Abstract
Objective:
To assess the effect of high-frequency repetitive transcranial magnetic stimulation (rTMS) on lower extremities motor score (LEMS) and gait in patients with motor incomplete spinal cord injury (SCI).
Method:
The prospective longitudinal randomized, double-blind study assessed 17 SCI patients ASIA D. We assessed LEMS, modified Ashworth Scale (MAS), 10-m walking test (10MWT), Walking Index for SCI (WISCI II) scale, step length, cadence, and Timed Up and Go (TUG) test at baseline, after the last of 15 daily sessions of rTMS and 2 weeks later. Patients were randomized to active rTMS or sham stimulation. Three patients from the initial group of 10 randomized to sham stimulation entered the active rTMS group after a 3-week washout period. Therefore a total of 10 patients completed each study condition. Both groups were homogeneous for age, gender, time since injury, etiology, and ASIA scale. Active rTMS consisted of 15 days of daily sessions of 20 trains of 40 pulses at 20 Hz and an intensity of 90% of resting motor threshold. rTMS was applied with a double cone coil to the leg motor area.
Results:
There was a significant improvement in LEMS in the active group (28.4 at baseline and 33.2 after stimulation; P = .004) but not in the sham group (29.6 at baseline, and 30.9 after stimulation; P = .6). The active group also showed significant improvements in the MAS, 10MWT, cadence, step length, and TUG, and these improvements were maintained 2 weeks later. Following sham stimulation, significant improvement was found only for step length and TUG. No significant changes were observed in the WISCI II scale in either group.
Conclusion:
High-frequency rTMS over the leg motor area can improve LEMS, spasticity, and gait in patients with motor incomplete SCI.
Keywords: gait, spasticity, spinal cord injury, transcranial magnetic stimulation
Ambulation is probably the most important goal of rehabilitation following spinal cord injury (SCI). Patients with an incomplete SCI have the potential to regain some ambulatory function; hence, a considerable effort is dedicated to gait training. After lesion, functional reorganization of remaining circuits, at the cortical and subcortical levels, can contribute to the recovery of functions.1-3 Following SCI, central nervous system plasticity can actually lead to maladaptive changes that prevent fuller recovery; therefore, the challenge is to guide this plasticity to optimize the functional outcome for a given individual.
Repetitive transcranial magnetic stimulation (rTMS) can modulate cortical excitability and induce changes over the descending corticospinal output.4 This modulation may be useful to promote active recovery of motor function to obtain functional benefit from gait rehabilitation. Belci et al5 using repetitive high-frequency rTMS applied to the primary motor cortex reported significant improvement in American Spinal Cord Injury Association (ASIA) sensory and motor scores in 4 chronic incomplete cervical SCI patients.
In this study, we hypothesized that high-frequency rTMS stimulation coupled with gait training can improve motor recovery in lower extremities and locomotion in incomplete SCI as compared to the same treatment with sham stimulation.
Methods
The study was a randomized, double-blind, sham-controlled trial. We recruited 17 patients with SCI who were randomized to undergo either active rTMS (n=7) or sham stimulation (n=10). Three patients initially randomized to the sham stimulation group were subsequently crossed over to the active rTMS group. This was done 3 weeks after they had completed the sham stimulation protocol. The results in these 3 patients did not differ from those in the other patients directly randomized to active rTMS, and results are thus reported for 10 patients in each of the study groups.
All patients underwent 15 (3 weeks) consecutive daily sessions of active or sham rTMS. Patients and investigators (except the technician who applied rTMS) were blind to the treatment arm.
All patients received standard of care for their SCI rehabilitation at the Institute Guttmann in Barcelona. The program comprises 5 hours of therapy, including training of activities of daily livng, occupational therapy for upper extremities, fitness, sports, hydrotherapy, and gait training. The session of overground gait training was scheduled just after the rTMS sesion for all patients. Therefore, the effects of rTMS (active or sham) should be considered as adjuncts to the SCI rehabiliation protocol in our hospital.
Patients
We included patients with (1) incomplete SCI with ASIA Impairment Scale (AIS)6 D; (2) cervical or thoracic SCI level; (3) time since SCI between 3 and 12 months; (4) no joint-related limitation of passive range of movement; (5) stable medical condition; (6) stable rehabilitation program; and (7) a written informed consent for the study, which had been approved by the Institutional Review Board.
