SCI: (1) What clinical approaches are used? Principles and techniques used in 3 independent programs – Project Walk, the Center for SCI Recovery at the Rehabilitation Institute of Michigan, and Shepherd Center’s Beyond Therapy program – are compared and contrasted. (2) Is there empirical evidence supporting efficacy of ABT in promoting neurological recovery and improving overall function, health, and quality of life? Preliminary findings from each program are presented. (3) What are the issues related to long-term viability of ABT? Discussion focused on determining who benefits from ABT, gauging the point of diminishing return with respect to functional recovery, and strategies for maintaining any gains achieved from therapy.
Description of Activity-Based Therapy Programs
Project Walk is an outpatient facility in Carlsbad, California, that has been treating persons with SCI since 1999. From its inception, Project Walk has seen more than 1,000 individuals with SCI from around the world. Requirements to participate in the program are an SCI C2 or lower, non– ventilator-dependent, and a physician’s clearance for intensive exercise.
Project Walk originated from a sports training philosophy of designing programs around returning athletes to their respective competition; for SCI, this would be walking. As the founders had no real prior knowledge of what could or could not be accomplished with SCI, the goal was always to attempt to regain function below the level of injury. This was initially accomplished through trial and error; what worked with some clients would not work with others. Over time, more structured guidelines were developed to assist with determining what modalities would be most appropriate for the functional abilities of each client. These guidelines became the basis for the 5 phases of recovery and associated intervention approaches:
Phase I/II: Reactivation/Reorganization & Development/Stabilization – Stimulate the nervous system with active assistive exercise and use developmental sequencing to develop joint stabilization.
Phase III: Strength – Initiate eccentric and concentric muscle contractions through positional movement or stimulation.
Phase IV: Function and Coordination – Improve coordinated movement through all planes of movement and motion. Most exercises are performed in a load-bearing position, mainly free standing.
Phase V: Gait Training – Focus on proper gait mechanics and the ability to move over ground in multiple planes of motion.
Several modalities are used to implement the phases of recovery. The most basic modality is active assistive exercise or what Project Walk terms active nervous system recruitment (ANSR). This is used when clients have little to no voluntary movement and consists of helping the client through different ranges of motion and providing a resistance less than gravity. Clients are instructed to attempt or visualize actively assisting or resisting the movement performed. ANSR attempts to provide a sensory stimulus and elicit a motor response. The goal is to generate patterned neural activity2 and ultimately, using a high number of repetitions, long-term potentiation.3 Load-bearing exercises or developmental movement patterns (DMPs) are used at all levels of motor function and are characterized by the hands or elbows and/or feet or knees in contact with the ground, with some percentage of body weight supported through the extremities. DMPs consist of pushing the torso up out of a prone position, sitting unsupported, elbows/hands and knees position (“on all fours”), high kneeling, standing, and walking. These movements and positions are designed to mimic the human developmental process. Project Walk also includes many technologies in clients’ programs including body weight support treadmill training (Robomedica; Robomedica Inc, San Viejo, California), partial body weight loading (Total Gym; Total Gym Fitness), whole body vibration (PowerPlate; Power Plate North America, Irvine, California), FES (RT300; Restorative Therapies, Baltimore, Maryland), assisted standing/ squats (EasyStand Evolv; Altimate Medical, and Keiser Power Rack; Keiser Corportation), and a full complement of resistance training equipment.
Each client is evaluated upon entry to the program and every 3 to 6 months thereafter. From the initial evaluation, it is determined in which of the 5 phases of recovery the client is placed. A client will provide one or more long-term goals, and the training specialist will break these down into short- and medium-term goals that align with the phase(s) in which the client has been placed. Although clients are formally evaluated every 3 to 6 months, they are also being informally evaluated on a daily basis. If a client shows new activity in a muscle or new functional abilities, the specialists have the freedom to change a client’s program.
