Abstract
Background and Purpose:
Gait deviations in individuals after incomplete spinal cord injury (ISCI) that are quantified using spatiotemporal (ST) parameters are often targeted during therapeutic interventions. The purpose of our study was to establish reliability and responsiveness of ST parameters of gait after ISCI using an instrumented walkway (GaitMat II).
Methods:
Sixteen individuals with ISCI participated in the study. Each subject completed at least 2 walking trials at self-selected (SS) walking speed. Intraclass correlation coefficients model 2, 1 (ICC2,1) with 95% confidence intervals (CIs), standard error of measurement (SEM), SEM percent change (SEM%), the minimal detectable change (MDC), and the MDC percent change (MDC%) were determined for 8 ST parameters including step length, single limb support, and double limb support time for the more and less impaired limb, cadence, and speed.
Results:
Excellent test-retest agreement (0.84-0.99) was observed in all ST parameters. SEM% ranged from 8% to 29%, while MDC% ranged from 21% (cadence) to 80% (double limb support time). MDC% values were relatively higher (5-12 MDC%) for the more versus less impaired limb.
Discussion:
SEM% results indicate that small to moderate changes were needed to indicate a real change in walking performance. Differences in MDC% values between limbs indicated that variability in parameters might be sensitive to level of motor impairment.
Conclusion:
In individuals with ISCI, different gait, balance, or strength training programs can be compared and contrasted based on a quantifiable and meaningful change in the ST parameter of interest.
Keywords: gait, intraclass correlation coefficient, measurement, minimal detectable change, spinal cord injury
After incomplete spinal cord injury (ISCI), some individuals are able to ambulate independently, although they often present with reduced gait speeds, asymmetrical stepping patterns, and poor balance.1 Gait deviations in these individuals are often quantified using spatiotemporal (ST) parameters, and therapeutic interventions are targeted at improving these measures.2 To assess the efficacy of clinical interventions, it is imperative to identify and accurately measure initial ST parameters of gait and their alterations following interventions.3
Development of instrumented walkways that can rapidly measure ST gait parameters eliminates the need for manual assessment, thereby decreasing the likelihood of random human error.4 Intrarater and test-retest reliability have been established with walkways such as the Gaitrite (CIR Systems, Inc, Havertown, Pennsylvania) and GaitMat II (EQ, Inc, Chalfont, Pennsylvania) in several neurological populations, but not yet in individuals with ISCI.4-6 These individuals have a range of neuromuscular impairments leading to marked variability in gait deviations, which necessitates independent investigation.1
In addition to issues of reliability of ST parameters, investigators have recently directed attention to estimating how changes in various gait parameters indicate clinically relevant differences in walking ability.7 Currently, identifying clinically relevant changes in walking function is often a matter of subjective interpretation for clinicians and researchers and lacks objective definition and quantification. Detailed assessment of the reproducibility of ST parameters of gait, by examining relative and absolute reliability of these measures, will help identify the parameters that are invariant from one test occasion to another versus those that are amenable to change with repeated measurement. Relative reliability, which examines the relationship between multiple repeated measurements, can be obtained by calculating the intraclass correlation coefficient (ICC). Absolute reliability, which describes the within-subject variability attributable to repeated measures, is estimated by calculating the standard error of measurement (SEM). The SEM then can be used to obtain the minimal detectable change (MDC) defined as the minimal amount of change that is required to distinguish a true performance change from a change due to variability in performance or measurement error.
Other researchers have investigated the MDC in various neurological populations performing specific upper and lower extremity tasks.8 Such data provide a foundation for identifying the minimal thresholds of alterations in motor activity with recovery following neurological injury or following a specific intervention. Determination of the MDC will therefore improve the interpretation of the relative changes observed in specific ST parameters and allow the clinician to draw more definitive conclusions regarding the clinical importance of a change post intervention in individuals with ISCI.