Clinical and functional assessment
All study outcomes were collected at baseline and after the last session of rTMS. Functional outcomes (gait) were also assessed 2 weeks after last rTMS session. Outcomes included the following:
Lower extremities motor score (LEMS) obtained from the standardized ASIA clinical exam 6
Modified Ashworth Scale (MAS)7 evaluated on both knees for spasticity assessment (average from both legs is reported)
Walking Index for SCI (WISCI) II scale8 to quantify walking ability
Ten-meter walking test (10MWT)9 for gait velocity
Step length and cadence assessed during the 10MWT
Timed Up and Go (TUG)10 test
rTMS protocol
Patients received 15 consecutive business daily rTMS sessions applied in the morning before gait training. We used a MagStim Super Rapid magnetic stimulator (Magstim Company, Whitland, Wales, UK) equipped with a commercially available double cone coil that was held over the vertex. All rTMS sessions were conducted with the patient lying supine.
For active rTMS, we applied 2-s bursts at 20 Hz (40 pulses/burst) with intertrain intervals of 28 s, for a total of 1800 pulses over 20 min. The intensity of stimulation was set as 90% of the resting motor threshold (RMT) intensity for induction of motor-evoked potentials in the lowest muscle threshold in the upper extremity. Motor threshold was defined as the intensity that evoked motor-evoked potentials of >50 µV peak-to-peak amplitude in at least 5 of 10 consecutive stimulations. The mean rTMS intensity used was 39.8 ± 5.8% of maximal stimulator output.
For sham stimulation, the double cone coil disconnected from the main stimulator unit was held over the vertex while a second coil (8-shaped) was connected with the MagStim stimulator and discharged under the patient’s pillow. Thus, no current was induced in the brain, and the patients had a coil on their head and were exposed to a similar clicking noise (from the second coil), though they did not experience a tapping sensation on their scalp. Eight of 10 patients in the sham stimulation group reported that they thought they had gotten active stimulation when explicitly asked at the end of the trial.
Data analysis
Data are presented as mean (±SD). Wilcoxon t was used for comparisons for data between baseline, after last session of rTMS, and 2 weeks after rTMS. Mann-Whitney U test and chi-square test were used to compare data between different groups of patients.
For all tests, significance level was set as P < .05, with Bonferroni correction for multiple comparisons.
Results
Table 1 sumarizes demographic and clinical characteristics of all patients. Both groups were homogeneous for age, gender, time since injury, etiology, and ASIA scale. LEMS was also homegeneous at baseline (Wilcoxon t, P = .068) for active and sham groups.
Table 1.
Clinical and demographical characteristics of patients
| rTMS | Sex | Age | Level oflesion | AIS | Time since injury(months) | Etiology |
| Active | M | 29 | T12 | D | 8 | Traumatic |
| Activea | M | 18 | T1 | D | 6 | Nontraumatic |
| Active | M | 47 | C6 | D | 8 | Traumatic |
| Active | M | 21 | C5 | D | 9 | Traumatic |
| Active | F | 51 | T7 | D | 12 | Nontraumatic |
| Active | M | 60 | T7 | D | 3 | Nontraumatic |
| Activea | M | 18 | T5 | D | 11 | Nontraumatic |
| Activea | M | 24 | C6 | D | 5 | Traumatic |
| Active | F | 40 | C4 | D | 8 | Traumatic |
| Active | M | 21 | T2 | D | 12 | Traumatic |
| Shama | M | 18 | T1 | D | 4 | Nontraumatic |
| Sham | M | 50 | C4 | D | 5 | Traumatic |
| Shama | M | 18 | T5 | D | 9 | Nontraumatic |
| Sham | M | 56 | C6 | D | 10 | Traumatic |
| Sham | M | 34 | T7 | D | 11 | Nontraumatic |
| Sham | M | 37 | T3 | D | 12 | Traumatic |
| Shama | M | 24 | C6 | D | 3 | Traumatic |
| Sham | F | 41 | T2 | D | 5 | Nontraumatic |
| Sham | M | 54 | T3 | D | 3 | Nontraumatic |
| Sham | F | 33 | C5 | D | 6 | Nontraumatic |
Note: AIS = American Spinal Cord Injury Association Impairment Scale; C = cervical; F = female; M = male; rTMS = repetitive transcranial magnetic stimulation; T = thoracic.
The 3 patients who received first sham stimulation and then received active stimulation after a washout of at least 3 weeks.
All patients tolerated the study without complications, and no adverse effects were reported, except for 6 patients who complained of twitching facial muscles during the first session of active stimulation.