The Center for SCI Recovery – Rehabilitation Institute of Michigan started in Detroit in 2004. The Center was developed as an extension of the continuum of care to provide people with SCI an opportunity to continue working toward functional recovery in an intense, recovery focused therapy program. Three major guidelines were used in developing the program: (1) an emphasis on recovery versus compensation, (2) a concentration of treatment below the level of injury, and (3) a high dosage/intensity of physical rehabilitation. The overall goal is to facilitate neural plasticity using treatment activities based on forced use of involved areas,4,5 central pattern generation,6,7 and an enriched environment. 8,9
Duration, session length, and intensity of rehabilitation need to be longer to promote neural plasticity compared to traditional rehabilitation, but effective dosage elements are far from clear. Therefore treatment parameters are based partly on practicality. Treatment intensity is 3 hours/ day, 3 to 5 days per week. Optimal duration of therapy varies, because progress is expected to be much slower and it is difficult to determine when a plateau is reached. The staffing model consists of physical therapists working with athletic trainers and exercise physiologists. This addresses the labor intensity and contributes to a desired fitness-type environment. The recovery focus of the program refers to potential neurological improvement, but functional recovery is expected. The program appeals to a sense of optimism and hope that is far from guaranteed and, therefore, care is taken in setting expectations for recovery.
Therapeutic techniques and equipment used are somewhat varied in keeping with the concept of an enriched environment. Locomotor activities include body weight support treadmill training and overground walking with or without assistive devices and with or without overhead support. Developmental activities include crawling with or without body support, kneel walking, and a variety of mat activities designed to build core strength and trunk stability. Whole body vibration is used to decrease spasticity or increase muscle activity prior to other activities, primarily walking. Supplemental activities include FES cycling, strengthening exercises with resistance or with active assistance using, among other equipment, an overhead strap/sling system, stretching, balance activities, and other specific interventions as indicated by individual needs.
Shepherd Center’s Beyond Therapy Program was developed in June 2005 in response to the growing number of requests from former patients to participate in an activity-based exercise program following their traditional course of rehabilitation. Optimizing functional recovery and decreasing the likelihood of secondary complications that typically occur among people with neurological disorders are central objectives of the program.
Program staff consists of therapists who specialize in exercise physiology and physical therapy. The program combines the concepts of athletic training and physical therapy to promote neural plasticity in the injured nervous system. Clinicians integrate the neural development and strength goals of each client into an intensive rehabilitation program, incorporating the use of innovative treatments and technologies designed to facilitate some level of recovery. The approaches used in the program originate from 3 primary concepts believed to be important to neurorecovery: (1) developmental sequencing/core rehabilitation focused on strengthening the primary stabilizing muscles of the trunk and pelvis because of their central role in core stability; (2) repetition and patterned motor activity; and (3) functional training and task specificity. Locomotor training is a task-specific practice and an important component in the training plan of those clients who present with an AIS C or D injury.
Each client entering the program is evaluated by a physical therapist who designs an intense 9 hour per week program that combines the 3 primary concepts described above in ratios that are appropriate for their current level of function. Even though each client receives individualized treatment, the program and subsequent progression is based on a clinical algorithm based on the client’s functional status (Table 1) and appropriate clinical decision making by a skilled therapist. Clients progress through the levels of treatment as they demonstrate neuromuscular and/or functional improvement. The treatment algorithm is based on current evidence as well as clinical experience and divides a client’s total treatment time into developmental sequencing, strengthening, and locomotor training.
Table 1.
Clinical algorithm for client placement and prescription of treatment
| Level | Client functional status | Development sequencing | Strengthening exercise | Locomotor training (LT) |
| 1 | Demonstrates complete loss of motor and sensory function below injury level(LOI) | 4 hours | 5 hours | None |
| 2 | Demonstrates complete loss of motor function but sacral sparing below LOI | 3 hours | 3 hours | 1 hour full weight bearing/standing using FES to appropriate muscle groups; 2 hours robotic LT |
| 3 | Able to initiate one step without assistance and walk less than 50 ft withphysical assistance | 2 hours | 3 hours | 2 hours robotic or manual LT; 2 hours over ground gait training using FES |
| 4 | Able to walk > 50 ft with 1-person assistance | 2 hours | 2 hours | 2 hours robotic or manual LT; 3 hours overground gait training using FES |
| 5 | Able to walk > 150 ft without physical assistance | 1 hour | 4 hours resistance and plyometric training | 2 hours LT with or w/out body weight support; 2 hours overground gait training using FES |
Note: FES = functional electrical stimulation.