The purpose of this study was 2-fold. First, we wished to assess test-retest reliability, SEM, and SEM percent change (SEM%) of clinically relevant ST gait parameters in persons with ISCI using the GaitMat II. Our second goal was to determine MDC and MDC percent change (MDC%) in each ST parameter.9 The SEM estimates the measurement error across repeated measurements for a group of individuals, while the SEM% indicates measurement error independent of the units of measurement. Similarly, the MDC and MDC% quantify responsiveness of the GaitMat II and provide the absolute and relative magnitude of change necessary to exceed the measurement error of 2 or more repeated measures at a specified confidence interval (CI).9 We focused on commonly studied ST parameters for persons with ISCI, such as step length, cadence, speed, and single limb support and double limb support time with additional emphasis placed on gait speed, which is a commonly reported outcome measure.10-13 Clinicians may use this information to distinguish a true performance change from an observed change due to variability in performance or measurement error.14,15
Method
Participants
Sixteen adults with ISCI (3 women, mean age 41 ± 11 years; 3-88 months post injury) participated in the study. Inclusion criteria for subjects included (1) age of 18 years or older; (2) diagnosis of firsttime SCI, including etiology from trauma, vascular, or orthopedic pathology at cervical or thoracic levels; (3) 3 months post SCI; (4) SCI defined by the American Spinal Injury Association Impairment Scale (AIS) as category C or D; (5) medically stable condition; (6) documented medical approval from the subject’s personal physician; (7) ability to ambulate at least 10 ft with or without an assistive device (and/or contact guard); (8) minimum of 30 minutes (total) each day spent standing or walking; and (9) ability to give informed consent. Exclusion criteria for subjects included (1) current participation in a rehabilitation program or another research protocol that could interfere with the outcome measures of our study and (2) history of congenital SCI or other degenerative spinal disorders. Subject demographics are listed in Table 1. The study protocol was approved by the University of Florida and the Northwestern University Institutional Review Board. All subjects provided informed consent prior to participation.
Table 1. Subject characteristics.
| Subject | Sex/Age, years | Time since injury, months | AIS | More impaired side | Assistive device used |
| 1 | M/43 | 35 | D | L | RPW |
| 2 | M/23 | 84 | D | R | Bilateral Loftstrand |
| Crutches | |||||
| 3 | M/55 | 3 | D | R | RW + AFO |
| 4 | F/46 | 10 | D | L | Cane + AFO |
| 5 | F/23 | 20 | C | R | RPW + AFO |
| 6 | M/21 | 12 | D | R | None |
| 7 | M/42 | 88 | D | L | None |
| 8 | M/52 | 4 | D | R | SBQC |
| 9 | M/48 | 21 | D | L | SC |
| 10 | M/51 | 3 | C | L | None |
| 11 | M/37 | 8 | D | R | Walker |
| 12 | M/45 | 5 | D | L | Walker |
| 13 | M/58 | 28 | D | L | Walker + Bilateral AFO |
| 14 | M/36 | 3 | D | R | SC |
| 15 | F/43 | 9 | D | L | None |
| 16 | M/47 | 8 | D | L | SC |
Note: AIS = American Spinal Injury Association Impairment Scale; RPW = rolling platform walker; RW = rolling walker; AFO = ankle foot orthosis; SBQC = small base quad cane; SC = straight cane; M = male, F = female, R = right, L = left.
Procedure
Each subject completed at least 2 walking trials over the GaitMat II at self-selected (SS) walking speed. To maintain steady-state gait speed, subjects began and stopped walking approximately 2 to 3 ft from the ends of the walking platform. Subjects were guarded during walking trials if safety was a concern, and they were provided a rest break in between trials as needed. To estimate test-retest reliability, the testing procedure was repeated after 1 to 2 weeks. A blinded physical therapist (PT) analyzed the data as described previously. 5 To further examine the influence of impairment severity on the ST parameters, the selected gait parameters (step length, single and double limb support time) were grouped according to the more and less involved limb based on lower extremity motor scores (LEMS).
Statistical analyses
Test-retest reliability, a measure of relative reliability, was estimated using the intraclass correlation coefficients model 2,1 (ICCs2,1) for the ST parameters of gait. A 95% confidence interval (95% CI) was constructed around the ICC 2,1 point estimate for each parameter. To interpret ICC2,1 values, benchmarks suggested by Fleiss were used.16 According to Fleiss’ classification, an ICC value above 0.75 indicates excellent reliability, between 0.40 and 0.75 are fair to good, and less than 0.40 indicates poor reliability.