Clinical assessment
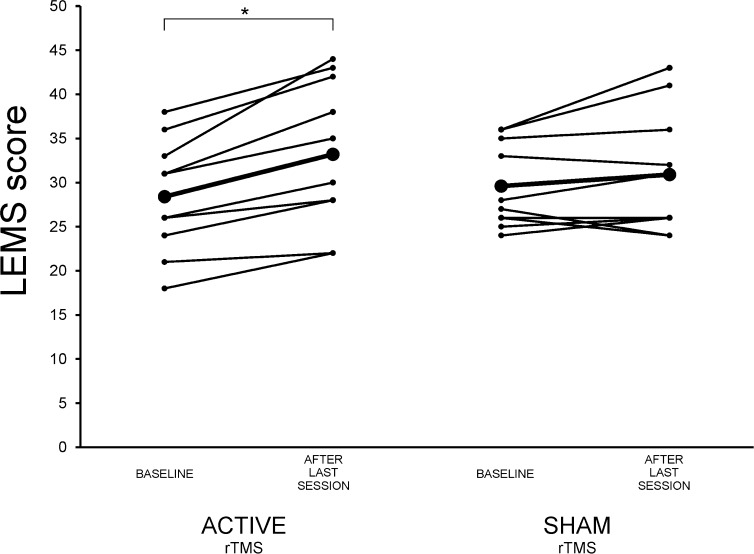
Total LEMS improved significantly at the end of the 15 rTMS sessions compared to baseline in active group (Wilcoxon t, P = .005), but not in sham group (Wilcoxon t, P = .258) (Figure 1).
Figure 1.
Change in lower extremities motor score (LEMS) from baseline to last repetitive transcranial magnetic stimulation (rTMS) session for each patient in active and sham groups. Mean values are shown in bold.
*Significant improvement was found in the active rTMS group.
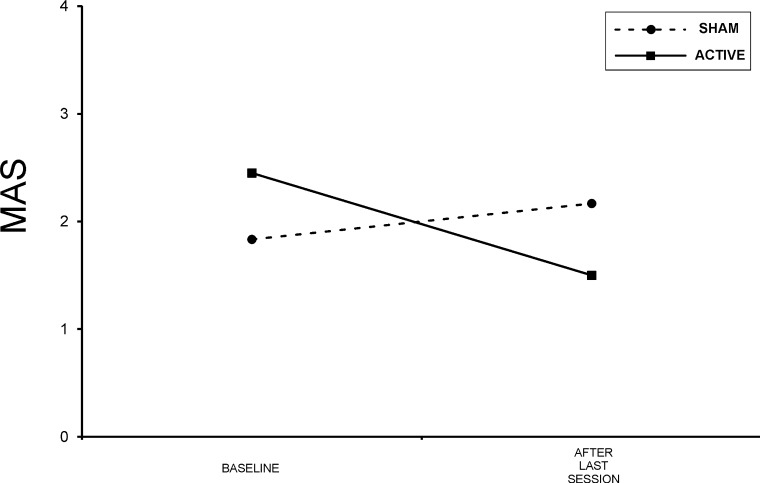
Patients undergoing active stimulation were found to have significantly less spasticity according to MAS at the end of the last rTMS session in comparison to their baseline (Wilcoxon t, P = .027) (Figure 2). However, there was no significant effect of sham stimulation on spasticity (Wilcoxon t, P = .066) (Figure 2).
Figure 2.
Group mean data for spasticity showing a significant improvement in the active repetitive transcranial magnetic stimulation (rTMS) group. MAS = modified Ashworth scale.
Functional (gait) assessment
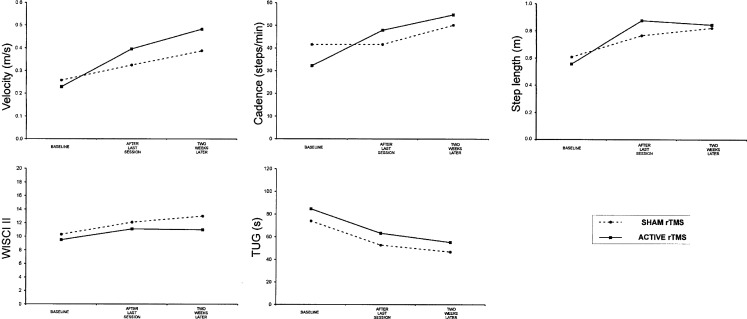
Velocity, cadence, step length, and TUG showed significant improvement in patients who received active rTMS at the end of the last rTMS session in comparison to baseline (Wilcoxon t, P = .005, P = .009, P = .013, and P = .017, respectively) (Figure 3). These effects were maintained 2 weeks after the last rTMS session. However, patients in the sham group only showed a significant improvement in step length and TUG after the last session of rTMS (Wilcoxon t, P = .018 and P = .043, respectively), and this effect was maintained 2 weeks later (Figure 3).
Figure 3.
Group mean data for functional outcomes showing a significant improvement in velocity, cadence, step length, and Timed Up and Go test (TUG) for the active repetitive transcranial magnetic stimulation (rTMS) group and for step length and TUG for the sham rTMS group. WISCI = Walking Index for Spinal Cord Injury.
No significant changes were observed in the WISCI II scale in either group (Wilcoxon t, P = .068 for active, and P = .109 for sham rTMS) (Figure 3).