Evidence of Clinical Efficacy
Several studies have been published on the ABT performed at Project Walk. Harness et al10 looked at 21 ABT subjects and 8 controls over a 6-month time course. Outcome measures pre and post were ASIA motor and sensory scores, CHART (Craig Handicap Assessment and Reporting Technique), and the EQ-5D thermometer. CHART and EQ-5D (self-rated general health assessment) scores were not significantly different between ABT and controls after 6 months. However, ABT subjects showed significantly greater gains than controls during the 6-month interval for total ASIA motor score (4.8±4.5 vs -0.1±1.45; P < .001); 71% of the ABT participants had an increase in total ASIA motor score. As noted in Figure 1, this change in score correlated significantly with total time spent in the ABT program (P < .02). The mean increase in total ASIA motor score was 4.8 points. Note that these total ASIA motor score gains in ABT subjects varied in relation to baseline deficits: subjects who were motor complete (AIS A or B, n=12), as compared to subjects who were motor incomplete (AIS C or D, n=9), had significantly less gains (2.8±4.32 vs 7.4±3.3; P < .02), a finding entirely attributable to changes in lower extremity motor scores.
Figure 1. Changes in ASIA Motor score as a function of exercise.

Yozbatiran et al11 found increases in lower extremity isometric strength in 21 participants after 6 months of training. This change in force production correlated significantly with changes in total ASIA motor score (P < .001). Alterations in lower extremity lean body mass (LBM) and bone density have also been observed over 6 months in a single C5 ASIA A participant .12 Lower extremity LBM increased by 11% and distal femur bone density increased by 10% with training.
The Rehabilitation Institute of Michigan has completed an analysis of outcomes for 23 participants in the CSCIR program. Average age at program initiation was 30.1 ± 9.8 years and time since injury averaged 5.1 ± 6.4 years. SCI levels ranged from C4 to T11; 13 participants experienced tetraplegia and 10 paraplegia. Fourteen participants had ASIA Impairment Scale (AIS) classification of A, 2 AIS B, 5 AIS C, 1 AIS D, and 1 cauda equina. Participants engaged in intensive ABT for an average of 7.1 ± 1.7 hours per week over an average of 4.6 ± 2.8 months. Total hours of therapy averaged 137.3 ± 83.9. Multiple outcome measures were assessed for each participant pre- and posttreatment. Results reported here include mat mobility, overground ambulation, life satisfaction, physical independence, and mobility changes over the course of therapy.Figure 1.
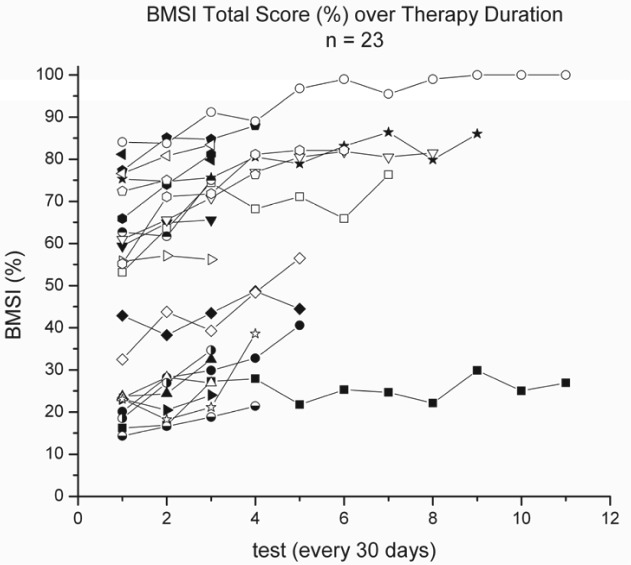
The Larson Basic Movement Skills Inventory (LBMSI) was used to assess mat mobility skills. Participants were observed while assuming, maintaining, and performing closed-chain activities in various positions for 25 mat mobility skills (eg, prone on elbows, sitting, hands and knees, kneeling and standing). Physical assistance (PA) required during the performance of each mat skill was recorded using a 7-point ordinal scale (FIM PA scale). From initial examination (IE) to discharge (DC) examination, all 23 subjects demonstrated significant improvement (Z = -4.1; P < .005) in total LBMSI scores (Wilcoxon signed ranks test), and in the assume, maintain, and closed-chain subscores (P < .005). Figure 2 shows changes in LBMSI scores for each participant over subsequent 30-day test intervals.
Figure 2. Total Larson Basic Movement Skills Inventory (BMSI).