Absolute reliability was assessed first by creating Bland Altman plots that reveal systematic changes in the mean values for each ST parameter and then by calculating the SEM and SEM%. The Bland Altman plots were constructed by plotting the between-session difference versus the mean value of the 2 sessions for each variable. These plots along with the 95% CI calculated for the between-session differences were then used to visualize systematic variations across the zero line. The 95% CI was calculated as the between session difference ± 1.96 multiplied by the standard error of the between session difference. The SEM accounts for within-subject variability and assesses how precisely a test measures a subjects true score.17 SEM was calculated by the square root of the within-subjects error variance (SEM = ).8 The SEM% was defined as (SEM/) x 100, where is the mean for all observations from test session 1 and 2. The SEM values obtained were utilized to measure MDCs for each ST parameter of gait.
The MDC can be used to assess the minimal magnitude of change required to be 95% confident that the observed change between the 2 tests reflects true change and not measurement error.18 The MDC was calculated as: 1.96 x SEM x . To calculate MDC independent of the units of measurement, the MDC% was defined as (MDC/) x 100.8 Significance was set at a = 0.05. Analyses were performed using SPSS 12.0 software for Windows (SPSS Inc., Chicago, Illinois).
Results
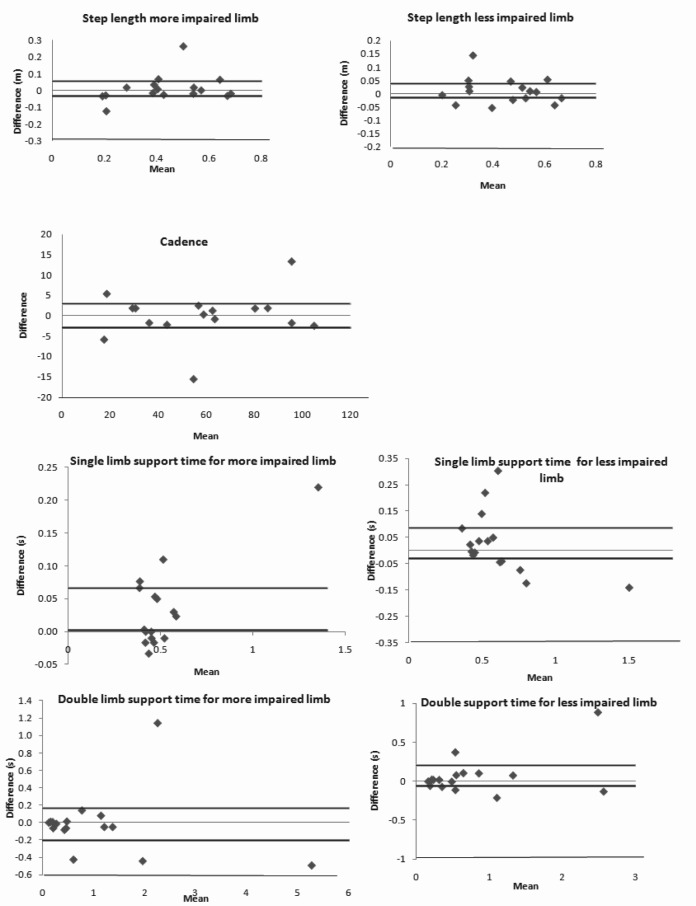
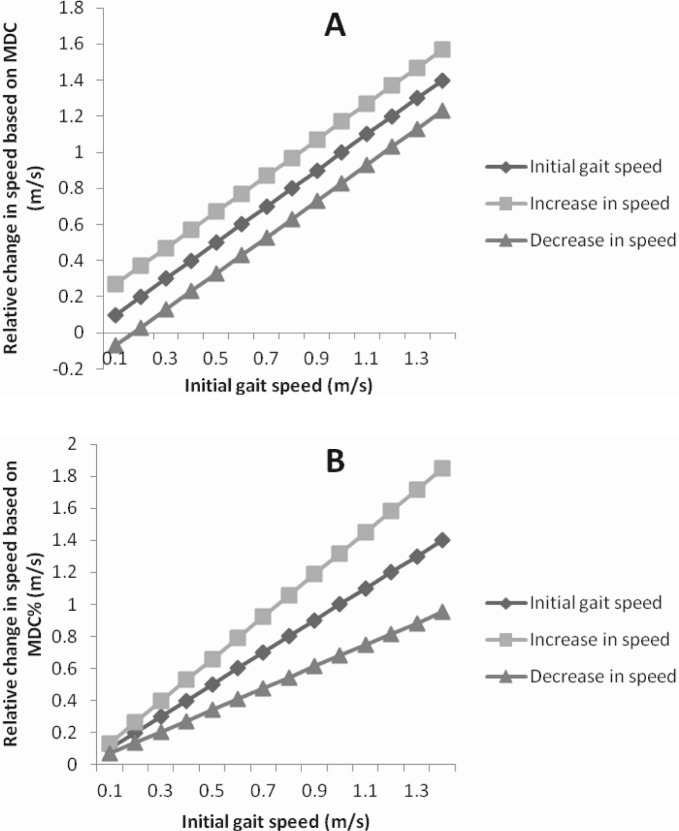
ICC2,1 values for the ST parameters based on impairment severity are listed in Table 2. All parameters demonstrated excellent agreement with ICC2,1 values ranging from 0.83-0.97 (95% CI, 0.72-.98). For gait speed, ICC2,1 was excellent (0.97). Bland Altman analysis revealed no systematic variance in performance from one session to the next for all of the ST parameters except for single limb support time on the more impaired limb (Figure 1). Given the values of the between-session difference, subjects spent slightly longer time in single limb support on the more impaired limb in the second session compared to the first. Furthermore, the plots reveal non-uniform variability across the means for single limb support time on the less impaired limb indicating that higher values of the mean suggest greater variability between the test sessions. The SEM, SEM%, MDC, and MDC% values for the ST parameters are also listed in Table 2. In general, the SEM% ranged from 8% to 29%, while MDC% ranged from 21% (cadence) to 80% (double limb support time). For gait speed, SEM% and MDC% were 12% and 32%, respectively. Figure 2 represents the MDC and MDC% changes in gait speed plotted