Discussion
Our main finding is the improvement in muscle strength, spasticity, and gait following 15 daily sessions of real rTMS in patients with incomplete SCI. The functional gains were maintained for at least 2 weeks after the rTMS course.
Our findings expand those of Belci et al5 who reported an improvement in motor score and upper extremity function in 4 patients with chronic incomplete cervical SCI after applying 5 days of rTMS over the motor cortex. The authors suggested that rTMS modifies corticospinal projections by increasing motor cortical excitability, resulting in an alteration of segmental spinal excitability; this may be a core mechanism for the observed functional effects.
In a different population, Lomarev et al11 showed the use of rTMS to improve gait in Parkinson’s disease, with a lasting effect for at least 1 month. Those patients were receiving levodopa therapy. As a potential explanation of rTMS single-session effect, they propose the increase of motor cortex excitability and a caudate dopamine release. The mechanism is probably different for the long-lasting rTMS effect, and they suggest the possible role of enhanced postsynaptic dopamine agonist effect, the upregulation of NMDA receptors, or an increase in active synapses. This “dopamine theory” to explain the effects of rTMS in our SCI population probably does not apply. Maric et al12 demonstrated that levodopa therapy in incomplete SCI had no greater effect on clinical and functional outcomes than placebo and physiotherapy.
Patients with real rTMS also showed significant improvement in spasticity according to MAS. In a previous study, Kumru et al4 showed similar clinical effects of rTMS on spasticity in patients with incomplete SCI that lasted for at least 1 week following a 5-day rTMS course. Kumru et al4 propose that the decrease in spasticity induced by rTMS was due to an enhancement of descending corticospinal projections and segmental effect on spinal interneurones.
Other authors have suggested that, in the multiple sclerosis population, after repeated daily sessions of repetitive magnetic stimulation over the spinal cord13 or rTMS,14 long-lasting modulation of spinal circuits may be related to long-lasting depression-like mechanisms.
Therefore, we hypothesized that rTMS could modify corticospinal projections, resulting in decreased spasticity, better motor control, and functional gains in incomplete SCI subjects. This makes rTMS a promising rehabilitation tool in SCI population.
References
- 1.Curt A, Schwab ME, Dietz V. Providing the clinical basis for new interventional therapies: refined diagnosis and assessment of recovery after spinal cord injury. Spinal Cord. 2004;42(1):1–6 [DOI] [PubMed] [Google Scholar]
- 2.Ditunno JF, Jr, Burns AS, Marino RJ. Neurological and functional capacity outcome measures: essential to spinal cord injury clinical trials. J Rehabil Res Dev. 2005;42 (3 suppl 1):35–41 [DOI] [PubMed] [Google Scholar]
- 3.Dobkin B, Barbeau H, Deforge D, et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized Spinal Cord Injury Locomotor Trial. Neurorehabil Neural Repair. 2007;21(1):25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumru H, Murillo N, Samso JV, et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury [published online ahead of print January 6, 2010]. Neurorehabil Neural Repair. 2010;24 (5): 435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belci M, Catley M, Husain M, Frankel HL, Davey NJ. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord. 2004;42 (7): 417–419 [DOI] [PubMed] [Google Scholar]
- 6.American Spinal Injury Association International Standards for Neurological Classification of Spinal Cord Injury, revised 2002. Chicago, IL: Author; 2002 [Google Scholar]
- 7.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1986;67: 206–207 [DOI] [PubMed] [Google Scholar]
- 8.Ditunno JF, Jr, Barbeau H, Dobkin BH, et al. Validity of the walking scale for spinal cord injury and other domains of function in a multicenter clinical trial. Neurorehabil Neural Repair. 2007;21: 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossier P, Wade DT. Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil. 2001;82: 9–13 [DOI] [PubMed] [Google Scholar]
- 10.van Hedel HJ, Wirz M, Dietz V. Standardized assessment of walking capacity after spinal cord injury: the European network approach. Neurol Res. 2008;30(1):61–73 [DOI] [PubMed] [Google Scholar]
- 11.Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord. 2006;21 (3): 325–331 [DOI] [PubMed] [Google Scholar]
- 12.Maric O, Zörner B, Dietz V. Levodopa therapy in incomplete spinal cord injury. J Neurotrauma. 2008;25: 1303–1307 [DOI] [PubMed] [Google Scholar]
- 13.Nielsen JF, Sinkjaer T. Long-lasting depression of soleus motoneurons excitability following repetitive magnetic stimuli of the spinal cord in multiple sclerosis patients. Mult Scler. 1997;3: 18–30 [DOI] [PubMed] [Google Scholar]
- 14.Centonze D, Koch G, Versace Vet al. Repetitive transcranial magnetic stimulation of the motor cortex amilorates spasticity in multiple sclerosis. Neurology. 2007;68: 1045–1050 [DOI] [PubMed] [Google Scholar]