The Satisfaction with Life Scale (SWLS) was used to measure life satisfaction and the CHART was used to measure physical independence (paid and unpaid care needed, assistance needed for grocery shopping, laundry, housekeeping, or medical needs), mobility (frequency of social interactions, in-home and transportation access, and assistance needed), and occupation (time spent on job, school, recreational, volunteer activities). From IE to DC examination, there was a nonsignificant trend (P = .06) for the group (n=21) to improve in SWL scores. From IE to DC, CHART physical independence subscores improved (P = .03), mobility subscores improved (P = .05), and occupation subscores did not improve (P = .59).
Six of the 23 participants succeeded in obtaining ambulation overground (4 paraplegic, 2 tetraplegic; 5 incomplete and 1 complete SCI). For participants who ambulated, 3- or 6-minute walk tests13,14 were administered every 30 days. Ambulation velocity did not improve (Z = -1.2; P = .25), but distance did improve (Z = -2.2; P = .02) over the course of therapy (Wilcoxon signed ranks test). Distance increased, on average, 16.7 ± 10.8 m every 30 days. Three of the 6 ambulatory participants progressed to a less restrictive assistive or orthotic device, and 4 of 6 decreased in the amount of physical assistance (PA) required.
In late 2008, Shepherd Center initiated a prospective, randomized clinical trial to evaluate empirically the effects of participation in an intensive, ABT program for individuals with chronic (> 1 year postinjury), motor incomplete SCI (AIS C or D). To date, 45 individuals have been enrolled in the trial, which is examining the effectiveness of an intensive (9 h/wk), 24-week ABT program targeting locomotor recovery. Individualized treatment and progression in treatment was provided based on the parameters presented in Table 1. Preliminary findings for the first 26 participants to complete the program are presented here. Table 2 presents basic demographic and baseline clinical information for this sample. The experimental and control groups varied significantly in age and gender but were essentially equivalent in baseline ASIA Motor and Spinal Cord Independence Measure (version II) scores.
Table 2.
Demographic and baseline information
| Experimental (n = 11) | Control (n = 15) | P | |
| Age, years | 44.9 ± 13.6 | 32.6 ± 11.9 | .02 |
| Gender | M = 11 / F = 0 | M = 7 / F = 8 | <.01 |
| Time postinjury, months | 107.0 ± 158.7 | 49.2 ± 56.0 | .21 |
| Tetraparesis/paraparesis | 11/0 | 13/2 | .21 |
| AIS Classification | C = 4 / D = 7 | C = 8 / D = 7 | .39 |
| ASIA Motor | 60.4 ± 16.7 | 59.6 ± 17.3 | .91 |
| ASIA LEMS | 26.6 ± 12.8 | 25.9 ± 11.8 | .88 |
| SCIM II | 59.0 ± 19.4 | 66.7 ± 21.9 | .36 |
Note: Values are mean ± SD or number of participants. AIS = ASIA Impairment Scale; LEMS = lower extremity motor score; SCIM II = Spinal Cord Independence Measure (version II).
A multitude of outcome measures have been collected for the trial. For experimental participants, outcome measures were collected before and after the 24-week intervention. For control participants, measures were collected before and after a 24-week delay in starting the intervention. During the delay, they were allowed to continue with any exercise or therapy activities they were already conducting. Several participants reported training to “get ready” for participation in the trial.
Presented in this preliminary analysis are pre- and posttest differences in motor function (ASIA Motor Score and LEMS), functional independence (SCIM II), and walking (10-Meter Walk, 6-Minute Walk, and Timed Up & Go tests). An adaptation of the Spinal Cord Injury Functional Ambulation Inventory (SCI-FAI)15 was used to account for changes in the use of assistive devices during walking and to assess qualitative aspects of gait. Video clips of participants taken during pre- and postwalking tests were simultaneously viewed and independently rated by 2 raters who were blinded to the timing of the video (pre- or posttest). The Walking Parameters (weight shift, step width, step rhythm, step height, foot contact, step length) and Assistive Devices (upper extremity balance/weightbearing; lower extremity orthosis) components of the SCI-FAI were used and scores summed for left and right side, for a maximum score of 34.
Table 3 presents pre- to posttest differences on each outcome measure. Although greater improvements were noted for experimental versus control participants on all measures, none of the mean differences were statistically significant and only changes in ASIA LEMS approached significance. Several factors contribute to the lack of significance noted thus far including limited sample size (about half of the final n needed based on our preliminary power analysis) and high variability in scores among participants.