against the initial gait speed, illustrating how the MDC% is effective in quantifying relative improvements in individuals with different initial walking speeds. It is interesting to note that the MDC% changes for some of the variables were relatively higher (eg, 5-12 MDC%) for the more impaired limb compared to the less impaired limb, indicating that the variability of measurements might be sensitive to the level of motor impairment.
Figure 1.
The Bland Altman plots the difference between the first and the second session against the mean of the 2 sessions for the ST parameters. The plots for step length, single limb support time, double limb support time, and for cadence demonstrate no systematic variation in group performance between sessions for all the ST parameters except for single limb support time on the more impaired limb and non-uniform relationships between the mean and the difference between the sessions for single limb support time on the less impaired limb. Solid black line represents the 95% confidence interval for the difference and the grey line represents zero.
Figure 2.
Graphical representation of a detectable increase or decrease in walking speed for a range of initial gait speeds in individuals with incomplete spinal cord injury when utilizing (A) minimal detectable change (MDC) values compared to (B) the MDC percent change (MDC%) values. While utilizing MDC values, the magnitude of change required for determining a clinically reasonable increase or decrease in walking speed is constant across initial walking speeds. In contrast, a detectable magnitude of change in walking speed is relative to the initial walking speed while considering MDC%.
Table 2. Values for the spatiotemporal (ST) parameters of gait at self-selected walking speed in individuals with incomplete spinal cord injury.
| Gait parameters | ICC2, 1 | 95% CI | SEMa | SEM% | MDCa | MDC% |
| Speed, m/s | 0.97 | (0.94,0.98) | 0.06 | 12 | 0.17 | 32 |
| Step length – MIL, m | 0.83 | (0.72,0.91) | 0.06 | 13 | 0.17 | 36 |
| Step length – LIL, m | 0.89 | (0.81,0.94) | 0.04 | 9 | 0.11 | 24 |
| Cadence, steps/min | 0.97 | (0.95,0.98) | 4.73 | 8 | 13 | 21 |
| Single limb support time – MIL, s | 0.86 | (0.76,0.92) | 0.08 | 15 | 0.22 | 42 |
| Single limb support time – LIL, s | 0.84 | (0.73,0.91) | 0.1 | 17 | 0.28 | 47 |
| Double limb support time – LIL, s | 0.96 | (0.93,0.98) | 0.25 | 29 | 0.69 | 80 |
| Double limb support time – LIL, s | 0.93 | (0.87,0.96) | 0.19 | 24 | 0.53 | 68 |
Note: ICC2, 1 = intraclass correlation coefficients, model (2, 1); LIL = less impaired limb; MDC = minimal detectable change; MIL = more impaired limb; 95% CI = 95% confidence interval; SEM = standard error of measurement.