Table 3.
Changes in primary outcome measures
| Experimental (n = 11) | Control (n = 15) | P | |
| ASIA Motor | 5.7 ± 7.3 | 1.7 ± 4.6 | .10 |
| ASIA LEMS | 3.7 ± 5.9 | 0.3 ± 3.4 | .08 |
| SCIM II | 2.7 ± 6.4 | 0.9 ± 4.5 | .39 |
| 10- Meter Walk (m/min) | 2.4 ± 7.4 | 0.6 ± 6.9 | .53 |
| 6-Minute Walk (ft) | 68.0 ± 108.8 | 0.6 ± 90.2 | .10 |
| Timed Up & Go (sec) | -27.6 ± 86.5 | -1.1 ± 5.9 | .24 |
| SCI-FAI (max = 34) | 3.9 ± 6.9 | -0.5 ± 3.0 | .11 |
Note: Values are mean ± SD. AIS = ASIA Impairment Scale; LEMS = lower extremity motor score; SCIM II = Spinal Cord Independence Measure (version II); SCI-FAI = Spinal Cord Injury Functional Ambulation Inventory.
Discussion
Differing in intensity and scope, the programs presented here share similar therapeutic approaches and modalities to promote functional recovery in individuals with chronic SCI. Taken together, results from preliminary analyses of effectiveness offer encouragement that ABT programs may be useful to improve functional status after SCI. Gains were noted in ASIA Motor scores, particularly lower extremity motor scores, which might be expected given the emphasis on walking recovery that is integral to these programs. Improvements in physical independence (SCIM II and CHART) and mobility (LBMSI) were noted, and modest improvements in walking were observed among a subset of program participants. Improvements in muscle strength and bone density were also reported, but participation in an ABT intervention had little effect on perceived health and life satisfaction.
Although the impact of ABT on functional recovery – and particularly walking – appears to be modest, it is important to note that these results are for individuals with chronic SCI. Conventional thinking is that functional recovery after the first year postinjury is limited. For example, Kirschblum et al16 reported that 27% of their sample classified as ASIA A and 58% classified ASIA B, C, or D showed an increase in ASIA Motor score from their 1-year to 5-year follow-up exam. The degree of improvement was modest, however, with an average increase of 1.38 ± 6.22. This is comparable with improvements noted by control group participants presented here and substantially lower than improvements noted for ABT participants.
Gauging efficacy of ABT is encumbered in part by the high variability noted in participants’ response to therapy. Preliminary results from the Beyond Therapy trial, for example, suggest that there are clear responders and nonresponders. Project Walk results suggest there may be a dose effect (Figure 1), whereas results from the CSCIR program (Figure 2) suggest there may also be an upper limit, beyond which therapeutic gains are diminished.
These findings point to the need to identify factors that predict response to ABT and also the optimal dosing “window” – the therapeutic threshold as well as point of diminishing returns. Failure to identify these parameters and establish clear expectations for ABT clients may lead to a loss in credibility of these nascent therapeutic programs. This is particularly important given the tenuous nature of funding for ABT. Because it is not considered to be a medical necessity by most health insurers, many ABT clients must pay out of pocket or seek funding from nonconventional sources such as fundraising; all the more reason to ensure that reasonable expectations for recovery are established and maintained. The work by Winchester and colleagues17 to establish a predictive model of responsiveness to locomotor training in SCI provides a useful blueprint for future research needed in this area.
Even for individuals who do achieve meaningful functional recovery from participation in an ABT program, there is little information available about the durability of outcomes achieved or what is needed to maintain gains. Do those who gain some degree of walking ability after participation in ABT continue to walk and progress in other areas of function? Do these gains translate into more meaningful participation, better health, fewer secondary complications, and improved perception of life satisfaction? Long-term follow-up studies are needed to determine the lasting impact of ABT participation and the active lifestyle needed to ensure longevity of outcomes.
Acknowledgments
Rehabilitation Institute of Michigan: Del Harder Rehabilitation Grant. Thank you to the following individuals who assisted in this project during their DPT studies at Oakland University: Lisa Dotson, PT, Ella Kishelova, PT, Tygre Whittington, PT, Nicole Wargo, PT, Lauren Yeomans, PT, Melanie Bevins, PT, Cassandra Ianni, PT, and Stefanie Collins Brewer, PT.
Shepherd Center: This work was funded in part by a grant (H133G080031) from the National Institute on Disability and Rehabilitation Research (NIDRR), US Department of Education. The opinions contained in this paper are those of the authors and do not necessarily reflect those of the US Department of Education or NIDRR. Sincere thanks are extended to Rebecca Washburn, Sarah Morrison, PT, Deborah Backus, PT, PhD, Keith Tansey, MD, PhD, and Jennifer Coker, MPH, for assistance in planning and execution of the study and initial analysis of findings.
References
- 1.Behrman AL, Harkema SJ, Locomotor training after human spinal cord injury: a series of case studies. Phys Ther. 2000;80(7): 688–700 [PubMed] [Google Scholar]
- 2.McDonald JW, Becker D, Sadowsky CL, et al. Late recovery following spinal cord injury. Case report and review of the literature. J Neurosurg. 2002;97(2 suppl):252–265 [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Sun T.Neural plasticity and functional recovery of human central nervous system with special reference to spinal cord injury. Spinal Cord. 2011;49: 486–492 [DOI] [PubMed] [Google Scholar]
- 4.Taub E, Uswatte G, Mark VW, Morris DM.The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42(3): 241–256 [PubMed] [Google Scholar]
- 5.Huang WC, Chen YJ, Chien CL, Kashima H, Lin KC.Constraint-induced movement therapy as a paradigm of translational research in neurorehabilitation: reviews and prospects. Am J Transl Res. 2010;3(1):4860. [PMC free article] [PubMed] [Google Scholar]
- 6.Bouyer LJ.Animal models for studying potential training strategies in persons with spinal cord injury. J Neurol Phys Ther. 2005;29(3): 117–125 [DOI] [PubMed] [Google Scholar]
- 7.Field-Fote EC, Roach KE.Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther. 2011;91(1): 48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Meeteren NL, Eggers R, Lankhorst AJ, Gispen WH, Hamers FP.Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J Neurotrauma. 2003;20(10): 1029–1037 [DOI] [PubMed] [Google Scholar]
- 9.Fischer FR, Peduzzi JD.Functional recovery in rats with chronic spinal cord injuries after exposure to an enriched environment. J Spinal Cord Med. 2007;30(2): 147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harness ET, Yozbatiran N, Cramer SC.Effects of intense exercise in chronic spinal cord injury. Spinal Cord. 2008;46: 733–737 [DOI] [PubMed] [Google Scholar]
- 11.Yozbatiran N, Harness ET, Le V, Luu D, Lopes CV, Cramer SC.A tele-assessment system for monitoring treatment effects in subjects with spinal cord injury. J Telemed Telecare. 2010;16(3): 152–157 [DOI] [PubMed] [Google Scholar]
- 12.Astorino TA, Witzke KA, Harness ET. Efficacy of multimodal training to alter bone mineral density and body composition in persons with spinal cord injury: a case study. Presented at: Annual Southwest Chapter Meeting of the American College of Sports Medicine; 2009; San Diego.
- 13.Shumway-Cook A, Woollacott M.Motor Control - Translating Research into Clinical Practice. 3rd ed. Lippincott Williams & Wilkins; 2007 [Google Scholar]
- 14.Kosak M, Smith T.Comparison of the 2-, 6-, and 12-minute walk tests in patients with stroke. J Rehabil Res Dev. 2005;42(1): 103–107 [DOI] [PubMed] [Google Scholar]
- 15.Field-Fote E, Fluet G, Schafer S, Schneider E, Smith R, Downey P, Ruhl C.The spinal cord injury functional ambulation inventory (SCI-FAI). J Rehabil Med. 2001;33: 177–181 [DOI] [PubMed] [Google Scholar]
- 16.Kirshblum S, Millis S, McKinley W, Tulsky D.Late neurologic recovery after traumatic spinal cord injury. Arch Phys Med Rehabil. 2004;85: 1811–1817 [DOI] [PubMed] [Google Scholar]
- 17.Winchester P, Smith P, Foreman N, Mosby J, Pacheco F, Querry R, Tansey K.A prediction model for determining over ground walking speed after locomotor training in persons with motor incomplete spinal cord injury. J Spinal Cord Med. 2009; 32(1): 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]