Provided in units of each parameter meters (m), seconds (s), or m/s.
Discussion
The present study examined the reproducibility and responsiveness of ST parameters of gait in individuals with ISCI. ICCs for test-retest reliability were good to excellent (>0.70) for all parameters, indicating that clinicians and researchers can measure the effect of an intervention without the substantial influence of labile measurements.
Bland Altman plots were constructed to evaluate systematic variance in ST parameters between sessions. Our data revealed that individuals spent slightly longer time in single limb support on the more impaired limb in the second versus the first testing session. It is important to note that the magnitude of the difference between the sessions were closer to zero suggesting that the systematic variance might not be due to a learning (testing) effect from one session to another. However, given the heterogeneity of the individuals in our study, it is likely that this variability could be due to one of several reasons including the type of assistive device used and/or the duration of injury. Closer analysis of the data revealed that the 5 individuals who demonstrated this difference between the testing sessions ranged between 3 to 35 months post injury and consisted of individuals with and without assistive devices. Therefore, the systematic variance observed in the Bland Altman plot for the single limb support time on the more impaired limb might not be due solely to the use of certain assistive devices and/or the subacute or chronic injury status of the individuals but could be due to a combination of these factors.
The plots also revealed non-uniform variability in the single limb support time for the less impaired limb. Further examination of the data revealed that greater differences were observed in the individuals who walked with either a walker or a rolling walker or a rolling platform walker, had a slow walking speed (less than 0.3 m/s), and spent longer time in single limb support on the less impaired limb. Thus, temporal variables may be more sensitive to the degree of motor impairment and to the type of assistive device used. Overall, our data suggest that the variability observed in our single limb support time plots might be due to the heterogeneity of our sample. A larger homogeneous sample of individuals with similar assistive devices, duration of injury, and motor impairment status might yield consistent results from one testing session to another compared to our findings.
Along with Bland Altman plots, the SEM was used to assess the measurement error of the ST parameters and MDC to detect the minimal threshold of change above the 95% CI for each ST parameter. These measures represent the limit for the smallest change that indicates a real or detectable improvement for individuals with ISCI. Small values of SEM for the gait parameters indicate that measurements made by GaitMat II were stable and reproducible over time thereby implying precision in measurement.15 SEM and SEM% have not been previously reported for this clinical population but the range of SEM% values (8%-29%) are comparable to those reported for gait performance measures in hemiparetic individuals.8 These results indicate that small to moderate changes were needed to indicate a real change in walking performance for a group of subjects with ISCI. Furthermore, the calculated MDC values for gait speed in individuals with ISCI from our study are comparable to those reported for individuals with chronic stroke (0.15 to 0.25 m/s)8 and Parkinson’s disease (0.18 m/s). 19
The calculated MDC% provides an assessment of a relative improvement or deterioration in the value of a parameter and would be beneficial to the clinician to determine whether performance has truly changed over time. Therefore, for a heterogeneous population of individuals with ISCI, the MDC% accounts for the variability across the population. For example, the MDC change for walking speed is 0.17 m/s, which represents a MDC% change of 32%. For an individual walking at 0.2 m/s at pre test, an improvement to 0.37 m/s would satisfy the requirements of the calculated MDC using absolute values, although this would represent a nearly 2-fold improvement in walking speed. In contrast, use of the MDC% would require only an improvement of 0.07 m/s for a detectable change in walking speed, which reflects a more realistic improvement during a brief bout of physical therapy. In contrast, for an individual walking at an initial SS speed of 0.8 m/s, an improvement of 0.17 m/s (ie, absolute MDC) would reflect a reasonable increase in walking speed as determined in previous studies in neurologically impaired populations following a brief (4 week) training period.20, 21 Thus, a true change in the parameter of interest can be reasonably determined not only by assessing statistical significance, but also by incorporating the MDC or MDC% into clinical decision making.
However, as Beckerman et al suggested, the MDC is a clinimetric property of a measurement tool and could be different from what clinicians and researchers judge as a clinically relevant change.15 Researchers and clinicians might deem an intervention to be beneficial based on its multifactorial impact across the continuum of health, functioning, and disability. Perera et al22 for example accounted for distribution-based methods that rely on the psychometric properties of a measure to define an MDC and anchorbased methods that rely on using the patient’s or provider’s perception of change as an external anchor to determine the corresponding magnitude of change in gait speed. According to their findings, the best initial estimate of small and substantial change in gait speed for community-dwelling older adults, adults with mobility issues, and a subacute stroke cohort was approximately 0.05 to 1.0 m/s.22 Similarly, Schmid et al examined the changes in velocity-based, community ambulation categories in relation to clinically meaningful changes in stroke-related function and quality of life.23 They reported that an increase in gait velocity that resulted in a transition to a higher class of ambulation resulted in better function and quality of life, especially for household ambulators. Similarly, for individuals with ISCI and other neurological conditions with gait dysfunction, an intervention may still be considered to be clinically meaningful if, for example, it led to a change in the assistive device used for walking or improved a person’s community ambulation, although there may have been no change in walking speed.24 Therefore, further research is needed to explore the correspondence between MDC and a clinically relevant change in individuals after ISCI so as to optimize outcome measurement post intervention.
ST parameters such as step length, single limb support, and double limb support time exhibited different MDC and MDC% values for the more and less impaired limbs. Since the impairment level for each limb was quantified based on the LEMS, the different values might imply that the change in magnitude of these ST parameters as a result of an intervention might correlate with a change in impairment status. Parameters such as speed or cadence that quantify overall gait performance alone might not be sensitive to a change in impairment status. The cumulative assessment of all the ST parameters might therefore reflect on the impairment status of the individual. Clinicians could use these parameters to their benefit by correlating them to the observed change in functional status or the quality of life post intervention.
Limitations
Our study has several limitations. First, the sample size for our analysis was small. It is possible that our results would have differed with a larger sample. Second, our sample was heterogeneous with regard to the type of assistive devices used for walking and the duration of injury (ie, subacute or chronic). It is possible that individuals in our study who were 3 months post injury or in the subacute phase of recovery might demonstrate true changes in ST parameters that might erroneously be included in the measurement variability from one testing session to another. A homogeneous sample of individuals with chronic injury would have been ideal for our study. Third, the type of assistive device used influenced the variability observed in the single limb support time for the less impaired limb. Fourth, although we have considered the ST parameters that are commonly used by clinicians, the results of our reliability analysis are not generalizable to other ST parameters. Therefore, although our sample is representative of the heterogeneous group of individuals after ISCI who demonstrate variable degrees of walking ability and are typically recruited for gait training, the heterogeneity of the group might threaten external validity of our study.
Conclusion
The present study utilizes a comprehensive set of statistical tools and serves as a first step in assessing the reliability and responsiveness of commonly assessed ST parameters of walking in a heterogeneous group of individuals with ISCI. A larger, homogeneous sample based on type of assistive device used and duration of injury will help further define MDC values for this population. Different gait training, balance, or strength training programs can thus be compared and contrasted based on a quantifiable and meaningful change in the parameter of interest.
Clinical message
A working knowledge of the MDC values for specific ST parameters will be useful for clinicians and researchers to determine whether an intervention has truly changed walking performance in individuals with ISCI.
Acknowledgments
We declare no proprietary, financial, professional, or any other personal interest of any kind in any product, service, or company that can be construed as influencing the subject of the work presented in or in the review of this article.
We thank all the volunteers who were subjects in this study and Dr. Mark Bishop for his helpful comments on the paper. We also acknowledge the financial support provided by the National Institute of Health/National Center for Medical Rehabilitation Research (grant K01 HD 01348-01) (PI: Behrman) and the National Institute of Health/National Institute of Child Health and Human Development (grant R21-HD046876-01A1) (PI: Hornby).
References
- 1.Van der Salm A, Nene AV, Maxwell DJ, Veltink PH, Hermens HJ, Ijzerman MJ.Gait impairments in a group of patients with incomplete spinal cord injury and their relevance regarding therapeutic approaches using functional electrical stimulation. Artificial Organs. 2005;29 (1): 8–14 [DOI] [PubMed] [Google Scholar]
- 2.Field-Fote EC, Fluet GG, Schafer SD, et al. The Spinal Cord Injury Functional Ambulation Inventory (SCI-FAI). J Rehabil Med. 2001;33 (4): 177–181 [DOI] [PubMed] [Google Scholar]
- 3.Dobbs RJ, Charlett A, Bowes SG, et al. Is this walk normal? Age Ageing. 1993;22 (1): 27–30 [DOI] [PubMed] [Google Scholar]
- 4.Pomeroy VM, Chambers SH, Giakas G, Bland M.Reliability of measurement of tempo-spatial parameters of gait after stroke using GaitMat II. Clin Rehabil. 2004;18 (2): 222–227 [DOI] [PubMed] [Google Scholar]
- 5.Barker SP.Changes in gait, balance, and function with vestibular rehabilitation [Biomed PhD]. School of Biomedical Engineering, Science and Health Systems, Drexel University; 2004 [Google Scholar]
- 6.Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture. 2004;20 (1): 20–25 [DOI] [PubMed] [Google Scholar]
- 7.Lewek MD, Randall EP.Reliability of spatiotemporal asymmetry during overground walking for individuals following chronic stroke. J Neurol Phys Ther. 2011;35 (3): 116–121 [DOI] [PubMed] [Google Scholar]
- 8.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J.Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37: 75–82 [DOI] [PubMed] [Google Scholar]
- 9.Haley SM, Fragala-Pinkham MA.Interpreting change scores of tests and measures used in physical therapy. Phys Ther. 2006;86 (5): 735–743 [PubMed] [Google Scholar]
- 10.Melis EH, Torres-Moreno R, Barbeau H, Lemaire ED.Analysis of assisted-gait characteristics in persons with incomplete spinal cord injury. Spinal Cord. 1999;37 (6): 430–439 [DOI] [PubMed] [Google Scholar]
- 11.van Hedel HJ, Dietz V, Curt A.Assessment of walking speed and distance in subjects with an incomplete spinal cord injury. Neurorehabil Neural Repair. 2007;21 (4): 295–301 [DOI] [PubMed] [Google Scholar]
- 12.Visintin M, Barbeau H.The effects of body weight support on the locomotor pattern of spastic paretic patients. Can J Neurol Sci. 1989;16 (3): 315–325 [DOI] [PubMed] [Google Scholar]
- 13.Gardner MB, Holden MK, Leikauskas JM, Richard RL.Partial body weight support with treadmill locomotion to improve gait after incomplete spinal cord injury: a single-subject experimental design. Phys Ther. 1998;78 (4): 361–374 [DOI] [PubMed] [Google Scholar]
- 14.Guyatt G, Walter S, Norman G.Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis. 1987;40 (2): 171–178 [DOI] [PubMed] [Google Scholar]
- 15.Beckerman H, Roebroeck ME, Lankhorst GJ, Becher JG, Bezemer PD, Verbeek AL.Smallest real difference, a link between reproducibility and responsiveness. Qual Life Res. 2001;10 (7): 571–578 [DOI] [PubMed] [Google Scholar]
- 16.Fleiss JL.Reliability of measurement In: The Design and Analysis of Clinical Experiments. New York: Wiley; 1986: 1–32 [Google Scholar]
- 17.Portney LG, Watkins MP.Foundations of Clinical Research. 2nd ed. Upper Saddle River, NJ: Prentice- Hall, Inc; 2000 [Google Scholar]
- 18.Stratford PW, Binkley J, Solomon P, Finch E, Gill C, Moreland J.Defining the minimum level of detectable change for the Roland-Morris questionnaire. Phys Ther. 1996;76 (4): 359–365 [DOI] [PubMed] [Google Scholar]
- 19.Steffen T, Seney M.Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88 (6): 733–746 [DOI] [PubMed] [Google Scholar]
- 20.Sullivan KJ, Knowlton BJ, Dobkin BH.Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002;83 (5): 683–691 [DOI] [PubMed] [Google Scholar]
- 21.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR.Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. 2008;39 (6): 1786–1792 [DOI] [PubMed] [Google Scholar]
- 22.Perera S, Mody SH, Woodman RC, Studenski SA.Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54 (5): 743–749 [DOI] [PubMed] [Google Scholar]
- 23.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38 (7): 2096–2100 [DOI] [PubMed] [Google Scholar]
- 24.Bowden MG, Hannold EM, Nair PM, Fuller LB, Behrman AL.Beyond gait speed: a case report of a multidimensional approach to locomotor rehabilitation outcomes in incomplete spinal cord injury. J Neurol Phys Ther. 2008;32 (3): 129–138 [DOI] [PubMed] [Google Scholar]